Abstract
In December 2016, the Union for International Cancer Control (UICC) published the 8th edition of the Tumor-Node-Metastasis (TNM) classification of malignant tumors, including a number of vital changes in the definitions of the T2 category, the N category and the stages of gallbladder cancer (GBC). The clinical value of this newly updated classification in patients with surgically treated GBC has not been rigorously validated. The present study aimed to analyze the prognosis of patients with GBC in a high-volume surgical unit, and to validate the prognostic value of the new UICC TNM classification, particularly the main changes in the stages of GBC. Data from 307 patients who were surgically treated and histopathologically diagnosed with GBC between January 2011 and July 2016 in The West China Hospital (Chengdu, Sichuan, China) were retrospectively collected and analyzed. The new UICC criteria distributed 32, 60, 99 and 116 eligible patients in stages I, II, III and IV, respectively. The differences in overall survival time between each stage (I–IV) demonstrated statistical significance (P<0.05). As a result of the main change of this classification, the novel definitions of T2a and T2b effectively stratified the prognosis of patients with T2 GBC (P<0.001). Furthermore, patients with stage IIa tumors also obtained significantly improved overall survival time compared with patients with stage IIb tumors (P=0.04), whereas the comparison between patients with stage IIb and IIIa tumors did not present any notable difference (P=0.20). Additionally, the new N category stratified the survival of the patients effectively (P<0.001). Together with curative resection, this latest classification was indicated to be an independent predictor of survival via multivariate analysis (hazard ratio, 6.25; 95% confidence interval, 3.81–10.26; P<0.001). In conclusion, the newly updated UICC TNM classification could effectively reflect the clinical outcome of patients with surgically treated GBC. Furthermore, tumor location could predict the survival of surgically treated T2 GBC. The novel classification of the N category by the number of lymph nodes involved was also demonstrated to be valid.
Keywords: gallbladder cancer, Union for International Cancer Control Tumor-Node-Metastasis classification, 8th edition, prognosis, tumor location
Introduction
Gallbladder cancer (GBC) is relatively rare among all the gastrointestinal tract cancer types, but is lethal, and can be characterized by a poor prognosis and lack of effective adjuvant therapy (1,2). However, worldwide, GBC is the most common and most aggressive malignant tumor of the biliary tract (3), with the shortest median survival time (MST) following diagnosis (4). The epidemiology of GBC varies widely, and the disease appears to be much more common in Japan, India and Chile (5,6). With 2.7% of all cases of biliary tract disease, the western area of China has witnessed an increase in the incidence of GBC in the last decade (7).
Aggressive surgical resection is the only potential curative treatment of this disease. With regard to surgical patients, the 5-year survival rate has improved from 5% to almost 38% in the last two decades (8–10). Surgery types and staging are considered to be two important prognostic factors (2,11), and appropriate surgical intervention may be estimated through use of the appropriate Tumor-Node-Metastasis (TNM) classification (12). Surgical styles ranging from simple cholecystectomy to hemi-hepatectomy should be adapted to different stages of GBC (13,14). For instance, simple cholecystectomy is the optimal choice for T1a GBC, whilst for T4 GBC, the efficiency of aggressive surgical resection has not been generally accepted (15,16). Due to the rarity of GBC, the correct type of surgery for advanced GBC and details certain of the TNM classification remain controversial (14,16–18).
As with the majority of other cancer types, the Union for International Cancer Control (UICC) classification stratifies GBC based on the evaluation of tumor depth of invasion (T stage), and metastasis to regional lymph nodes (N stage) and distant organs (M stage). In December 2016, the 8th edition of the UICC TNM Classification of malignant tumors was published, including vital changes in the T2 category, the N category and the stages of GBC (19).
As a reasoned and accepted classification, the UICC classification has been applied to the evaluation of outcomes from different institutions (19). Furthermore, it is practical to validate the predictive value of the newly updated UICC 8th edition from different institutions and regions of the world. To the best of our knowledge, the current study represents the first attempt to validate the latest UICC TNM classification of GBC. Based on the data of the eligible patients in The West China Hospital (Chengdu, China), the aim of the present study was to analyze the clinical characteristics of patients with surgically treated GBC by applying the newly updated UICC criteria in a high-volume surgical unit, to validate the potential predictors and to assess the prognostic value of the 8th edition TNM classification of GBC by survival analysis.
Patients and methods
Patient selection
Between January 2011 and July 2016, there were 413 patients with the diagnosis of GBC at The West China Hospital, and 337 of these had been surgically treated. All types of resection margins (R0, R1 and R2) and all cases with palliative surgeries were included in the study cohort, whereas those without surgical intervention at The West China Hospital were not enrolled. Parameters, including the demographics, laboratory examinations, surgical data and pathological diagnosis reports on the patients, were retrospectively collected. Postoperative morbidity and mortality were also retrospectively reviewed from the patient medical records, and patients with postoperative in-hospital mortality (6 patients) were excluded from further evaluation in order to focus on malignancy-associated outcome. A total of 24 patients were lost to follow-up. Thus, a total of 307 patients were considered available for survival analyses.
Tumor characteristics and definition of tumor location
The diagnosis of GBC was confirmed by pathologists via immunohistochemical staining of a surgical specimen or biopsy sample. Tumor features, including quantity, size, location, lymph node invasion, distant metastasis and surgical margin, were based on intraoperative data and pathological analyses. The N and M factors were clinically determined on the basis of either histopathological data or imaging modalities. The new 8th edition UICC TNM classification (19) was used to assess the clinical outcome of the patients with GBC.
The location of T2 and T3 tumors was defined histopathologically according to the study by Shindoh et al (18). For T2 GBC, the tumors were classified as being located on the peritoneal side when a tumor infiltrated only the free serosal side of the gallbladder, and on the hepatic side when at least part of a tumor infiltrated the region of the gallbladder wall attached to the liver. For T3 GBC, tumors were classified as being located on the hepatic side when at least a part of a tumor invaded directly into the liver parenchyma. All other tumors (without direct invasion into the liver parenchyma) were classified as being located on the peritoneal side regardless of the distribution of the tumor within the gallbladder wall.
Surgical procedures
Surgical resection was divided into two types: Curative resection and palliative surgery. Curative resection included simple cholecystectomy (only for stage I), known as the ‘standard’ resection for GBC, and the extended resections for GBC. Standard resection refers to the cholecystectomy plus combined resection of the bile duct and/or liver bed resection. Extended resections included the following surgical procedures: Hepatectomy more than subsegmentectomy; pancreaticoduodenectomy; hepatopancreaticoduodenectomy. Radical resection was achieved if R0 resection was performed based on different stages. Palliative surgeries, including biliary diversion, explorative laparotomy and gastrointestinal bypass, were performed for patients with T4 GBC or for those in a poor general condition.
Follow-up and survival
Telephone calls, office visits and outpatient clinic appointments were conducted for follow-up of the remaining 307 eligible patients between December 2016 and March 2017 for all patients, providing a potential follow-up time in months. Patients who were lost to follow-up were not enrolled in the present study. Overall survival (OS) time was defined as the number of months from the date of resection to the time of mortality or last contact (March 2017). Cases with mortality classified as not being associated with GBC were also excluded when selecting the patients.
Statistical analyses
Continuous data are expressed as mean ± standard error of the mean. Categorical data are presented as numbers and their frequencies as proportions (%), which were compared by Pearson χ2 tests wherever possible. Kaplan-Meier curves were plotted and log-rank tests were performed to analyze and compare OS. Multivariate analyses were finally applied to assess the prognostic value of UICC 8th edition staging for GBC and other potential predictors using Cox regression proportional hazards model. P<0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed by IBM SPSS 21.0 statistical software (IBM, Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 307 eligible and consecutive patients who were surgically treated and histologically diagnosed with GBC between January 2011 and July 2016 in The West China Hospital were enrolled in the present study. Clinicopathological data, including demographics, surgical procedures, tumor characteristics and staging, are summarized in detail in Table I. The analyses consist of 109 males (35.5%) and 198 females (64.5%), with a median age at initial diagnosis of 60 years (range, 24–96 years). A total of 160 (52.1%) patients underwent macroscopic curative resection and the remaining 147 (47.9%) patients underwent palliative surgery. The majority of the patients in the present study were classified as stage III and IV (70.1%).
Table I.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Male | 109 (35.5) |
| Female | 198 (64.5) |
| Median age in years at diagnosis (range) | 60 (24–96) |
| History of gallstones, n (%) | 122 (39.7) |
| Preoperative tumor markers, mean ± SEM | |
| AFP in ng/ml | 22.5±6.8 |
| CEA in ng/ml | 23.3±4.3 |
| CA19-9 in U/ml | 281.1±21.6 |
| Surgical procedure | |
| Macroscopically curative resection | 160 (52.1) |
| Palliativesurgery | 147 (47.9) |
| Ta by UICC 8th edition | |
| 1 | 32 (10.4) |
| 2 | 82 (26.7) |
| 3 | 114 (37.1) |
| 4 | 79 (25.7) |
| Staging by UICC 8th edition | |
| I | 32 (10.4) |
| II | 60 (19.5) |
| III | 99 (32.3) |
| IV | 116 (37.8) |
T factor was clinically determined on the basis of histopathological data combined with surgical data and radiographic evaluation. SEM, standard error of mean; UICC, Union of International Cancer Control; T, primary tumor; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; SEM, standard error of the mean.
T2 and T3 tumor location
As depicted in Table II, T2 and T3 patients were assigned through the newly updated UICC TNM classification, with a distribution of 82 and 114 patients, respectively. Tumors of T2 and T3 occurred more frequently on the peritoneal side of the gallbladder (56.1 and 60.5%, respectively). Applying the latest classification, tumors with ≥4 regional lymph nodes involved or any distant metastasis were classified as stage IVb, which accounted for 7.3 and 27.2% of T2 and T3 tumors, respectively. Finally, 46.3% of patients with T2 tumors succumbed, whereas 81.6% patients succumbed in the T3 group.
Table II.
Distribution of T2 and T3 tumors with different tumor locations.
| T2 (n=82) | T3a (n=114) | |||
|---|---|---|---|---|
| Factors | Peritoneal-side (n=46) | Hepatic-side (n=36) | Peritoneal-side (n=69) | Hepatic-side (n=45) |
| N1 and N2b | 6 | 16 | 35 | 30 |
| N2 | 1 | 2 | 5 | 11 |
| Mb | 1 | 5 | 13 | 15 |
| Staging | ||||
| IIa | 40 | NA | NA | NA |
| IIb | NA | 20 | NA | NA |
| IIIa | NA | NA | 33 | 11 |
| IIIb | 5 | 11 | 23 | 16 |
| IVb | 1 | 5 | 13 | 18 |
| Status | ||||
| Alive | 32 | 12 | 13 | 8 |
| Succumbed | 14 | 24 | 56 | 37 |
For T3 GBC tumors, only if a region of a tumor invaded directly into the liver parenchyma, tumors were classified as being located on the hepatic-side. All other tumors were classified as being located on the peritoneal-side
N factor and M factor were clinically determined on the basis of either histopathological data or radiographic evaluation. NA, not applicable; N, regional lymph nodes; M, distant metastasis.
According to the 8th edition of the UICC TNM classification, T2 GBC was stratified into T2a and T2b, which are peritoneal-side and hepatic-side tumors, respectively. In the present cohort, patients with T2b tumors were significantly associated with a higher incidence of nodal involvement and distant metastasis compared with patients with T2a tumors (44.4 vs. 13.0%, P=0.002; 13.9 vs. 2.2%, P=0.043; respectively). Amongst the 114 patients with T3 tumors, the patients with hepatic-side tumors had a higher incidence of nodal involvement and distant metastasis compared with those with peritoneal-side GBC; however, the comparisons did not present any significant difference (66.7 vs. 50.7%, P=0.122; 33.3 vs. 18.8%, P=0.118; respectively). The incidence of N2 involvement (metastases to ≥4 regional lymph nodes) was significantly higher in patients with hepatic-side T3 GBC (24.4 vs. 7.2%, P=0.001).
Survival analyses by UICC 8th edition TNM classification
Follow-up began in December 2016 and finished in March 2017 for the included patients (from January 2011 to July 2016) with a median follow-up time of 39 months (range, 6–76 months). A total of 209 patients (68.1%) succumbed to mortality. TNM stage was assigned to each patient according to the new UICC 8th edition TNM classification, and is also described in detail in Table I. There were 32, 60, 99 and 116 patients from stages I, II, III and IV, respectively, according to these criteria. The MST was recorded as 19 months [95% confidence interval (CI), 14.8–23.2 months] for the entire cohort, and as not applicable. 49, 24 and 8 months for stages I, II, III and IV, respectively. The 3- and 5-year survival rates of the entire cohort were estimated as 31.9 and 22.1%, respectively.
In the present cohort, differences between the survival of patients with stage I and the other stages (II–IV) were statistically significant (P=0.002, P<0.001 and P<0.001, respectively). Similar results occurred in comparisons between stage II, III and IV (P<0.001, for all comparisons). By applying the latest 8th edition UICC classification, stage IIa patients obtained a significantly improved OS compared with stage IIb patients (P=0.041; Fig. 1), whereas OS comparison between Stage IIb and IIIa did not present any significant differences (P=0.198; Fig. 1).
Figure 1.
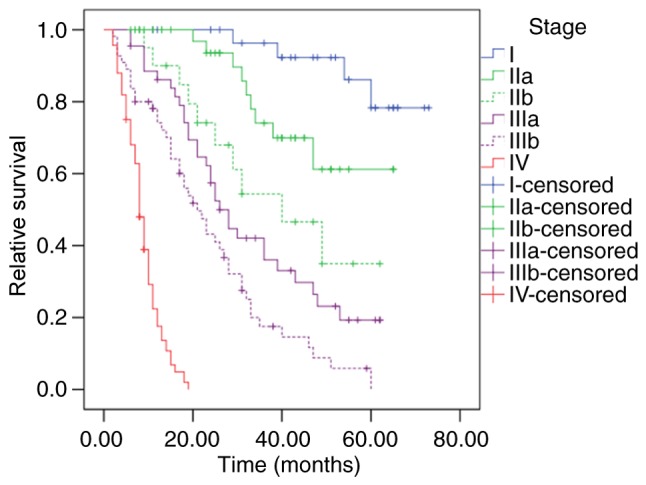
Survival of patients with gallbladder cancer at different stages according to the Union for International Cancer Control 8th edition Tumor-Node-Metastasis classification. Differences in the survival of patients between stage I and other stages were significant (all P<0.05), as well as the differences between patients of stage IV and the other stages (all P<0.001). Comparisons of the survival between stage IIa and stage IIb patients, and stage IIIa and stage IIIb patients were also significant (P=0.041 and P=0.011, respectively), whereas the difference between the survival of stage IIb and stage IIIa patients was not statistically significant (P=0.198).
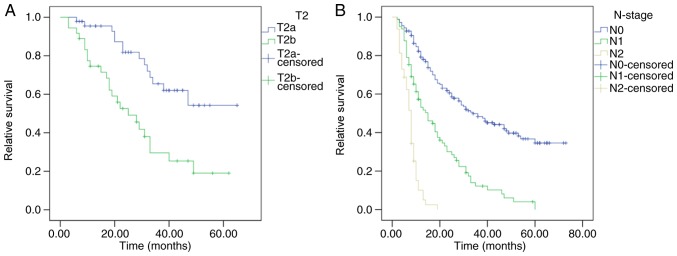
Compared with the 7th edition (20), the latest 8th edition UICC staging manual has produced a number of changes in the definitions of the T2 category, the N category and the stages of GBC, which were aforementioned. When long-term survival times were compared between patients with T2a (peritoneal-side) and T2b (hepatic-side) GBC in the present cohort, a significant prognostic difference was observed for MSTs, which were 49 and 25 months, respectively (P<0.001; Fig. 2A). Patients in the present cohort were classified into 3 groups by the new definition of the N category, and with MSTs of 36, 15 and 8 months, respectively, the differences in OS between patients with N0, N1 and N2 GBC were significantly different (P<0.001; Fig. 2B).
Figure 2.
Survival according to the main changes in the UICC 8th edition of the Tumor-Node-Metastasis classification. (A) Survival of patients with T2 GBC in different tumor locations. According to the latest UICC classification, patients with T2a experienced significantly improved survival compared with patients with T2b (P<0.001). (B) Survival of patients with GBC in different N-stages. Comparisons of the survival of patients with GBC in different N-stages were statistically significant (P<0.001). GBC, gallbladder cancer; UICC, Union for International Cancer Control; T2a, peritoneal-side T2; T2b, hepatic-side T2.
Additionally, to investigate the prognostic value of the tumor location in patients with T3 tumors, survival analysis was performed for patients with T3h (hepatic-side) and T3p (peritoneal-side) tumors. However, no significant difference was determined between the survival of patients with T3p and T3h tumors (19.0 vs. 12.0 months, P=0.379).
Prognostic factors
To identify prognostic factors for patients with GBC, multivariate analysis was performed for the entire cohort. A total of six potential confounders were selected: Age (≥60 vs. <60 years), sex (male vs. female), surgical procedure (curative resection vs. palliative), nodal involvement (N1 and N2 vs. N0), distant metastasis (M1 vs. M0) and UICC 8th edition staging (stage III and IV vs. stage I and II). Among these confounders, curative resection was associated with improved survival [hazard ratio (HR), 0.11; 95% CI, 0.07–0.17], whereas nodal involvement (HR, 1.56; 95% CI, 1.14–2.14), distant metastasis (HR, 2.37; 95% CI, 1.64–3.43) and more advanced UICC 8th stages (HR, 6.25; 95% CI, 3.81–10.26) were considered independent predictors for reduced survival (Table III).
Table III.
Multivariate analysis of potential prognostic factors for GBC.
| Variables | Hazards ratio | 95% CI | P-valuea |
|---|---|---|---|
| Stage by UICC 8th (19) | 6.25 | 3.81–10.26 | <0.001 |
| Stage III and IV vs. Stage I and II | |||
| Age, years | |||
| ≥60 vs. <60 | 1.09 | 0.92–1.29 | 0.316 |
| Sex | |||
| Male vs. female | 0.077 | 0.57–1.02 | 0.072 |
| Surgical procedure | |||
| Curative vs. palliative | 0.11 | 0.07–0.17 | <0.001 |
| Lymph nodes involvement | |||
| N1 and N2 vs. N0 | 1.56 | 1.14–2.14 | 0.003 |
| Distant metastasis | |||
| M1 vs. M0 | 2.37 | 1.64–3.43 | <0.001 |
Multivariate analyses were applied to assess the prognostic value of UICC 8th edition staging for GBC and other potential predictors using Cox regression proportional hazards model.
Discussion
GBC represents an increasing proportion of all biliary tract diseases, with an incidence that is increasing yearly in the western area of China (7). As the largest medical center of West China, the West China Hospital produces data that reflects the epidemiological characteristics of GBC in this region. In the present study, the male:female ratio was 1.0:1.82, indicating that females are more susceptible to GBC, which may be mediated by estrogen levels. Pandey and Shukla (21) determined that multiple pregnancies significantly increased the risk of GBC, which is associated with the higher levels of progesterone and endogenous estrogen during pregnancy. Of the 307 eligible patients in the present study, 39.7% had gallstones, indicating cholelithiasis as a vital risk factor of GBC. Additionally, an incidental diagnosis of GBC commonly occurs following simple cholecystectomy for benign diseases (18,22); therefore, patients with a long-term history of gallbladder stones should be highly recommended to undergo surgery.
In December 2016, the UICC published its latest edition of the TNM staging manual, including a number of changes in the T2 category, the N category and the stages of GBC. T2 has been stratified by the location of the tumor for the first time. Furthermore, the number, instead of the location, of the lymph nodes involved has been taken into account for the N category. Accompanying that, a number of vital changes have been introduced to the TNM staging (19). In the present study, based on the data of the eligible patients in the West China Hospital, the clinical characteristics of patients with surgically treated GBC were analyzed using the newly updated UICC 8th edition TNM classification. Furthermore, the prognostic value of this newly updated edition was validated by survival analyses of a large cohort from a developing country for the first time.
With a region of the gallbladder wall being attached to the liver, the unique position of the gallbladder generates anatomical differences in the venous/lymphatic drainage route between hepatic-side and peritoneal-side GBC (23,24). Ito et al (22) determined that incidental T2 gallbladder tumors with residual liver disease were similar to T3 tumors in terms of survival. The Japanese Biliary Surgical Society staging system for GBC has taken hepatic invasion as a vital factor for accurate staging (2); however, the 7th edition of the UICC/AJCC staging system did not take into account the impact of tumor location (20). Following the publishing of the UICC 8th edition staging manual, T2 (tumor invades perimuscular connective tissue; no extension beyond serosa or into liver) was stratified into T2a (peritoneal-side) and T2b (hepatic-side). Furthermore, T2aN0M0 and T2bN0M0 were classified as stage IIa and IIb, respectively (19). The present study indicated that patients could be successfully classified into 4 stages by the UICC 8th staging manual. Furthermore, as a result of the main change of this edition, patients in stage IIa demonstrated a significantly improved survival time compared with those in stage IIb in this cohort, which validated the prognostic value of tumor location in patients with T2 GBC. Differences in stage IIb and IIIa were not statistically significant.
As one of the strongest predictors, the N category was classified into N1 (hilar nodes) and N2 (other regional nodes) based on the location of lymph node metastasis in the 7th edition of the UICC TNM classification (20); however, the number of lymph nodes involved in metastasis has been reported to be a vital factor for clinicians to make predictions regarding the long-term survival of patients with GBC (17,25,26). In the latest 8th edition of the UICC staging manual, N1 was defined as metastases to 1–3 regional nodes, and N2 was defined as metastases to ≥4 regional nodes. Subsequently, T1N1M0, T2N1M0 and T3N1M0 were classified as stage IIIa, and AnyTN2M0 was defined as stage IVb (19). As indicated in the survival analyses, the new definition of the N category effectively stratified the prognosis of the patient. Furthermore, in agreement with previous studies (17,25,26), analyses by Cox multivariate regression proportional hazards model confirmed that regional node involvement was an independent predictor for patients with GBC.
The newly updated UICC TNM classification has taken into account the tumor location for stage II; however, classifications of stage I, III and IV were not influenced by the tumor location in this edition (19). Theoretically, survival of stage I patients could not be stratified well by the tumor location for the limited infiltrating range of T1 tumors, and the majority of T1 tumors were associated with a favorable prognosis following radical resection (11,27,28). Furthermore, all T4 tumors were considered as hepatic-side tumors (18). Thus, a comparison was produced between hepatic-side and peritoneal-side location for all the patients with T2 and T3 tumors, respectively, in this cohort. Amongst the patients with T2 tumors, the hepatic-side tumor location was significantly associated with a higher incidence of distant metastasis and regional lymph node involvement. Notably, it was observed that in the present study, patients with T3 hepatic-side tumors may obtain a higher incidence of nodal involvement and distant metastasis, although this association did not achieve any statistical significance. Nevertheless, as aforementioned, the incidence of N2 involvement was significantly higher in the patients with hepatic-side T3 tumors. With an MST of 19 and 12 months for peritoneal-side and hepatic-side T3 GBCs, respectively (P=0.379), location (hepatic-side vs. peritoneal-side) did not influence the prognosis of the patients with T3 GBC in the present study. Due to the reduced prognosis, we suggest that more aggressive surgical procedures should be performed for patients with suspicious lesions on the hepatic-side of the gallbladder. Additionally, simple cholecystectomy, instead of just follow-up, should be highly recommended for patients diagnosed with polyps or other benign lesions on the hepatic-side of the gallbladder.
The present study had a number of limitations, the most significant of which was its retrospective nature, with potential error and variations when collecting information, including the details of the surgery and follow-up. In addition, all patients were surgically treated and diagnosed with GBC histologically, while those without surgical intervention were not enrolled, which inevitably meant missing a number of cases. Additionally, methods to improve prognosis and prevent recurrence, including adjuvant therapies, novel technologies, and molecular and genetic features, were not taken into account for the survival and multivariate analyses in the present cohort.
In conclusion, the present study conducted the first attempt to validate the utility of the prognostic value of the newly updated UICC 8th edition TNM classification for patients with surgically treated GBC. The data indicated that applying the latest definition of the T2 category, the N category and the stages of GBC for the survival analysis of patients who were surgically treated is appropriate and promising. Additionally, the location of the tumor on the gallbladder may not influence the prognosis of patients with T3 tumors. Application of the UICC 8th edition of the TNM classification would enhance the ability to risk-stratify patients and predict the prognosis of patients with GBC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- UICC
Union for International Cancer Control
- GBC
gallbladder carcinoma
- TNM
Tumor-Node-Metastasis
- OS
overall survival
- MST
median survival time
- CI
confidence interval
- HR
hazard ratio
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LW designed the study, and was a major contributor in data collecting and writing the manuscript. PD was a major contributor in data collecting and analysis of data. YZ, MY and YC were major contributors in patient follow-up. BLT was a major contributor in study design and revision of the manuscript.
Ethics approval and consent to participate
The present study was in accordance with the ethical standards of the Human Subjects Institutional Committee of West China Hospital (Chengdu, China).
Patient consent for publication
All patients provided informed consent for inclusion in this study and the publication of any associated data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lai CH, Lau WY. Gallbladder cancer-a comprehensive review. Surgeon. 2008;6:101–110. doi: 10.1016/S1479-666X(08)80073-X. [DOI] [PubMed] [Google Scholar]
- 2.Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder cancer in the 21st Century. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, La Vecchia C. Epidemiology of biliary tract cancers: An update. Ann Oncol. 2009;20:146–159. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 4.Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi F, Lucchini F, Negri E, La Vecchia C. The recent decline in gallbladder cancer mortality in europe. Eur J Cancer Prev. 2003;12:265–267. doi: 10.1016/S0959-8049(02)00533-6. [DOI] [PubMed] [Google Scholar]
- 6.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 7.Shen HX, Song HW, Xu XJ, Jiao ZY, Ti ZY, Li ZY, Ren B, Chen C, Ma L, Zhao YL, et al. Clinical epidemiological survey of gallbladder carcinoma in northwestern China, 2009–2013: 2379 cases in 17 centers. Chronic Dis Transl Med. 2017;3:60–66. doi: 10.1016/j.cdtm.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French surgical association survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: Comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon E, Vollmer CM, Jr, Sahajpal A, Cattral M, Grant D, Doig C, Hemming A, Taylor B, Langer B, Greig P, Gallinger S. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: A 12-year study at a north american center. Ann Surg. 2005;241:385–394. doi: 10.1097/01.sla.0000154118.07704.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hari DM, Howard JH, Leung AM, Chui CG, Sim MS, Bilchik AJ. A 21-year analysis of stage I gallbladder carcinoma: Is cholecystectomy alone adequate? HPB (Oxford) 2013;15:40–48. doi: 10.1111/j.1477-2574.2012.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilgrim C, Usatoff V, Evans PM. A review of the surgical strategies for the management of gallbladder carcinoma based on T stage and growth type of the tumour. Eur J Surg Oncol. 2009;35:903–907. doi: 10.1016/j.ejso.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Yip VS, Gomez D, Brown S, Byrne C, White D, Fenwick SW, Poston GJ, Malik HZ. Management of incidental and suspicious gallbladder cancer: Focus on early referral to a tertiary centre. HPB (Oxford) 2014;16:641–647. doi: 10.1111/hpb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang JY, Heo JS, Han Y, Chang J, Kim JR, Kim H, Kwon W, Kim SW, Choi SH, Choi DW, et al. Impact of type of surgery on survival outcome in patients with early gallbladder cancer in the era of minimally invasive surgery: Oncologic safety of laparoscopic surgery. Medicine (Baltimore) 2016;95:e3675. doi: 10.1097/MD.0000000000003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim CS, Jang JY, Lee SE, Kang MJ, Kim SW. Reappraisal of hepatopancreatoduodenectomy as a treatment modality for bile duct and gallbladder cancer. J Gastrointest Surg. 2012;16:1012–1018. doi: 10.1007/s11605-012-1826-5. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto Y, Nara S, Kishi Y, Esaki M, Shimada K, Kokudo N, Kosuge T. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 2013;153:794–800. doi: 10.1016/j.surg.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Amini N, Spolverato G, Kim Y, Gupta R, Margonis GA, Ejaz A, Pawlik TM. Lymph node status after resection for gallbladder adenocarcinoma: Prognostic implications of different nodal staging/scoring systems. J Surg Oncol. 2015;111:299–305. doi: 10.1002/jso.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, Javle M, Conrad C, Maru DM, Aoki T, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: An international multicenter study. Ann Surg. 2015;261:733–739. doi: 10.1097/SLA.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brierley JD, Gospodarowicz MK, Wittekind C, editors. UICC TNM Classification of Malignant Tumours. 8th edition. Wiley Blackwell; New York, NY: 2017. [Google Scholar]
- 20.Sobin LH, Gospodarowicz MK, Wittekind C, editors. UICC TNM Classification of Malignant Tumours. 7th edition. Wiley; New York: 2009. [Google Scholar]
- 21.Pandey M, Shukla VK. Lifestyle, parity, menstrual and reproductive factors and risk of gallbladder cancer. Eur J Cancer Prev. 2003;12:269–272. doi: 10.1097/00008469-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: Implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–325. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 23.Fahim RB, Mc DJ, Richards JC, Ferris DO. Carcinoma of the gallbladder: A study of its modes of spread. Ann Surg. 1962;156:114–124. doi: 10.1097/00000658-196207000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo I, Shimada H, Takimoto A, Fujii Y, Miura Y, Sugita M, Morioka D, Masunari H, Tanaka K, Sekido H, Togo S. Microscopic liver metastasis: Prognostic factor for patients with pT2 gallbladder carcinoma. World J Surg. 2004;28:692–696. doi: 10.1007/s00268-004-7289-4. [DOI] [PubMed] [Google Scholar]
- 25.Sakata J, Shirai Y, Wakai T, Ajioka Y, Hatakeyama K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol. 2010;17:1831–1840. doi: 10.1245/s10434-009-0899-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu GJ, Li XH, Chen YX, Sun HD, Zhao GM, Hu SY. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J Gastroenterol. 2013;19:5150–5158. doi: 10.3748/wjg.v19.i31.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloia TA, Jarufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: Expert consensus statement. HPB (Oxford) 2015;17:681–690. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon YS, Han HS, Cho JY, Choi Y, Lee W, Jang JY, Choi H. Is laparoscopy contraindicated for gallbladder cancer? A 10-year prospective cohort study. J Am Coll Surg. 2015;221:847–853. doi: 10.1016/j.jamcollsurg.2015.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.