Each minute of large vessel occlusion (LVO), 1.9 million neurons are lost, and for each hour of ischemia, the brain ages 3.6 years.[1] The recent advances in stroke care due to (LVO) stand in stark contrast to the results of trials only 5 years prior. PROACT II and MERCI trials published in 1999 and 2005 started the era of impactful endovascular stroke intervention. These trials, however, had high rates of mortality and intracranial hemorrhage.[2,3] In 2013, three trials reporting negative results for mechanical thrombectomy (SYNTHESIS,[4] IMS 3,[5] and MR RESCUE[6]) were published, and it seemed as if this therapy should be abandoned. These failures were, in hind sight, to be attributed to the lack of strict patient selection protocols, small percentage of patients receiving vascular imaging, and early generation thrombectomy device use (if at all a device was used). In 2015, just 2 years later, 5 randomized controlled trials reported on the efficacy of mechanical thrombectomy for LVO defining the standard of care we now utilize today.[7,8,9,10,11] The number needed to treat to reduce the mRS by one level per patient is 2.6, which is extremely low for any intervention. In addition, a recent meta-analysis found robust effects in favor of intervention, even in populations that were assumed to have poor prognosis, namely those >80 years old, those randomized after 300 min, and those ineligible for alteplase.[12] More recently, perfusion imaging has allowed surgeons to increase the 6–8 h time window for high NIH Stroke Scale (NIHSS) anterior circulation strokes out to 24 h. In 2018, the DAWN trial and DEFUSE-3 trial both utilized perfusion imaging to extend the interventional time window. DAWN investigators compared 107 thrombectomies to 99 control patients presenting 6–24 h from their last known well. The trial was stopped early after 31 months since 49% of patients in the intervention group achieved a rate of functional independence (mRS 0–2) at 90 days compared to 13% in the control group. Furthermore, the DAWN trial defined mismatch criteria pertaining to the ischemic core size and clinical deficit for patients <80 and over 80 years old. Patients >80 years old needed to have NIHSS >10 and core infarct size <21 cc for inclusion, those patients <80 years old needed NIHSS >10 and core size <31 cc, and patients <80-years old with the very high NIHSS >20 could have core sizes 31–50 cc.[13] While clinical benefit was shown out to 24 h, post hoc analysis has shown that a decay in efficacy is still seen with each hour of delay continuing to negatively affect functional independence. The absolute risk difference for mRS 0–2 at 8 h was 24.4% versus 35.5% at 24 h.[14] DEFUSE-3 enrolled 92 patients from 38 centers in the thrombectomy arm and 90 patients in the medical arm. For inclusion in the trial, the core size was required to be <70 cc and the ratio of ischemic tissue to infarct core 1.8 on perfusion imaging. 45% of thrombectomy patients and 17% of medical group patients achieved mRS 0–2 at 90 days. 90-day mortality was 14% in the thrombectomy arm versus 26% in the medical arm. Symptomatic hemorrhages were 7% and 4% in the surgical versus medical arms, respectively, and were not significant.[15]
There are challenges to operating and caring for impoverished populations. While Detroit is on the upswing in many ways, unemployment, education, violence, substance abuse, and homelessness still remain major issues and can negatively affect the time of presentation to the emergency room. Examining a prospectively maintained database of LVO patients treated with thrombectomy in our institution during this academic year (July 2017–May 2018), we identified 51 patients with an average age 66 years old (29–91 range), 45% female, presenting with an average NIHSS 16.6 (anterior and posterior circulation treated). Comorbidities include about 10% of patient with IV drug abuse, 38% with atrial fibrillation, 95% with high blood pressure, 50% with tobacco use, and 24% with diabetes. Only 36% of our patients received tPA either presenting outside the time window for administration or having contraindications. For those patients undergoing CTP, an average core size of 6 cc was noted (range 0–50 cc). LKW to room time was on average 8.2 h (0.5–24 h for anterior circulation). TICI-2b/3 recanalization rates were 75%. We use predominately strentrievers with balloon guide catheters. Intermediate large-bore aspiration catheters are used for bailout or as needed for support when significant extracranial arterial tortuosity is present. 24 h postintervention, the NIHSS fell to on average 8.4. Long-term follow up remains a problem, given socioeconomic factors, as does postprocedure medication compliance, with at least two patients presenting with re-occlusion, probably due to not taking prescribed antiplatelets on discharge. Mortality rates are around 10%. M-3 occlusions presenting with high NIHSS (>6) are considered for thrombectomy.
There is no doubt that the newly expanded time windows have benefited the LVO patients in Detroit with at least 9 patients being treated beyond 11 h this academic year. One such example is the 75-year-old patient below, found down with an unknown last known well [Figure 1]. Perfusion imaging and the DAWN/DEFUSE trial clinical/core mismatch criteria allow for wake up strokes and patients with unknown last known wells to be treated using the standard of care. Some deficits with larger core infarcts may not correct, and while the benefit of thrombectomy may not be initially reflected in the NIHSS, likely the benefits will be found in disability and mortality reduction, quality-adjusted life years, cost savings, and possible avoidance of further surgical procedures such as hemicraniectomy. Of course, not all patients require perfusion imaging prior to thrombectomy-2018 updated American Heart Association/American Stroke Association guidelines recommend no advanced imaging for suspected LVO strokes with favorable ASPECTs scores on plain computed tomography (CT) in the first 6 h in order not to delay thrombectomy.[16] [Figure 2]. Looking outside Detroit we feel the newly expanded time windows will have a similar impact on stroke care nationwide and ideally globally. Nationally one of the most important changes in our opinion will be updating both community and tertiary practitioners alike regarding changes in time windows for patients considered salvageable. Stroke team activation guidelines will need to reflect these changes with activations for patients presenting up to 24 h out (longer with posterior circulation ischemia). LVO care once considered relatively refractory to standard treatment with IV-tPA has become a treatable entity with a new standard of care accepted. Each geographic area globally has its own struggle with bringing this treatment modality in reach of patients. Some countries such as Australia (State of Victoria) have a top down method of care with drip and ship to two large 24/7 facilities.[17] In contrast, within the US there are pockets of stroke specialists usually near major metropolitan areas such as Detroit, however more rural areas maybe and are likely underserved.
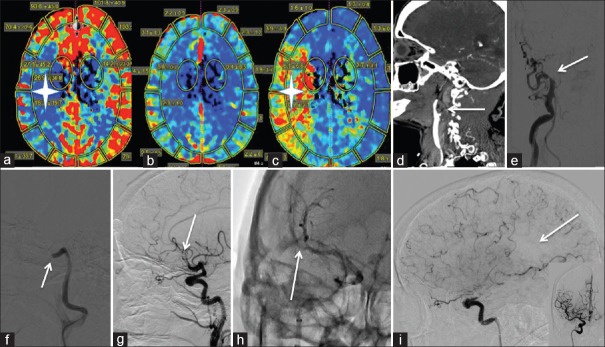
Figure 1.
Wake up Stroke: (a-c) represents perfusion imaging on a 75-year-old patient presenting with a wakeup stroke and NIH Stroke Scale 17. (a) The white star is in the area of low cerebral blood flow. (b) Preserved cerebral blood volume is noted. (c) The white star is in the ischemic penumbra as demonstrated by elevated mean time to transit. (d) A right internal carotid artery cervical occlusion extending to the supraclinoidal internal carotid artery is noted on this sagittal computed tomography angiography (white arrow). (e) Anteroposterior right common carotid artery roadmap with internal carotid artery occlusion at the takeoff (white arrow). (f) Lateral right common carotid artery roadmap. A large bore aspiration catheter was utilized to aspirate the clot to the level of the cavernous internal carotid artery (white arrow). (g) Lateral right internal carotid artery subtracted run revealing an M2 cutoff after recanalization of the internal carotid artery with 1 pass of a 6 mm × 30 mm stentriever (white arrow). (h). Anteroposterior native right internal carotid artery run with a 4 mm × 40 mm stentriever in the M2 (white arrow). (i) thrombolysis in cerebral infarction 2b recanalization with a medium size parietal perfusion deficit (white arrow). Inset is the anteroposterior view right internal carotid artery postthrombectomy. 24 h postprocedure the patient was NIH Stroke Scale 3
Figure 2.
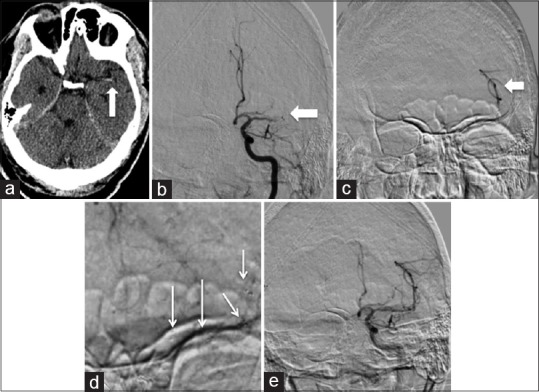
Acute presentation of a 66-year-old male on Xarelto with atrial fibrillation, NIH Stroke Scale 17, LKW 2 h prior: (a) A hyperdense left middle cerebral artery sign is noted on plain computed tomography head (white arrow). (b) Anteroposterior subtracted left internal carotid artery run reveals a distal middle cerebral artery segment one (M1) occlusion (white arrow). (c) Anteroposterior subtracted left M3 microinjection to ensure the microcatheter is beyond the lesion (white arrow). (d) Anteroposterior subtracted left internal carotid artery run with the 4 mm × 40 mm device deployed from the M2 to M1 segment revealing trace recanalization (white arrow). (e) Postthrombectomy anteroposterior subtracted left internal carotid artery run reveals thrombolysis in cerebral infarction 2b recanalization after 1 pass. 24 h postprocedure the patients right sided strength improved, and 48 h post his aphasia resolved. NIH Stroke Scale 0 at discharge
Among the next steps to optimize systems of care on a national scale will be preparing for the influx of patients who will now fall within the acute and subacute intervention windows. This may require more personnel, expediting triage and transfer protocols, designating resources for centers performing thrombectomy, and software to analyze, and postprocess CT angiogram and perfusion maps instantaneously and communicate this among stroke team members. A 2016 study estimated the incidence of LVO to be 24/100,000 person years. Thrombectomy was performed at a rate of 3/100,000 persons at that time, with 10, 284 thrombectomies performed in 2015.[18] Thus, as we capture more patients via extending the time windows of intervention and patient/practitioner education, the volumes of thrombectomies may increase significantly. Hopefully, all will be ready.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saver JL. Time is brain – Quantified. Stroke. 2006;37:263–6. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M, et al. PROACT: A phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT investigators Prolyse in acute cerebral thromboembolism Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke. 2005;36:1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 4.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. Atrial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 10.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira RG, Haussen DC, Jadhav A, Liebeskind DS, Budzik RF, Bonafe A. Time to Endovascular Treatment and Outcomes in the DAWN Trial, in: American Heart Association. 2018 [Google Scholar]
- 15.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Davis SM, Campbell BC, Donnan GA. Endovascular thrombectomy and stroke physicians: Equity, access, and standards. Stroke. 2017;48:2042–4. doi: 10.1161/STROKEAHA.117.018208. [DOI] [PubMed] [Google Scholar]
- 18.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017;9:722–6. doi: 10.1136/neurintsurg-2016-012515. [DOI] [PMC free article] [PubMed] [Google Scholar]