Abstract
OBJECTIVES:
Exogenously administered recombinant human erythropoietin (rhEPO) has been reported to exhibit neuroprotective effects in animal models. However, there are still have some controversies that combination of EPO and tissue plasminogen activator (tPA) in acute ischemic stroke. In the present study, we investigated the effects of local intra-arterial infusion of low-dose EPO in combination with tPA on focal cerebral ischemic stroke.
MATERIALS AND METHODS:
Sixty adult male Sprague–Dawley rats were randomly divided into five groups, including sham, vehicle, EPO, tPA, and EPO+tPA groups. Rats were subjected to middle cerebral artery occlusion (MCAO) and administrated with EPO (800 U/kg, middle cerebral artery injection), tPA (10 mg/kg, tail vein injection), EPO+tPA, or saline (vehicle) onset of reperfusion. Neurobehavioral deficits, infarct volume, brain edema, the expression of tight junction proteins (Claudin-5, Occludin), and AQP4 were assessed following 2 h ischemia and 24 h reperfusion. The number of apoptotic cells in the periinfarct region was detected by the terminal deoxyribonucleotide transferase dUTP nick end labeling (TUNEL) staining.
RESULTS:
The neurobehavioral deficits, brain infarct volume, edema volume, TUNEL-positive cells and downregulation of Claudin-5 and Occludin were alleviated by EPO or EPO plus tPA, following the ischemia/reperfusion (I/R) in rats. The EPO and EPO plus tPA both reduced the upregulation of AQP4 in the ischemic brain tissue.
CONCLUSION:
Our data demonstrate local intra-arterial infusion of low-dose EPO in combination with tPA protected against focal cerebral ischemia in rats manifested by a decrease in brain edema and blood-brain barrier (BBB) disruption after 2 h ischemia and 24 h reperfusion.
Keywords: Blood-brain barrier (BBB), brain ischemia, edema, erythropoietin (EPO)
Introduction
Acute stroke is one of the major causes of death and disabilities. However, to date the only approved drug tissue plasminogen activator (tPA) represents a thrombolytic and nonneuroprotective approach and also increases the incidence of hemorrhagic transformation.[1] Experimental studies show that neuroprotective agents used in conjunction with tPA increase safety and efficacy of thrombolytic therapy in experimental stroke.[2,3,4] One of the strategies is based on erythropoietin (EPO). Exogenously administered EPO has been reported to exhibit neuroprotective effects in animal models, through the anti-apoptotic, anti-oxidant, and anti-inflammatory effects as well as through the stimulation of angiogenic and neurogenic events.[5,6,7] The first clinical trial (phase I/II) ‘Gottingen EPO Stroke Study’ using recombinant human EPO (rhEPO) published in 2002 showed that 3-days treatment of rhEPO was safe and effective in acute ischemic stroke patients.[8] However, the contradictory information emerged from the ‘German Multicenter EPO Stroke Trial’ in 2009, which indicated that there were limited benefits in clinical outcomes following EPO treatment, especially that the combination of rtPA and EPO was associated with increased risk of serious complications including death, bleeding, edema, and thromboembolic events.[9] A study in rats showed that the administration of a combination tPA and EPO early after stroke (2 h) is neuroprotective, however, exacerbates hemorrhagic transformation and negates any benefit of EPO therapy outside the therapeutic window (6 h).[10] Another investigation in mice reported that combined treatment with tPA and EPO after 90 min of intraluminal MCAO facilitates extracellular matrix breakdown therefore increasing brain swelling.[11] The combination treatment-induced serious complications might be avoided through mending the dosage and route of EPO administration following stroke.
Because of the low brain-blood barrier (BBB) permeability of systematic administration of EPO, the effective dose of EPO treatment for stroke in humans (70 kg body weight, 33,000 U daily) is much higher than that of clinical treatment of chronic kidney disease or patients with anemia (70 kg body weight, 1,050-3,500 U daily). Some clinical studies have shown that the concentration of EPO when it reaches 50-150 U/kg (70 kg body weight, 3,500-10,500 U daily) can significantly increase the risk of thrombosis and cerebral injury,[9,12,13,14] which severely limited the clinical application in cerebral infarction. It is remarkable to note that the administration of high-doses EPO may be one of the main reasons for serious side effects of its combination with tPA. To solve the above problems, one potential way is to reorganize the molecular structure of EPO, that will not increase the formation of red blood cells, and preserves a wide range of neuroprotective effects,[15,16] but the side effects and the feasibility of clinical application need further study. Another possible solution is to change the route of administration. It has been reported previously that EPO could efficiently bypass the BBB by intranasal delivery,[17] however it is difficult to be applied in clinical treatment. In our previous study, low-dose EPO treatment via middle cerebral artery infusion was showed to be neuroprotective against focal cerebral ischemia-reperfusion injury in rats.[18] The present study was designed to test whether local intra-arterial infusion of low-dose EPO at 800 U/kg in combination with tPA through middle cerebral artery could protect against ischemic stroke.
Materials and Methods
Animal
Male Sprague–Dawley (SD) rats (280 ± 30 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All experimental protocols in this study were in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Animal grouping
A total of sixty adult male Sprague–Dawley rats were randomly divided into five groups (N = 12), including sham, vehicle, EPO, tPA, and EPO+tPA groups, as follows:
sham group, the rats underwent the same procedure as other groups without insertion of a nylon filament;
vehicle group, following MCAO rats were administrated saline through MCAI at the beginning of reperfusion;
EPO group, following MCAO rats were treated with low-dose rhEPO (800 U/kg) via middle cerebral artery infusion (MCAI) at the beginning of reperfusion;
tPA group, following MCAO rats were administrated the tPA (10 mg/kg) through tail vein at the beginning of reperfusion;
tPA+EPO group: Rats were subjected to MCAO and administrated 800 U/kg EPO plus 10 mg/kg tPA at the beginning of reperfusion.
A PE-50 catheter was used for middle cerebral artery infusion, which was modified to a filament with a 0.2-mm outer diameter and a 0.1-mm inner diameter. The catheter was inserted into the right external carotid artery immediately after the nylon suture was just withdrawn, then passed into the intracranial circulation and lodged in the MCA origin. EPO (400 μL) or tPA (400 μL) or saline (400 μL) were injected at a constant rate of 100 uL/min with a microinfusion pump.
Twelve animals were enrolled in each group; all rats except sham evaluated the neurological function, among which three for 2,3,5-triphenyl-tetrazolium chloride (TTC) staining to evaluate the brain infarction volume and edema volume, three for immunofluorescence and TUNEL staining and six for Western blot assessments.
Model of focal cerebral ischemia
The rats were housed in laboratory cages and maintained on a 12 h light–dark cycle, with free access to food and water throughout the period of study. Focal cerebral ischemia was induced by transient occlusion of middle cerebral artery, as previously described.[19] Briefly, rats were anaesthetized with enflurane. After a median neck incision, the right common and external carotid arteries were exposed. A nylon filament was inserted through the puncture of the external carotid artery and gently advanced along the internal carotid artery. The thread was removed 2 h after ischemia allowing reperfusion. To confirm the proper occlusion, the local cerebral blood flow in the area supplied by the middle cerebral artery was observed by transcranial laser Doppler (LDF, PeriFlux System 5000; Perimed, Sweden). Body temperature was monitored continuously with a rectal probe and maintained at 37.0 ± 0.5°C using a heating lamp during the surgery and MCAO.
Evaluation of neurological deficits
Neurological deficits were assessed at 24 h after reperfusion using the methods of Ludmila Belayev and forelimb placing tests.[18,20] The behavioral tests were performed by a blinded observer. For the Ludmila Belayev score, the neurological function was graded on a scale of 0-12, with 0 representing normal function and 12 representing maximum neurological deficits. For the foot-fault tests, rats were placed on and traversed along a platform with 9 × 9 cm2 grids. The number of contralateral forelimb foot faults made per meter within 1 min was calculated, high scores represents more neurological function deficits.
Determination of infarct size and brain edema
Rats were intracardially perfused with cold phosphate-buffered saline (PBS, pH 7.4) under deep anesthesia at 24 h after MCAO. Following decapitation, brains were quickly removed and cut into six 2-mm-thick sections. The sections were stained with 1.5% 2,3,5-TTC at 37°C for 10 min and then fixed in 4% paraformaldehyde for 24 h. Infraction and edema volume were expressed as a percentage of the contralateral area for each section that outlined with Image-J ProPlus Analysis Software (Media Cybernetics, USA). The extent of infarct volume was calculated by the following formula: (volume of contralateral hemisphere–volume of nonlesioned ipsilateral hemisphere)/volume of contralateral hemisphere.[21] The extent of edema was calculated using the following equation: (volume of ipsilateral hemisphere – volume of contralateral hemisphere)/volume of contralateral hemisphere.[22]
Immunofluorescence staining and terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick-end labeling (TUNEL)
Rats were deeply anesthetized, perfused with cold PBS and 4% paraformaldehyde. The brain were postfixed in 4% paraformaldehyde for 24 h, and then immersed in 30% sucrose solution in PBS for 24 h. The coronal brain sections of 10 μm thick were cut using a cryostat vibratome (Ultapro 5000, USA) at –25°C. The serial frozen sections were used for immunofluorescence staining to detect the expression of anti-Claudin-5 (1:500, Invitrogen, USA) and anti-Occludin (1:500, Invitrogen, USA). Briefly, the frozen sections were fixed in acetone at –4°C for 20 min. Then the sections were blocked in 0.3% bovine serum albumin (Sigma-Aldrich, USA) in PBS at room temperature for 1 h, and incubated with primary antibodies against Claudin-5 and Occludin (Invitrogen, USA) diluted 1:100 in PBS overnight at 4°C. Following three washes in PBS, the sections were incubated with fluorescent-conjugated secondary antibodies. Negative controls were performed using PBS to replace primary antibodies. To identify the apoptosis induced by I/R injury, tissue sections were stained using the TUNEL assay kit (Roche, Germany) according to the manufacturer's instructions. After labeling, all sections were counterstained with 4¢,6-diaminido-2-phenylidole (DAPI) and cover-slipped. Fluorescent images were acquired using an Olympus Fluoview FV1000 microscope with FV10-ASW 2.0 software (Olympus, USA).
Isolation of brain microvessels
Cerebral microvessels were isolated from rat cortex in the ischemic region based on a previously described method.[23] Briefly, the rats were intracardially perfused with 0.1 M PBS under deep anesthesia, and brains were removed and homogenized with a Dounce homogenizer (Kimble Chase, USA) at 4°C. Tissue fragments were filtrated through a 40-μm nylon mesh (Spectrum, USA), the microvessels retained on the mesh were suspended in PBS and centrifuged for 10 min at 3,500 g. Then the precipitation was suspended in 10% and 15% dextran T-500, the microvessels were collected after centrifuged for 10 min at 25,000g.
Western blot analysis
The rats were killed and the brains were removed immediately under deep anesthesia. The tissues were homogenized in lysis buffer with further sonication and centrifugation at 12,000 g for 30 min. Western blot was carried out as previously described.[24] The following antibodies were used in the present study: Anti-AQP4 (1:1000, Abcam, USA), anti-Claudin-5 (1:500, Invitrogen, USA), anti-Occludin (1:500, Invitrogen, USA). Following washes in Tris-buffer saline Tween-20 (TBST), the membranes were incubated with secondary antibodies (1:5000, Abgent, USA) for 30 min at room temperature. Immunoblots were then probed with an ECL kit (Millipore, USA) and visualized with a computerized image analysis system (Fluro Chen 2.0). IDV were calculated with the software Quantity One and normalized to β-actin (1:1000, Santa, USA).
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Difference between two groups was statistically analyzed using one-way ANOVA analysis, followed by the Tukey post-hoc test analysis. P ≤ 0.05 was considered statistically significant.
Results
Treatment with EPO plus tPA attenuates neurological deficits following cerebral I/R injury
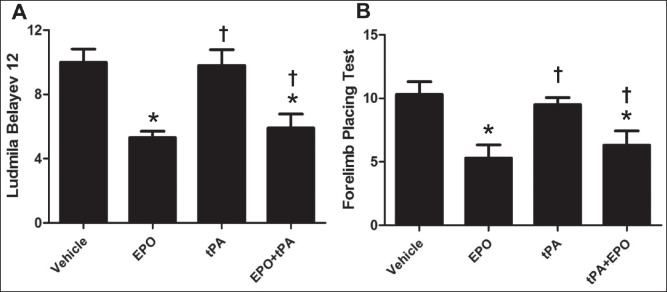
Neurological deficits was evaluated using the Ludmila Belayev and forelimb placing tests, the results are showed in Figure 1a and b. Treatment with EPO alone and EPO plus tPA markedly attenuated the neurological deficits compared to vehicle group (P < 0.05). Treatment with tPA alone did not show effect, whereas tPA combination with EPO treatment improved neurological function 24 h after cerebral reperfusion (P < 0.05).
Figure 1.
Treatment with EPO plus tPA attenuates neurological deficits following 2 h ischemia and 24 h reperfusion in rats. (a) The neurological function evaluated by Ludmila Belayev test; (b) forelimb placing's neurological scores at 24 h. N = 12. Data are expressed as means ± SEM. *P < 0.05 versus vehicle group; †P < 0.05 versus tPA group
Treatment with EPO plus tPA reduces brain infarction and edema following cerebral I/R injury
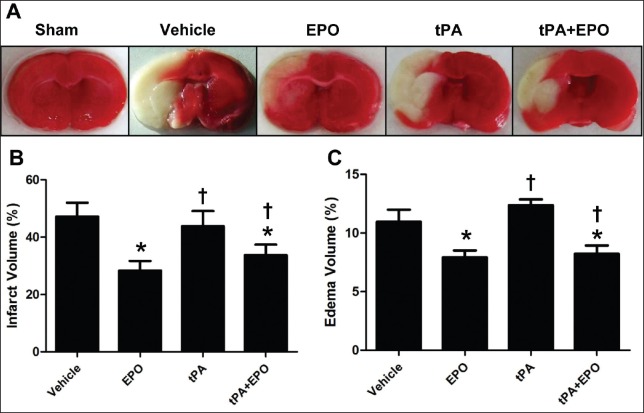
Treatment with EPO plus tPA reduced cerebral I/R injury was assessed in terms of infarction volume and brain edema, the results are showed in Figure 2. The brain infarction and edema volume were determined by TTC staining. The results indicated that EPO and EPO plus tPA treatment significantly reduced brain infarction and edema volume compared to vehicle group (P < 0.05). Importantly, tPA alone treatment had no effect on brain infarction and edema after I/R, while compared with the tPA group, EPO plus the tPA attenuated the infarction, and edema (P < 0.05), similar as the EPO group.
Figure 2.
Treatment with EPO plus tPA reduces brain infarction and brain edema following 2 h ischemia and 24 h reperfusion in rats. (a) Representative coronal sections stained by 2,3,5TTC; (b and c) the schematic diagram of brain infarction, brain edema in different groups. N = 3. Data are expressed as means ± SEM. *P < 0.05 versus vehicle group; †P < 0.05 versus tPA group
Treatment with EPO plus tPA attenuates the number of apoptotic cells in periinfarct region following cerebral I/R injury
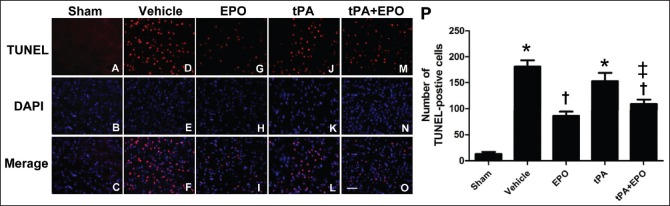
After the rats were subjected to 2 h ischemia and 24 h reperfusion, we detected the apoptotic cells of periinfarct region using TUNEL staining in different groups [Figure 3a]. The number of apoptotic cells was counted, and the mean value was calculated [Figure 3b]. TUNEL staining indicated that the cells in the periinfarct region of rats with I/R showed obvious apoptotic cells, and the occurrence of apoptosis was ameliorated by the EPO and EPO plus tPA treatments after cerebral ischemia (P < 0.05), whereas treatment with tPA alone did not show anti-apoptosis effect. The trend of cell apoptosis in different groups was consistent with cerebral infarction volume.
Figure 3.
Treatment with EPO plus tPA attenuates the number of apoptotic cells in periinfarct region after I/R injury. Brain tissues were processed for TUNEL. (A-O) The levels of TUNEL-positive cells (red) merged with DAPI (blue) in different groups were showed in the figures. (P) The schematic diagram of numbers of TUNEL-positive cells in different groups. N = 3. Data are expressed as means ± SEM. *P < 0.05 versus sham group; †P < 0.05 versus vehicle group; ‡P < 0.05 versus tPA group
Treatment with EPO plus tPA reduces AQP4 expression following I/R injury
To gain insights into the mechanism for the beneficial role of EPO in cerebral I/R injury, we first assessed the expression of AQP4 by western blot [Figure 4]. The result showed a significant increase in AQP4 expression in both the vehicle and tPA monotherapy groups at 24 h after I/R injury (P < 0.05), as compared with sham group. Noticeably, EPO plus tPA or EPO alone treatments attenuated cerebral I/R-enhanced AQP4 expression (P < 0.05), which may contribute to its role in reducing brain edema. In addition, compared with the tPA group, EPO plus tPA reduced the expression of AQP4 following 2 h cerebral ischemia and 24 h reperfusion (P < 0.05).
Figure 4.
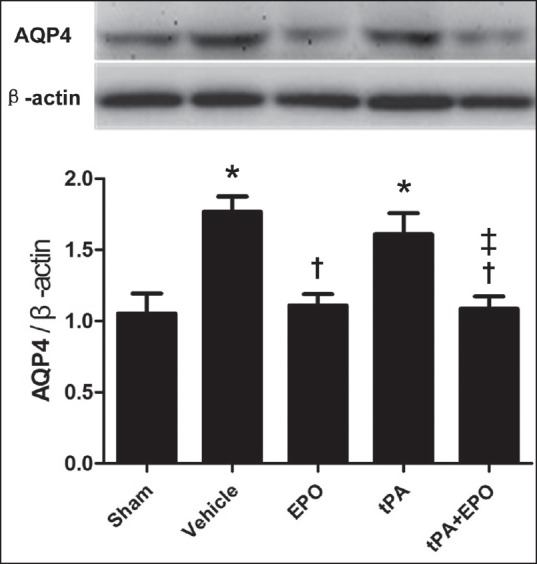
Treatment with EPO plus tPA prevents the upregulation of AQP4 in cerebral tissue induced by I/R injury. Rats were subjected to 2 h ischemia and 24 h reperfusion, and ischemic cerebral tissues were collected for western blot. The protein levels of AQP4 in different groups. β-actin served as a loading control. N = 6. Data are expressed as means ± SEM. *P < 0.05 versus sham group; †P < 0.05 versus vehicle group; ‡P < 0.05 versus tPA group
Treatment with EPO plus tPA prevents tight junction proteins in brain microvessels from decrease after I/R
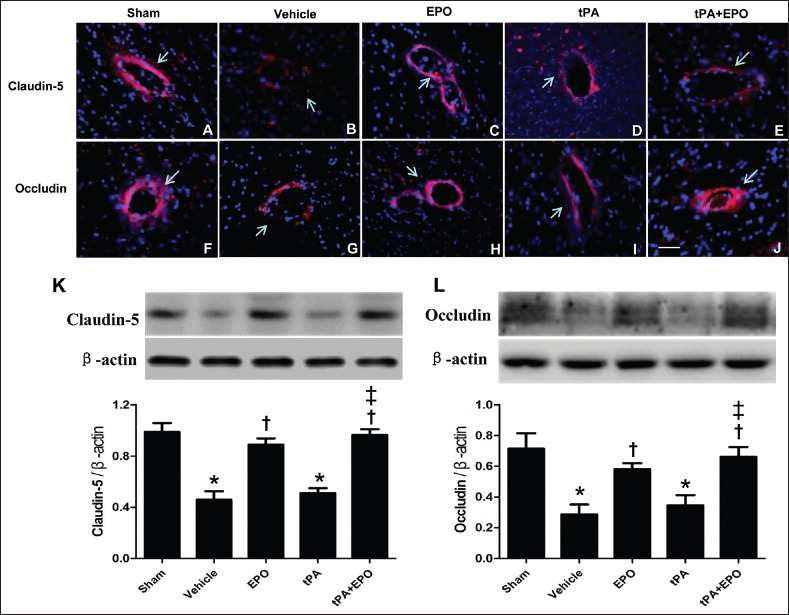
BBB disruption-induced brain edema is reported as an important adverse effect of EPO combined with tPA after ischemia. To examine whether the present strategy of cotreatment with EPO and tPA influences the BBB after ischemia-reperfusion, the expression and distribution of tight junction proteins Claudin-5 and Occludin in brain microvessels were determined by immunofluorescence staining and western blot. The immunofluorescence staining demonstrated that the expression of Claudin-5 and Occludin in endothelial cells markedly declined in vehicle and tPA groups, implying a significant disruption of the vascular endothelium, whereas the EPO alone or combination treatment of EPO and tPA prevented the decrease of Claudin-5 and Occludin after I/R [Figure 5a–j]. Consistent with immunofluorescence staining, the western blot indicated that vehicle and tPA groups demonstrated a markedly decrease of Claudin-5 and Occludin in isolated microvessels compared with sham operated animal [Figure 5k and l, P < 0.05]. EPO alone or in combination with tPA significantly prevented the decrease of Claudin-5 and Occludin (P < 0.05), whereas tPA alone did not show effect.
Figure 5.
Treatment with EPO plus tPA prevents the downregulation of Claudin-5 and Occludin in brain microvessels after I/R injury. The immunofluorescence staining of Claudin-5 (a-e) and Occludin (f-j) in periinfarct cortex (red), nuclear staining with DAPI (blue). Arrows indicate immunofluorescence staining positive portion of microvessels. N = 3. Scale bar = 50 μm. The level of tight junction protein Claudin-5 (K) and Occludin (L) in brain vessels detected by western blot. β-actin served as a loading control. N = 4. Data are expressed as means ± SEM. *P < 0.05 versus sham group; †P < 0.05 versus vehicle group; ‡P < 0.05 versus tPA group
Discussion and Conclusion
Controversy exists so far regarding the efficacy of combination treatment of EPO and tPA for ischemic stroke. It is noteworthy that, in the “German Multicenter EPO Stroke Trial,” some patients in EPO-treated but non-rtPA-treated groups showed beneficial effects similar to those observed in the first clinical trial “Gottingen EPO Stroke Study.” Such discrepancies between the two studies may result from potential risks in EPO and tPA interaction not found in previously preclinical experiments. In our previously studies,[18,25] the optimized the dosage and route of administration for EPO (low dose EPO treatment via MCA infusion) has been reported, the neuroprotective effects of EPO against cerebral I/R injury, such as reduced the neurological deficits, brain infarction and BBB disruption were confirmed in the present study. Importantly, we pay more attention to the efficacy of combination treatment of EPO and tPA for ischemic stroke in the present study. Interestingly, consistent with EPO alone treatment, the combination treatment with low-dose EPO (800 U/kg) and tPA through MCAI at the onset of reperfusion not only decreased brain edema, but also reduced BBB disruption following 2 h ischemia and 24 h reperfusion in rats. Because the dosage of EPO used in this study was much lower than that commonly used in prior studies (2,500-5,000 U/kg), the finding in this study may provide an effective and safe strategy for cerebral ischemia–reperfusion injury.
Brain edema can be vasogenic and/or cellular in origin. The edema that develops after ischemia and trauma is primarily of cellular origin, and astroglial cells possess the predominant water channel AQP4 in the mammalian brain. Several lines of evidence suggest that water influx via AQP4 contributes to inappropriate water uptake,[26,27] resulting in cell swelling. In vitro and in vivo study demonstrated that EPO treatment significantly reduces the hypoxia-ischemia-induced neural swelling and upregulation of AQP4.[28,29] Consistent with these results, the present study showed the potential of EPO to attenuate the upregulation of AQP4 in brain tissue after I/R, which may account for, at least partly, the effect of EPO on brain edema. On the other hand, BBB disruption brings about vasogenic brain edema. BBB is consisted of endothelial cells a basal lamina. Tight junction between endothelial cells is believed to play a critical role in maintaining BBB integrity, while MMP9 is known to contribute to the BBB disruption via degradation of basal lamina. The present study showed that EPO, either alone or in combination with tPA, is able to attenuate I/R-elicited downregulation of tight junction proteins Claudin-5 and Occludin, highly suggesting the protective role of EPO in BBB disruption. This result is in disagreement with a previous clinical trial[9] and a study in mice[11] showing that exacerbating BBB disruption and brain edema is a main side effect of EPO in combination with tPA after brain ischemia, but is in keeping with a study by Jia et al. in rats.[10] This controversy may arise due to the difference in time, dosage, and route of administration. In addition, the species of animals used in different studies may also affect the outcomes. Nonetheless, much works remain to be done before the translation of the results to the clinic.
In summary, the administration of low-dose EPO in combination with tPA through MCA infusion at the onset of reperfusion enhanced its neuroprotective effect, which may be correlated with the potential of EPO to antagonize the brain edema and BBB disruption. This result suggests the mode of EPO in combination with tPA administration reported in this study could be useful for establishing new therapeutic strategy for patients with acute ischemic stroke.
Financial support and sponsorship
This project was supported by Natural Science Foundation in China (81071058, 81271461), and Beijing Nova Program (Z151100000315065).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Jinhuan Gao (Xuanwu Hospital of Capital Medical University) for her excellent technical assistance.
References
- 1.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28:2109–18. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 2.Wang CX, Ding X, Shuaib A. Treatment with melagatran alone or in combination with thrombolytic therapy reduced ischemic brain injury. Exp Neurol. 2008;213:171–5. doi: 10.1016/j.expneurol.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: Generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–5. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 5.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 6.Villa P, Bigini P, Mennini T, Agnello D, Laraqione T, Caqnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–5. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewcuzuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and benificial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. EPO Stroke Trial Group. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–56. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41:2071–6. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zechariah A, ElAli A, Hermann DM. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 2010;41:1008–12. doi: 10.1161/STROKEAHA.109.574418. [DOI] [PubMed] [Google Scholar]
- 12.Stohlawetz PJ, Dzirlo L, Hergovich, N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95:2983–9. [PubMed] [Google Scholar]
- 13.Beguin Y. Erythropoietin and platelet production. Haematologica. 1999;84:541–7. [PubMed] [Google Scholar]
- 14.Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: Comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–94. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gan Y, Xing J, Jing Z, Stetler RA, Zhang F, Luo Y, et al. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke. 2012;43:3071–7. doi: 10.1161/STROKEAHA.112.663120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hand CC, Brines M. Promises and pitfalls in erythopoietin-mediated tissue protection: Are nonerythropoietic derivatives a way forward? J Investig Med. 2011;59:1073–82. doi: 10.231/JIM.0b013e3181ed30bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez Cruz Y, Mengana Támos Y, Muñoz Cernuda A, Subirós Martines N, González-Quevedo A, Sosa Testé I, et al. Treatment with nasal neuro-EPO improves the neurological, cognitive, and histological state in a gerbil model of focal ischemia. ScientificWorldJournal. 2010;10:2288–330. doi: 10.1100/tsw.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang S, Liu X, Fu P, Gong W, Yan F, Han P, et al. Neuroprotection by local intra-arterial infusion of erythropoietin after focal cerebral ischemia in rats. Neurol Res. 2011;33:520–8. doi: 10.1179/016164111X13007856084287. [DOI] [PubMed] [Google Scholar]
- 19.Qi ZF, Luo YM, Liu XR, Wang RL, Zhao HP, Yan F, et al. AKT/GSK3β-Dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci Ther. 2012;18:965–73. doi: 10.1111/cns.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–23. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 21.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 22.Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- 23.Biegel D, Spence DD, Pachter JS. Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro . Brain Res. 1995;692:183–9. doi: 10.1016/0006-8993(95)00511-n. [DOI] [PubMed] [Google Scholar]
- 24.Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, et al. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–71. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Wu X, Liang J, Qi Z, Liu X, Min L, et al. Intra-artery infusion of recombinant human erythropoietin reduces blood-brain barrier disruption in rats following cerebral ischemia and reperfusion. Int J Neurosci. 2015;125:693–702. doi: 10.3109/00207454.2014.966354. [DOI] [PubMed] [Google Scholar]
- 26.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100:2106–11. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–63. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 28.Gunnarson E, Song Y, Kowalewski JM, Brismar H, Brines M, Cerami A, et al. Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc Natl Acad Sci U S A. 2009;106:1602–7. doi: 10.1073/pnas.0812708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z, Sun X, Huo G, Xie Y, Shi Q, Chen S, et al. Protective effects of erythropoietin on astrocytic swelling after oxygen-glucose deprivation and reoxygenation: Mediation through AQP4 expression and MAPK pathway. Neuropharmacology. 2013;67:8–15. doi: 10.1016/j.neuropharm.2012.10.017. [DOI] [PubMed] [Google Scholar]