The process of angiogenesis is paramount in blood vessel recovery and regeneration after a cerebrovascular accident. The reparative role of angiogenesis in the maintenance of brain circulation after an acute ischemic stroke (AIS) episode is mediated by cellular signaling cascades orchestrated through many molecules including an essential protein regulator of angiogenesis known as vascular endothelial growth factor (VEGF).[1] The use of VEGF as a molecular target for therapeutic treatment of AIS events is complex, and its application involves an intricate timeline to minimize nervous system tissue damage and maximize blood vessel restoration. There are numerous ongoing investigations aimed at better understanding the cellular and molecular mechanisms of VEGF in the setting of AIS. These clinical and scientific studies have similar long-term goals, such as the development of rational drug therapies, including monoclonal antibodies and stem cells, and the improvement of patients’ functional outcomes and rehabilitative times.
In mammals, the VEGF family consists of five members, including Placental Growth Factor and VEGF-A/B/C/D. The outstanding molecule in this family is certainly VEGF-A due to its ability to promote angiogenesis and neurogenesis along with neuroprotection.[2,3,4,5,6,7] After AIS, VEGF induces astrocyte transdifferentiation into new neurons, thus promoting neurogenesis.[8] In theory, the supportive role of VEGF-A in both angiogenesis and neurogenesis makes it an attractive molecular target for rational drug therapy in the setting of AIS. Contrariwise, under these circumstances, the increase in endogenous VEGF levels and the use of exogenous VEGF in ischemic cerebral tissue is actually injurious in the early recovery phase after AIS.[9,10] Perhaps, these inconsistencies are best reconciled through realizing the dual role of VEGF-A in AIS; it establishes the endothelial network through enhancing endothelial cell migration and proliferation, but also induces vascular leakiness and permeability.[11,12]
The phosphorylation of tyrosine residues within VEGF-A activates its receptor tyrosine kinase activity and causes activation of its downstream target proteins in three important signal transduction pathways: (1) the Akt and phosphatidylinositol-3-kinase (PI3K) pathways, (2) the mitogen-activated protein kinase (MAPK) pathway, and (3) matrix metalloproteinases (MMPs).[13,14,15,16] The VEGF-A molecule supports neuron survival in cell culture models of AIS, including the excitotoxicity model[12,13] and the oxygen-and-glucose deprivation model.[15] In the AIS setting, the upregulation of the first and second signaling pathways are beneficial for neuron proliferation and survival while increased MMP levels lead to VEGF-A-mediated cerebrovascular edema and inflammatory damage.[13,14,15,16] A recent research study in a rodent middle cerebral artery occlusion (MCAO) model of AIS made four key conclusions: (1) the early secretion of VEGF contributes to endothelial leakiness after AIS, (2) the early inhibition of VEGF may reduce neurovascular permeability along with (3) decreasing infarct volume and increasing neurological functioning, and (4) these neuroprotective effects may decrease MMP 2 and 9 levels and simultaneously increase tight junction proteins such as occludin.[16]
When implementing these treatments, there are important temporal constraints for physicians and scientists to consider. For example, the application of intravenous VEGF-A is contraindicated at both the 1–3 h up to 24 h time intervals after an episode of AIS.[13,17] Instead the use of VEGF-A 1 day after AIS appears to confer neuroprotection through decreasing infarct volume and increasing vascular volume.[18,19] In humans, the efficacy and timing of VEGF-A administration still need to be fully resolved to ensure its safe use in the recovery phase of AIS.
There have been recent therapeutic efforts to molecularly target VEGF-A through using stem cells, chemical agents, and alternative medicines to improve neurological outcomes in the recovery phase of AIS. Foremost, the injection of stem cells overexpressing VEGF-A has been demonstrated to induce angiogenesis in nervous system tissue.[20] Similarly, transplantation of human neural stem cells into rat cerebral cortex 1-week post-MCAO decreased behavioral deficits and infarct volume and these positive effects were diminished through administration of bevacizumab, a human monoclonal antibody against VEGF-A.[21] Next, the application of the chemical agent dl-3-n-butylphthalide in eighty human patients after AIS was recently shown to be salubrious because it decreased neurological deficits and increased serum VEGF levels.[22] Furthermore, the application of a traditional Chinese medicinal herb, xueshuantong (Panax notoginseng), is reputed to be beneficial in the treatment of AIS in humans.[23] In a recent investigation, rodents that underwent transient MCAO were given xueshuantong and were found to experience three advantages: (1) enhanced fibroblast growth factor, nerve growth factor (NGF), and VEGF expression, (2) improved neurological functioning, and (3) increased vascular density in penumbral areas.[24] Finally, there are studies that have observed synergistic effects with combined treatment strategies that use VEGF-A. For instance, Yang et al. measured decreased expression of the pro-apoptotic protein caspase 3 and increased levels of the anti-apoptotic protein Bcl2 (B-cell lymphoma 2) in rabbits treated with both VEGF and NGF 5–8 h post-MCAO.[25]
Many of these research studies implementing VEGF-A in AIS have been performed mostly on animal models. There are increases in VEGF-A in humans after stroke, but the consequences of this correlation remain to be fully resolved.[26,27] The authors Lee et al. determined that increased VEGF-A levels relate to improved stoke recovery outcomes.[28] However, Matsuo et al. found that VEGF-A concentration positively correlated with stroke severity for cardioembolic infarcts and negatively correlated with to neurological severity for atherothrombotic infarcts.[27] These conflicting reports need to be reconciled before realizing the potency of VEGF-A in human patients. The advancements in our understanding of VEGF-A's signal transduction activity after AIS need to be experimentally manipulated in the near future to maximize the positive effects of the Akt-PI3K and MAPK pathways and to minimize the negative effect of MMPs, including cerebral edema and inflammation [Figure 1].
Figure 1.
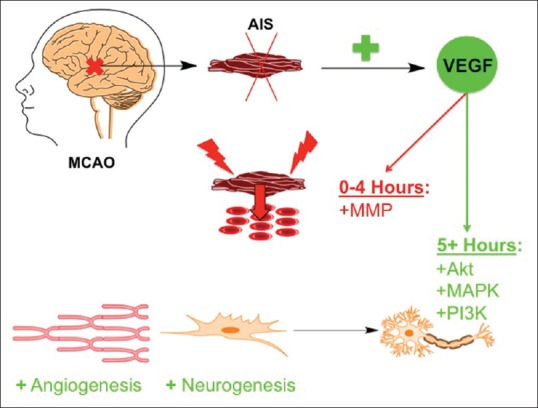
The temporal effects of vascular endothelial growth factor in acute ischemic stroke. In middle cerebral artery occlusion models of acute ischemic stroke there are increases in vascular endothelial growth factor levels. In the first 4 h after acute ischemic stroke, there is vascular endothelial growth factor-mediated activation of matrix metalloproteinases causing cerebral edema and localized inflammation, thus damaging neurons and nervous system tissue. After this acute inflammatory phase, the positive effects of vascular endothelial growth factor signaling to its downstream target proteins, such as Akt, mitogen-activated protein kinase, and phosphatidyl-inositol-3-kinase, include angiogenesis and neurogenesis
Financial support and sponsorship
This work was partially supported by Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–96. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 2.Namiecińska M, Marciniak K, Nowak JZ. VEGF as an angiogenic, neurotrophic, and neuroprotective factor. Postepy Hig Med Dosw (Online) 2005;59:573–83. [PubMed] [Google Scholar]
- 3.Góra-Kupilas K, Jośko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–9. [PubMed] [Google Scholar]
- 4.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 5.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, et al. Vascular endothelial growth factor – A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Zechariah A, Qu Y, Hermann DM. Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res. 2012;90:1873–82. doi: 10.1002/jnr.23088. [DOI] [PubMed] [Google Scholar]
- 8.Shen SW, Duan CL, Chen XH, Wang YQ, Sun X, Zhang QW, et al. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology. 2016;108:451–61. doi: 10.1016/j.neuropharm.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–76. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31:1461–74. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: Signal transduction cascades. FASEB J. 2001;15:1218–20. [PubMed] [Google Scholar]
- 14.Svensson B, Peters M, König HG, Poppe M, Levkau B, Rothermundt M, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–5. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 15.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HT, Zhang P, Gao Y, Li CL, Wang HJ, Chen LC, et al. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol Med Rep. 2017;15:57–64. doi: 10.3892/mmr.2016.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaya D, Gürsoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T, et al. VEGF protects brain against focal ischemia without increasing blood – Brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab. 2005;25:1111–8. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg. 2010;23:149–55. doi: 10.3109/08941930903469482. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang SC, Luo CJ, Zhang KH, Li K, Fan XH, Ning LP, et al. Effects of dl-3-n-butylphthalide on serum VEGF and bFGF levels in acute cerebral infarction. Eur Rev Med Pharmacol Sci. 2017;21:4431–6. [PubMed] [Google Scholar]
- 23.Gao L, Zhao H, Liu Q, Song J, Xu C, Liu P, et al. Improvement of hematoma absorption and neurological function in patients with acute intracerebral hemorrhage treated with xueshuantong. J Neurol Sci. 2012;323:236–40. doi: 10.1016/j.jns.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Guo H, Adah D, James PB, Liu Q, Li G, Ahmadu P, et al. Xueshuantong injection (Lyophilized) attenuates cerebral ischemia/Reperfusion injury by the activation of Nrf2-VEGF pathway. Neurochem Res. 2018;43:1096–103. doi: 10.1007/s11064-018-2523-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Yang B, Xiu B, Qi J, Liu H. Effect of combination therapy with neuroprotective and vasoprotective agents on cerebral ischemia. Can J Neurol Sci. 2018;45:325–31. doi: 10.1017/cjn.2018.8. [DOI] [PubMed] [Google Scholar]
- 26.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–70. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo R, Ago T, Kamouchi M, Kuroda J, Kuwashiro T, Hata J, et al. Clinical significance of plasma VEGF value in ischemic stroke – Research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013;13:32. doi: 10.1186/1471-2377-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH, Lee YJ, et al. Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur J Neurol. 2010;17:45–51. doi: 10.1111/j.1468-1331.2009.02731.x. [DOI] [PubMed] [Google Scholar]


