Abstract
Ischemic stroke as an initial presentation of malignancy is extremely rare and the underlying etiology is often ignored. The aim of this study is to outline the clues to occult malignancy in patients presenting with cerebral infarction initially. The clinical characteristics of total 19 patients with Trousseau's Syndrome presenting with cerebral infarction initially were analyzed. Among those patients, no conventional vascular risk factors were detected in 68% (13/19) of patients, and infarction occurring in multiple vascular distributions was found in 84% (16/19). Blood test showed thrombophilia in 79% (15/19) of patients with significantly elevated D-dimer, disseminated intravascular coagulopathy (DIC) in 59% (11/19), and elevated levels of tumor makers in 47% (9/19). The prognosis of the 19 patients was poor, with 68% (13/19) of patients undergoing a relapse of stroke in short interval, and 84% (16/19) being reportedly to die in 6 months. In patients, who developed unexplained recurrent brain infarction involving multiple arterial territory, with laboratory evidence suggesting hypercoagulability (higher level of D-dimer, or DIC), Trousseau's Syndrome should be considered, and investigation for an occult malignancy was required.
Keywords: Cerebral infarction, disseminated intravascular coagulopathy, hypercoagulability, ischemic stroke, malignant tumor, Trousseau's syndrome
Introduction
It was reported that thrombosis may occur in patients with known cancer. In an autopsy study, 7.4% and 14.6% of those patients with cancer suffered from clinically or pathologically cerebrovascular events attacks, respectively.[1] Some patients even have concomitant disseminated intravascular coagulation (DIC). However, little is known that thrombotic episodes can precede the diagnosis of malignancy by months or years or appear concomitantly, which is named as Trousseau's syndrome.[2] Trousseau's syndrome presenting with ischemic stroke is exceedingly exceptional and the underline stroke etiology is often ignored. The present study aimed to outline the clues to occult malignancy in ischemic stroke patients by summarizing the characteristics of 19 cases (including three cases in our institution) with cerebral infarction as the presenting manifestation of Trousseau's Syndrome.
Case Reports
Case 1
A 65-year-old male presented with memory decline and behavioral disturbance for 11 days, weakness in left limbs for 2 days. Past medical history included mild hypertension and ischemic stroke 3 months ago with slight residual weakness (mRs = 1). Physical examination on admission revealed somnolence, gaze paralysis, and left hemiplegia. Massive ischemic infarcts with hemorrhagic transformation in the right cerebral hemisphere, along with multiple punctate infarcts in the brainstem, bilateral cerebellum, and left cerebral hemisphere were shown on brain magnetic resonance imaging [Figure 1]. Transthoracic echocardiography revealed no atrial enlargement, vegetation, or emboli. Ultrasound showed only thick intima within bilateral carotid arteries. No intracranial artery stenosis was found by transcranial color-coded duplex (TCCD) sonography. His complete blood counts were normal except elevating white blood cell count (13.21 × 109/L). Blood coagulation studies showed extending activated partial prothrombin time (7.5 s), increasing international neutralization ratio (1.52), and D-dimer (20ug/ml). Other thrombophilic screen was normal.
Figure 1.
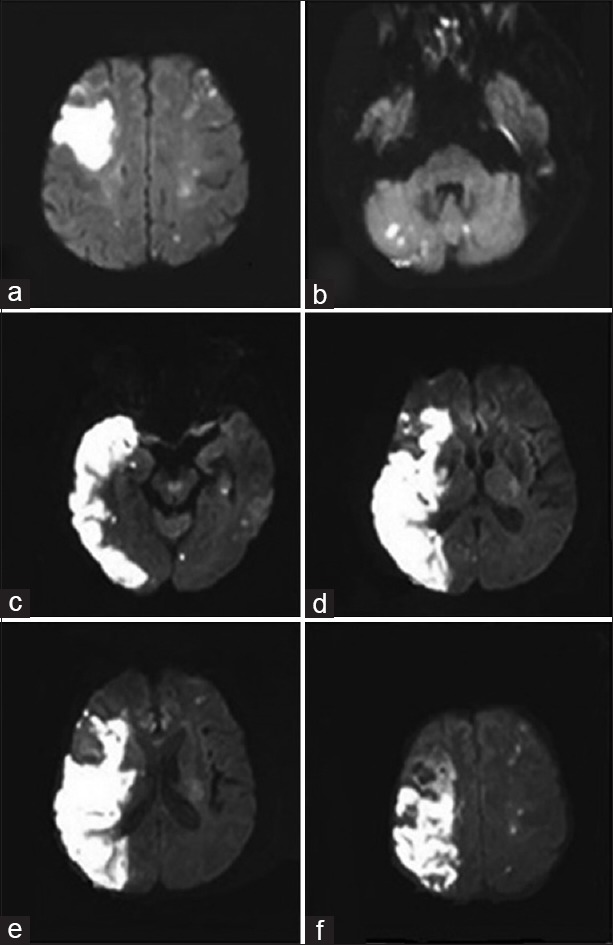
(a-b) Diffusion weighted imaging showed multiple lesions in bihemispheric territories and cerebellum (2016/2/4) (c-f) Diffusion weighted imaging showed massive ischemic infarction in the right cerebral hemisphere as well as multiple punctate infarcts in the brainstem, bilateral cerebellum and left cerebral hemisphere (2016/2/15)
After admission, the patient was treated with aspirin and atorvastatin, but his condition continued to deteriorate and he lapsed into unconsciousness. Extremely, high level of tumor markers such as carcinoembryonic antigen (1000 ng/ml), carcinoma antigen 125 (90 U/ml), carcinoma antigen 19-9 (1000U/ml), carcinoma antigen 15-3 (119U/ml), neuron-specific enolase (196 ng/ml), cytokeratin fragment 19 (72 ng/ml), and carcinoma antigen 72-4 antigen (300 U/ml) were noticed, which reminded us to investigate concealed cancer. The contrast-enhanced computer tomography showed multiple enlarged lymph nodes in the abdominal cavity [Figure 2]. Bone marrow biopsy revealed unknown classification of cells suggestive of metastases from occult tumor [Figure 3]. Flow cytometry and immune phenotype showed P3 accounted for 1.7% of marrow nucleated cells, with expression of CD19, CD20, CD22, and lambda, with no expressing of CD10, CD5, FMC7, CD23, Kappa, CD103, CD25, and Monoclonal B cells accounted for 1.7% in bone marrow. The genetic testing of leukemia showed monoclonal gene rearrangement of IgH tube B, IgH tube C, Igκ tube A, Igκtube B, and Igλ. Malignant monoclonal proliferative disease of B cells was indicated. Thereafter, he experienced bleeding in the airway, nasal mucosal, urinary tract, intestinal, and skin. Abnormal blood coagulation with decreased platelet (PLT) counts (45 × 109/l) and fibrinogen (FIB) (1.1 g/L), along with prolonged APPT (96.1S), INR (1.91), and D-dimer (>20 ug/ml), confirmed DIC. The patient was given plasma and PLT infusion. However, he deteriorated continuously and died after the third attack of brainstem infarction on the 46th day of onset.
Figure 2.
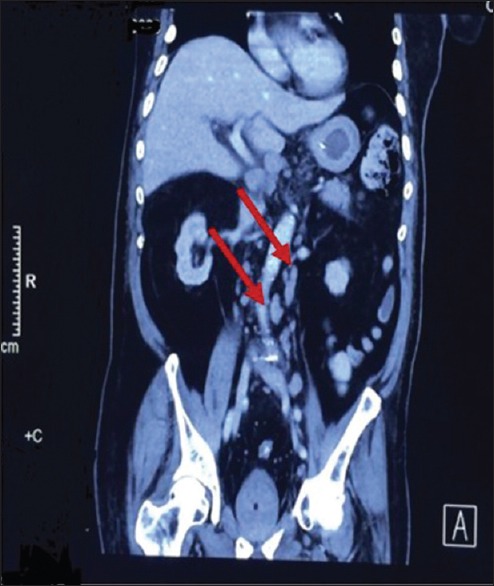
A contrast-enhanced abdominal computed tomography showed multiple swollen mediastinal lymph nodes (arrows)
Figure 3.
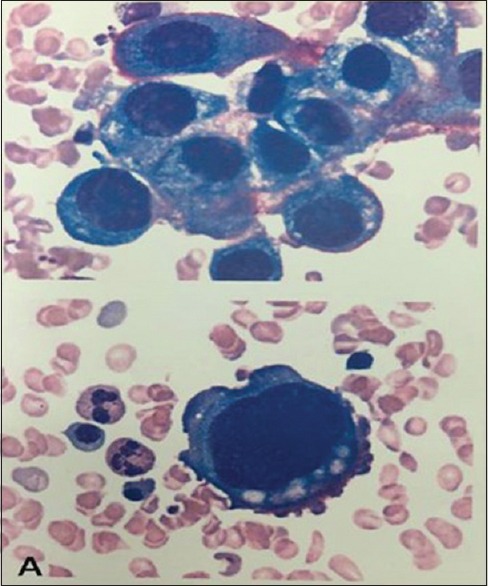
Morphology of bone marrow cell showed cells of unidentified classification
Case 2
A 56-year-old male presented with dizziness for 1½ months. He had a transient loss of consciousness 1 week ago. When consciousness recovered, he was found weakness in the left limbs and paresthesia in the right face. However, all of her symptoms had fully resolved 3 days later. No cerebrovascular risk factors were identified. Medical history revealed resection of lung adenocarcinoma in the left lung lobe 2 years ago. Neurological examination on admission showed nothing special. However, the chest percussion was dull with no breath sounds in the left chest, which was confirmed as pleural effusion associated with severe lung infection. Multiple punctate infarcts in the bilateral cerebral hemispheres were revealed by brain computed tomography (CT) scan. No vascular stenosis was found by TCCD and ultrasound. Blood test showed normal except for elevated white blood cells (WBC) (12 × 109/L). He was given anti-infective therapy, thoracentesis drainage, and mechanical ventilation. On the 5th day after admission, he developed a hematoma in the location of right jugular vein catheterization. Blood testing showed the increased level of WBC (22 × 109/L), but decreased level of PLT (38 × 109/L), hemoglobin (105 g/L), and FIB (0.6 g/L). Coagulation parameters were also extremely abnormal (INR 4.66, APTT 67.8s, and D-dimer 4.8 mg/L). He was given FIB intravenously. However, oozing at the access of deep venous catheter happened on the 6th day. The results of the blood tests deteriorated; furthermore, the diagnosis of DIC (INR 7.01, APTT 91.8, WBC 46 × 109/L, FIB < 0.6 g/L, and PLT 28 × 109/L) was confirmed. The patient deteriorated progressively and died on the 6th day.
Case 3
A 50-year-old male was admitted with a 4-day history of sternal pain, cough, sputum, and fever. He had a transient weakness in the right limbs 2 weeks ago. No conventional cerebrovascular risk factors were identified. Blood test showed elevated levels of WBC (66 × 109/l) and D-dimer (2.2 mg/L), and decreased levels of PLT (36 × 109/l) and FIB (1.153 g/L). Bone marrow biopsy showed hyperplasia, myeloid hyperplasia, in which 4% of primary granulocyte, 87% of promyelocytic with irregular nuclear, cytoplasmic particles, Amer bodies, erythroid suppression, and megakaryocytes less were observed. About 20% of primary granulocytes and 74% of promyelocytic were in plains of peripheral blood. The patient was diagnosed as an acute nonlymphocytic leukemia M3 and DIC. He was administrated with PLT, FIB, and plasma intravenously; leukemia regimen (dexamethasone, arsenic) and hydroxyurea orally; and low-molecular-weight heparin (LMWH) subcutaneously for DIC. However, the patient developed global aphasia and weakness on the right limbs. Physical examination revealed somnolence, Broca's aphasia, and right hemiplegia. Cranial CT scan showed massive infarct in the left middle cerebral artery territory. Dehydration was given, but herniation still happened on the 4th day.
Furthermore, we searched in PubMed/MEDLINE using the MeSH term of “Trousseau's syndrome, cerebral infarction/ischemic stroke, and tumor/cancer/malignancy” for literature concerning about Trousseau's Syndrome presenting with cerebral infarction and summarized 19 cases including the three cases in our institution [Table 1].[3,4,5,6,7,8,9,10,11,12,13,14,15,16]
Table 1.
Clinical features of trousseau's syndrome in 19 patients presenting with cerebral infarction
| Patient number/author | Sex/age | Conventional vascular RF | frequency of recurrence | Imaging | DIC | D-dimer | Tumor marker | Other hints | Diagnosis of tumor | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/present cases | Male/65 | + | 3 | Multiple infarcts in multiregional artery with HT | + | High | CA125, CA155, CA199, CEA | No vascular stenosis in TCCD and ultrasound | Malignant monoclonal proliferative disease of B cells | Died on the 46th day after the 3rd onset |
| 2/present cases | Male/56 | - | 2 | Multiple punctate infarcts in the bilateral cerebral hemispheres | + | High | NS | No vascular stenosis in TCCD and ultrasound | Lung cancer | Died on the 6th day |
| 3/present cases | Male/50 | - | 2 | Massive infarct in the left MCA territory | + | High | NS | NS | Acute nonlymphocytic leukemia M3 | Died on the 4th day |
| 4/Thalin et al.[3] | Male/67 | - | 3 | Multiple infarcts of in multiregional artery with HT | - | High | NS | With concomitant cerebral and myocardial microthrombosis | Adenocarcinoma of prostate | Died in 2 weeks after the 1st onset |
| 5/Yamane et al.[4] | Female/62 | - | 2 | Multiple infarcts in bilateral cerebral and cerebellar | - | High | CEA | TEE showed NBTE; MRA showed occlusion of RICA | Gallbladder tumor | Survival after tumor surgically resected |
| 6/Woo et al.[5] | Male/37 | - | 1 | Massive left MCA territory infarction with HT | + | High | Normal | With concomitant DVT and PE | Adenocarcinoma | Died in less than 1 month |
| 7/Tsai and Wu[6] | Male/46 | + | 3 | Multiple infarcts in bilateral cerebral and cerebellar with HT | - | High | CEA | MDCT showed NBTE; MRA showed no focal stenosis | Adenocarcinoma of colon | Died in 3 weeks after the 1st onset |
| 8/Chen et al.[7] | Female/66 | + | 3 | Large infarction left hemisphere and right occipital lobe, and brainstem | - | High | CA125 | Ultrasonographic duplex of carotid vessels; TTE showed normal; With concomitant DVT | Malignant struma ovarii | Died in 6 weeks after the 1st onset |
| 9/Zis et al.[8] | Female/38 | + | 1 | Infarction in the right cerebellum | - | NS | NS | MRA showed no focal stenosis; TTE was negative | Hepatic heman gioendothelioma | Survival at discharge |
| 10/Yoshida et al.[9] | Male/70 | - | 2 | Multiple infarcts in cerebellar and cerebral hemisphere | - | High | Cytokeratin 19 | TTE showed no NBTE; With concomitant arterial thrombosis | Lung cancer | Died 6 months later |
| 11/Giray et al.[10] | Male/54 | - | 3 | Multiregional infarcts in both cerebral and cerebellar | + | High | CA199 CA155 | MRA showed no stenosis; Thromboembolic lesions in multiple organs | Liver adenocarcinoma | Died of cardiac arrest 1 month later |
| 12/Ikeda et al.[11] | Male/80 | - | 1 | Left cerebrum and multiple small areas of bilateral cerebral cortices with HT | + | High | NS | MRA showed no stenosis; TTE showed no embolism | Lung cancer | Died on the 136th days after the onset |
| 13/Yeh and Lin[12] | Female/62 | - | 1 | Infarction in the right MCA and left PCA territories | + | High | CA 125 | TTE, TEE and carotid duplex revealed normal findings | Ovarian tumor | Died one month after the onset |
| 14/Yeh and Lin[12] | Female/42 | - | 1 | Multiple infarctions on bilateral MCA, left PCA, and ACA territories | + | High | CA 199 CA 125 | TTE and TEE findings were unremarkable | Endometrioid carcinoma with liver metastasis | Died before any antineoplastic therapy was given |
| 15/Yeh and Lin[12] | Male/63 | - | 2 | Infarctions in the right MCA and left ACA territories | + | High | NS | TTE and TEE studies also disclosed no abnormalities | Lung cancer | Died |
| 16/Goedee et al.[13] | Female/58 | - | 1 | Multiple bilateral infarcts | - | NS | CA 125 | CTA revealednormal; TEE showed no signs of endocarditis | Ovarian tumor | Died 42 days after the initial hospitalization |
| 17/Gundersen and Moynihan[14] | Male/59 | + | 2 | Multiple bihemispheric infarcts | + | NS | NS | Postmortem examination revealed NBTE of the aortic valve | Adenocarcinoma of lung | Died within a week of his initial presentation |
| 18/Chen et al.[15] | Female/81 | + | 2 | Left posterior parietal infarcts and right ACA occlusion | + | NS | NS | Autopsy revealed vegetations of NBTE attached to the mitral valve | Pancreatic Adenocarcinoma | Died 2 weeks after the initial presentation |
| 19/Suri et al.[16] | Female/56 | NS | 2 | Multiple infarcts in the left temporal and thalamic regions, along with bilateral fronto-parietal regions and cerebellar | NS | High | NS | TEE showed vegetation; CTA revealed normal | Bronchogenic adenocarcinoma | NS |
DIC: Disseminated intravascular coagulopathy, MCA: Middle cerebral artery, PCA: Posterior cerebral artery, ACA: Anterior cerebral artery, MDCT: Multidetector computed tomography, NBTE: Nonbacterial thrombotic endocarditis, RICA: Right internal carotid artery, PE: Pulmonary embolism, DVT: Deep vein thrombosis, TTE: Transthoracic echocardiography, TEE: Transesophageal echocardiography, CT: Computed tomography, TCCD: Transcranial color-coded duplex sonography, MRA: Magnetic resonance angiography, CTA: Computed tomography angiography, CA125: Carcinoma antigen 125, CA155: Carcinoma antigen 155, CA199: Carcinoma antigen 199, CEA: Carcinoembryonic antigen, HT: Hemorrhagic transformation, L: Low, NS: Not stated, RF: Risk factor, +: Positive, -: Negative
Discussion
In this study, we reported three cases of Trousseau's syndrome presented with ischemic stroke as the first manifestation of malignant tumor and reviewed 16 such entities. In another report, among 5106 cases admitted for ischemic stroke, 24 patients (0.4%) had an underlying malignancy.[17] That is to say, a cerebrovascular thromboembolic event may precede the identification of cancer and be the first clinical evidence of an underlying malignancy, which is an extremely rare kind of Trousseau's syndrome.
Patients with ischemic stroke as the first manifestation of malignant tumor had less conventional stroke risk factors.[18] In this study, vascular risk factors were found only in 32% (6/19) of patients. Furthermore, 84% (16/19) of patients had infarction occurring in multiple vascular distributions. Similarly, multi-vessel lesions were found in 77% of the ischemic stroke patients who had occult lung cancer by Mai et al.,[18] and in 90% (9/10) of stroke cases with infarct lesions in the bihemispheric territories as the first manifestation of concealed cancer by Kwon et al.[19] In short, when extensive stroke evaluation failed to identify a primary cerebrovascular explanation in those patients whose imaging manifested as multiple infarct lesion that exceed the territory of a single vessel, it was a key clue for Trousseau's syndrome.
In our cases series, high value of D-dimer were observed in 79% (15/19) of patients. Mai et al.[18] reported that all of the patients with occult lung cancer, who suffered from ischemic stroke, were accompanied by significantly increased D-dimer. Kwon's study showed that the proportion with elevated D-dimer was 60%.[19] Kim et al.[20] suggest that increased D-dimer (with cutoff value of 2.15 mg/L), along with multi-infarct region, were potential predictors of tumor. The Guo's study showed the sensitivity and specificity of elevated D-dimer levels (>5.5 mg/L) in patients of ischemic stroke with occult cancer will exceed 93%.[21] Development of DIC was also common, which was observed in 59% (11/19) of patients in this study. High value of D-dimer and DIC in these patients with multiple ischemic infarct regions is suggestive of a thrombophilia, and a screen for occult cancers is necessary.
The cases series reviewed here indicated that the principal mechanisms of stroke pathogenesis were hypercoagulable states (9/19), nonbacterial thrombotic endocarditis (5/15), and chronic DIC (5/19), which is in according to the previous study.[6,17,22,23,24,25] Hypercoagulable state is the most common mechanism in patients with cancer or potential cancer. The molecular mechanisms inducing a prothrombotic state in patients with malignancy include tumoral production of procoagulants such as tissue factor and cancer procoagulant and inflammatory cytokines, and the interaction between tumor cells, blood, and endothelial cells.
Approaches for treating Trousseau's syndrome are to eliminate the causative tumor first to improve the hypercoagulable state. Systemic anticoagulation, if there are no contraindications, may ameliorate symptoms and prevent further thromboembolic episodes to a certain extent.[17] Studies suggest that warfarin is less effective than heparin in reducing the rate of recurrent embolization.[26] A trend toward decreased mortality with LMWH has recently been reported as compared to standard heparin and the reduction in mortality appears to be independent of the reduction in thromboembolism and bleeding.[10] The novel oral anticoagulant dabigatran is also reported to be effective for the treatment of the Trousseau's syndrome.[27] However, in Yoshida's case report, the patient, who was considered as with stroke caused by cardiogenic embolism, still endured recurrent stroke although using dabigatran.[9]
The prognosis of the 19 patients was poor. As Kwon reported, half of these patients had recurrent strokes within 6 months.[19] Mai et al.[18] also reviewed that 92% (12 patients) of such entities had recurrent episodes of stroke and more than half of the patients (58%) had a relapse within one month.[17,18] In this study, 68% (13/19) of patients underwent a relapse of stroke in short intervals, which verified the prothrombotic state caused by the occult malignancy. Most of our case (84%, 16/19) were reportedly to die in 6 months (5 days–6 months). The 3-month mortality rates are higher in patients with cancer metastasis compared to those without metastasis.[28] The published mortality rate in cancer-related stroke ranges from 25% to 30%, compared to 14% in stroke patients without cancers.[29,30,31] Guo's study showed that the 1-year mortality rate in patients with active cancer was more than 50%.[21] In Taccone's study, the mortality was even up to 79% (19 of 24) with a median survival of 58 days after the index event. Mean follow-up of surviving patients was 29 months (3–60).[17] In other words, once stroke occurs in a patient with cancer, regardless of etiology, the overall prognosis was poor.[29]
Conclusion
Our cases emphasize that when extensive stroke evaluation failed to identify a primary cerebrovascular explanation, particularly when the patients have repeated occurring stroke in short intervals in which the lesions exceed the territory of a single vessel, with hypercoagulability (higher level of D-dimer or DIC), a search for an underlying malignancy is suggested. Ischemic stroke might be the first manifestation of an undiagnosed cancer, in which elimination of the causative tumor and systemic anticoagulation is suggested, but the outcomes are poor with early stroke recurrence and high mortality rate.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
This study was financially supported by Beijing Municipal Administration of Hospitals’ Youth Programme, the People's Republic of China (No. QML20160801).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors greatly appreciate the support of Beijing Municipal Administration of Hospitals’ Youth Programme (No. QML20160801).
References
- 1.Arboix A. Cerebrovascular disease in the cancer patient. Rev Neurol. 2000;31:1250–2. [PubMed] [Google Scholar]
- 2.Varki A. Trousseau's syndrome: Multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thalin C, Blomgren B, Mobarrez F, Lundstrom A, Laska AC, von Arbin M, et al. Trousseau's syndrome, a previously unrecognized condition in acute ischemic stroke associated with myocardial injury. J Investig Med High Impact Case Rep. 2014;2:2324709614539283. doi: 10.1177/2324709614539283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamane A, Sadahiro H, Goto H, Inamura A, Ishihara H, Oka F, et al. Multiple ischemic strokes caused by nonbacterial thrombotic endocarditis because of gallbladder cancer: A case report. J Stroke Cerebrovasc Dis. 2014;23:1727–9. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Woo PY, Chan DT, Cheung TC, Zhu XL, Poon WS. Middle cerebral artery infarction in a cancer patient: A fatal case of Trousseau's syndrome. Hong Kong Med J. 2014;20:74–7. doi: 10.12809/hkmj133780. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CC, Wu MN. Frequent ischemic stroke as first manifestation of occult colon cancer: A rare case. Am J Case Rep. 2015;16:723–7. doi: 10.12659/AJCR.895130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YL, Lin KH, Lin MC, Chen CA, Cheng WF. Malignant struma ovarii complicated by Trousseau's syndrome and repeated episodes of cerebral ischemic strokes: A case report. Gynecol Oncol Case Rep. 2011;2:35–8. doi: 10.1016/j.gynor.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zis P, Assi A, Kravaritis D, Sevastianos VA. Ischemic stroke as the first manifestation of hepatic epithelioid hemangioendothelioma. J Stroke Cerebrovasc Dis. 2014;23:e237–40. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Kimura T, Aburakawa Y, Suzuki Y, Kuroda K, Yahara O, et al. Recurrent ischemic stroke in a patient with the trousseau syndrome treated with dabigatran. J Stroke Cerebrovasc Dis. 2014;23:1724–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Giray S, Sarica FB, Arlier Z, Bal N. Recurrent ischemic stroke as an initial manifestation of an concealed pancreatic adenocarcinoma: Trousseau's syndrome. Chin Med J (Engl) 2011;124:637–40. [PubMed] [Google Scholar]
- 11.Ikeda H, Enatsu R, Yamana N, Nishimura M, Saiki M. Multiple extra-ischemic hemorrhages following intravenous thrombolysis in a patient with Trousseau syndrome: Case study. Springerplus. 2015;4:141. doi: 10.1186/s40064-015-0920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh PS, Lin HJ. Cerebrovascular complications in patients with malignancy: Report of three cases and review of the literature. Acta Neurol Taiwan. 2004;13:34–8. [PubMed] [Google Scholar]
- 13.Goedee S, Naber A, Rovers JM, Roks G. Ischaemic stroke as initial presentation of systemic malignancy. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2013-202122. pii: bcr2013202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gundersen H, Moynihan B. An uncommon cause of stroke: Non-bacterial thrombotic endocarditis. J Stroke Cerebrovasc Dis. 2016;25:e163–4. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Li Y, Gebre W, Lin JH. Myocardial and cerebral infarction due to nonbacterial thrombotic endocarditis as an initial presentation of pancreatic adenocarcinoma. Arch Pathol Lab Med. 2004;128:1307–8. doi: 10.5858/2004-128-1307-MACIDT. [DOI] [PubMed] [Google Scholar]
- 16.Suri V, Sharma S, Jadhav N, Rastogi R. Bronchogenic adenocarcinoma masquerading as recurrent embolic strokes and myocardial infarction due to nonbacterial thrombotic endocarditis. Neurol India. 2013;61:80–2. doi: 10.4103/0028-3886.108020. [DOI] [PubMed] [Google Scholar]
- 17.Taccone FS, Jeangette SM, Blecic SA. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis. 2008;17:169–74. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Mai H, Xia J, Wu Y, Ke J, Li J, Pan J, et al. Clinical presentation and imaging characteristics of occult lung cancer associated ischemic stroke. J Clin Neurosci. 2015;22:296–302. doi: 10.1016/j.jocn.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HM, Kang BS, Yoon BW. Stroke as the first manifestation of concealed cancer. J Neurol Sci. 2007;258:80–3. doi: 10.1016/j.jns.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Park JH, Lee MJ, Park YG, Ahn MJ, Bang OY, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959. doi: 10.1371/journal.pone.0044959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo YJ, Chang MH, Chen PL, Lee YS, Chang YC, Liao YC, et al. Predictive value of plasma (D)-dimer levels for cancer-related stroke: A 3-year retrospective study. J Stroke Cerebrovasc Dis. 2014;23:e249–54. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2004;24:453–60. doi: 10.1055/s-2004-861539. [DOI] [PubMed] [Google Scholar]
- 23.Khorana AA. Cancer-associated thrombosis: Updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–30. doi: 10.1182/asheducation-2012.1.626. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, et al. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–34. doi: 10.1161/STROKEAHA.112.658625. [DOI] [PubMed] [Google Scholar]
- 25.Franchini M, Montagnana M, Favaloro EJ, Lippi G. The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread. Semin Thromb Hemost. 2009;35:644–53. doi: 10.1055/s-0029-1242718. [DOI] [PubMed] [Google Scholar]
- 26.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: Pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–23. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 27.Beyer-Westendorf J, Werth S, Folprecht G, Weiss N. Trousseau's syndrome in a patient with adenocarcinoma of unknown primary and therapy-resistant venous thrombosis treated with dabigatran and fondaparinux. Br J Clin Pharmacol. 2011;72:715–6. doi: 10.1111/j.1365-2125.2011.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong CT, Tsai LK, Jeng JS. Patterns of acute cerebral infarcts in patients with active malignancy using diffusion-weighted imaging. Cerebrovasc Dis. 2009;28:411–6. doi: 10.1159/000235629. [DOI] [PubMed] [Google Scholar]
- 29.Cestari DM, Weine DM, Panageas KS, Segal AZ, DeAngelis LM. Stroke in patients with cancer: Incidence and etiology. Neurology. 2004;62:2025–30. doi: 10.1212/01.wnl.0000129912.56486.2b. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YY, Cordato D, Shen Q, Sheng AZ, Hung WT, Chan DK, et al. Risk factor, pattern, etiology and outcome in ischemic stroke patients with cancer: A nested case-control study. Cerebrovasc Dis. 2007;23:181–7. doi: 10.1159/000097639. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ. Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand. 2006;114:378–83. doi: 10.1111/j.1600-0404.2006.00709.x. [DOI] [PubMed] [Google Scholar]


