Abstract
Excessive oxygen and its chemical derivatives, namely reactive oxygen species (ROS), produce oxidative stress that has been known to lead to cell injury in ischemic stroke. ROS can damage macromolecules such as proteins and lipids and leads to cell autophagy, apoptosis, and necrosis to the cells. This review describes studies on the generation of ROS, its role in the pathogenesis of ischemic stroke, and recent development in therapeutic strategies in reducing oxidative stress after ischemic stroke.
Keywords: Ischemic stroke, mitochondria, oxidative stress, reactive oxygen species
Introduction
Reactive oxygen species (ROS) are a group of reactive oxygen-containing molecules that readily react with macromolecules resulting in irreversible functional alterations or even complete destruction. While ROS play crucial roles in human physiological processes, ROS overproduction is a noteworthy feature of ischemic stroke and ROS is an important mediator of ischemic damage. Significant progress has been made in understanding the mechanisms underlying ROS-induced brain damage after ischemic stroke. Accordingly, anti-ROS approaches have been extensively explored for the treatment of ischemic stroke, including both upstream and downstream strategies. The upstream strategies focus on attenuating the ROS production from different sources after ischemic stroke while the downstream strategies target neutralizing ROS and/or disabling the subsequent detrimental actions. Although the protective effects of antioxidants against ischemic stroke have been demonstrated in experimental ischemic stroke models in numerous studies, all antioxidant treatments have failed to provide therapeutic effects in clinical trials. Despite their detrimental effects, ROS play very important roles in normal physiological process and homeostasis, such as synaptic activity, vascular tone regulation, and inflammatory response. Further studies on the mechanism of ROS in ischemic damage should lead to more specific targeting or combination treatments that may reduce their detrimental effects without interfering their normal functions.
Functions of Reactive Oxygen Species under Normal Physiological Conditions
Under normal physiological conditions, ROS play important roles in many biological processes including cell signaling, gene transcription regulation, immune response, and apoptosis. ROS work as the second messengers during signal transduction of many growth factors, such as epidermal growth factor, platelet-derived growth factor, and NK1.[1,2] They also regulate the activities of many transcription factors, such as of p53 and nuclear factor-kappa B (NF-κB), by oxidative modification of the proteins.[3,4] Oxidative burst, which is the massive production of ROS in immune cells such as neutrophils and macrophages, is an important defense mechanism against foreign pathogens. Insufficient ROS production caused by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase deficiency is responsible for the recurrent infections in patients with chronic granulomatous disease.[5,6] Apoptosis, which is programmed cell death, is important during development and for clearance of unhealthy cells. ROS can initiate both extrinsic and intrinsic apoptosis through various signaling pathways, such as activation of death receptors, damage to mitochondrial DNA, and activation of c-Jun N-terminal kinases.[7] However, during ischemic stroke, the amount of ROS is much more than what is needed for normal physiological functions and generates detrimental effects in the brain.
Reactive Oxygen Species Generation in Ischemic Stroke
ROS, including superoxide anion, peroxide, hydroxyl radical, and singlet oxygen, are reactive molecules; thus, their concentration is difficult to measure directly in the brain.[8,9] Excessive ROS generation after stroke is mainly indirectly supported by the protective effects of ROS scavengers.[10,11,12,13,14] In human stroke patients, oxidation of serum albumin was increased, which can be attributed to the oxidation of amino acid residues by ROS.[15] Direct measurement of ROS using an in vivo chemiluminescence method through a closed cranial window in a rat middle cerebral artery occlusion (MCAO) model found a steady increase of ROS production after occlusion.[16] Reperfusion may induce the second phase of ischemia/reperfusion injury and precipitate the generation of ROS that is fueled by the reintroduction of oxygen molecules to the ischemic tissue. The second peak of ROS generation was detected in rodents during reperfusion after MCAO.[16,17,18]
The mitochondrial electron transport chain is an important source of ROS.[19] Under normal conditions, mitochondria reduce O2 to H2 O by cytochrome c oxidase in Complex IV of the electron transport chain. In isolated mitochondria, only 0.1-2% of the oxygen is reduced by the mitochondria to generate ROS.[20] At least seven sites in the mitochondria have been identified that can partially reduce oxygen to generate ROS.[21,22,23,24,25,26] Ubiquinone-cytochrome b region of the electron transport chain has been proposed as the major site for ROS production during ischemia.[27] A recent study identified succinate accumulated during ischemia as a potential mitochondrial metabolite that drives extensive ROS production.[28]
NADPH oxidase (NOX) is an enzyme complex on the cell membrane. It is another important source of ROS generation in ischemic stroke.[29] NOX is an enzyme made up of six subunits that generate superoxide by transferring electrons from NADPH across the cell membrane to oxygen molecules. ROS generation by NOX has been known for decades to contribute to the respiratory burst in neutrophils that provide a defense against bacteria.[30,31] A family of NOX has been identified: NOX1, NOX2, NOX3, NOX4, NOX5, and Dual Oxidase 1 and 2 (Duo × 1 and Duo × 2). NOX subunits are widely expressed in different regions of the brain.[32,33,34] Upregulation of NOX2 and NOX4 expression was observed after ischemic stroke.[35,36] NOX2 drives neuronal ROS production in ischemic stroke[37] and is the major contributor of N-methyl-D-aspartate receptor activated superoxide production.[38] Both NOX1 and NOX2 knockout in mice reduced lesion volume after stroke.[39,40,41] Increased NOX expression and activity after MCAO also mediated matrix metalloproteinase-9 (MMP-9) upregulation and contributed to blood-brain barrier (BBB) damage.[42] Proteolytic degradation of zonula occludens by MMP-9 contributed to BBB damage in ischemic stroke.[43] NOX2 knockout also attenuated MMP-9 upregulation in ischemic stroke and reduced BBB damage.[44] NOX4 knockout also protected the brain from oxidative stress after stroke.[45]
Xanthine oxidase (XO) is also a source of ROS during ischemic stroke. XO is a molybdo-flavin enzyme that catalyzes the conversion of hypoxanthine to xanthine and xanthine to urate. This enzyme exists in two interconvertible forms: an NAD-dependent dehydrogenase (xanthine dehydrogenase) and oxygen-dependent superoxide production oxidase (XO). XO has higher affinity to O2 than NAD+ and hydrogen peroxide is the major product of XO.[46,47] Ischemia increased the activity of XO in rat brain.[48,49] XO is an important source of superoxide anion radicals in blood after forebrain ischemia/reperfusion in rat[50] and hydrogen peroxide derived from XO contributed brain edema induced by ischemia/reperfusion in gerbils.[51]
Other intracellular enzymes that catalyze the production of ROS include cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450 enzymes. These enzymes are involved in the metabolism of free arachidonic acid released from cell membrane phospholipids during ischemia. COX metabolism of arachidonic acid has been proposed as a major source of superoxide generation during reperfusion in ischemic piglet brain.[52]
Functions of Reactive Oxygen Species in Ischemic Stroke
Under normal conditions, ROS production in the brain is balanced by the endogenous enzymatic and nonenzymatic antioxidative mechanisms. The enzymes include superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT). SOD catalyzes dismutation of superoxide to hydrogen peroxide, providing the first line against ROS damage.[53,54] GPX and CAT further metabolize hydrogen peroxide to water and oxygen.[55,56] Nonenzymatic endogenous antioxidative small molecules also play very important roles in defending against oxidative stress, especially in extracellular spaces where the enzymes are absent or in very low levels.[57] Small-molecule antioxidants can be water-soluble or lipid-soluble, and these molecules include glutathione (GSH), Vitamins E and C (inhibits oxidation of membrane lipid), N-acetylcysteine (NAC), and melatonin. In humans, levels of most antioxidants (Vitamins A, E, and C) were reduced immediately after an acute ischemic stroke,[58] probably due to the larger amount of ROS produced that cannot be balanced by endogenous antioxidants. In normal conditions, ROS play beneficial roles in regulating many important cellular processes, such as gene expression, cell proliferation and migration, and immune response.[59,60] However, when ROS produced during ischemic stroke exceed the need for maintaining normal functions and cannot be balanced by endogenous antioxidants, they can cause excessive damage.
ROS can interact with amino acids in protein molecules and cause protein modification or degradation. It can also react with the side chains and the backbone of protein, which can lead to protein oxidation, peptide bond cleavage, and protein degradation.[61,62,63] ROS oxidation of protein can lead to protein-protein cross-linkage and aggregation.[64,65] Protein oxidation can lead to functional changes of the proteins, such as enzyme inactivation[66] or ion channel activity modification.[67] Ischemic stroke causes extensive protein oxidation in human[68] and animal models of stroke.[69] Oxidative inactivation of glutamine synthetase, which catalyzes the conversion of glutamate to glutamine in astrocytes to protect neurons against excitotoxicity,[70] has been proposed as an important factor in the neurotoxicity caused by cerebral ischemia in gerbil brains.[71]
Lipid peroxidation, which is the oxidative degradation of lipids, by ROS is more damaging than protein oxidant to cells during ischemic stroke.[72,73] Lipid peroxidation by ROS leads to a self-propagation of free radical reaction. ROS attack lipids containing carbon-carbon double bonds, especially polyunsaturated fatty acids, producing lipid radicals. Lipid radical is not stable and can react with oxygen and form lipid peroxyl radical; lipid peroxyl radical can react with other lipid acids to generate another lipid radical and lipid peroxide.[74,75] Two lipid radicals react to form end products of lipid peroxidation-reactive aldehydes, such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). MDA and HNE have been used as markers for lipid peroxidation.[76,77,78] MDA can react with amino acids in proteins and other molecules to form its adducts such as malondialdehyde-acetaldehyde and advanced lipid peroxidation end-products, which can produce secondary deleterious effects by promoting intra- or inter-molecular protein or DNA crosslinking to cause protein modification and DNA damage/mutation.[79,80,81,82,83] 4-HNE is a very reactive compound with three reactive groups: an aldehyde, a double-bond, and a hydroxyl group.[84] 4-HNE is a second messenger that can regulate several transcription factors such as nuclear factor erythroid 2-related factor 2, activating protein-1, NF-κB, and peroxisome-proliferator-activated receptors.[85,86,87,88,89] 4-HNE also regulates major cell signaling pathways, such as MAPK and PI3K/AKT.[90,91,92,93] Lower nontoxic concentrations of 4-HNE are beneficial to cells by promoting cell proliferation, differentiation, antioxidant defense, and anti-inflammation while high concentrations of 4-HNE induce cell apoptosis.[94,95,96] Increased lipid peroxidation has been found in human stroke patients[97,98,99,100,101] as well as rat cerebral ischemia models[102,103] and has been proposed to play an important role in cell death by ischemic stroke.
ROS break DNA double strands, cause intra- and inter-strand crosslinks, protein-DNA crosslinks, DNA mutations, and DNA structural changes.[104] 8-hydroxy-2V-deoxyguanosine (8OHdG) is one of the most common products of oxidative damage of DNA[105,106] and increased levels of 8OHdG suggested extensive DNA oxidation, which precedes DNA fragmentation, in ischemic stroke in rat.[69,107,108]
Therapeutic Strategies to Reduce Oxidative Stress for Treatment of Ischemic Stroke
In ischemic stroke, oxidative stress is created by the excessive ROS, whose effects cannot be balanced by endogenous antioxidants, resulting in wide-spread damages by oxidation of lipid acid, protein, and DNA, which lead to cell death. To counteract this oxidative stress, different strategies have been proposed to target the pathways from upstream ROS production to their downstream effects on macromolecules.
Reactive oxygen species scavenger
ROS scavenger, such as vitamins, NAC, and lipoic acid (LA), are the most commonly used antioxidants. Vitamin E is a potent, lipid-soluble antioxidant. It can interrupt the chain reaction of free radical production during lipid peroxidation by ROS. Vitamin E has been reported to be protective in rodent ischemic stroke models as shown by reduced lesion volume and lessened behavioral impairments.[109,110,111] MDL 74,722, a Vitamin E analog, has been reported to reduce lesion volume in the rat MCAO model.[112] It has been reported that Vitamin C is protective in both rodent and primate models of ischemic stroke.[113,114,115] However, a follow-up study in human showed that food supplement of Vitamin E and Vitamin C did not reduce the risk for ischemic stroke.[116] Vitamin C did not improve functional recovery in ischemic stroke patients.[117] High dose of Vitamin E has been suggested to increase all-cause mortality.[118,119] Dehydroascorbic acid (DHA), a blood-brain barrier transportable form of Vitamin C, has shown potent protective effects in ischemic stroke in mice.[120] However, preclinical investigation of DHA in an ischemic stroke model in adult baboons did not show any protective effect.[121] EPC-K1, a phosphate diester of Vitamins C, has been found to reduce lesion size and lipid peroxidation in the rat MCAO model.[122,123] The disappointing results from clinical trials of vitamins in ischemic stroke may suggest that food supplementation or intake of vitamins has little effect on the tightly regulated endogenous pathways.
Edaravone (5-methyl-2-phenyl-4H-pyrazol-3-one) is a free radical scavenger that has been approved for the treatment of stroke in Asia since 2002. Edaravone is lipophilic and can readily cross the BBB.[124] Several clinical trials in Japan have shown that Edaravone treatment was beneficial for a subset of stroke patients.[125,126,127,128] Decreased lesion size, attenuated MMP-9 activation, and reduced BBB damage after ischemia were reported in rodent stroke models treated with Edaravone.[129] Edaravone also reduced recombinant tissue plasminogen activator (rtPA)-induced BBB damage in rodents, suggesting Edaravone as a promising candidate to expand the time window of rtPA treatment.[130,131] Future clinical trials may expand the use of Edaravone for the treatment of ischemic stroke in other countries.
NXY-059 is (Disufenton sodium) a broad-spectrum nitrone-based free radical scavenger.[132] This compound reduced lesion volume in rats after permanent MCAO.[133] NXY-059 also improved motor function of monkeys after permanent MCAO.[134] A Phase III clinical trial published in 2006 reported that NXY-059 administered within 6 h after acute ischemic stroke reduced disability at 90 days.[135,136] However, a following larger clinical trial failed to support the efficacy of NXY-059 for acute ischemic stroke.[137,138]
NAC is an antioxidant that has a free thiol group capable of reacting with ROS; it is a GSH precursor which can exert an indirect antioxidant effect.[139] NAC has been reported to reduce lesion volume and improve neurological score in rat MCAO models;[140,141] it also increased hippocampal neuron survival in a transient forebrain ischemia model in rat.[142] GSH monoethyl ester, which can be effectively transported into cells and converted to GSH, has been reported to be protective in a rat ischemic stroke model.[143]
LA can react with ROS and it also recycles Vitamin E and Vitamin C.[144] LA reduced mortality rate of rats after cerebral ischemia.[145] LA pretreatment reduced lesion volume in rat MCAO model when administered 30 min before the collusion.[145] A recent study further indicated that infusion of LA through the jugular vein immediately after reperfusion reduced lesion volume and promoted functional recovery in rat MCAO model.[146]
Tirilazad (U-74006F) is a synthetic lipid-soluble nonglucocorticoid. It is an inhibitor of lipid peroxidation as well as an antioxidant and free radical scavenger.[147] Tirilazad reduced lesion volume and attenuated neurological deficits in a rat permanent MCAO model;[148] another study reported that tirilazad reduced lesion volume after transient but not permanent focal cerebral ischemia in rats.[149] A meta-analysis of the efficacy of tirilazad in experimental stroke concluded from 18 studies that tirilazad reduced infarct volume by 29.2% and improved neurobehavioral score by 48.1%.[150] A clinical trial (RANTTAS) with 660 patients found that 6 mg/kg per day for 3 days dose did not improve overall functional outcome.[151] A following clinical trial of higher dose (12-15 mg/kg per day) in acute ischemic stroke found that tirilazad treatment reduced mortality and increased functional recovery in both men and women.[152] However, a meta-analysis of 4 published and 2 unpublished clinical trials concluded that tirilazad increased death and disability in acute ischemic stroke patients, which precludes future trials of the drug.[153]
Citicoline is a natural compound that is an intermediate in the generation of phosphatidylcholine from choline.[154] Citicoline can stabilize cell membranes and reduce free fatty acid release caused by lipid peroxidation during ischemia.[155] Many studies have examined its protective effects in animal models of stroke. A meta-analysis of fourteen studies concluded that citicoline reduced lesion volume by 27.8%.[156] Several clinical trials of citicoline for the treatment of stroke have been conducted.[157,158,159] One of the trials reported that citicoline improved functional outcome and reduced neurologic deficit.[157] Although the other two studies concluded that citicoline was ineffective in improving functional outcomes,[158,159] a pooled analysis of data from four clinical trials found that citicoline was safe and promoted recovery after acute ischemic stroke.[160] In 2012, the International Citicoline Trial on Acute Stroke reported that citicoline was not efficacious for moderate-to-severe acute ischemic stroke.[161] GM1-ganglioside, which may also stabilize membranes, was tested in clinical trials; however, the results did not support improved outcome after treatment.[162,163,164]
Reactive oxygen species degradation
ROS can be degraded by SOD and CAT, which makes them candidates for stroke treatment. Intravenous administration of polyethylene glycol-conjugated SOD (PEG-SOD) and CAT (PEG-CAT) reduced infarct volume in rats.[10] PEG-SOD or recombinant human SOD alone also reduced ischemic damage in animals.[165,166,167] Synthetic combined superoxide dismutase/CAT mimetics EUK-134 and EUK-8 reduced infarct volume when administered 3 h after MCAO.[14] A SOD mimetic, M40401, generated a protective effect in gerbil ischemic stroke models;[168] it also reduced infract size and improved neurological score when administered either before or after MCAO in rat.[169]
Reducing reactive oxygen species generation in ischemic stroke
Failure of ROS scavengers in ischemic stroke clinical trials suggests that it may be very difficult to eliminate the detrimental effects of ROS when it is already generated. Attenuating excessive ROS production after onset of ischemic stroke might provide a more effective strategy for treatment of ischemic stroke. In animal stroke models, excessive ROS generation persisted through occlusion and there is even a second perk of ROS generation after reperfusion, suggesting that there could be a time windows for treatment to reducing ROS generation after occurrence of occlusion.
NOX inhibition has been proposed as a strategy to reduce oxidative stress in ischemic stroke by reducing ROS generation.[170,171] NOX inhibitor apocynin has been extensively studied for stroke treatment, and several studies have reported its protective effect against ischemic stroke.[172,173,174,175] NOX inhibitor diphenyleneiodonium (DPI) was protective in a rat MCAO model when administered with dimethyl sulfoxide;[176] however, DPI is not a specific NOX inhibitor.[177] A more specific NOX inhibitor, VAS2870, has been found to reduce stroke lesion volume and improve long-term neurological functions in mice.[45] However, recent study indicated that VAS2870 has significant off-target effects.[178] NOX inhibition is an important strategy to reduce ROS production in ischemic stroke; however, it is still not clear which NOX isoform and what cell types play a major role in NOX ROS production during ischemic stroke.[45,170] Further studies are warranted to examine the underlying mechanism and develop/test more specific NOX inhibitors, such as gp91ds-tat[179] and GKT136901,[180] for the treatment of ischemic stroke.
XO inhibitor allopurinol has shown some beneficial effects on inflammatory indices in ischemic stroke patients in clinical trial[181] although a following clinical trial was not able to find any beneficial effect in patients with subcortical stroke.[182] A recent clinical trial indicated that allopurinol was well tolerated and improved the 3-month functional status of acute ischemic stroke patients with high levels of serum uric acid.[183] Beneficial effects of allopurinol in ischemic stroke have been found in many studies using different animal stroke models.[51,184,185,186,187,188] As a drug that has been approved by the Food and Drug Administration and has been used in humans for many years, allopurinol is a very promising candidate for stroke treatment. However, allopurinol can also reduce XO and generate superoxide when inhibiting its activity.[189] Many other small molecule XO inhibitors have been developed, such as TEI-6720,[190] febuxostat,[191] Y-700,[192] and BOF-4272;[193] it may be interesting to test their effects in ischemic stroke considering their reported improved potency and/or efficacy compared to allopurinol.
COX-2 knockout in mice decreased infarct volume after MCAO[194] while COX-2 overexpression increase infarct volume.[195] COX-2 inhibitor NS-398 reduced infarct volume and behavioral deficits in mice after MCAO model.[196,197] 12/15-LOX knockout mice also exhibited smaller lesion volume after transient MCAO.[198] 12/15-LOX inhibitor LOXBlock-1 reduced infarct size in mouse MCAO model; it also reduced rtPA-induced hemorrhage in a distal MCAO clot stroke model.[199] Baicalein, a natural product and specific inhibitor of 12/15-LOX, reduced lesion volume and behavioral deficits in rodent stroke models.[198,200,201]
Mitochondrion is an important source of ROS. CoQ10 is a component of the mitochondrial electron transport chain. When administered, CoQ10 can accumulate in the mitochondria[202] and has been found to be protective against ischemia in various animal models of stroke, which can be attributed to its role as a potent antioxidant and ROS scavenger in mitochondria.[203,204,205] CoQ10 belongs to the mitochondria-targeted antioxidant (MTA) family.[206] CoQ10 is also an endogenous antioxidant.[207,208] Therefore, CoQ10 has dual therapeutic benefits by enhancing electron transport chain efficiency and simultaneously acting as an ROS scavenger. However, recent clinical trial for Parkinson's showed that CoQ10 did not slow disease progression.[209]
Another MTA, MitoQ10, can accumulate in the mitochondria[210] and has been reported to reduce mitochondrial oxidative damage. MitoQ10 has been reported to be effective in many disorders, including Alzheimer's disease, Parkinson Disease, cardiac ischemia, and hypertension.[211,212,213,214] It is an interesting candidate for stroke treatment. Mild uncoupling of mitochondrial respiration and phosphorylation has been proposed as a strategy to reduce mitochondrial ROS production.[215,216] A cationic uncoupler SkQR1 has been shown to reduce lesion volume after ischemic stroke in rat.[216]
We have reported that methylene blue (MB) can shuttle electrons between NADH and cytochrome c and bypass Complex I/III blockage, which reduced electron leakage and ROS generation.[217] Our study and other studies have indicated that MB is protective against ischemic stroke.[217,218,219,220] MB is a small molecular that can easily cross the BBB,[221] and it can be reoxidized by cytochrome c and reused for electron shuttling. MB and its derivatives as regenerable antioxidants that target mitochondria to reduce ROS production and provide neuroprotection are promising candidates for the treatment for ischemic stroke.[217,222]
Conclusion
ROS are generated from various sources during ischemia-reperfusion, with mitochondrial electron transport chain as one of the most important sources. While most previous studies and clinical trials for ischemic stroke focused on ROS scavengers, more studies should be conducted to develop and test agents that can reduce ROS generation after onset of stroke, especially from mitochondria. The combination of the upstream and downstream therapeutic strategies should also be considered in the future studies [Figure 1].
Figure 1.
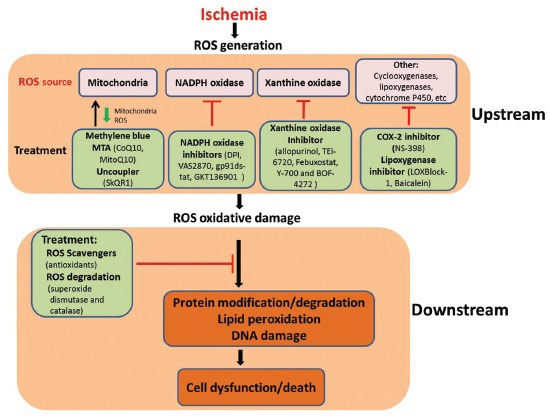
Upstream and downstream strategies targeting oxidative stress for the treatment of ischemic stroke. MTA: mitochondria-targeted antioxidant
Financial support and sponsorship
This work was partly supported by National Institutes of Health grants R01NS054651 (SY) and R01NS088596 (SY).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–21. [PubMed] [Google Scholar]
- 2.Lei H, Kazlauskas A. A reactive oxygen species-mediated, self-perpetuating loop persistently activates platelet-derived growth factor receptor α. Mol Cell Biol. 2014;34:110–22. doi: 10.1128/MCB.00839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–85. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPhail LC, DeChatelet LR, Shirley PS, Wilfert C, Johnston RB, Jr, McCall CE. Deficiency of NADPH oxidase activity in chronic granulomatous disease. J Pediatr. 1977;90:213–7. doi: 10.1016/s0022-3476(77)80632-x. [DOI] [PubMed] [Google Scholar]
- 6.Hohn DC, Lehrer RI. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975;55:707–13. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Egashira T, Takayama F, Yamanaka Y. Detection and characterization of free radicals, radical scavenging activity, and lipid peroxides in cerebral ischemia-reperfusion injury by electron spin resonance and chemiluminescence high-performance liquid chromatography. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997;17:153–8. [PubMed] [Google Scholar]
- 9.Dugan LL, Lin TS, He YY, Hsu CY, Choi DW. Detection of free radicals by microdialysis/spin trapping EPR following focal cerebral ischemia-reperfusion and a cautionary note on the stability of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) Free Radic Res. 1995;23:27–32. doi: 10.3109/10715769509064016. [DOI] [PubMed] [Google Scholar]
- 10.Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989;256(2 Pt 2):H589–93. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–604. [PubMed] [Google Scholar]
- 12.Oh SM, Betz AL. Interaction between free radicals and excitatory amino acids in the formation of ischemic brain edema in rats. Stroke. 1991;22:915–21. doi: 10.1161/01.str.22.7.915. [DOI] [PubMed] [Google Scholar]
- 13.Abe K, Yuki S, Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke. 1988;19:480–5. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- 14.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: A key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–21. [PubMed] [Google Scholar]
- 15.Moon GJ, Shin DH, Im DS, Bang OY, Nam HS, Lee JH, et al. Identification of oxidized serum albumin in the cerebrospinal fluid of ischaemic stroke patients. Eur J Neurol. 2011;18:1151–8. doi: 10.1111/j.1468-1331.2011.03357.x. [DOI] [PubMed] [Google Scholar]
- 16.Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, et al. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Yamato M, Egashira T, Utsumi H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic Biol Med. 2003;35:1619–31. doi: 10.1016/j.freeradbiomed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Abe K, Tonomura M, Ito M, Takai N, Imamoto N, Rokugawa T, et al. Imaging of reactive oxygen species in focal ischemic mouse brain using a radical trapping tracer [(3) H] hydromethidine. EJNMMI Res. 2015;5:115. doi: 10.1186/s13550-015-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–31. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–72. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–31. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 23.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–35. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–7. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 25.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 26.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 27.Cino M, Del Maestro RF. Generation of hydrogen peroxide by brain mitochondria: The effect of reoxygenation following postdecapitative ischemia. Arch Biochem Biophys. 1989;269:623–38. doi: 10.1016/0003-9861(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 28.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–63. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–7. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem J. 2005;386(Pt 3):401–16. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013;10:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radermacher KA, Wingler K, Langhauser F, Altenhöfer S, Kleikers P, Hermans JJ, et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18:1418–27. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–8. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CJ, Weston RM, Dusting GJ, Roulston CL. NADPH oxidase and angiogenesis following endothelin-1 induced stroke in rats: role for nox 2 in brain repair. Brain Sci. 2013;3:294–317. doi: 10.3390/brainsci3010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–8. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–63. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–63. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, et al. NADPH oxidase Nox 1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 2010;40:185–92. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–6. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 41.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–8. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 42.Tang X, Zhong W, Tu Q, Ding B. NADPH oxidase mediates the expression of MMP-9 in cerebral tissue after ischemia-reperfusion damage. Neurol Res. 2014;36:118–25. doi: 10.1179/1743132813Y.0000000266. [DOI] [PubMed] [Google Scholar]
- 43.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Chen Q, Liu J, Liu KJ. Normobaric hyperoxia protects the blood brain barrier through inhibiting No × 2 containing NADPH oxidase in ischemic stroke. Med Gas Res. 2011;1:22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. pii: E1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishino T, Okamoto K, Eger BT, Pai EF, Nishino T. Mammalian xanthine oxidoreductase – Mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–89. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 47.Harris CM, Massey V. The oxidative half-reaction of xanthine dehydrogenase with NAD; reaction kinetics and steady-state mechanism. J Biol Chem. 1997;272:28335–41. doi: 10.1074/jbc.272.45.28335. [DOI] [PubMed] [Google Scholar]
- 48.Oka H, Kanemitsu H, Nihei H, Nakayama H, Tamura A, Sano K. Change of xanthine dehydrogenase and xanthine oxidase activities in rat brain following complete ischaemia. Neurol Res. 1992;14:321–4. doi: 10.1080/01616412.1992.11740077. [DOI] [PubMed] [Google Scholar]
- 49.Kinuta Y, Kimura M, Itokawa Y, Ishikawa M, Kikuchi H. Changes in xanthine oxidase in ischemic rat brain. J Neurosurg. 1989;71:417–20. doi: 10.3171/jns.1989.71.3.0417. [DOI] [PubMed] [Google Scholar]
- 50.Ono T, Tsuruta R, Fujita M, Aki HS, Kutsuna S, Kawamura Y, et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009;1305:158–67. doi: 10.1016/j.brainres.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 51.Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ, et al. Xanthine oxidase-derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Invest. 1988;81:1556–62. doi: 10.1172/JCI113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstead WM, Mirro R, Busija DW, Leffler CW. Postischemic generation of superoxide anion by newborn pig brain. Am J Physiol. 1988;255(2 Pt 2):H401–3. doi: 10.1152/ajpheart.1988.255.2.H401. [DOI] [PubMed] [Google Scholar]
- 53.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 54.Greenlund LJ, Deckwerth TL, Johnson EM., Jr Superoxide dismutase delays neuronal apoptosis: A role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–15. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 55.Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, et al. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283–97. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 56.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 57.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988;85:9748–52. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherubini A, Polidori MC, Bregnocchi M, Pezzuto S, Cecchetti R, Ingegni T, et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31:2295–300. doi: 10.1161/01.str.31.10.2295. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–48. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 60.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Garrison WM, Jayko ME, Bennett W. Radiation-induced oxidation of protein in aqueous solution. Radiat Res. 1962;16:483–502. [PubMed] [Google Scholar]
- 62.Garrison WM. Reaction mechanisms in the radiolysis of peptide, polypeptides, and proteins. Chem Rev. 1987;87:381–98. [Google Scholar]
- 63.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–6. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 64.Grune T, Reinheckel T, Joshi M, Davies KJ. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of the multicatalytic proteinase complex, proteasome. J Biol Chem. 1995;270:2344–51. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- 65.Gray WA, Feiring A, Cioppi M, Hibbard R, Gray B, Khatib Y, et al. S.M.A.R.T. self-expanding nitinol stent for the treatment of atherosclerotic lesions in the superficial femoral artery (STROLL): 1-year outcomes. J Vasc Interv Radiol. 2015;26:21–8. doi: 10.1016/j.jvir.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Fucci L, Oliver CN, Coon MJ, Stadtman ER. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: Possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983;80:1521–5. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–6. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- 68.Domínguez C, Delgado P, Vilches A, Martín-Gallán P, Ribó M, Santamarina E, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke. 2010;41:653–60. doi: 10.1161/STROKEAHA.109.571935. [DOI] [PubMed] [Google Scholar]
- 69.Li S, Zheng J, Carmichael ST. Increased oxidative protein and DNA damage but decreased stress response in the aged brain following experimental stroke. Neurobiol Dis. 2005;18:432–40. doi: 10.1016/j.nbd.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Suárez I, Bodega G, Fernández B. Glutamine synthetase in brain: Effect of ammonia. Neurochem Int. 2002;41:123–42. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 71.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–7. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6:303–13. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 73.Watson BD, Busto R, Goldberg WJ, Santiso M, Yoshida S, Ginsberg MD. Lipid peroxidation in vivo induced by reversible global ischemia in rat brain. J Neurochem. 1984;42:268–74. doi: 10.1111/j.1471-4159.1984.tb09728.x. [DOI] [PubMed] [Google Scholar]
- 74.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev. 2011;111:5944–72. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 75.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(5 Suppl):715S–24S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 76.Serteser M, Ozben T, Gumuslu S, Balkan S, Balkan E. Lipid peroxidation in rat brain during focal cerebral ischemia: Prevention of malondialdehyde and lipid conjugated diene production by a novel antiepileptic, lamotrigine. Neurotoxicology. 2002;23:111–9. doi: 10.1016/s0161-813x(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 77.Picaud JC, Steghens JP, Auxenfans C, Barbieux A, Laborie S, Claris O. Lipid peroxidation assessment by malondialdehyde measurement in parenteral nutrition solutions for newborn infants: A pilot study. Acta Paediatr. 2004;93:241–5. [PubMed] [Google Scholar]
- 78.Guéraud F, Peiro G, Bernard H, Alary J, Créminon C, Debrauwer L, et al. Enzyme immunoassay for a urinary metabolite of 4-hydroxynonenal as a marker of lipid peroxidation. Free Radic Biol Med. 2006;40:54–62. doi: 10.1016/j.freeradbiomed.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slatter DA, Avery NC, Bailey AJ. Identification of a new cross-link and unique histidine adduct from bovine serum albumin incubated with malondialdehyde. J Biol Chem. 2004;279:61–9. doi: 10.1074/jbc.M310608200. [DOI] [PubMed] [Google Scholar]
- 81.Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011;650:184–94. doi: 10.1016/j.ejphar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 82.Feng Z, Hu W, Marnett LJ, Tang MS. Malondialdehyde, a major endogenous lipid peroxidation product, sensitizes human cells to UV- and BPDE-induced killing and mutagenesis through inhibition of nucleotide excision repair. Mutat Res. 2006;601:125–36. doi: 10.1016/j.mrfmmm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–33. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 84.Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–59. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Sano M, Shinmura K, Tamaki K, Katsumata Y, Matsuhashi T, et al. 4-hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol Cell Cardiol. 2010;49:576–86. doi: 10.1016/j.yjmcc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 86.Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro . Free Radic Biol Med. 2007;42:1838–50. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 87.Braithwaite EK, Mattie MD, Freedman JH. Activation of metallothionein transcription by 4-hydroxynonenal. J Biochem Mol Toxicol. 2010;24:330–4. doi: 10.1002/jbt.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem. 2000;74:159–68. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 89.Lim JH, Lee JC, Lee YH, Choi IY, Oh YK, Kim HS, et al. Simvastatin prevents oxygen and glucose deprivation/reoxygenation-induced death of cortical neurons by reducing the production and toxicity of 4-hydroxy-2E-nonenal. J Neurochem. 2006;97:140–50. doi: 10.1111/j.1471-4159.2006.03715.x. [DOI] [PubMed] [Google Scholar]
- 90.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–50. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, et al. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicol Appl Pharmacol. 2013;272:345–55. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SJ, Kim CE, Yun MR, Seo KW, Park HM, Yun JW, et al. 4-Hydroxynonenal enhances MMP-9 production in murine macrophages via 5-lipoxygenase-mediated activation of ERK and p38 MAPK. Toxicol Appl Pharmacol. 2010;242:191–8. doi: 10.1016/j.taap.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Shearn CT, Fritz KS, Reigan P, Petersen DR. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–96. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 94.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/360438. 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zarkovic N, Zarkovic K, Schaur RJ, Stolc S, Schlag G, Redl H, et al. 4-Hydroxynonenal as a second messenger of free radicals and growth modifying factor. Life Sci. 1999;65:1901–4. doi: 10.1016/s0024-3205(99)00444-0. [DOI] [PubMed] [Google Scholar]
- 96.Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, et al. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–75. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferretti G, Bacchetti T, Masciangelo S, Nanetti L, Mazzanti L, Silvestrini M, et al. Lipid peroxidation in stroke patients. Clin Chem Lab Med. 2008;46:113–7. doi: 10.1515/CCLM.2008.011. [DOI] [PubMed] [Google Scholar]
- 98.Selakovic VM, Jovanovic MD, Jovicic A. Changes of cortisol levels and index of lipid peroxidation in cerebrospinal fluid of patients in the acute phase of completed stroke. Vojnosanit Pregl. 2002;59:485–91. doi: 10.2298/vsp0205485s. [DOI] [PubMed] [Google Scholar]
- 99.Iavorskaia VA, Belous AM, Mokhamed AN. The level of middle mass molecules and lipid peroxidation in blood of patients with different forms of stroke. Zh Nevrol Psikhiatr Im S S Korsakova. 2000;100:48–51. [PubMed] [Google Scholar]
- 100.Imre SG, Fekete I, Farkas T. Increased proportion of docosahexanoic acid and high lipid peroxidation capacity in erythrocytes of stroke patients. Stroke. 1994;25:2416–20. doi: 10.1161/01.str.25.12.2416. [DOI] [PubMed] [Google Scholar]
- 101.Sonecki P, Olczyk K, Gralewski Z, Ogrodowska-Hamankiewicz E. Superoxide dismutase and lipid peroxidation in cerebrospinal fluid of patients with ischemic brain stroke. Acta Biochim Pol. 1994;41:148–50. [PubMed] [Google Scholar]
- 102.Bromont C, Marie C, Bralet J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20:918–24. doi: 10.1161/01.str.20.7.918. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto M, Shima T, Uozumi T, Sogabe T, Yamada K, Kawasaki T. A possible role of lipid peroxidation in cellular damages caused by cerebral ischemia and the protective effect of alpha-tocopherol administration. Stroke. 1983;14:977–82. doi: 10.1161/01.str.14.6.977. [DOI] [PubMed] [Google Scholar]
- 104.Jena NR. DNA damage by reactive species: Mechanisms, mutation and repair. J Biosci. 2012;37:503–17. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 105.Dizdaroglu M, Rao G, Halliwell B, Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch Biochem Biophys. 1991;285:317–24. doi: 10.1016/0003-9861(91)90366-q. [DOI] [PubMed] [Google Scholar]
- 106.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 107.Cui J, Holmes EH, Greene TG, Liu PK. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000;14:955–67. doi: 10.1096/fasebj.14.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacManus JP, Fliss H, Preston E, Rasquinha I, Tuor U. Cerebral ischemia produces laddered DNA fragments distinct from cardiac ischemia and archetypal apoptosis. J Cereb Blood Flow Metab. 1999;19:502–10. doi: 10.1097/00004647-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 109.van der Worp HB, Bär PR, Kappelle LJ, de Wildt DJ. Dietary Vitamin E levels affect outcome of permanent focal cerebral ischemia in rats. Stroke. 1998;29:1002–5. doi: 10.1161/01.str.29.5.1002. [DOI] [PubMed] [Google Scholar]
- 110.Mishima K, Tanaka T, Pu F, Egashira N, Iwasaki K, Hidaka R, et al. Vitamin E isoforms alpha-tocotrienol and gamma-tocopherol prevent cerebral infarction in mice. Neurosci Lett. 2003;337:56–60. doi: 10.1016/s0304-3940(02)01293-4. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Estrada J, Gonzalez-Perez O, Gonzalez-Castaneda RE, Martinez-Contreras A, Luquin S, de la Mora PG, et al. An alpha-lipoic acid-vitamin E mixture reduces post-embolism lipid peroxidation, cerebral infarction, and neurological deficit in rats. Neurosci Res. 2003;47:219–24. doi: 10.1016/s0168-0102(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 112.van der Worp HB, Thomas CE, Kappelle LJ, Hoffman WP, de Wildt DJ, Bär PR. Inhibition of iron-dependent and ischemia-induced brain damage by the alpha-tocopherol analogue MDL 74,722. Exp Neurol. 1999;155:103–8. doi: 10.1006/exnr.1998.6968. [DOI] [PubMed] [Google Scholar]
- 113.Henry PT, Chandy MJ. Effect of ascorbic acid on infarct size in experimental focal cerebral ischaemia and reperfusion in a primate model. Acta Neurochir (Wien) 1998;140:977–80. doi: 10.1007/s007010050201. [DOI] [PubMed] [Google Scholar]
- 114.Ranjan A, Theodore D, Haran RP, Chandy MJ. Ascorbic acid and focal cerebral ischaemia in a primate model. Acta Neurochir (Wien) 1993;123:87–91. doi: 10.1007/BF01476291. [DOI] [PubMed] [Google Scholar]
- 115.Sciamanna MA, Lee CP. Ischemia/reperfusion-induced injury of forebrain mitochondria and protection by ascorbate. Arch Biochem Biophys. 1993;305:215–24. doi: 10.1006/abbi.1993.1414. [DOI] [PubMed] [Google Scholar]
- 116.Ascherio A, Rimm EB, Hernán MA, Giovannucci E, Kawachi I, Stampfer MJ, et al. Relation of consumption of Vitamin E, Vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999;130:963–70. doi: 10.7326/0003-4819-130-12-199906150-00003. [DOI] [PubMed] [Google Scholar]
- 117.Rabadi MH, Kristal BS. Effect of Vitamin C supplementation on stroke recovery: A case-control study. Clin Interv Aging. 2007;2:147–51. doi: 10.2147/ciia.2007.2.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage Vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 119.Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: Current status and future prospects. Curr Med Chem. 2011;18:3871–88. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 120.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, et al. Dehydroascorbic acid, a blood-brain barrier transportable form of Vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci U S A. 2001;98:11720–4. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ducruet AF, Mack WJ, Mocco J, Hoh DJ, Coon AL, D’Ambrosio AL, et al. Preclinical evaluation of postischemic dehydroascorbic Acid administration in a large-animal stroke model. Transl Stroke Res. 2011;2:399–403. doi: 10.1007/s12975-011-0084-2. [DOI] [PubMed] [Google Scholar]
- 122.Takamatsu H, Kondo K, Ikeda Y, Umemura K. Hydroxyl radical generation after the third hour following ischemia contributes to brain damage. Eur J Pharmacol. 1998;352:165–9. doi: 10.1016/s0014-2999(98)00353-7. [DOI] [PubMed] [Google Scholar]
- 123.Kato N, Yanaka K, Nagase S, Hirayama A, Nose T. The antioxidant EPC-K1 ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischaemia. Acta Neurochir (Wien) 2003;145:489–93. doi: 10.1007/s00701-003-0036-z. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: From bench to bedside. Cardiovasc Ther. 2008;26:101–14. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 125.Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–9. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 126.Mishina M, Komaba Y, Kobayashi S, Tanaka N, Kominami S, Fukuchi T, et al. Efficacy of edaravone, a free radical scavenger, for the treatment of acute lacunar infarction. Neurol Med Chir (Tokyo) 2005;45:344–8. doi: 10.2176/nmc.45.344. [DOI] [PubMed] [Google Scholar]
- 127.Ohta Y, Takamatsu K, Fukushima T, Ikegami S, Takeda I, Ota T, et al. Efficacy of the free radical scavenger, edaravone, for motor palsy of acute lacunar infarction. Intern Med. 2009;48:593–6. doi: 10.2169/internalmedicine.48.1871. [DOI] [PubMed] [Google Scholar]
- 128.Shinohara Y, Saito I, Kobayashi S, Uchiyama S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial) Cerebrovasc Dis. 2009;27:485–92. doi: 10.1159/000210190. [DOI] [PubMed] [Google Scholar]
- 129.Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, et al. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–31. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 130.Lukic-Panin V, Deguchi K, Yamashita T, Shang J, Zhang X, Tian F, et al. Free radical scavenger edaravone administration protects against tissue plasminogen activator induced oxidative stress and blood brain barrier damage. Curr Neurovasc Res. 2010;7:319–29. doi: 10.2174/156720210793180747. [DOI] [PubMed] [Google Scholar]
- 131.Zhang W, Sato K, Hayashi T, Omori N, Nagano I, Kato S, et al. Extension of ischemic therapeutic time window by a free radical scavenger, Edaravone, reperfused with tPA in rat brain. Neurol Res. 2004;26:342–8. doi: 10.1179/016164104225014058. [DOI] [PubMed] [Google Scholar]
- 132.Lapchak PA, Araujo DM. Development of the nitrone-based spin trap agent NXY-059 to treat acute ischemic stroke. CNS Drug Rev. 2003;9:253–62. doi: 10.1111/j.1527-3458.2003.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Z, Cheng M, Maples KR, Ma JY, Buchan AM. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909:46–50. doi: 10.1016/s0006-8993(01)02618-x. [DOI] [PubMed] [Google Scholar]
- 134.Marshall JW, Duffin KJ, Green AR, Ridley RM. NXY-059, a free radical - Trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke. 2001;32:190–8. doi: 10.1161/01.str.32.1.190. [DOI] [PubMed] [Google Scholar]
- 135.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 136.Lees KR, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, et al. Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial. Stroke. 2006;37:2970–8. doi: 10.1161/01.STR.0000249410.91473.44. [DOI] [PubMed] [Google Scholar]
- 137.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–71. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 138.Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–8. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 139.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 140.Carroll JE, Howard EF, Hess DC, Wakade CG, Chen Q, Cheng C. Nuclear factor-kappa B activation during cerebral reperfusion: Effect of attenuation with N-acetylcysteine treatment. Brain Res Mol Brain Res. 1998;56:186–91. doi: 10.1016/s0169-328x(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 141.Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 142.Knuckey NW, Palm D, Primiano M, Epstein MH, Johanson CE. N-acetylcysteine enhances hippocampal neuronal survival after transient forebrain ischemia in rats. Stroke. 1995;26:305–10. doi: 10.1161/01.str.26.2.305. [DOI] [PubMed] [Google Scholar]
- 143.Anderson MF, Nilsson M, Eriksson PS, Sims NR. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci Lett. 2004;354:163–5. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 144.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–50. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 145.Panigrahi M, Sadguna Y, Shivakumar BR, Kolluri SV, Roy S, Packer L, et al. alpha-Lipoic acid protects against reperfusion injury following cerebral ischemia in rats. Brain Res. 1996;717:184–8. doi: 10.1016/0006-8993(96)00009-1. [DOI] [PubMed] [Google Scholar]
- 146.Choi KH, Park MS, Kim HS, Kim KT, Kim HS, Kim JT, et al. Alpha-lipoic acid treatment is neurorestorative and promotes functional recovery after stroke in rats. Mol Brain. 2015;8:9. doi: 10.1186/s13041-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fleishaker JC, Peters GR, Cathcart KS, Steenwyk RC. Evaluation of the pharmacokinetics and tolerability of tirilazad mesylate, a 21-aminosteroid free radical scavenger: II. Multiple-dose administration. J Clin Pharmacol. 1993;33:182–90. doi: 10.1002/j.1552-4604.1993.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 148.Park CK, Hall ED. Dose-response analysis of the effect of 21-aminosteroid tirilazad mesylate (U-74006F) upon neurological outcome and ischemic brain damage in permanent focal cerebral ischemia. Brain Res. 1994;645:157–63. doi: 10.1016/0006-8993(94)91649-7. [DOI] [PubMed] [Google Scholar]
- 149.Xue D, Slivka A, Buchan AM. Tirilazad reduces cortical infarction after transient but not permanent focal cerebral ischemia in rats. Stroke. 1992;23:894–9. doi: 10.1161/01.str.23.6.894. [DOI] [PubMed] [Google Scholar]
- 150.Sena E, Wheble P, Sandercock P, Macleod M. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007;38:388–94. doi: 10.1161/01.STR.0000254462.75851.22. [DOI] [PubMed] [Google Scholar]
- 151.A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). The RANTTAS Investigators. Stroke. 1996;27:1453–8. doi: 10.1161/01.str.27.9.1453. [DOI] [PubMed] [Google Scholar]
- 152.Haley EC., Jr High-dose tirilazad for acute stroke (RANTTAS II). RANTTAS II Investigators. Stroke. 1998;29:1256–7. [PubMed] [Google Scholar]
- 153.Tirilazad mesylate in acute ischemic stroke: A systematic review. Tirilazad International Steering Committee. Stroke. 2000;31:2257–65. doi: 10.1161/01.str.31.9.2257. [DOI] [PubMed] [Google Scholar]
- 154.Kennedy EP. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem. 1956;222:185–91. [PubMed] [Google Scholar]
- 155.Trovarelli G, de Medio GE, Dorman RV, Piccinin GL, Horrocks LA, Porcellati G. Effect of cytidine diphosphate choline (CDP-choline) on ischemia-induced alterations of brain lipid in the gerbil. Neurochem Res. 1981;6:821–33. doi: 10.1007/BF00965041. [DOI] [PubMed] [Google Scholar]
- 156.Bustamante A, Giralt D, Garcia-Bonilla L, Campos M, Rosell A, Montaner J. Citicoline in pre-clinical animal models of stroke: A meta-analysis shows the optimal neuroprotective profile and the missing steps for jumping into a stroke clinical trial. J Neurochem. 2012;123:217–25. doi: 10.1111/j.1471-4159.2012.07891.x. [DOI] [PubMed] [Google Scholar]
- 157.Clark WM, Warach SJ, Pettigrew LC, Gammans RE, Sabounjian LA. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology. 1997;49:671–8. doi: 10.1212/wnl.49.3.671. [DOI] [PubMed] [Google Scholar]
- 158.Clark WM, Wechsler LR, Sabounjian LA, Schwiderski UE Citicoline Stroke Study Group. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–602. doi: 10.1212/wnl.57.9.1595. [DOI] [PubMed] [Google Scholar]
- 159.Clark WM, Williams BJ, Selzer KA, Zweifler RM, Sabounjian LA, Gammans RE. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke. 1999;30:2592–7. doi: 10.1161/01.str.30.12.2592. [DOI] [PubMed] [Google Scholar]
- 160.Dávalos A, Castillo J, Alvarez-Sabín J, Secades JJ, Mercadal J, López S, et al. Oral citicoline in acute ischemic stroke: An individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–7. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- 161.Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, et al. Citicoline in the treatment of acute ischaemic stroke: An international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–57. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 162.Lenzi GL, Grigoletto F, Gent M, Roberts RS, Walker MD, Easton JD, et al. Early treatment of stroke with monosialoganglioside GM-1. Efficacy and safety results of the Early Stroke Trial. Stroke. 1994;25:1552–8. doi: 10.1161/01.str.25.8.1552. [DOI] [PubMed] [Google Scholar]
- 163.Ganglioside GM1 in acute ischemic stroke. The SASS Trial. Stroke. 1994;25:1141–8. doi: 10.1161/01.str.25.6.1141. [DOI] [PubMed] [Google Scholar]
- 164.Candelise L, Ciccone A. Gangliosides for acute ischaemic stroke. Cochrane Database Syst Rev. 2001;4:CD000094. doi: 10.1002/14651858.CD000094. [DOI] [PubMed] [Google Scholar]
- 165.He YY, Hsu CY, Ezrin AM, Miller MS. Polyethylene glycol-conjugated superoxide dismutase in focal cerebral ischemia-reperfusion. Am J Physiol. 1993;265(1 Pt 2):H252–6. doi: 10.1152/ajpheart.1993.265.1.H252. [DOI] [PubMed] [Google Scholar]
- 166.Uyama O, Shiratsuki N, Matsuyama T, Nakanishi T, Matsumoto Y, Yamada T, et al. Protective effects of superoxide dismutase on acute reperfusion injury of gerbil brain. Free Radic Biol Med. 1990;8:265–8. doi: 10.1016/0891-5849(90)90072-q. [DOI] [PubMed] [Google Scholar]
- 167.Tagaya M, Matsumoto M, Kitagawa K, Niinobe M, Ohtsuki T, Hata R, et al. Recombinant human superoxide dismutase can attenuate ischemic neuronal damage in gerbils. Life Sci. 1992;51:253–9. doi: 10.1016/0024-3205(92)90083-2. [DOI] [PubMed] [Google Scholar]
- 168.Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Modesti A, et al. The protective effect of M40401, a superoxide dismutase mimetic, on post-ischemic brain damage in Mongolian gerbils. BMC Pharmacol. 2003;3:8. doi: 10.1186/1471-2210-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shimizu K, Rajapakse N, Horiguchi T, Payne RM, Busija DW. Protective effect of a new nonpeptidyl mimetic of SOD, M40401, against focal cerebral ischemia in the rat. Brain Res. 2003;963:8–14. doi: 10.1016/s0006-8993(02)03796-4. [DOI] [PubMed] [Google Scholar]
- 170.Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34:338–45. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCann SK, Roulston CL. NADPH Oxidase as a Therapeutic Target for Neuroprotection against Ischaemic Stroke: Future Perspectives. Brain Sci. 2013;3:561–98. doi: 10.3390/brainsci3020561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–22. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 173.Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–62. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang XY, Zhao N, Liu YY, Hu BH, Sun K, Chang X, et al. Inhibition of NADPH Oxidase Mediates Protective Effect of Cardiotonic Pills against Rat Heart Ischemia/Reperfusion Injury. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/728020. 728020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 176.Nagel S, Genius J, Heiland S, Horstmann S, Gardner H, Wagner S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–7. doi: 10.1016/j.brainres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 177.Williams HC, Griendling KK. NADPH oxidase inhibitors: New antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 178.Sun QA, Hess DT, Wang B, Miyagi M, Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl) thio-1,2,3-triazolo[4,5-d] pyrimidine (VAS2870) Free Radic Biol Med. 2012;52:1897–902. doi: 10.1016/j.freeradbiomed.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P) H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–14. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 180.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox 4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–30. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 181.Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, et al. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: A randomized, double-blind, placebo-controlled trial. Stroke. 2008;39:3303–7. doi: 10.1161/STROKEAHA.108.519793. [DOI] [PubMed] [Google Scholar]
- 182.Dawson J, Quinn TJ, Harrow C, Lees KR, Walters MR. The effect of allopurinol on the cerebral vasculature of patients with subcortical stroke; a randomized trial. Br J Clin Pharmacol. 2009;68:662–8. doi: 10.1111/j.1365-2125.2009.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taheraghdam AA, Sharifipour E, Pashapour A, Namdar S, Hatami A, Houshmandzad S, et al. Allopurinol as a preventive contrivance after acute ischemic stroke in patients with a high level of serum uric acid: A randomized, controlled trial. Med Princ Pract. 2014;23:134–9. doi: 10.1159/000355621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martz D, Rayos G, Schielke GP, Betz AL. Allopurinol and dimethylthiourea reduce brain infarction following middle cerebral artery occlusion in rats. Stroke. 1989;20:488–94. doi: 10.1161/01.str.20.4.488. [DOI] [PubMed] [Google Scholar]
- 185.Itoh T, Kawakami M, Yamauchi Y, Shimizu S, Nakamura M. Effect of allopurinol on ischemia and reperfusion-induced cerebral injury in spontaneously hypertensive rats. Stroke. 1986;17:1284–7. doi: 10.1161/01.str.17.6.1284. [DOI] [PubMed] [Google Scholar]
- 186.Phillis JW, Perkins LM, Smith-Barbour M, O’Regan MH. Oxypurinol-enhanced postischemic recovery of the rat brain involves preservation of adenine nucleotides. J Neurochem. 1995;64:2177–84. doi: 10.1046/j.1471-4159.1995.64052177.x. [DOI] [PubMed] [Google Scholar]
- 187.Shadid M, Moison R, Steendijk P, Hiltermann L, Berger HM, van Bel F. The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr Res. 1998;44:119–24. doi: 10.1203/00006450-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 188.Akdemir H, Asik Z, Pasaoglu H, Karaküçük I, Oktem IS, Koç RK. The effect of allopurinol on focal cerebral ischaemia: An experimental study in rabbits. Neurosurg Rev. 2001;24:131–5. doi: 10.1007/pl00012397. [DOI] [PubMed] [Google Scholar]
- 189.Miyamoto Y, Akaike T, Yoshida M, Goto S, Horie H, Maeda H. Potentiation of nitric oxide-mediated vasorelaxation by xanthine oxidase inhibitors. Proc Soc Exp Biol Med. 1996;211:366–73. doi: 10.3181/00379727-211-43982. [DOI] [PubMed] [Google Scholar]
- 190.Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem. 2003;278:1848–55. doi: 10.1074/jbc.M208307200. [DOI] [PubMed] [Google Scholar]
- 191.Becker MA, Kisicki J, Khosravan R, Wu J, Mulford D, Hunt B, et al. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004;23:1111–6. doi: 10.1081/NCN-200027372. [DOI] [PubMed] [Google Scholar]
- 192.Fukunari A, Okamoto K, Nishino T, Eger BT, Pai EF, Kamezawa M, et al. Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy) phenyl]-1H-pyrazole-4-carboxylic acid]: A potent xanthine oxidoreductase inhibitor with hepatic excretion. J Pharmacol Exp Ther. 2004;311:519–28. doi: 10.1124/jpet.104.070433. [DOI] [PubMed] [Google Scholar]
- 193.Naito S, Nishimura M, Tamao Y. Evaluation of the pharmacological actions and pharmacokinetics of BOF-4272, a xanthine oxidase inhibitor, in mouse liver. J Pharm Pharmacol. 2000;52:173–9. doi: 10.1211/0022357001773823. [DOI] [PubMed] [Google Scholar]
- 194.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001;98:1294–9. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Doré S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, et al. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54:155–62. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- 196.Nagayama M, Niwa K, Nagayama T, Ross ME, Iadecola C. The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab. 1999;19:1213–9. doi: 10.1097/00004647-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 197.Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res. 2003;960:273–6. doi: 10.1016/s0006-8993(02)03805-2. [DOI] [PubMed] [Google Scholar]
- 198.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 199.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol. 2013;73:129–35. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–91. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 201.Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–12. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 202.Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998;95:8892–7. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grieb P, Ryba MS, Sawicki J, Chrapusta SJ. Oral coenzyme Q10 administration prevents the development of ischemic brain lesions in a rabbit model of symptomatic vasospasm. Acta Neuropathol. 1997;94:363–8. doi: 10.1007/s004010050720. [DOI] [PubMed] [Google Scholar]
- 204.Ostrowski RP. Effect of coenzyme Q10 (CoQ10) on superoxide dismutase activity in ET-1 and ET-3 experimental models of cerebral ischemia in the rat. Folia Neuropathol. 1999;37:247–51. [PubMed] [Google Scholar]
- 205.Li G, Zou L, Jack CR, Jr, Yang Y, Yang ES. Neuroprotective effect of Coenzyme Q10 on ischemic hemisphere in aged mice with mutations in the amyloid precursor protein. Neurobiol Aging. 2007;28:877–82. doi: 10.1016/j.neurobiolaging.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 206.Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: Preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–94. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl):S154–67. doi: 10.1016/j.mito.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 208.Sarter B. Coenzyme Q10 and cardiovascular disease: A review. J Cardiovasc Nurs. 2002;16:9–20. doi: 10.1097/00005082-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 209.Parkinson Study Group QE Investigators. Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014;71:543–52. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 210.James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–18. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 211.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, et al. Neuroprotection by a mitochondria-targeted drug in a Parkinson's disease model. Free Radic Biol Med. 2010;49:1674–84. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–8. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 214.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–95. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 215.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–8. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 216.Plotnikov EY, Silachev DN, Jankauskas SS, Rokitskaya TI, Chupyrkina AA, Pevzner IB, et al. Mild uncoupling of respiration and phosphorylation as a mechanism providing nephro- and neuroprotective effects of penetrating cations of the SkQ family. Biochemistry (Mosc) 2012;77:1029–37. doi: 10.1134/S0006297912090106. [DOI] [PubMed] [Google Scholar]
- 217.Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–15. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang Z, Watts LT, Huang S, Shen Q, Rodriguez P, Chen C, et al. The effects of methylene blue on autophagy and apoptosis in MRI-defined normal tissue, ischemic penumbra and ischemic core. PLoS One. 2015;10:e0131929. doi: 10.1371/journal.pone.0131929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shen Q, Du F, Huang S, Rodriguez P, Watts LT, Duong TQ. Neuroprotective efficacy of methylene blue in ischemic stroke: An MRI study. PLoS One. 2013;8:e79833. doi: 10.1371/journal.pone.0079833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rojas J, Plautz E. Ann Stowe and Mark Goldberg. Neurology. 2014;82(10 Supplement P1):104. [Google Scholar]
- 221.O’Leary JL, Petty J, Harris AB, Inukai J. Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol. 1968;43:197–201. doi: 10.3109/10520296809115068. [DOI] [PubMed] [Google Scholar]
- 222.Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, et al. Neuroprotective actions of methylene blue and its derivatives. PLoS One. 2012;7:e48279. doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]


