Abstract
Maintenance of adequate tissue perfusion through a dense network of cerebral microvessels is critical for the perseveration of normal brain function. Regulation of the cerebral blood flow has to ensure adequate delivery of nutrients and oxygen with moment-to-moment adjustments to avoid both hypo- and hyper-perfusion of the brain tissue. Even mild impairments of cerebral blood flow regulation can have significant implications on brain function. Evidence suggests that chronic stress and depression elicits multifaceted functional impairments to the cerebral microcirculation, which plays a critical role in brain health and the pathogenesis of stress-related cognitive impairment and cerebrovascular events. Identifying the functional and structural changes to the brain that are induced by stress is crucial for achieving a realistic understanding of how related illnesses, which are highly disabling and with a large economic cost, can be managed or reversed. This overview discusses the stress-induced alterations in neurovascular coupling with specific attention to cerebrovascular regulation (endothelial dependent and independent vasomotor function, microvessel density). The pathophysiological consequences of cerebral microvascular dysfunction with stress and depression are explored.
Keywords: Depression, metabolic syndrome, stress, vascular
Introduction
The meaning of “stress” is ambiguous and often refers to life events that are seen as predominantly negative. Stress has been defined as a subjective perception of an adverse environmental change, which usually leads to a stress response allowing for adaptation to the new condition.[1] Different stress stimuli (i.e., physical or psychological in nature) innervate separate regions of the central nervous system to elicit an appropriate response.[2] Activation of the autonomic nervous system and hypothalamic–pituitary–adrenal (HPA) axis are prominent features of a stress response, which in turn impacts immune and metabolic mediators. These physiological responses operate non-linearly and promote adaptation through “allostasis,” i.e., reestablishing homeostasis.[3] In the short term, these changes may be adaptive; but chronic stress exposure (i.e., allostasis overload) leads to maladaptive responses in various body organs and activates pathophysiological mechanisms. Indeed, chronic stress acts as a pre-disposing and participating factor in the onset of depression in humans.[4] Over 350 million people worldwide suffer from depressive disorders, establishing depression as a leading cause of disease burden.[5,6] Importantly, both stress and depression are linked to cardio[7,8] and cerebrovascular diseases.[9]
The brain is the key organ of stress reactivity, coping, and recovery processes. Within the brain, a distributed neural circuitry determines what is threatening and thus stressful to the individual. As such, there is tight coupling between neural activity and cerebral blood flow to meet metabolic demands. The brain receives energy substrates from its blood supply, however, the brain lacks a reservoir to store fuel, thus the brain must dynamically regulate blood flow to quickly meet the metabolic needs. Failure to do so has disastrous consequences, and if blood flow is not well matched to energy demands, then more subtle brain alterations ensue, leading to chronic brain injury. The moment-to-moment matching of blood flow to metabolic demand is ensured by the signaling mechanisms of neurovascular coupling. The neurovascular unit consists of the cerebral microvessels, glial cells (astroglia, microglia, and oligodendroglia), and neurons. Harmonious interactions between neuronal and endothelial cells must be maintained to ensure normal cerebral circulation. For example, when neurons become active, they signal to local arterioles through astrocytes which generate a calcium wave in response to neuronal activity to evoke dilation through the release of potassium ions and other vasodilators to the arteriolar smooth muscle cells.[10,11,12] This response increases local blood flow and provides the nutrients necessary to support neuronal function. It has been suggested that stress impairs this balance.
To date, the effects of stress on neurovascular coupling has mainly focused on its effects on neurotransmitters, hormones, and neuronal plasticity. In postmortem biopsies of patients with a history of depressive symptoms, the morphology of neurons was altered leading to a loss in number and synaptic connectivity causing impairment in synaptic function.[13] More recent work has established that astrocytes are also an important target of stress, with both acute and chronic stressors altering the morphology and the expression of several astrocyte-specific proteins. We direct the readers to excellent reviews describing the impact of stress on neurons and astrocytes.[14,15,16] However, there is limited information as to what effect stress has on the cerebrovasculature. The following review will focus on how stress affects the cerebral vessels. We will describe briefly the hormonal changes due to stress, the effects on the cerebrovasculature that seem to be sex-dependent, and how pre-existing cardiovascular disease further impacts the stress-induced cerebrovascular dysfunction. We will also describe the relationship between cerebrovasculature dysfunction and the upregulation of oxidative stress and proinflammatory cytokines.
Hormonal Changes during Stress
Acute exposure to stress results in the release of catecholamines through the sympathetic-adrenomedullary system and an increase in the stress hormones through the HPA axis [Figure 1].[17] Stressful stimuli can produce plasma levels of epinephrine to 10,000 pg/ml.[18] The release of epinephrine and norepinephrine into the bloodstream increases heart rate, blood pressure, and respiratory rate, and reflects the typical “fight-or-flight response.” In order to do this, epinephrine triggers the releases of glucose from temporary storage sites within the body such as adipose tissue. When faced with physical stressors, the pituitary gland also releases growth hormones to enhance metabolic activity. However, changes in growth hormone responses are seldom seen when faced with psychological stress.
Figure 1.
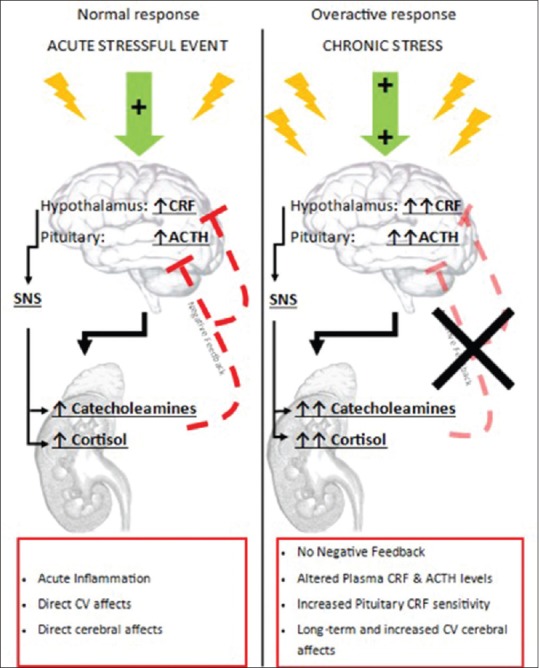
Acute and chronic hormonal response to stress. Normal physiological response to stressful stimuli leads to activation of the hypothalamic–pituitary–adrenal axis. This activation is characterized by hypothalamic release of corticotropin-releasing factor which elicits pituitary release of adrenocorticotropic hormone followed by the adrenocorticotropic hormone dependent release of cortisol and catecholamines. The production of corticotropin-releasing factor and adrenocorticotropic hormone is typically inhibited by negative feedback from increasing levels of catecholamines and cortisol. When exposed to chronic stressful stimulus, the normal physiological response is altered. The negative feedback mechanism is removed and the physiological system becomes oversensitive to stress. This leads to an increase in circulating stress hormones that compound rather than returning to homeostatic levels
The activation of the HPA axis results in the secretion of corticotropin-releasing hormone, which acts to regulate the anterior pituitary adrenocorticotropic hormone to stimulate the adrenal cortex to secrete cortisol (in humans) and corticosterone (in humans, rats, and mice). Plasma levels of these hormones can increase two- to five-fold during stress in humans.[19] Cortisol reaches every organ by way of the circulation and facilitates a cascade of physiological changes, including increased metabolism, blood flow, and gluconeogenesis to facilitate the fight-or-flight response. Thus, circulating cortisol permits coordination between the brain and body functions that are geared toward coping, recovering, and adapting to the stressful stimulus. Cortisol also plays a key role in regulating the stress response by providing inhibitory feedback at the HPA axis, thereby terminating the stress response once the stressor has subsided. Chronic stress, on the other hand, has been seen to decrease neurogenesis and increase oligodendrogenesis from neuronal stem cells in regions of the hippocampus.[20] This means there is an increase in the white matter in this brain area and an altered cellular composition, preventing the hippocampus from properly executing its role within the brain.
Stress and the Cerebral Vasculature
Regulation of cerebral blood flow in a normal brain is determined by a variety of intrinsic control mechanisms (myogenic, chemical, metabolic, and neurogenic) that match cerebral blood flow to metabolic demand. Overall, the diameter of the cerebral vessels (vascular tone) is maintained by the sympathetic nervous system. This sympathetic innervation ensures that fluctuations in blood pressure do not result in an overstretching of the cerebral arterioles, or lead to blood–brain barrier leakage. During acute psychological stresses, the norepinephrine released improves the ability to couple blood volume to oxygen demand and thus can stimulate an increase in cerebral blood flow to increase perfusion in areas of heightened neuronal activity.[21] Corroborating evidence for this stress mechanism came in the form of perfusion functional MRI revealing an increase in cerebral blood flow in the ventral right prefrontal cortex and left insula/putamen area during acute psychological stress.[22] Further, in individuals with a high-stress level response, the physiological response to a mild-to-moderate stressor is sustained even when the stress/task is completed.[22] Indeed, it can take minutes for heart rate and hours for cortisol to return to their baseline levels, despite a return to normal behavioral.[17,23] It is also interesting to note that some studies have suggested that psychophysiological stressors might compromise the blood–brain barrier.[24,25] However, a recent study has shown that acute psychophysiological stress does not increase blood–brain barrier permeability.[26] Thus, the acute cerebral vascular responses to stress are likely emergent to protect the brain and improve cerebral blood flow to metabolic demand. Whereas, a chronically stressed brain may be incrementally and deleteriously remodeled through the repeated neural activation pattern and sustained hyperactivity of the HPA axis.[27]
Chronic activation of the HPA axis and ANS can have significant detrimental effects that impact the integrated responses of the immune, metabolic, and cardiovascular systems. The breakdown of this integrated stress response is referred to as allostatic overload. The neurovascular endothelium plays a key role in regulating the influx of essential nutrients, the efflux of toxic substances, ionic homeostasis of the brain interstitial fluid, and prevent the brain influx of peripheral substances, neurotransmitters, etc.[28] Neurovascular endothelial dysfunction contributes to blood–brain barrier hyperpermeability. Thus, it is important to ask to what extent chronic stress leads to endothelial dysfunction in the cerebral circulation. Figure 2 provides a summary of the main downstream effects of chronic stress exposure on cerebrovascular dysfunction.
Figure 2.
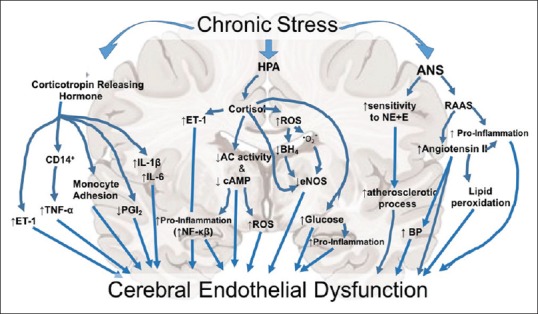
Systematic representation of the potential downstream effects of chronic stress exposure on the cerebrovasculature system. HPA: Hypothalamic–pituitary–adrenal axis, ANS: Autonomic nervous system, CD14+: Cluster of differentiation 14, ET-1: Endothelin 1, TNF-α: Tumor necrosis factor alpha, PGI2: Prostaglandin I2, IL-1 β: Interleukin 1 beta, IL-6: Interleukin six. ROS: Reactive oxygen species, BH4, THB: Tetrahydrobiopterin, O2−: Superoxide, AC: Adenylyl cyclase, cAMP: Cyclic adenosine monophosphate, NF-kb: Nuclear factor kappa-light-chain-enhancer of activated B cells, eNOS: Endothelial nitric oxide synthase, RAAS: Renin–angiotensin–aldosterone system, BP: Blood pressure, E: Epinephrine, NE: Norepinephrine
Overstimulation of the ANS and HPA axis is associated with an overactivation of the renin–angiotensin system, which leads to increased levels of homocysteine and elevated cardiovascular activity accompanied by various degrees of endothelial damage. Further, glucocorticoids regulate vascular reactivity by acting on both endothelial and vascular smooth muscle cells. Increased cortisol levels decrease nitric oxide (NO; a powerful vasodilator) bioavailability directly by inhibiting endothelial NO synthase directly,[29] and indirectly through increasing the production of oxidative stress through cortisol.[30] An increase in cortisol has also been shown to decrease cyclic adenosine monophosphate expression, an important second messenger mediating vascular function.[31] Cortisol-induced dysregulations of NO production in the endothelium may, therefore, induce some of the deleterious effects associated with stress. Acute stress raises the circulatory levels of inflammatory cytokines interleukin tumor necrosis factor (IL-6 and TNF-α)[32] and results in platelet activation.[33] Thus, it is not surprising to note that from a peripheral vascular perspective, chronic psychological stress can contribute to intimal-medial thickening,[34] the development of arterial stiffness[35] and atherosclerosis.[36,37]
There are few studies that have attempted to understand the effects of stress on the vascular system in the brain. Previous studies, including our own, have used the unpredictable chronic mild stress (UCMS) model to induce depression-like behaviors in rodents.[38] The UCMS model exposes rodents to mild daily stresses that are randomized. The UCMS protocol is considered to be the most appropriate rodent model for human clinical depression, based on its ability to reproduce the development of many human clinical depressive symptoms, including anhedonia and learned helplessness.[39] Recently, our laboratory, using the UCMS model, has established significant pathological adaptations in the large proximal arteries, which represent up to 40% of the total cerebrovascular resistance and their functional response are critical for preventing pressure fluctuations from reaching the distal regions of the cerebral circulation.[40,41] Specifically, we identified that 8 weeks of chronic stress in lean male rats reduced middle cerebral artery (MCA) vasodilation to acetylcholine (a potent endothelial-dependent dilator) and an exaggerated MCA constriction response to serotonin (a potent cerebrovascular constrictor).[42] These adaptations to chronic stress were coupled with increased levels of oxidative stress and reduced NO bioavailability in the cerebral vessels. Indeed, pro-oxidative conditions, such as UCMS, can alter the balance between constricting and dilating metabolites, by shifting arachidonic acid metabolism through cyclooxygenase to the production of vasoconstricting metabolites (e.g., thromboxane) that can compete against the vasodilatory stimulus from NO, prostacyclin, or other vasodilators.[43] Others have shown that chronic stress, induced by immobilization, decreased the cerebral vascular hemodynamic response to electrical stimulation, a reduction and delay in the blood volume recruitment through the pila arterioles, implying overall alterations to the cerebrovascular system, including arteries and capillaries.[44] Further, the impaired vascular responses were accompanied by a downregulation in neuron NO synthase and heme oxygenase-2 and enhanced inducible NO synthase (iNOS) expression. The increased iNOS expression may indicate changes in inflammation-mediated signaling pathways and increased neurotoxicity.[44] In addition to the endothelial damage, 7 days of stress (induced by a variety of stress stimuli: foot shocks, forced swim, restraint, and oscillation) greatly reduced vasodilation of parenchymal arterioles to neuronal stimulation and impaired dilation of isolated parenchymal arterioles to external potassium ions.[45] These data would suggest a defect in smooth muscle inwardly rectifying potassium ions channel function with stress. It was postulated that the chronic stress resulted in a glucocorticoid-mediated decrease in functional potassium channels in the parenchymal arterioles myocytes, which rendered the arterioles less responsive to potassium ions released from astrocytic endfeet during neurovascular coupling, leading to impairment of this process.[45]
The total length of capillaries in the human brain is approximately 600 km and almost all neuron is supplied blood and nutrients by its own capillary. Neovascularization is an innate physiologic response by which tissues respond to various stimuli through arteriogenesis (collateral remodeling) and angiogenesis (new vessel formation from existing vessels). Our laboratory has recently demonstrated a significant reduction in cerebral microvessel density in male lean rats after 8 weeks of UCMS.[42] In contrast, using the social defeat model of chronic stress, Pearson-Leary et al.[46] indicated that microvessel density in the brain was increased. It was postulated that increase in microvessel density would provide metabolic support following increased neural activity. These differing results may be a consequence of different stress models (social stress vs. UCMS), or more likely the duration of stress imposed (7 days vs. 8 weeks), and the different animal model utilized (Lean Zucker rat vs. Sprague Dawley rat). Irrespective, the mechanism by which chronic stress results in decreased angiogenesis in the brain is not fully understood. A previous study has shown that the balance between oxidant stress and endothelial function (e.g., NO bioavailability and altered arachidonic acid metabolism) were key factors involved in the progression and severity of microvascular rarefaction.[47] Both arteriogenesis and angiogenesis are tightly modulated by environmental cues and in likely differ under physiologic and disease conditions. Further, these processes are critically dependent on expression of vascular endothelial growth factors (VEGF).[48,49,50] While VEGF is an essential trigger to initiate angiogenesis, evidence suggests that thrombospondin-1 (TSP-1) is an important factor for capillary regression and/or pathologically mediated rarefaction.[51] Indeed, a fine bidirectional interaction exists between TSP-1, VEGF, and NO. For example, TSP-1 can negatively regulate NO signaling,[52,53] and in turn, decreased NO production can induce TSP-1 expression.[54] TSP-1 can also interfere with VEGF binding to its receptor.[55,56] When TSP-1 is elevated, the VEGF signal pathways can be endogenously inhibited, regardless of whether VEGF levels are elevated. Further, NO simultaneously induces vasodilatation and enhances VEGF expression.[57] Evidence suggests that the processes of oxidative stress and inflammation on microvascular rarefaction are interconnected. Reactive oxygen species stimulate the induction of VEGF expression in endothelial cells, smooth muscle cells, and macrophages.[58,59] In addition, up-regulation of TSP-1 is mediated by superoxides.[60] Our laboratory has made a similar observation in obese Zucker rats (OZR; a model for metabolic syndrome, MetS), where TSP-1 expression can be elevated up to 2-fold in OZR whole brain extracts, with either VEGF protein expression being unchanged or slightly elevated in OZR compared to similar-aged healthy lean controls (PD Chantler and IM Olfert unpublished data).
Sex-specific differences
Sex differences have been established in the stress-related hormonal secretion, whereby females, regardless of age, have an increased hormonal secretion in comparison to males.[61] In premenopausal women with an ovariectomy, a significant reduction of ACTH and cortisol are found.[62] Further, there are sex differences in the cognitive consequences of repeated stress, with males showing impairment of hippocampal-dependent memory, whereas females do not.[63,64,65] In men and women, neural activation patterns to the same tasks are quite different between the sexes even when performance is similar.[66] This leads to the concept that men and women often use different strategies to approach and deal with issues in their daily lives, in part because of the subtle differences in brain architecture.
Recent data demonstrated important sex-specific differences in the response to chronic stress. Using a mild UCMS protocol, female Wistar rats were found to have a reduced serotonergic activity in the hippocampus and reduced dopaminergic activity in the prefrontal cortex. This disparity was not identified in male rats.[67] Sex-differences have also been reported in the dysregulation of the HPA axis, whereby higher serum corticosterone concentrations were reported in female versus male rats.[68,69] These data would suggest that females may be more vulnerable to the UCMS model than the male sex. Indeed, we have previously shown that female rats display a more phenotypically severe depressive state compared to male rats.[70] At the neural level, exposure to chronic stress results in dendritic atrophy of neurons in the prefrontal cortex in male rats but dendritic hypertrophy in female rats.[71] However, dendritic hypertrophy was absence in ovariectomized female rats, suggesting that ovarian hormones modulate the morphological changes with stress.
These data, both in human and preclinical models, would suggest that chronic stress may affect the cerebrovasculature differently in men and women or to a different magnitude of response. However, despite a more phenotypically severe depressive state in female compared to male rats, female rats demonstrate a better MCA endothelial-dependent dilation (albeit lower to female nonstressed rats) compared to male rats exposed to UCMS.[70] The vascular protection from UCMS in females appeared to reflect a superior maintenance of endothelial function, with more normal levels of NO, hydrogen peroxide (another endothelium-dependent vasodilator), prostacyclin, and thromboxane. However, the protective effect in female rats was dependent on the maintenance of a normal sex hormone profile, as ovariectomized females before UCMS abolished the protective effect and resulted in cerebrovascular outcomes being virtually identical to those in males. Previous studies have shown not only the protective effects of estrogen on vascular endothelial function through its action on promoting the bioavailability of NO, prostacyclin, and potentially other dilator metabolites,[72,73] but also the ability of estrogen to blunt a pro-oxidant or pro-inflammatory environment.[74,75]
The role of preexisting cardiovascular disease
The presence of preexisting cardiovascular and metabolic diseases (obesity, MetS, and diabetes) has been shown to dysregulate the HPA axis with neuroendocrine hyperresponsiveness to different neuropeptides and acute stress challenges.[76,77,78] With diabetes, in addition to an increased plasma adrenocorticotrophic hormone, RNA expression of corticosterone, and hypothalamic corticotrophin-releasing hormone are upregulated.[79] We have also established that circulating corticosterone was elevated in MetS compared to lean control rats, and that circulating corticosterone concentrations was further elevated with exposure to chronic stress.[42,80] Such data point toward vascular dysfunction. Indeed, there is strong evidence linking existing vascular dysfunction, due to the presence of metabolic/cardiovascular diseases, to the development of major depressive disorders especially with the presence of two or more classic risk factors.[81,82] The MetS affects ~20%–25% of adults worldwide and has a strong association with depression.[83,84] The normal development of MetS is characterized by chronic low-grade inflammation and a pro-oxidative environment that negatively affects multiple vascular beds, including in the brain.[85,86] We have recently shown that the cerebrovascular dysfunction (impaired endothelial function and microvascular rarefaction) with UCMS was enhanced in the presence of MetS.[42,70] Interestingly, these cerebrovascular adaptions to UCMS in lean healthy rats reflect what we see in a MetS rats (without UCMS), i.e., 8 weeks of UCMS transformed a healthy cerebrovasculature to one with similar dysfunction seen in the MetS. However, when chronic exposure to stress was performed in rats that were developing the MetS phenotype resulted in greater pathological cerebrovascular adaptations. Unlike lean rats exposed to UCMS, the MetS rats also developed impaired smooth muscle dilation (endothelial-independent dilation) coupled with increased MCA stiffness. The profile of vasoactive metabolites was further altered with the combined presentation of MetS and UCMS, such that the evolving pro-oxidant and pro-inflammatory environment within the cerebrovasculature nearly abolishes NO and prostacyclin levels and further elevated thromboxane production.[80] These results suggest that MetS results in greater pathological cerebrovascular alterations compared to lean rats when exposed to chronic stress. Obesity, a major epidemic in Western societies and an important component of the MetS, increases the severity of cerebrovascular dysfunction.[87] Adipose tissue conditioned media derived from lean subjects protected neuronal cells from oxidative stress damage, however conditioned media from adipose tissue obtained from obese subjects lacked this protection.[88] Adipokines by altering neuroinflammation and oxidative stress can modulate cerebral endothelial function.[89] For example, hypoadiponectinemia leads to endothelial dysfunction and is a predictor of cerebrovascular events.[90,91]
Inflammatory and Oxidative Stress
Neuroinflammation and oxidative stress are implicated in the cerebrovascular adaptations to chronic stress. The brain is particularly susceptible to oxidative stress due to its high levels of peroxidizable polyunsaturated fatty acids and transition metals, the low antioxidant defense mechanisms, and the brains high oxygen demand.[92,93] Data from postmortem human studies have provided strong evidence of neurovascular dysfunction with blood-brain barrier hyperpermeability in association with oxidative stress and neuroinflammation.[82,94,95,96,97] Brain samples from individuals with major depressive disorders have documented decreased levels of antioxidants,[93,98] and increased levels of lipid peroxidation end products.[99] Furthermore, an increase in serum concentrations of malondialdehyde coupled with decreased serum levels of nitrite, ascorbic acid, and superoxide dismutase were noted in patients diagnosed with unipolar disorders, compared to healthy controls.[100] Similarly, peripheral markers of oxidation are altered in individuals with major depressive disorders. For example, decreased activity of antioxidant enzymes (glutathione peroxidase, catalase, and superoxide dismutase 1), with increased activity of pro-oxidant enzymes (xanthine oxide), and increased iNOS and superoxides.[92,93] Further, support for the role of pro-inflammatory cytokines in stress-induced vascular damage comes from its actions on the peripheral vasculature. In various animal models, pro-inflammatory mediators IL-6, TNF-α, soluble ICAM-1, and C-reactive protein (CRP) have been increased following psychological stress.[101,102] Similarly, pro-inflammatory cytokines (IL-6, IL-8, IL-2, and CRP) have been reported to be increased in the presence of psychological stress in several human studies.[103,104,105] There is also increased activation of microglia,[106,107] upregulation of T-helper 1 cells and pro-inflammatory cytokines,[108] coupled with decreased anti-inflammatory cytokines.[109] There is a close relationship and a bidirectional interaction between Inflammation and oxidative stress, whereby a pro-oxidative stress environment can activate microglia and exacerbate the pro-inflammatory reactions through the NF-κB pathway.[110] In turn, activated microglia and pro-inflammatory cytokines can perpetuate oxidative stress.[99]
A major mechanism for the impact of oxidative stress on vascular function is the decrease of NO bioavailability and/or signaling. The oxidative stress induced by stressful conditions can shift the functional balance of NO from the beneficial endothelial NO synthase (eNOS) generation of NO to the harmful superoxide generation from NO. NO synthase is expressed in endothelial cells and astrocytes as eNOS (regulates vascular smooth muscle tone), and in the neurons as nNOS (regulates neurotransmission). However, iNOS which occurs in the glial and inflammatory cells is induced by pathological inflammatory states.[94] The eNOS production of NO increases cellular cyclic guanosine monophosphate levels, which can increase cerebral blood flow through endothelial-dependent dilation.[94] However, when combined with superoxides, the production of NO from iNOS and nNOS can impair vascular endothelial function and disrupt blood-brain barrier integrity.[94] In addition, reactive oxygen species activate the PI3K/ras/Akt/MAPK pathway leading to redox gene expression, which results in inhibition of eNOS mRNA expression and eNOS activity. Increased oxidative stress has important consequences with respect to smooth muscle cell signaling by the soluble guanylate cyclase and the cyclic guanosine monophosphate-dependent kinase I, whose activity and expression has been shown to be regulated in a redox-sensitive manner.[111,112] It is well reported that pro-inflammatory cytokines can impact endothelial function through its actions on decreasing eNOS activity, expression, and protein content.[113] Interestingly, CRP at concentrations known to predict cardiovascular disease downregulates eNOS and destabilizes eNOS mRNA, with resultant decreases in both basal and stimulated NO release.[114] However, pro-inflammatory cytokines can also alter calcium channel expression and activity, and upregulation of Rho-kinase expression and function.[115,116]
In addition to an upregulation of the oxidative stress pathway with stress, there is a decrease in the antioxidant pathway. Seven days of exposure to stress, induced by the social defeat model, significantly reduced protein levels of antioxidant enzymes (glutathione reductase, magnesium, and copper/zinc superoxide dismutase) in the rat hippocampus but not in the amygdala or prefrontal cortex. Concurrently, protein levels of glyoxalase-1 (the main enzymatic detoxification of methylglyoxal) were also reduced in the hippocampus and amygdala after social defeat-induced stress.[117] It was also noted that key second messengers, such as calcium/calmodulin-dependent protein kinase type (CAMK) IV and extracellular signal-regulated kinase (ERK)-1/2 were decreased and increased respectively in the hippocampus after social stress.[117] Calcium (Ca2+) is a universal second messenger that regulates many diverse cellular processes including cell proliferation, development, motility, secretion, learning, and memory, and there is converging signaling between CaMKII and CaMKIV. This congregated signaling has significant physiological actions, ranging from regulating contractile state and cellular proliferation to Ca2+ homeostasis and cellular permeability.[118] Furthermore, ERK1/2 plays an important role in vascular smooth muscle constriction,[119] and thus, an upregulation of ERK1/2 in accordance with stress could lead to an increase in vascular tone. Thus, it may not be surprising to note that the changes in the oxidative and anti-oxidative markers were associated with impaired cognition function with stress.[117] Overall, these data would suggest that social defeat stress alters ERK1/2, IL-6, GLO1, GSR1, and CAMKIV levels in specific brain areas, leading to oxidative stress-induced anxiety-depression-like behaviors and as well as memory impairment in rats.
In addition to vessel reactivity, our previous efforts into the physiological mechanisms contributing to microvascular rarefaction in the brain[47] have implicated the balance between oxidant stress and endothelial function (e.g., NO bioavailability and altered arachidonic acid metabolism) as a key contributor to the progression and severity of microvascular rarefaction. For example, interventions aimed at improving vascular NO bioavailability and reducing oxidant stress (e.g., tempol, captopril, metformin, and rosiglitazone) were the most effective at blunting the severity of the cortical microvascular rarefaction.[47] With the degree of loss in NO bioavailability reflecting the severity of the rarefaction that followed.[47] As such the reduction in NO bioavailability, with a corresponding higher oxidative environment with stress in the brain, might have directly contributed to the loss of cerebral microvessel density.
Clinical Implications
The pathophysiological adaptations that manifest in the presence of chronic stress, especially with the co-occurrence of metabolic and cardiovascular diseases could have important implications on cardiovascular disease, cognitive impairment, Alzheimer's disease, and an increased risk of a cerebral infarction (stroke)[120,121,122,123,124] for which impaired cerebrovascular endothelial function is likely a contributory factor.[125] Vessel diameter and functional hyperemic responses play a key role in maintaining adequate blood flow to each region of the brain, and must be able to respond accordingly to accommodate increases in flow during periods of higher neural activity. Impairments in any/all of these processes would likely have significant effects on neuronal metabolism and activity. Further, the reduction in cerebral microvessel density (cortex and striatum) with UCMS could have important clinical consequences. Microvascular rarefaction affects spatial hemodynamics and induces a non-uniform blood flow distribution, and has been implicated in reducing the capillary transport of small solutes.[126] Such pathological adaptations could also damage cerebral autoregulation and cerebral blood flow reserve, favoring the occurrence of cognitive impairment, and ischemic stroke.[127,128]
Lifestyle modifications such as changes in dietary fat[129] and moderate exercise[42,130] has shown to improve stress-associated symptoms and lower risk to cerebrovascular dysfunction. The impaired cognitive function of high-fat fed mice was improved by reduction of dietary fat.[129] We recently showed that aerobic exercise can protect the cerebrovascular function in obese Zucker rats exposed to UCMS.[42] This protection was attributed to changes in oxidative stress within the cerebral microvasculature. Although antioxidants can blunt most of the redox-mediated events leading to cerebrovascular dysfunction in animal models,[131,132] its use clinically is still questionable.[133]
Conclusion
Chronic exposure to stress conditions leads to significant pathophysiological alterations to the cerebrovasculature and the creation of depressive symptoms in otherwise healthy rats. However, these pathophysiological responses to stress are somewhat sex-specific and dependent on the preexistence of metabolic and cardiovascular diseases. Evidence would suggest that endothelial-dependent dysfunction is a primary manifestation coupled with a reduction in cerebral microvessel density of the stress response. However, in female rats, a vascular protection exists against chronic stress that is dependent on normal sex hormone levels (abolished in ovariectomized mice) that better maintains cerebral vasodilator reactivity compared to males or females with an ovariectomy following chronic stress. In the presence of MetS, the cerebrovascular dysfunction and elevated depressive symptoms to chronic stress are amplified. Further, female rats with MetS were no longer somewhat protected from the chronic stress response. The mechanisms behind the cerebrovascular dysfunction are partly linked to the increased pro-oxidative and inflammatory environment in the brain.
Financial support and sponsorship
This study was supported by the AHA pre-doctoral fellowship 14PRE 721 20380386, and the National Institute of General Medical Sciences of the National 722 Institutes of Health under Award Numbers U54GM104942, and 5P20GM109098.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scharf SH, Schmidt MV. Animal models of stress vulnerability and resilience in translational research. Curr Psychiatry Rep. 2012;14:159–65. doi: 10.1007/s11920-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 4.Mah L, Szabuniewicz C, Fiocco AJ. Can anxiety damage the brain? Curr Opin Psychiatry. 2016;29:56–63. doi: 10.1097/YCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 5.CDC.gov. Current depression among adults-United States, 2006 and 2008. Morb Mortal Wkly Rep. 2010;59:1229–35. [PubMed] [Google Scholar]
- 6.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the national comorbidity survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 7.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg J, Yaroslavsky I, Carney RM, Freedland KE, George CJ, Baji I, et al. The association between major depressive disorder in childhood and risk factors for cardiovascular disease in adolescence. Psychosom Med. 2014;76:122–7. doi: 10.1097/PSY.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everson-Rose SA, Roetker NS, Lutsey PL, Kershaw KN, Longstreth WT, Jr, Sacco RL. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2318–23. doi: 10.1161/STROKEAHA.114.004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–69. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–37. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 13.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender CL, Calfa GD, Molina VA. Astrocyte plasticity induced by emotional stress: A new partner in psychiatric physiopathology? Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:68–77. doi: 10.1016/j.pnpbp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses?. Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 18.Wortsman J. Role of epinephrine in acute stress. Endocrinol Metab Clin North Am. 2002;31:79–106. doi: 10.1016/s0889-8529(01)00024-x. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves KM. Neuroendocrine markers of stress. Anesth Prog. 1990;37:99–105. [PMC free article] [PubMed] [Google Scholar]
- 20.Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–83. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012;32:2135–45. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–9. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘trier social stress test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 24.Sharma HS, Cervós-Navarro J, Dey PK. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res. 1991;10:211–21. doi: 10.1016/0168-0102(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 25.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–6. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 26.Roszkowski M, Bohacek J. Stress does not increase blood-brain barrier permeability in mice. J Cereb Blood Flow Metab. 2016;36:1304–15. doi: 10.1177/0271678X16647739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Mladinov D, Pietrusz JL, Usa K, Liang M. Glucocorticoid response elements and 11 beta-hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc Res. 2009;81:140–7. doi: 10.1093/cvr/cvn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, et al. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–7. doi: 10.1161/01.res.0000050588.35034.3c. [DOI] [PubMed] [Google Scholar]
- 31.Borski RJ, Hyde GN, Fruchtman S. Signal transduction mechanisms mediating rapid, nongenomic effects of cortisol on prolactin release. Steroids. 2002;67:539–48. doi: 10.1016/s0039-128x(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Musumeci V, Baroni S, Cardillo C, Zappacosta B, Zuppi C, Tutinelli F, et al. Cardiovascular reactivity, plasma markers of endothelial and platelet activity and plasma renin activity after mental stress in normals and hypertensives. J Hypertens Suppl. 1987;5:S1–4. [PubMed] [Google Scholar]
- 34.Whipple MO, Lewis TT, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Powell LH, et al. Hopelessness, depressive symptoms, and carotid atherosclerosis in women: The Study of Women's Health Across the Nation (SWAN) heart study. Stroke. 2009;40:3166–72. doi: 10.1161/STROKEAHA.109.554519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bomhof-Roordink H, Seldenrijk A, van Hout HP, van Marwijk HW, Diamant M, Penninx BW, et al. Associations between life stress and subclinical cardiovascular disease are partly mediated by depressive and anxiety symptoms. J Psychosom Res. 2015;78:332–9. doi: 10.1016/j.jpsychores.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 37.von Känel R, Hepp U, Traber R, Kraemer B, Mica L, Keel M, et al. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158:363–73. doi: 10.1016/j.psychres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet. 2003;33:513–9. doi: 10.1023/a:1025770616068. [DOI] [PubMed] [Google Scholar]
- 39.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 40.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 41.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11:710–7. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks S, Brnayan KW, DeVallance E, Skinner R, Lemaster K, Sheets JW, et al. Psychological stress-induced cerebrovascular dysfunction: The role of metabolic syndrome and exercise. Exp Physiol. 2018;103:761–76. doi: 10.1113/EP086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O’Donnell J, et al. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav. 2011;103:540–6. doi: 10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Kang BM, Shin MK, Min J, Heo C, Lee Y, et al. Chronic stress decreases cerebrovascular responses during rat hindlimb electrical stimulation. Front Neurosci. 2015;9:462. doi: 10.3389/fnins.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A. 2014;111:7462–7. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson-Leary J, Eacret D, Chen R, Takano H, Nicholas B, Bhatnagar S, et al. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl Psychiatry. 2017;7:e1160. doi: 10.1038/tp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chantler PD, Shrader CD, Tabone LE, d’Audiffret AC, Huseynova K, Brooks SD, et al. Cerebral cortical microvascular rarefaction in metabolic syndrome is dependent on insulin resistance and loss of nitric oxide bioavailability. Microcirculation. 2015;22:435–45. doi: 10.1111/micc.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Audet GN, Meek TH, Garland T, Jr, Olfert IM. Expression of angiogenic regulators and skeletal muscle capillarity in selectively bred high aerobic capacity mice. Exp Physiol. 2011;96:1138–50. doi: 10.1113/expphysiol.2011.057711. [DOI] [PubMed] [Google Scholar]
- 49.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation. 2014;21:301–14. doi: 10.1111/micc.12117. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol. 2005;288:H759–68. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- 51.Olfert IM, Baum O, Hellsten Y, Egginton S. Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2016;310:H326–36. doi: 10.1152/ajpheart.00635.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou L, Isenberg JS, Cao Z, Roberts DD. Type I collagen is a molecular target for inhibition of angiogenesis by endogenous thrombospondin-1. Oncogene. 2006;25:536–45. doi: 10.1038/sj.onc.1209069. [DOI] [PubMed] [Google Scholar]
- 53.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–93. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 54.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: A physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–42. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–32. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J, et al. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–18. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benoit H, Jordan M, Wagner H, Wagner PD. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J Appl Physiol (1985) 1999;86:1513–8. doi: 10.1152/jappl.1999.86.5.1513. [DOI] [PubMed] [Google Scholar]
- 58.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–31. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 2013;91:323–8. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF, et al. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56:1137–44. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, et al. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol Aging. 1994;15:227–31. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 62.De Leo V, la Marca A, Talluri B, D’Antona D, Morgante G. Hypothalamo-pituitary-adrenal axis and adrenal function before and after ovariectomy in premenopausal women. Eur J Endocrinol. 1998;138:430–5. doi: 10.1530/eje.0.1380430. [DOI] [PubMed] [Google Scholar]
- 63.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinol. 2007;19:743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 64.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–70. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 65.Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–89. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- 66.Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, Schneider F, et al. Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: Are females more vulnerable? Neuroscience. 2005;135:703–14. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 68.Xing Y, He J, Hou J, Lin F, Tian J, Kurihara H, et al. Gender differences in CMS and the effects of antidepressant venlafaxine in rats. Neurochem Int. 2013;63:570–5. doi: 10.1016/j.neuint.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Harpaz I, Abutbul S, Nemirovsky A, Gal R, Cohen H, Monsonego A, et al. Chronic exposure to stress predisposes to higher autoimmune susceptibility in C57BL/6 mice: Glucocorticoids as a double-edged sword. Eur J Immunol. 2013;43:758–69. doi: 10.1002/eji.201242613. [DOI] [PubMed] [Google Scholar]
- 70.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, et al. Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol. 2018;314:H1070–84. doi: 10.1152/ajpheart.00647.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, et al. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1713–23. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liochev SI, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med. 2007;42:1465–9. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 74.Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 76.Pasquali R, Vicennati V. The abdominal obesity phenotype and insulin resistance are associated with abnormalities of the hypothalamic-pituitary-adrenal axis in humans. Horm Metab Res. 2000;32:521–5. doi: 10.1055/s-2007-978680. [DOI] [PubMed] [Google Scholar]
- 77.Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111–28. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- 78.Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143:1761–8. doi: 10.1210/endo.143.5.8809. [DOI] [PubMed] [Google Scholar]
- 79.Chan O, Chan S, Inouye K, Shum K, Matthews SG, Vranic M, et al. Diabetes impairs hypothalamo-pituitary-adrenal (HPA) responses to hypoglycemia, and insulin treatment normalizes HPA but not epinephrine responses. Diabetes. 2002;51:1681–9. doi: 10.2337/diabetes.51.6.1681. [DOI] [PubMed] [Google Scholar]
- 80.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, et al. Protection from chronic stress- and depressive symptom-induced vascular endothelial dysfunction in female rats is abolished by preexisting metabolic disease. Am J Physiol Heart Circ Physiol. 2018;314:H1085–97. doi: 10.1152/ajpheart.00648.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serlin Y, Levy J, Shalev H. Vascular pathology and blood-brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovasc Psychiatry Neurol. 2011;2011:609202. doi: 10.1155/2011/609202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biol Psychiatry. 2013;73:406–13. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 83.Pratt LA, Brody DJ. Depression and Obesity in the U.S. Adult Household Population, 2005–2010. National Health and Nutrition Examination Survey Data. NCHS Data Brief No. 167. [Last accessed on 2018 Feb 12]. Available from: https://www.cdc.gov/nchs/products/databriefs/db167. htm 2014 . [PubMed]
- 84.Kahl KG, Schweiger U, Correll C, Müller C, Busch ML, Bauer M, et al. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. 2015;5:e00306. doi: 10.1002/brb3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brooks SD, DeVallance E, d’Audiffret AC, Frisbee SJ, Tabone LE, Shrader CD, et al. Metabolic syndrome impairs reactivity and wall mechanics of cerebral resistance arteries in obese zucker rats. Am J Physiol Heart Circ Physiol. 2015;309:H1846–59. doi: 10.1152/ajpheart.00691.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–30. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- 87.Letra L, Sena C. Cerebrovascular disease: Consequences of obesity-induced endothelial dysfunction. Adv Neurobiol. 2017;19:163–89. doi: 10.1007/978-3-319-63260-5_7. [DOI] [PubMed] [Google Scholar]
- 88.Little JP, Safdar A. Adipose-brain crosstalk: Do adipokines have a role in neuroprotection? Neural Regen Res. 2015;10:1381–2. doi: 10.4103/1673-5374.165222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre d’Hellencourt C, et al. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflammation. 2016;13:67. doi: 10.1186/s12974-016-0530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. J Am Coll Cardiol. 2011;57:761–70. doi: 10.1016/j.jacc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Shimabukuro M, Higa N, Masuzaki H, Sata M, Ueda S. Impact of individual metabolic risk components or its clustering on endothelial and smooth muscle cell function in men. Cardiovasc Diabetol. 2016;15:77. doi: 10.1186/s12933-016-0394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maes M, Mihaylova I, Kubera M, Leunis JC, Geffard M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: New pathways that underpin the inflammatory and neuroprogressive pathophysiology. J Affect Disord. 2011;135:414–8. doi: 10.1016/j.jad.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 93.Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants: Fact or fiction? CNS Drugs. 2012;26:477–90. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 94.Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–64. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 95.Betzen C, White R, Zehendner CM, Pietrowski E, Bender B, Luhmann HJ, et al. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radic Biol Med. 2009;47:1212–20. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 96.Yapislar H, Aydogan S, Ozüm Ü. Biological understanding of the cardiovascular risk associated with major depression and panic disorder is important. Int J Psychiatry Clin Pract. 2012;16:27–32. doi: 10.3109/13651501.2011.620127. [DOI] [PubMed] [Google Scholar]
- 97.Le Mellédo JM, Mahil N, Baker GB. Nitric oxide: A key player in the relation between cardiovascular disease and major depressive disorder? J Psychiatry Neurosci. 2004;29:414–6. [PMC free article] [PubMed] [Google Scholar]
- 98.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–30. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 99.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 100.Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. 2014;8:CC04–7. doi: 10.7860/JCDR/2014/10258.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in apoE-/- mice. Atherosclerosis. 2012;221:359–65. doi: 10.1016/j.atherosclerosis.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 104.Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, Uemura H, et al. Association of serum cytokine concentrations with psychological symptoms in midlife women. J Reprod Immunol. 2007;75:56–62. doi: 10.1016/j.jri.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 105.von Känel R, Bellingrath S, Kudielka BM. Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J Psychosom Res. 2008;65:51–9. doi: 10.1016/j.jpsychores.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–92. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- 108.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67:1211–24. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, et al. Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation. 2013;20:39–50. doi: 10.1159/000343100. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto K, Ando J. New molecular mechanisms for cardiovascular disease: blood flow sensing mechanism in vascular endothelial cells. J Pharmacol Sci. 2011;116:323–31. doi: 10.1254/jphs.10r29fm. [DOI] [PubMed] [Google Scholar]
- 111.Brüne B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Biochem. 1990;192:683–8. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 112.Mülsch A, Bauersachs J, Schäfer A, Stasch JP, Kast R, Busse R, et al. Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br J Pharmacol. 1997;120:681–9. doi: 10.1038/sj.bjp.0700982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J, Patel JM, Li YD, Block ER. Proinflammatory cytokines downregulate gene expression and activity of constitutive nitric oxide synthase in porcine pulmonary artery endothelial cells. Res Commun Mol Pathol Pharmacol. 1997;96:71–87. [PubMed] [Google Scholar]
- 114.Järvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–8. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 115.Tiwari S, Zhang Y, Heller J, Abernethy DR, Soldatov NM. Atherosclerosis-related molecular alteration of the human CaV1.2 calcium channel alpha1C subunit. Proc Natl Acad Sci U S A. 2006;103:17024–9. doi: 10.1073/pnas.0606539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hiroki J, Shimokawa H, Higashi M, Morikawa K, Kandabashi T, Kawamura N, et al. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol. 2004;37:537–46. doi: 10.1016/j.yjmcc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toussaint F, Charbel C, Allen BG, Ledoux J. Vascular CaMKII: Heart and brain in your arteries. Am J Physiol Cell Physiol. 2016;311:C462–78. doi: 10.1152/ajpcell.00341.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhattacharya I, Damjanović M, Dominguez AP, Haas E. Inhibition of activated ERK1/2 and JNKs improves vascular function in mouse aortae in the absence of nitric oxide. Eur J Pharmacol. 2011;658:22–7. doi: 10.1016/j.ejphar.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 120.Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, et al. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: Rationale, design, and baseline characteristics. Am Heart J. 2017;192:1–2. doi: 10.1016/j.ahj.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Booth J, Connelly L, Lawrence M, Chalmers C, Joice S, Becker C, et al. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: A meta-analysis. BMC Neurol. 2015;15:233. doi: 10.1186/s12883-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26:9047–56. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, et al. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer's disease model. FASEB J. 2006;20:729–31. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 124.May M, McCarron P, Stansfeld S, Ben-Shlomo Y, Gallacher J, Yarnell J, et al. Does psychological distress predict the risk of ischemic stroke and transient ischemic attack?. The caerphilly study. Stroke. 2002;33:7–12. doi: 10.1161/hs0102.100529. [DOI] [PubMed] [Google Scholar]
- 125.Balkaya M, Prinz V, Custodis F, Gertz K, Kronenberg G, Kroeber J, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke. 2011;42:3258–64. doi: 10.1161/STROKEAHA.110.607705. [DOI] [PubMed] [Google Scholar]
- 126.Henrich HA, Romen W, Heimgärtner W, Hartung E, Bäumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klin Wochenschr. 1988;66:54–60. doi: 10.1007/BF01713011. [DOI] [PubMed] [Google Scholar]
- 127.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39:2712–9. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 128.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: A potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 129.Johnson LA, Zuloaga KL, Kugelman TL, Mader KS, Morré JT, Zuloaga DG, et al. Amelioration of metabolic syndrome-associated cognitive impairments in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine. 2016;3:26–42. doi: 10.1016/j.ebiom.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fornaro M, Solmi M, Veronese N, De Berardis D, Buonaguro EF, Tomasetti C, et al. The burden of mood-disorder/cerebrovascular disease comorbidity: Essential neurobiology, psychopharmacology, and physical activity interventions. Int Rev Psychiatry. 2017;29:425–35. doi: 10.1080/09540261.2017.1299695. [DOI] [PubMed] [Google Scholar]
- 131.Katusic ZS, Austin SA. Neurovascular protective function of endothelial nitric oxide - recent advances. Circ J. 2016;80:1499–503. doi: 10.1253/circj.CJ-16-0423. [DOI] [PubMed] [Google Scholar]
- 132.Holloway PM, Gillespie S, Becker F, Vital SA, Nguyen V, Alexander JS, et al. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vascul Pharmacol. 2016;85:29–38. doi: 10.1016/j.vph.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De Silva TM, Miller AA. Cerebral small vessel disease: Targeting oxidative stress as a novel therapeutic strategy? Front Pharmacol. 2016;7:61. doi: 10.3389/fphar.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]


