Abstract
This paper addresses how hormesis, a biphasic dose response, can protect and affect performance of neural systems. Particular attention is directed to the potential role of hormesis in mitigating age-related neurodegenerative diseases, genetically based neurological diseases, as well as stroke, traumatic brain injury, seizure, and stress-related conditions. The hormetic dose response is of particular significance since it mediates the magnitude and range of neuroprotective processes. Consideration of hormetic dose-response concepts can also enhance the quality of study designs, including sample size/statistical power strategies, selection of treatment groups, dose spacing, and temporal/repeat measures’ features.
Keywords: Biphasicdose response, hormesis, hormetic dose response, neuroprotection, postconditioning, preconditioning
Introduction
Ongoing neuroscientific research is focused on preventing and/or reducing the occurrence and extent of neuropsychiatric disease and neurological injury-based processes/events, mitigating age-related decrements in neurocognitive performance and improving defined aspects of cognitive performance in healthy individuals. These research domains share a grounding impetus to access, assess, and affect the nervous system in attempts to enhance health and performance throughout the lifespan.
Achieving these key ends remains somewhat problematic, given the diversity of functions, substrates, and mechanisms putatively involved, and the defined limitations of extant approaches commonly employed. When assessing gaps in such approaches, it becomes apparent that a common goal would be to achieve an optimal biological response that evokes desired effects in a number of neurological substrates and processes. To date, most interventions have been characteristically engaged and evaluated within a dose-response context that is considered to enable the pharmacokinetic and pharmacodynamic effects desired. However, it is becoming clear that such dose-response parameters may be less than optimal in exploiting the amplification mechanisms of the nervous system,[1] and thus may tend to produce unwanted, or in some cases adverse effects, while in other cases, may appear to be ineffective (due to paradoxical actions). In light of this, we propose that response optimization can positively affect neurobiological functions toward evoking a variety of desirable end points and that such effects are both defined and mediated by hormesis. This paper assesses the concept of hormetic dose responses, the occurrence and significance of these processes in and to the protection and functional optimization of neurological systems, and the putative molecular and cellular mechanisms subserving such responses and effects.
Hormesis: A Historical Overview
The term hormesis, originating from the Greek “to excite,” was first introduced into the biomedical lexicon by Southam and Ehrlich[2] to describe the effects of extracts of the Red Cedar tree on wood-rotting fungi. These investigators found that several species of fungi display a low-dose stimulation and a high-dose inhibition for cell metabolism and survival. Southam and Ehrlich were aware of historically old descriptions of this type of biphasic dose response, with reports dating from the 1880s by Schulz,[3,4] who assessed the effects of multiple disinfectants on yeast metabolism and survival. By the 20th century, such research became increasingly more widespread, especially in microbiology and botany, wherein the biphasic dose response was seen as a general biological phenomenon. Detailed summarization and overview of the historicity and canonical development and dissemination of hormesis research is provided by Calabrese and Baldwin,[5,6,7,8,9] and Calabrese[10] also provides an assessment of controversies and challenges surrounding the validity, viability, and value of this dose-response model within the conventional biological and medical paradigms.
Despite such challenges and controversies, what has become clear is that hormesis represents a biphasic dose response that occurs following either direct stimulation by a chemical or physical agent or as an overcompensatory response to toxic or homeostatically disruptive insult.[11] Regardless of how the hormetic dose response is induced, its quantitative features (i.e., amplitude and width of the stimulation) are similar[12,13] and appear to be independent of mechanism. Furthermore, the highly generalizable amplitude of hormetic stimulation suggests that the quantitative features of the hormetic dose response may be a measure of biological plasticity, which reflects the relative “gain” of the system affected.[14] Quantitative evaluations indicate that hormetic stimulation conforms to an allometrically estimable parameter.[14,15] In this light, hormetic dose responses can be seen as evolutionarily based, adaptive, and highly generalizable,[16] reflecting a biological process of optimizing and managing the use of cellular resources under a variety of temporal, environmental, and stress-related conditions and effects.[17]
Hormesis and Preconditioning: A Role in Neuroprotection
First reports in the biomedical literature
While it has been over 130 years since Schulz made his first presentation on hormesis to the Greifswald Medical Society in 1884,[18] the first report associating hormesis with neuroprotection appeared in the biomedical literature in 1999; Jonas et al.[19] described how a prior low dose of glutamate may protect against subsequent higher and toxic doses of glutamate in a preconditioning (PC) experiment. Hormetic neuroprotection was subsequently reported by Andoh et al.[20] [Table 1].
Table 1.
The historical listing of hormesis and neuroscience and hormesis and neuroprotection in PubMed and web of science
| Hormesis and Neuroscience |
| Arumugam TV, Gleichmann M, Tang SC, Mattson MP. 2006. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 5(2):165-78. |
| Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, Guo Z, Lee J, Furukawa K. 2002. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging 23(5):695-705. |
| Mattson MP, Chan SL, Duan W. 2002. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 82(3):637-72. |
| Hormesis and Neuroprotection |
| *Andoh T, Chock PB, Chiueh CC. 2002. The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J Biol Chem. 22;277(12):9655-60. |
| Jonas, W; Lin, Y; Tortella, F. 2001. Neuroprotection from glutamate toxicity with ultra-low dose glutamate. NeuroReport 12(2): 335-339. |
| Jonas, W; Lin, Y; Williams, A; et al. 1999. Treatment of experimental stroke with low-dose glutamate and homeopathic Arnica montana. Perfusion 12(11): 452-+ |
| Preconditioning and Neuroprotection/Neuroscience |
| Chen J, Graham SH, Zhu RL, Simon RP. 1996. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 16(4):566-577. |
| Gage AT, Stanton PK. 1996. Hypoxia triggers neuroprotective alterations in hippocampal gene expression via a heme-containing sensor. Brain Res. 719(1-2):172-178. |
| Matsushima K, Hakim AM. 1995. Transient forebrain ischemia protects against subsequent focal cerebral ischemia without changing cerebral perfusion. Stroke 26(6):1047-1052. |
| Gidday JM, Fitzgibbons JC, Shah AR, Park TS. 1994. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 168(1-2):221-224. |
| Liu Y, Kato H, Nakata N, Kogure K. 1993. Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience. 56(4):921-927. |
*First paper that linked hormesis, preconditioning and neuroprotection
Initial correlation of PC to neuroprotection appeared in the biomedical literature in the journal Neuroscience, in a study examining HSP70 synthesis in ischemic tolerance induced by PC effects in the rat hippocampus.[15] This paper preceded by 15 years the publication of Calabrese et al.[21] which integrated the concept of PC with hormesis, and proposed a common set of terms to describe biological stress responses within hormetic contexts.
Despite the fact that a role for hormesis in neuroprotection has explicitly emerged within the research community within the past two decades, review of PubMed/Web of Science listings (i.e., for hormesis) revealed that the historical study of hormesis and the possibility of hormetic PC to affect neurological function had heretofore not been well depicted and fully recognized in the neuroscientific literature [Table 1]. For example, while there are >1700 citations in the Web of Science for PC and neuroprotection, there are only 40 citations for hormesis and neuroprotection, regardless of the evolving appreciation that PC is a manifestation of hormesis.[22,23,24] This lack of recognition, and a failure to integrate the hormesis concept into the neuroscientific/neuroprotection literature, appears to be due to many factors, including, but not limited to a lack of understanding of the quantitative aspects of hormetic dose-response features that persisted until the late 1990s,[25,26,27,28] the use of multiple terms to describe hormetic dose responses (e.g., biphasic, U-shaped, J-shaped, Arndt-Schulz Law, Hueppe's Rule, bitonic, hormoligosis, rebound effect, repeat bout effect, etc.), lack of mechanistic understanding,[29] difficulty in assessment and replication of hormetic effects due to the modest nature of the low dose stimulatory response, and lack of strategy in end point selection.
A role for hormesis in the performance and protection of neurobiological systems
Neurobiological performance and neuroprotection mediated by hormetic mechanisms and manifested within the framework of hormetic-biphasic dose-response relationships were the foci of a thematic issue of Critical Reviews in Toxicology in 2008.[30] This issue included 14 papers devoted to diverse areas of neuroscience, in which hormetic dose responses were observed to play a significant role in reducing damage during normal aging,[30,31] slowing the onset of major neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease, Huntington's disease, etc.),[31,32,33] and reducing damage from stroke and traumatic brain injury.[34] Hormetic effects were also shown to facilitate neurite outgrowth,[35] modulate pain,[36] mediate stress responses,[37] and enhance adaptive responses in astrocytes.[38] Hormetic responses were extensively observed in studies assessing pharmacological interventions to enhance memory,[33] decrease anxiety,[39] prevent seizure onset, and reduce seizure severity.[40] Each of these papers was subjected to independent evaluations and critiques.[41,42,43,44,45]
These papers demonstrated the occurrence and commonality of hormetic responses in neurological systems and extended the generality of hormetic dose responses to neuroprotective processes at molecular, cellular, and organismal levels of biological organization. The analyses indicated that quantitative features of the adaptive/protective responses in neuroprotection studies were similar, regardless of end point measured, biological model, mechanism, therapeutic agent employed, or disease process(es) studied.[29,46] Of particular interest was the demonstration that hormetic-like biphasic dose-response relationships in neurobiological systems had been reported in the literature for nearly a century[47] without recognition that such dose responses were similar to those reported in other fields of the biological sciences, and without placing such findings in broader neuroscientific context.
As potentially novel as these findings of hormetic responses were for neuroscience, it is important to note that they were in fact wholly consistent with the existing and rather extensive literature demonstrating hormesis in other domains of the biological sciences.[5,6,7,8,9,12,13,48,49,50,51,52] For example, decades prior to the 2008 Critical Reviews in Toxicology issue on neuroscience and hormesis, a German language journal, Cell Stimulation Research (Zell Stimulations-Forschungen, 1924–1930)[53] published findings on hormesis during the 1920s, and reports of hormesis were also provided in a journal-like publication, the Stimulation Newsletter.[54] Luckey[55,56] published two books that provided considerable documentation of ionizing radiation-induced hormesis within a variety of biological models. Likewise, Stebbing[57,58,59] published substantial research addressing toxicology and hormesis, with particular emphasis on effects in the marine environment.
The first conference addressing hormesis was held in August 1985 in Oakland, California, with peer-reviewed proceedings published in the journal Health Physics 2 years later. Subsequently, Calabrese extended these efforts and conducted a series of conferences, which began in the 1990s[60,61,62] and which continue to the present. These meetings convene an international cadre of researchers who have studied, assessed, and documented evidence demonstrating hormesis in immunologic systems,[63] tumor cell biology,[64] mediating effects of pharmacological interventions inclusive of adrenergic agents,[65] prostaglandins,[66] xanthines,[67] nitric oxide,[68] serotonin (5-hydroxytryptamine),[69] opioids,[70] dopamine,[71] estrogens,[72] androgens,[73] as well as heavy metals[74] and mediating apoptosis.[75]
Following this consolidation of information, Calabrese et al.[21] proposed that biological stress terminology, including the concepts of pre- and post-conditioning, be incorporated within a hormetic framework. These developments may provide a scientific foundation upon which to structure the integration of new findings on hormesis and pre- and post-conditioning to the current and future knowledge of processes and mechanisms of neuroprotection,[12] and to enable greater understanding of frequency and temporal aspects of hormetic-biphasic dose-response relationships in neural systems.[76,77,78,79,80,81,82]
Hormetic dose-response effects appear to be involved in the action of pharmacological agents that have been shown to enhance social interactions,[39] decrease anxiety,[39] reduce pain,[36] and enhance memory[33] [Figure 1]. In this latter regard, it is noteworthy that all drugs currently approved by the United States Food and Drug Administration to relieve symptoms of Alzheimer's disease and to reduce seizures have been shown to display hormetic dose responses during preclinical phases of testing in animal models.[33,40]
Figure 1.
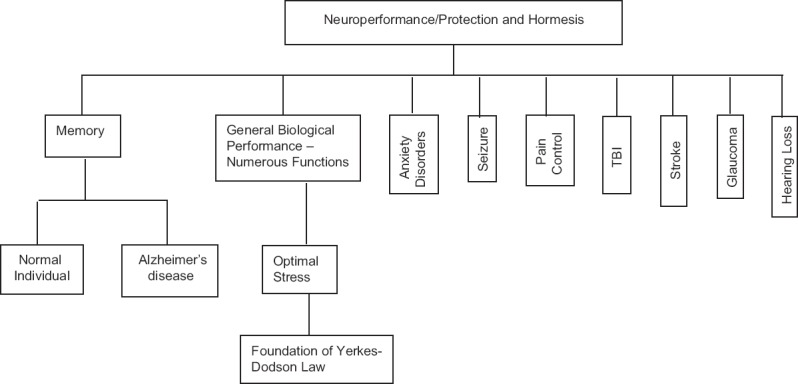
Partial listing of neuroperformance/protection domains affected by hormesis
Hormetic responses in these models do not constitute neuroprotection per se, but increasing certain aspects of neural function can and frequently does exert influence upon neuroprotective mechanisms and effects. For example, if neural mechanisms are impaired (e.g., via aging processes, genetic predisposition, injury, etc.), it is likely that a decrement in the performance of particular cognitive and/or behavioral capabilities and tasks will result. Pre- and/or post-conditioning processes may provide some modicum of protection against these insults and effects. Conversely, if and when these functions are maintained and/or facilitated in a healthy individual, such effects would be considered to be a type of neurological performance optimization.[83] This is not merely semantics; rather, terms and definitions used and their meanings employed in medical, social, and legal contexts are important to establishing standards and guidelines that can influence, if not direct, research agenda and the relative view and value of research outcomes for translational use in practice.[84,85,86]
An experimental approach to assessing hormesis
The assessment of hormesis in experimental contexts can be challenging given that the magnitude of the low-dose stimulation is modest, typically being only 30%–60% greater than the control group.[12] This biphasic dose response is also temporally dependent, making the hormetic response a dose-time response. This necessitates conducting experiments to assess an adequate dose-range within a repeated measures experimental design. It also requires strong statistical power and appropriate experimental replication. Knowledge of control group variation is extremely important when assessing hormetic dose responses. The use of a control group with low variability is critical to the protocol. These factors are essential for creating the necessary conditions in which hypotheses regarding hormetic dose-response effects can and should be effectively tested and evaluated.
These experimental parameters have important implications for many types of low-dose assessments. For example, they may place specific constraints on high throughput studies that utilize thousands of compounds. Such a range of compounds may have a wide spectrum of physiochemical properties that differentially affect the uptake and action of ligands at particular subcellular substrates, which could then elicit physiological effects and outcomes at a variety of levels within a biological system. Since high throughput systems often test compounds at only one-time point, hormetic dose responses can be masked. Further, hormetic evaluations typically entail the requirement to first estimate a threshold response so that subthreshold doses can be tested in subsequent evaluations, and this needs to be considered (and implemented) in any/all research that aims to assess putative hormetic effects.
An example of a study design that effectively evaluated several hormetic hypotheses is provided by Zhang et al.,[87] whose research studied both direct stimulation-induced hormesis and the relation of hormetic responses to PC effects. As shown in Figure 2, Zhang et al.[87] presented effects of camptothecin (CPT) on PC12 cells (a rat pheochromocytoma cell line, which produces dopamine and exhibits characteristics that are consistent with neuronal cells), across 11 concentrations (i.e., 1400-fold concentration range). CPT is a monoterpene indole alkaloid that inhibits a topoisomerase-1 by stabilizing the enzyme-DNA complex. Topoisomerases are enzymes that affect supercoiling during the DNA replication process. These studies revealed a hormetic biphasic dose response with a maximum stimulation of ~40%, and a stimulatory range of ~44-fold (0.01–0.44 uM). After completing and replicating the experiment, the authors then evaluated CPT within a PC protocol in which CPT was administered 24 h before an oxidizing challenge induced by perfusion with hydrogen peroxide (H2O2). Of note was that H2O2 diminished the response of the control group and each of the four treatment groups in a manner that was approximately proportional to the response seen in the direct stimulatory experiment. Thus, although the PC treatment was not able to fully protect the PC12 (i.e., prevent any decrease from the original control group values), it did incur an increase in response that was 40% greater than that seen in the (H2O2-treated) control group.
Figure 2.
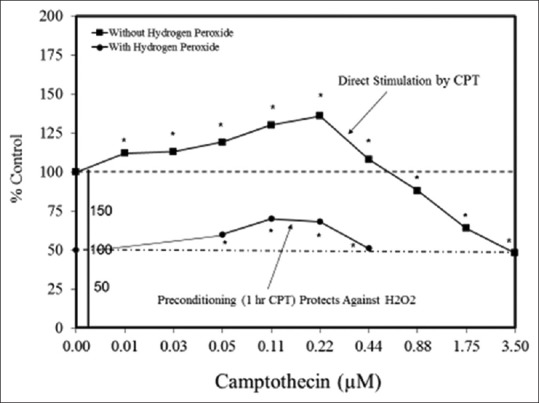
Effects of camptothecin on PC12 cell viability using direct stimulation and preconditioning protocols (adapted from: Zhang et al.)[87]
Zhang et al.[87] additionally addressed the mechanism(s) by which CPT enhances the viability of PC12 cells via direct stimulation and/or PC. Low concentrations of CPT enhanced cell proliferation by upregulating p-P13k, p-AKT, and p-mTOR, as well as the expression of several proteins, including HO-1 and Nrf2. CPT also downregulated PTEN expression. These findings strengthen the hypothesis that the hormetic and neuroprotective effects of low concentrations of CPT in PC12 cells occurred via upregulation of P13k/AKT/mTOR and Nrf2/HO-1 pathways. The capacity of CPT to enhance MTT at low concentrations was also shown, suggesting a putative role for mitochondrial metabolism in the hormetic process. The administration of the P13 inhibitor, LY294002, blocked the low-dose stimulation, further suggesting that the P13k pathway is involved in hormetic and neuroprotective effects of CPT in PC12 cells.
Figure 3(a–y) provides several examples of hormetic dose responses in neurobiological models. These examples were selected to illustrate the range, diversity, and generality of the hormetic dose-response in neural systems. As well, these examples illustrate the generally consistent quantitative features of the hormetic dose response, which is especially evident with respect to the amplitude of response. In some cases, detailed mechanistic findings are represented and/or summarized in the figures, as related to either specific receptors and/or cell signaling pathways that have been shown to mediate the hormetic response.
Figure 3(a).
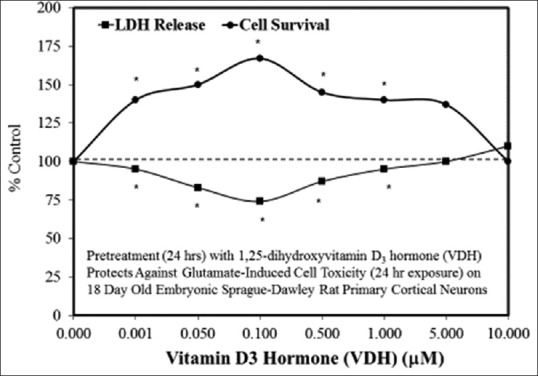
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(y).
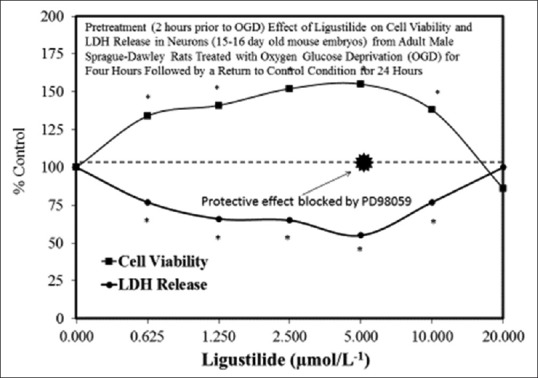
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(e).
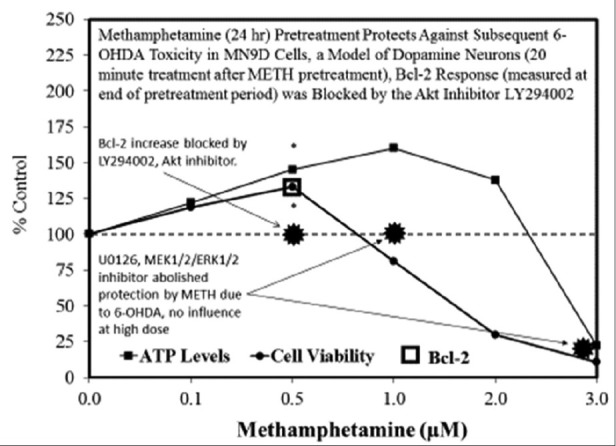
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(f).
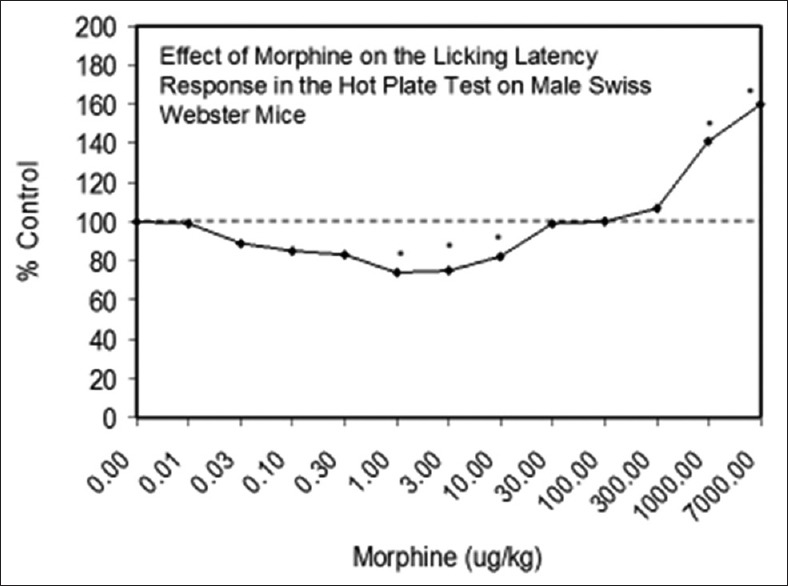
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(g).

Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(h).
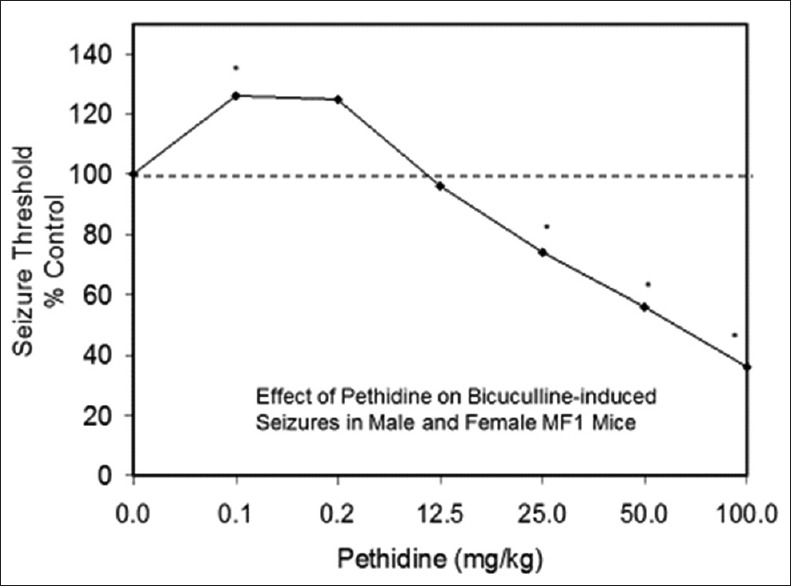
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(i).
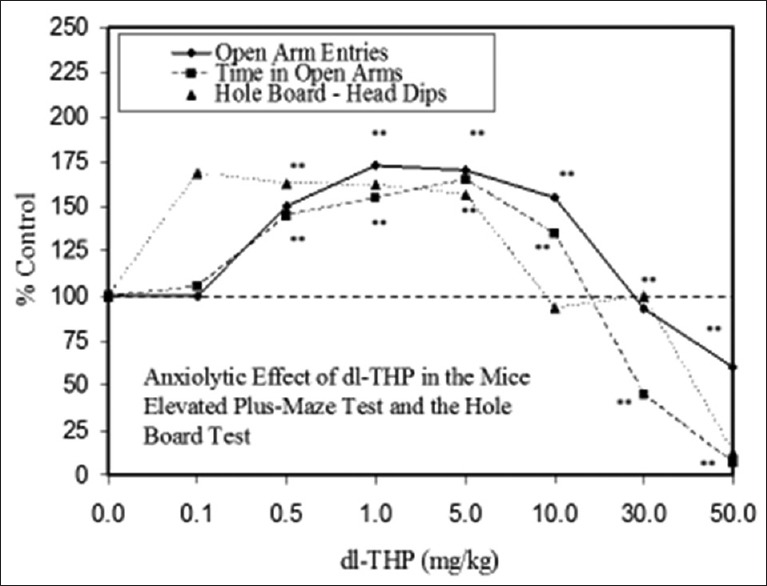
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(j).
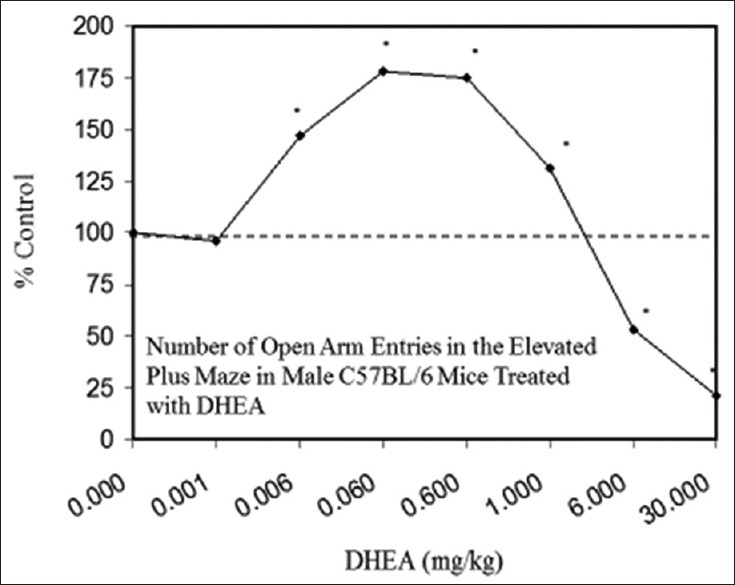
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(k).
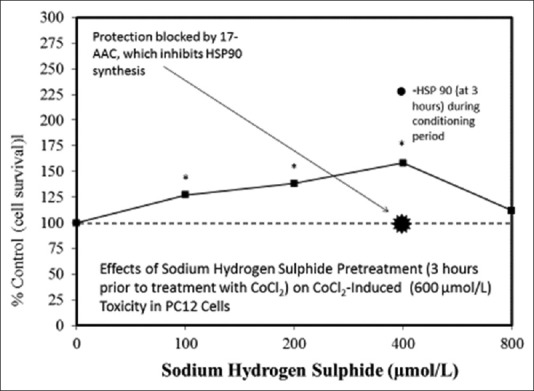
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(l).
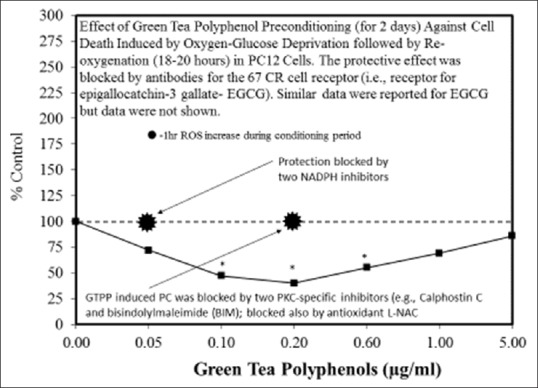
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(m).
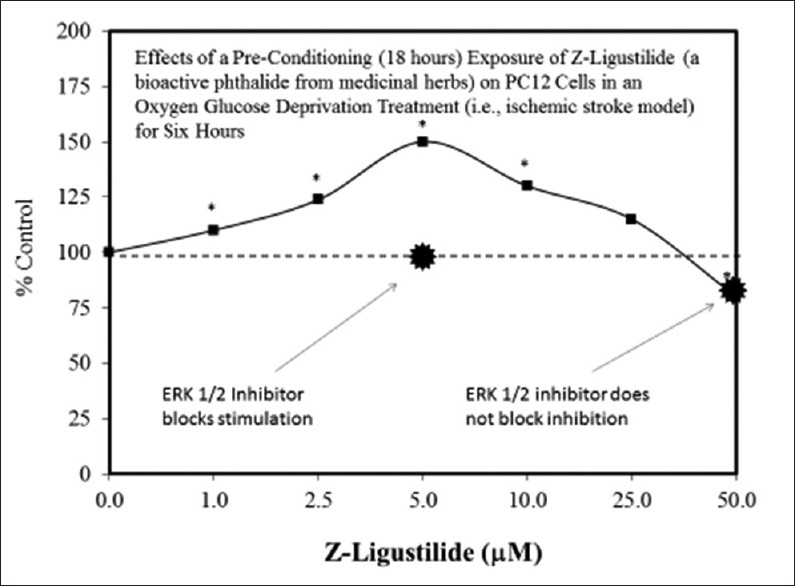
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(n).
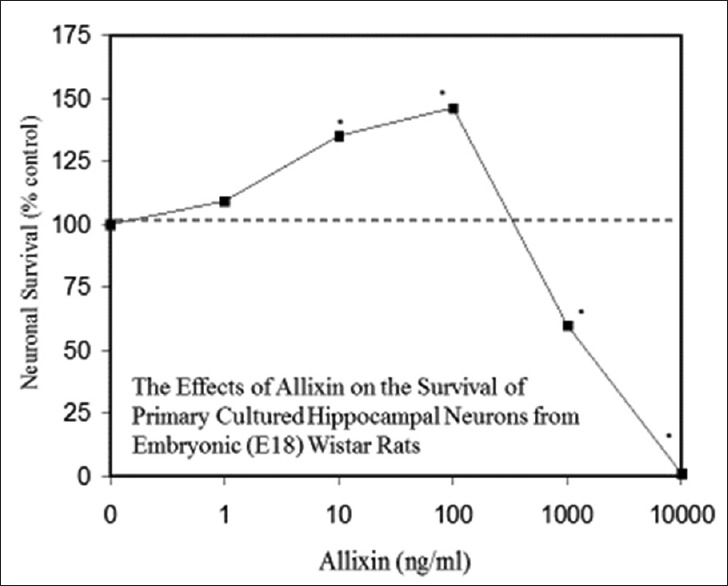
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(o).

Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(p).
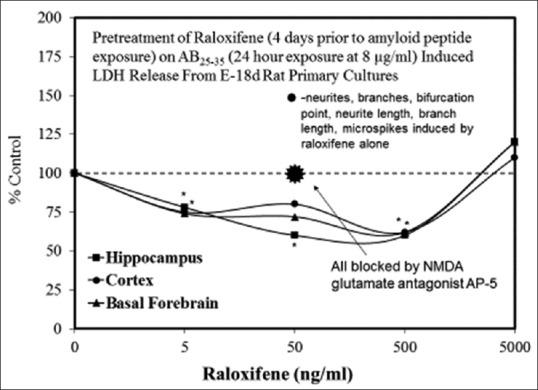
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(q).
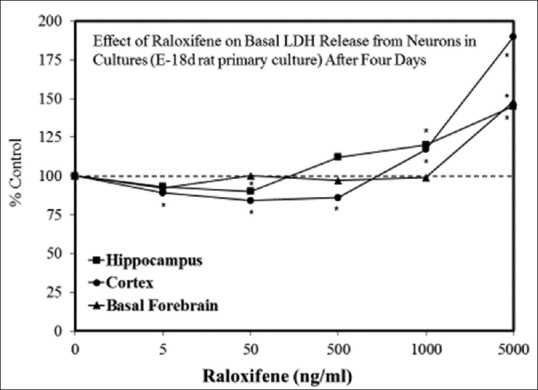
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(r).
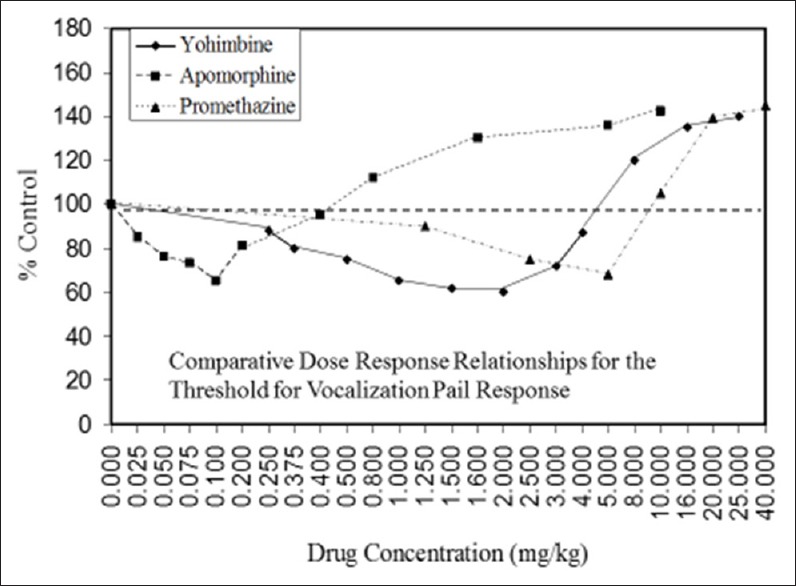
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(s).
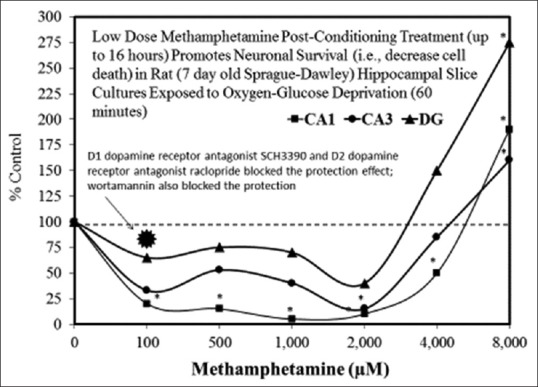
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(t).
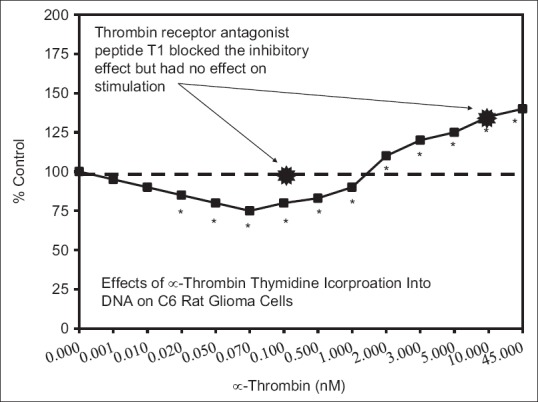
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(u).
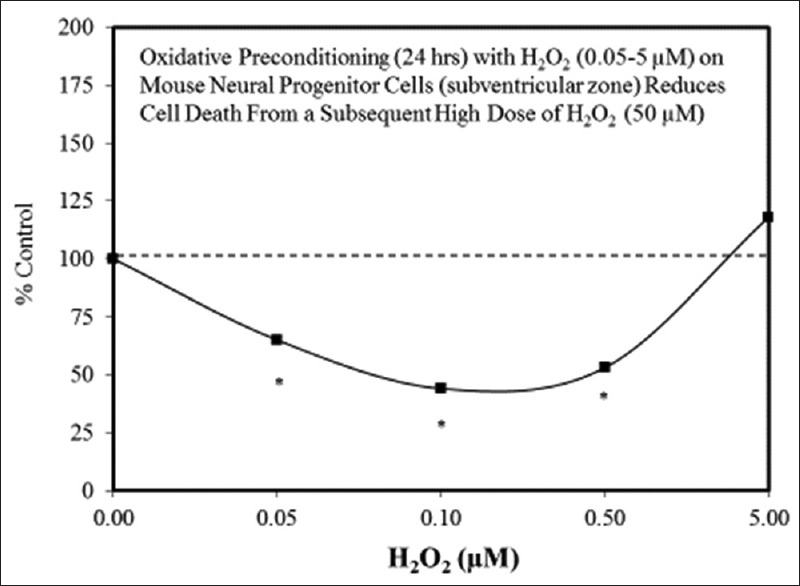
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(v).
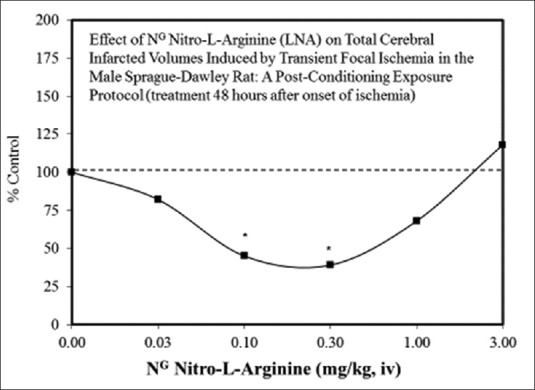
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(w).
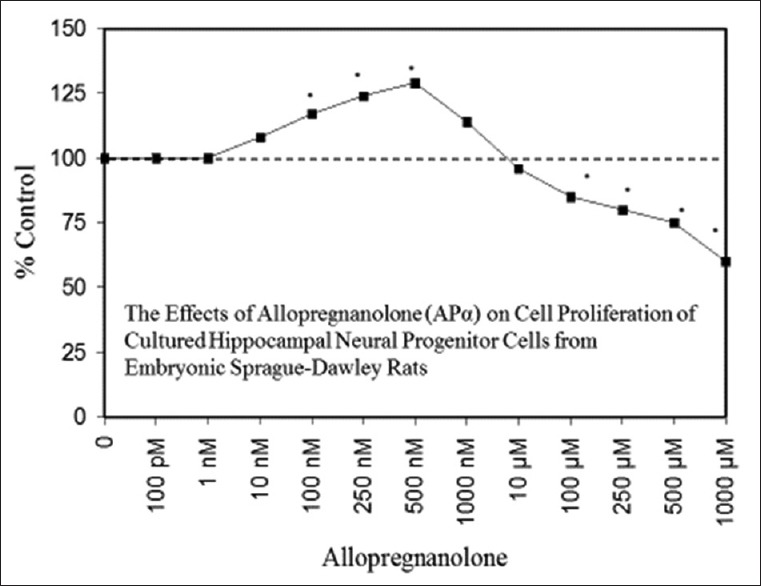
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(x).

Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Support for Hormetic Effects in Neural Systems: The Hormesis Data Base
Within the hormesis data base,[13] there are almost three hundred entries for hormetic dose-response effects in neural systems and models. Assessment of these entries reveals that ~87% were from in vitro studies, and ~13% were from in vivo studies. Of these studies, 80% have >3 doses below the zero equivalent point (ZEP) (i.e., threshold), and 42.5% have >5 doses below the ZEP [Figure 4]. Consistent with other end points (i.e., as shown in non-neurobiologically based studies), <20% of the dose responses in neurobiological systems exhibited maximum response in an inverted U-shaped dose response that was greater than twice the control group value, while approximately 80% had a maximum response between 10% and 100% greater than the control group [Figure 5]. While nearly 85% of the in vivo studies displayed a width of stimulation within 100-fold of the ZEP, this was the case for only 52.6% for the in vitro studies, with 21% of these displaying a stimulating width of >1000-fold [Figure 6]. This marked contrast may be an artifact of in vitro studies, which lack more complex biological regulatory controls or may be an issue related to the limited sample size in the in vivo studies. Hormetic dose responses have been shown in a variety of neural systems and models (e.g. a range of PC12, MN9D cells [a dopamine neuron model], HT-22 cells [mouse hippocampal cells], rat glioma cells, and RBE-4 [rat brain endothelial cells]) [Figure 3]. To reiterate, these findings indicate that hormetic dose responses in neural systems display quantitative characteristics that are similar to those occurring in other biological models and end points, suggesting that hormetic responses may be a broad, general physiologically adaptive process.
Figure 3(b).
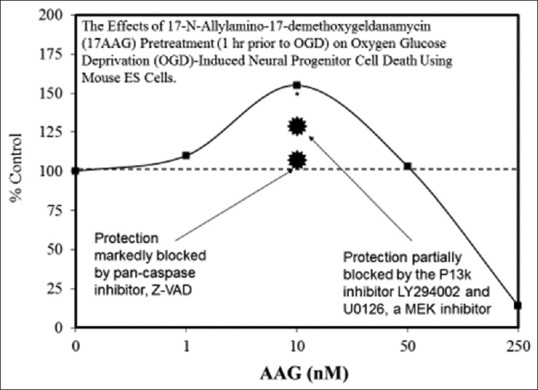
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(c).
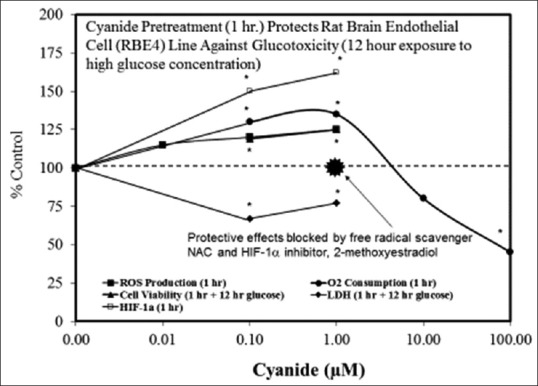
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 3(d).
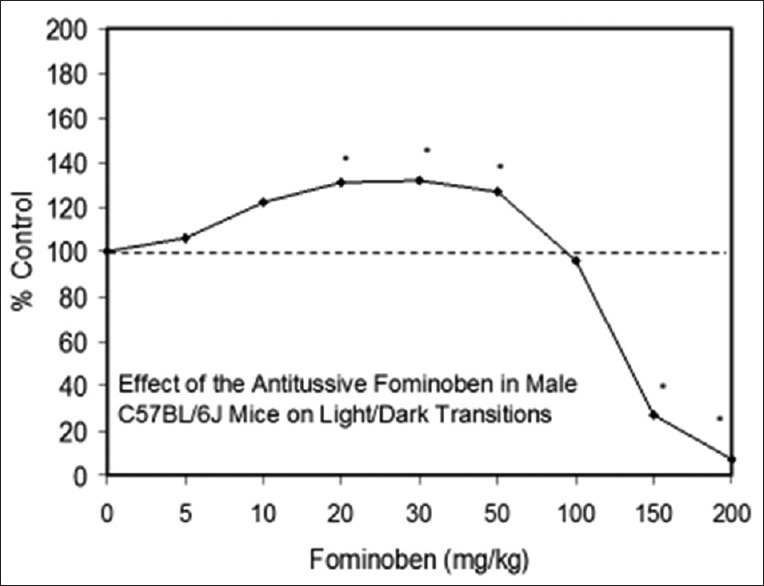
Examples of neuroprotective effects displaying hormetic dose responses [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]
Figure 4.
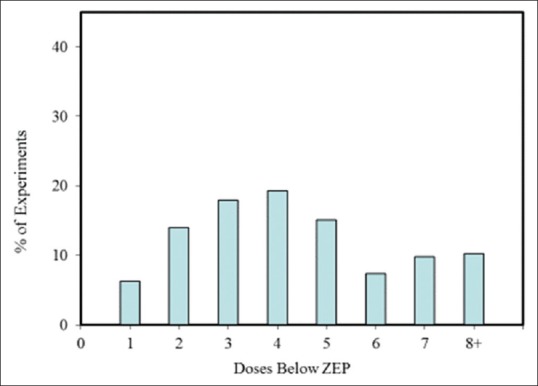
Percentage Of Neuroscience Dose-Response Experiments In The Hormetic Database With A Specific Number Of Doses Below The Zero Equivalent Point
Figure 5.
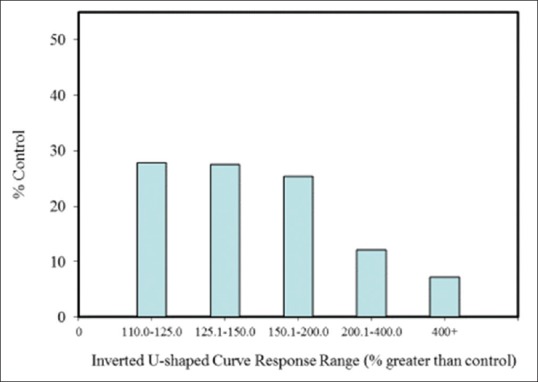
Percentage of total neuroscience dose-response experiments within a specific maximum stimulatory response range in the hormetic database
Figure 6.
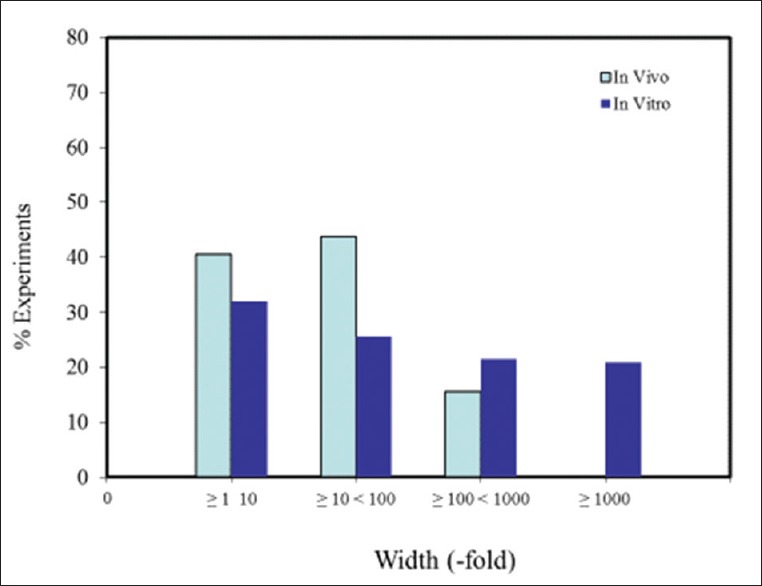
Dose-response relationships by width of stimulation range in neuroscience experiments in the hormetic database
Discussion
Hormetic dose responses occur in a number of neural systems and models. We posit that such hormetic effects sustain the function of neurological systems under normal conditions, fortify and optimize certain neural functions, and when taken together, may serve to protect neural systems (viz., the brain) from a variety of metabolic, neurodegenerative and traumatic insults. In neural systems (as in other biological systems), the hormetic response is constrained by limits of plasticity which, in turn, reflect the quantitative features of the hormetic dose response. These findings suggest that the hormetic dose response likely plays a fundamental role in neural performance and neuroprotection, which may be applicable and of value in experimental and clinical contexts.
In general, the most significant and consistent observation of hormetic dose responses is that the magnitude of the stimulation/protective effect is modest, being at maximum only 30%–60% greater than the control group. While this response defines, and perhaps restricts potential benefit, it also reveals that demonstrating beneficial effects in highly heterogeneous experimental treatment groups may be challenging. In this light, it is equally important to note that there is little evidence to suggest that experimental approaches using simultaneous multiple treatments (e.g., pharmacological/mechanical, etc.) and/or specific temporal sequence treatment approaches will impart improvements that exceed this 30%–60% maximum protective response.[33] However, while it may be difficult to exceed the apparent bounds of biological plasticity, some success has been achieved in extending the period of protection from days to a few months via manipulation of PC methods employed.[88] Thus, it will be important to direct current and future research to find reliable and practical ways to achieve protection >30%–60%, to reliably extend the neuroprotection period, and to more fully define and detail mechanisms and effects of hormetic responses in neural systems.
Financial support and sponsorship
The present work was supported, in part, by funding from the AEHS Foundation, the United States Air Force Office of Research, and the William H and Ruth Crane Schaefer Endowment (JG).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The prior work on hormesis by EC that has provided the basis for much of the information contained in this manuscript has been supported by the United States Air Force and ExxonMobil Foundation. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing those of supporting organizations, or as representative of expressed or implied endorsements or policies.
References
- 1.Calabrese Ej, Ives Ja, Giordano J. Neuroprotective Agents Commonly Display Hormesis: Implications For Nanoneuropharmacology. In: Giordano J, editor. Neurotechnology: Premises, Potential And Problems. Boca Raton, Fl: Crc Press; 2012. pp. 69–92. [Google Scholar]
- 2.Southam CM, Ehrlich J. Effects of extract of Western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–24. [Google Scholar]
- 3.Schulz H. Zur lehre von der arzneiwirkung. Arch Pathol Anat Physiol Klin Med. 1887;108:423–45. [Google Scholar]
- 4.Schulz H. About yeast poisons. Arch Gesamte Physiol Menschen Tiere. 1888;42:517–41. [Google Scholar]
- 5.Calabrese EJ, Baldwin LA. Chemical hormesis: Its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19:2–31. doi: 10.1191/096032700678815585. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese EJ, Baldwin LA. The marginalization of hormesis. Hum Exp Toxicol. 2000;19:32–40. doi: 10.1191/096032700678815594. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese EJ, Baldwin LA. Radiation hormesis: Its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19:41–75. doi: 10.1191/096032700678815602. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese EJ, Baldwin LA. Radiation hormesis: The demise of a legitimate hypothesis. Hum Exp Toxicol. 2000;19:76–84. doi: 10.1191/096032700678815611. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese EJ, Baldwin LA. Tales of two similar hypotheses: The rise and fall of chemical and radiation hormesis. Hum Exp Toxicol. 2000;19:85–97. doi: 10.1191/096032700678815620. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese EJ. Getting the dose-response wrong: Why hormesis became marginalized and the threshold model accepted. Arch Toxicol. 2009;83:227–47. doi: 10.1007/s00204-009-0411-5. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21:91–7. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Blain RB. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: Accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–60. doi: 10.1016/j.envpol.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Parsons PA. Radiation hormesis: An evolutionary expectation and the evidence. Int J Rad Appl Instrum A. 1990;41:857–60. doi: 10.1016/0883-2889(90)90063-m. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese EJ. Toxicology rewrites its history and rethinks its future: Giving equal focus to both harmful and beneficial effects. Environ Toxicol Chem. 2011;30:2658–73. doi: 10.1002/etc.687. [DOI] [PubMed] [Google Scholar]
- 18.Jonas W, Lin Y, Williams A, Tortella F, Tuma R. Treatment of experimental stroke with low-dose glutamate and homeopathic Arnica montana. Perfusion. 1999;12:452. [Google Scholar]
- 19.Andoh T, Chock PB, Chiueh CC. The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J Biol Chem. 2002;277:9655–60. doi: 10.1074/jbc.M110701200. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Kato H, Nakata N, Kogure K. Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience. 1993;56:921–7. doi: 10.1016/0306-4522(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–8. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese EJ. Preconditioning is hormesis part I: Documentation, dose-response features and mechanistic foundations. Pharmacol Res. 2016;110:242–64. doi: 10.1016/j.phrs.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese EJ. Preconditioning is hormesis part II: How the conditioning dose mediates protection: Dose optimization within temporal and mechanistic frameworks. Pharmacol Res. 2016;110:265–75. doi: 10.1016/j.phrs.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese EJ. Pre- and post-conditioning hormesis in elderly mice, rats, and humans: Its loss and restoration. Biogerontology. 2016;17:681–702. doi: 10.1007/s10522-016-9646-8. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese EJ, Baldwin LA. The dose determines the stimulation (and poison): Development of a chemical hormesis database. Int J Toxicol. 1997;16:545–59. [Google Scholar]
- 26.Calabrese EJ, Baldwin LA. A quantitatively-based methodology for the evaluation of chemical hormesis. Hum Ecol Risk Assessment. 1997;3:545–54. [Google Scholar]
- 27.Calabrese EJ, Baldwin LA. A general classification of U-shaped dose-response relationships in toxicology and their mechanistic foundations. Hum Exp Toxicol. 1998;17:353–64. doi: 10.1177/096032719801700701. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese EJ, Baldwin LA. Hormesis as a biological hypothesis. Environ Health Perspect. 1998;106(Suppl 1):357–62. doi: 10.1289/ehp.98106s1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43:580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese EJ. Neuroscience and hormesis: Overview and general findings. Crit Rev Toxicol. 2008;38:249–52. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese EJ. Dose-response features of neuroprotective agents: An integrative summary. Crit Rev Toxicol. 2008;38:253–348. doi: 10.1080/10408440801981965. [DOI] [PubMed] [Google Scholar]
- 32.Calabrese EJ. Pharmacological enhancement of neuronal survival. Crit Rev Toxicol. 2008;38:349–89. doi: 10.1080/10408440801981973. [DOI] [PubMed] [Google Scholar]
- 33.Calabrese EJ. Alzheimer's disease drugs: An application of the hormetic dose-response model. Crit Rev Toxicol. 2008;38:419–51. doi: 10.1080/10408440802003991. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese Ej. Drug Therapies For Stroke And Traumatic Brain Injury Often Display U-Shaped Dose Responses: Occurrence, Mechanisms, And Clinical Implications. Crit Rev Toxicol. 2008;38:557–77. doi: 10.1080/10408440802014287. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese EJ. Enhancing and regulating neurite outgrowth. Crit Rev Toxicol. 2008;38:391–418. doi: 10.1080/10408440801981981. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese EJ. Pain and U-shaped dose responses: Occurrence, mechanisms, and clinical implications. Crit Rev Toxicol. 2008;38:579–90. doi: 10.1080/10408440802026281. [DOI] [PubMed] [Google Scholar]
- 37.Calabrese EJ. Stress biology and hormesis: The Yerkes-Dodson law in psychology – a special case of the hormesis dose response. Crit Rev Toxicol. 2008;38:453–62. doi: 10.1080/10408440802004007. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese EJ. Astrocytes: Adaptive responses to low doses of neurotoxins. Crit Rev Toxicol. 2008;38:463–71. doi: 10.1080/10408440802004023. [DOI] [PubMed] [Google Scholar]
- 39.Calabrese EJ. An assessment of anxiolytic drug screening tests: Hormetic dose responses predominate. Crit Rev Toxicol. 2008;38:489–542. doi: 10.1080/10408440802014238. [DOI] [PubMed] [Google Scholar]
- 40.Calabrese EJ. Modulation of the epileptic seizure threshold: Implications of biphasic dose responses. Crit Rev Toxicol. 2008;38:543–56. doi: 10.1080/10408440802014261. [DOI] [PubMed] [Google Scholar]
- 41.Diamond DM. The search for hormesis in the nervous system. Crit Rev Toxicol. 2008;38:619–22. doi: 10.1080/10408440802026331. [DOI] [PubMed] [Google Scholar]
- 42.Giordano J, Ives JA, Jonas WB. Hormetic responses in neural systems: Consideration, contexts, and caveats. Crit Rev Toxicol. 2008;38:623–7. doi: 10.1080/10408440802026356. [DOI] [PubMed] [Google Scholar]
- 43.Kastin AJ, Pan W. Peptides and hormesis. Crit Rev Toxicol. 2008;38:629–31. doi: 10.1080/10408440802026372. [DOI] [PubMed] [Google Scholar]
- 44.Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit Rev Toxicol. 2008;38:633–9. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stark M. Hormesis, adaptation, and the sandpile model. Crit Rev Toxicol. 2008;38:641–4. doi: 10.1080/10408440802026422. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese EJ, Hoffmann GR, Stanek EJ, Nascarella MA. Hormesis in high-throughput screening of antibacterial compounds in E.coli. Hum Exp Toxicol. 2010;29:667–77. doi: 10.1177/0960327109358917. [DOI] [PubMed] [Google Scholar]
- 47.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–82. [Google Scholar]
- 48.Calabrese EJ. Hormesis: Why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27:1451–74. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- 49.Calabrese EJ, Blain RB. Hormesis and plant biology. Environ Pollut. 2009;157:42–8. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: Why it is important to biogerontologists. Biogerontology. 2012;13:215–35. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- 51.Calabrese EJ, Dhawan G, Kapoor R, Iavicoli I, Calabrese V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16:693–707. doi: 10.1007/s10522-015-9601-0. [DOI] [PubMed] [Google Scholar]
- 52.Rattan SI, Le Bourg E. Hormesis in Health and Disease. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 53.Popoff M, Gleisberg W, editors. Cell Stimulation Research. Berlin: 1924-1930. [Google Scholar]
- 54.Ayengar AR, Glubrecht H, Fendrik I, Bhattacharya S, editors. Stimulation Newsletter. Germany: 1970-1976. [Google Scholar]
- 55.Luckey TD. Hormesis with Ionizing Radiation. Boca Raton, FL: CRC Press, Inc; 1980. [Google Scholar]
- 56.Luckey TD. Radiation Hormesis. Boca Raton, FL: CRC Press, Inc; 1991. [Google Scholar]
- 57.Stebbing AR. Hormesis – Stimulation of colony growth in campanularia-flexuosa (hydrozoa) by copper, cadmium and other toxicants. Aquat Toxicol. 1981;1:227–38. [Google Scholar]
- 58.Stebbing AR. The kinetics of growth-control in a colonial hydroid. J Mar Biol Assoc UK. 1981;61:35–63. [Google Scholar]
- 59.Stebbing AR. Hormesis – the stimulation of growth by low levels of inhibitors. Sci Total Environ. 1982;22:213–34. doi: 10.1016/0048-9697(82)90066-3. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese EJ, editor. Biological Effects of Low Level Exposures to Chemicals and Radiation. Chelsea: Lewis Publishers, Inc; 1992. [Google Scholar]
- 61.Calabrese EJ, editor. Boca Raton: Lewis Publisher/CRC Press, Inc; 1994. Biological Effects of Low Level Exposures Dose-Response Relationships. [Google Scholar]
- 62.Calabrese EJ. Toxicological defense mechanisms and the shape of dose-response relationships. Environ Health Perspect. 1998;106(Suppl 1):276–394. [Google Scholar]
- 63.Calabrese EJ. Hormetic dose-response relationships in immunology: Occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit Rev Toxicol. 2005;35:89–295. doi: 10.1080/10408440590917044. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese EJ. Cancer biology and hormesis: Human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35:463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- 65.Calabrese EJ. Adrenergic receptors: Biphasic dose responses. Crit Rev Toxicol. 2001;31:523–38. doi: 10.1080/20014091111802. [DOI] [PubMed] [Google Scholar]
- 66.Calabrese EJ. Prostaglandins: Biphasic dose responses. Crit Rev Toxicol. 2001;31:475–87. doi: 10.1080/20014091111767. [DOI] [PubMed] [Google Scholar]
- 67.Calabrese EJ. Adenosine: Biphasic dose responses. Crit Rev Toxicol. 2001;31:539–51. doi: 10.1080/20014091111811. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese EJ. Nitric oxide: Biphasic dose responses. Crit Rev Toxicol. 2001;31:489–501. doi: 10.1080/20014091111776. [DOI] [PubMed] [Google Scholar]
- 69.Calabrese EJ. 5-Hydroxytryptamine (serotonin): Biphasic dose responses. Crit Rev Toxicol. 2001;31:553–61. doi: 10.1080/20014091111820. [DOI] [PubMed] [Google Scholar]
- 70.Calabrese EJ. Opiates: Biphasic dose responses. Crit Rev Toxicol. 2001;31:585–604. doi: 10.1080/20014091111848. [DOI] [PubMed] [Google Scholar]
- 71.Calabrese EJ. Dopamine: Biphasic dose responses. Crit Rev Toxicol. 2001;31:563–83. doi: 10.1080/20014091111839. [DOI] [PubMed] [Google Scholar]
- 72.Calabrese EJ. Estrogen and related compounds: Biphasic dose responses. Crit Rev Toxicol. 2001;31:503–15. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- 73.Calabrese EJ. Androgens: Biphasic dose responses. Crit Rev Toxicol. 2001;31:517–22. doi: 10.1080/20014091111794. [DOI] [PubMed] [Google Scholar]
- 74.Calabrese EJ, Blain R. Metals and hormesis. J Environ Monit. 2004;6:14N–9N. [Google Scholar]
- 75.Calabrese EJ. Apoptosis: Biphasic dose responses. Crit Rev Toxicol. 2001;31:607–13. doi: 10.1080/20014091111866. [DOI] [PubMed] [Google Scholar]
- 76.Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62:330–8. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–50. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese EJ, Staudenmayer JW, Stanek EJ, 3rd, Hoffmann GR. Hormesis outperforms threshold model in National Cancer Institute antitumor drug screening database. Toxicol Sci. 2006;94:368–78. doi: 10.1093/toxsci/kfl098. [DOI] [PubMed] [Google Scholar]
- 79.Calabrese EJ, Stanek EJ, 3rd, Nascarella MA, Hoffmann GR. Hormesis predicts low-dose responses better than threshold models. Int J Toxicol. 2008;27:369–78. doi: 10.1080/10915810802503735. [DOI] [PubMed] [Google Scholar]
- 80.Calabrese EJ, Stanek EJ, 3rd, Nascarella MA. Evidence for hormesis in mutagenicity dose-response relationships. Mutat Res. 2011;726:91–7. doi: 10.1016/j.mrgentox.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Calabrese EJ. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol Environ Saf. 1999;42:135–7. doi: 10.1006/eesa.1998.1729. [DOI] [PubMed] [Google Scholar]
- 82.Calabrese EJ. Overcompensation stimulation: A mechanism for hormetic effects. Crit Rev Toxicol. 2001;31:425–70. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- 83.Shook JR, Giordano J. Neuroethics beyond normal. Performance enablement and self-transformative technologies. Camb Q. Healthc Ethics. 2016;25:121–40. doi: 10.1017/S0963180115000377. [DOI] [PubMed] [Google Scholar]
- 84.Gini A, Rossi J, Giordano J. Considering enhancement and treatment: On the need to regard contingency and develop dialectic evaluation. Am J Bioeth Neurosci. 2010;1:25–7. [Google Scholar]
- 85.Shook JR, Galvagni L, Giordano J. Cognitive enhancement kept within contexts: Neuroethics and informed public policy. Front Syst Neurosci. 2014;8:228. doi: 10.3389/fnsys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giordano J, Shook JR. Minding brain science in medicine: On the need for neuroethical engagement for guidance of neuroscience in clinical contexts. Ethics Biol Eng Med. 2015;6:37–42. [Google Scholar]
- 87.Zhang C, Chen S, Bao J, Zhang Y, Huang B, Jia X, et al. Low doses of camptothecin induced hormetic and neuroprotective effects in PC12 cells. Dose Response. 2015;13:1–7. doi: 10.1177/1559325815592606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gidday JM. Extending injury- and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Front Neurol. 2015;6:42. doi: 10.3389/fneur.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with Vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;15:328–36. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradley E, Zhao X, Wang R, Brann D, Bieberich E, Wang G. Low dose Hsp90 inhibitor 17AAG protects neural progenitor cells from ischemia induced death. J Cell Commun Signal. 2014;8:353–62. doi: 10.1007/s12079-014-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Correia Sc, Santos Rx, Cardoso Sm, Santos Ms, Oliveira Cr, Moreira Pi. Cyanide Preconditioning Protects Brain Endothelial And Nt2 Neuron-Like Cells Against Glucotoxicity: Role Of Mitochondrial Reactive Oxygen Species And Hif-1A. Neurobiol Dis. 2012;45:206–18. doi: 10.1016/j.nbd.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Crawley JN, Blumstein LK, Baldino F., Jr Anxiolytic-like properties of fominoben. Eur J Pharmacol. 1984;97:277–81. doi: 10.1016/0014-2999(84)90460-6. [DOI] [PubMed] [Google Scholar]
- 93.El Ayadi A, Zigmond MJ. Low concentrations of methamphetamine can protect dopaminergic cells against a larger oxidative stress injury: Mechanistic study. PLoS One. 2011;6:e24722. doi: 10.1371/journal.pone.0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Honar H, Riazi K, Homayoun H, Sadeghipour H, Rashidi N, Ebrahimkhani MR, et al. Ultra-low dose naltrexone potentiates the anticonvulsant effect of low dose morphine on clonic seizures. Neuroscience. 2004;129:733–42. doi: 10.1016/j.neuroscience.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 96.Lauretti GR, Ahmad I, Pleuvry BJ. The activity of opioid analgesics in seizure models utilizing N-methyl-DL-aspartic acid, kainic acid, bicuculline and pentylenetetrazole. Neuropharmacology. 1994;33:155–60. doi: 10.1016/0028-3908(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 97.Leung WC, Zheng H, Huen M, Law SL, Xue H. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:775–9. doi: 10.1016/s0278-5846(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 98.Melchior CL, Ritzmann RF. Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacol Biochem Behav. 1994;47:437–41. doi: 10.1016/0091-3057(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 99.Meng JL, Mei WY, Dong YF, Wang JH, Zhao CM, Lan AP, et al. Heat shock protein 90 mediates cytoprotection by H2S against chemical hypoxia-induced injury in PC12 cells. Clin Exp Pharmacol Physiol. 2011;38:42–9. doi: 10.1111/j.1440-1681.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- 100.Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, Gopalakrishna R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Ce. J Biol Chem. 2012;287:34694–708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qi H, Han Y, Rong J. Potential roles of PI3K/Akt and Nrf2-Keap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology. 2012;62:1659–70. doi: 10.1016/j.neuropharm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 102.Moriguchi T, Matsuura H, Itakura Y, Katsuki H, Saito H, Nishiyama N. Allixin, a phytoalexin produced by garlic, and its analogues as novel exogenous substances with neurotrophic activity. Life Sci. 1997;61:1413–20. doi: 10.1016/s0024-3205(97)00687-5. [DOI] [PubMed] [Google Scholar]
- 103.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–95. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Neill K, Chen S, Brinton RD. Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer's disease. Exp Neurol. 2004;185:63–80. doi: 10.1016/j.expneurol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 105.Paalzow GH, Paalzow LK. Promethazine both facilitates and inhibits nociception in rats: Effect of the testing procedure. Psychopharmacology (Berl) 1985;85:31–6. doi: 10.1007/BF00427318. [DOI] [PubMed] [Google Scholar]
- 106.Rau TF, Kothiwai A, Zhang L, Ulatowski S, Jacobson S, Brooks DM, et al. Low dose methamphetamine mediates neuroprotection through a P13K-Akt pathway. Neuropharmacology. 2011;61:677–86. doi: 10.1016/j.neuropharm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Schafberg H, Nowak G, Kaufmann R. Thrombin has a bimodal effect on glioma cell growth. Br J Cancer. 1997;76:1592–5. doi: 10.1038/bjc.1997.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma RK, Zhou Q, Netland PA. Effect of oxidative preconditioning on neural progenitor cells. Brain Res. 2008;1243:19–26. doi: 10.1016/j.brainres.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 109.Spinnewyn B, Cornet S, Auguet M, Chabrier PE. Synergistic protective effects of antioxidant and nitric oxide synthase inhibitor in transient focal ischemia. J Cereb Blood Flow Metab. 1999;19:139–43. doi: 10.1097/00004647-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–18. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei H, Ming QZ, Li Z, Qian C, Fang D, Ho YW, et al. Ginkgolides mimic the effects of hypoxid preconditioning to protect C6 cells against ischemic injury by up-regulation of hypoxia-inducible factor-1 alpha and erythropoietin. Int J Biochem Cell Biol. 2008;40:651–62. doi: 10.1016/j.biocel.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 112.Wu XM, Qian ZM, Zhu L, Du F, Yung WH, Gong Q, et al. Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br J Pharm. 2011;164:332–43. doi: 10.1111/j.1476-5381.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


