Abstract
Wake-up stroke or stroke with unclear onset of symptoms is known to occur in one-fourth of ischemic stroke patients. These patients are not considered for thrombolytic therapy based on time designation of their symptom onset as per the current guidelines. Observational studies have investigated the pathophysiology and suggested actual onset of symptoms to be approximate to the awakening time for these patients. Use of advanced imaging modalities in these patients tends to identify favorable patient profiles for thrombolysis. Results of the ongoing trials will likely beckon a seminal juncture in stroke therapy and deliver critical modifications in the current treatment guidelines for thrombolysis in this substantial, yet neglected, group of stroke patients. In this article, we have reviewed the predisposing factors, preferred imaging modalities and various ongoing thrombolytic and endovascular trials to date for patients with unclear time of symptom onset or who wake up with stroke symptoms.
Keywords: Diffusion-fluid-attenuated inversion recovery mismatch, multimodal imaging, wake-up stroke
Introduction
Acute ischemic stroke (AIS) has a recurrence rate of 13% by 1 year that accounts for an increasing trend toward elevated global burden of stroke.[1,2] Around a quarter of AIS patients notice stroke symptoms on awakening (wake-up stroke [WUS]),[3,4] and no clear time of symptom onset could be ascertained in these cases. Use of intravenous tissue plasminogen activator (IV tPA) has been approved within 3 h and can be safely administered up to 4.5 h[5,6] while the risk of harm increases beyond 4.5 h.[7] Duration of clinical symptoms determines the eligibility for thrombolysis[8] while narrow therapeutic window of IV tPA precludes its usage for patients with unknown time last seen well (TLSW) or WUS.[9,10] Thrombolysis in this subgroup of patients has been studied in detail although the final consensus on the benefit remains to be unraveled.[11]
Obtaining critical data of TLSW has been the primary information that delineates therapeutic management in each AIS case. Investigators in the stroke community have questioned the reliability of TLSW in a hustling emergency department setting, which determines the inclusion or exclusion of patient for IV tPA.[12] This concept of relatively arbitrary onset time designation has been implicated as cause of admission delays of AIS patients.[13]
Majority of ischemic stroke subtypes have a predilection for early morning onset.[14] Similar analogy has been well-studied in WUS patients with a crude dogma that patients likely wake up with their respective clinical symptoms and could very likely be within therapeutic window for IV tPA.[9] WUS patients usually present with severe NIH stroke scale (NIHSS), secondary clinical deterioration with prolonged hospital admission, and poor clinical outcomes.[15] The rigid time stamp involving TLSW for this subgroup seems to be incongruent due to the dynamic process of ischemia evolution. Poor clinical outcomes further reiterate the focus of stroke community toward this subgroup of stroke patients for consideration of reperfusion therapy.
Recent advancement in neuroimaging, especially with the inclusion of multimodal imaging, has opened up new horizon to further investigate patients with unclear onset of stroke symptoms. Irrespective of the time of symptom onset, various neuroimaging patterns render exquisite details of hemodynamics that guide physicians for thrombolytic therapy. Using Alberta Stroke Program Early CT Score (ASPECTS) for computed tomography (CT) or diffusion-weighted imaging (DWI) for magnetic resonance imaging (MRI), similar early ischemic changes (EICs) have been observed in WUS patients when compared with patients presenting within 3 h[16] or 6 h[17] of symptom onset. In this review, we discuss predisposing factors involved in the pathophysiology of WUS and various neuroimaging modalities available to detect viable tissue that supports therapeutic decisions in the most meticulous time fashion. We have also summarized various clinical trials that are investigating reperfusion therapies in this subclass of stroke population.
Predisposing Factors for Wake-up Stroke
Various studies have observed stroke incidence during early morning hours with symptom manifestation on awakening or compelling patients to wake up due to symptoms.[9,18] Circadian variation plays a major role in the alteration of cerebral blood flow (CBF) and contributes to the pathophysiology of WUS.[19] These hemodynamic variations in congruence to any downstream vascular stenosis likely play a critical role in AIS, especially in WUS patients. Kim et al. studied the association of ischemic stroke during sleep with clinical outcomes and observed high prevalence of large vessel atherosclerosis in WUS.[20]
Several hemodynamic alterations are associated during sleep and are postulated as potential risk factors that predispose cerebral ischemia. Reduction of blood pressure, heart rate, sympathetic and metabolic drive occurs during sleep.[21] These hemodynamic alterations potentially contribute toward cerebral ischemia during sleep or WUS.[22] Various sleep disorders such as obstructive sleep apnea (OSA) are independently associated with cerebral infarction.[23] OSA is chronic disorder with a prevalence of 49% and 23% in men and women, respectively, and the estimated prevalence rate has substantially increased over the last few decades.[24] Uncorrected OSA results in frequent hypoxic episodes and generates negative intrathoracic pressure that further increases cardiac afterload.[22] OSA also encompasses elevation of systemic blood pressure due to hypoxia, elevated sympathetic nervous system activity, and sleep arousals. These alterations increase cardiac afterload and predispose to cardiac ischemia and arrhythmias. This further reduces the cardiac output and CBF, especially in patients with reduced cerebrovascular reserve due to proximal steno-occlusive carotid disease.[21] Transcranial Doppler (TCD) is a noninvasive method to measure blood flow velocities and is an effective tool to detect the presence of patent foramen ovale (PFO).[25] Increased prevalence of PFO has been associated with OSA using TCD.[26] Recurrent apneic episodes in OSA are associated with elevated right cardiac chamber pressures that tend to increase right-to-left shunting through PFO[27] and predispose for paradoxical embolism.
Commonly used Imaging Sequences in Wake-up Stroke
Neuroimaging is the most critical aspect to assess the viability of cerebral tissue in the therapeutic management of ischemic stroke.[28] Cerebral ischemia is a dynamic process that demonstrates heterogeneous imaging patterns in AIS patients. Recent advent of comprehensive imaging techniques, especially multimodal CT or MRI including perfusion and angiography, provides multifaceted critical details of cerebral hemodynamics. Utilization of multimodal imaging has provided substantial support to physicians to investigate thrombolysis in patients with unclear time of symptom onset.[29,30]
Various authors have compared different aspects of patients presenting with unclear onset of symptoms or WUS and patients with accurate time of symptom onset. They have reported similar imaging profiles in both the groups based on either EICs using ASPECTS,[31] CT perfusion (CTP) mismatch,[9] or diffusion-perfusion-related mismatch[18] imaging protocols.
Noncontrast computed tomography
Noncontrast CT (NCCT) has the benefit of wide availability and easy access in the stroke community. ASPECTS refers to a 10-point grading system that scores EICs in the middle cerebral artery (MCA) territory for AIS patients.[32] This scoring system has been shown to be a reliable method to assess EICs using NCCT, with lower scores correlating to poor clinical outcomes. The incidence of EICs was shown to be comparable between WUS patients and patients presenting within 3 h[16] or 6 h[17] of symptom realization. Other authors have also corroborated with similar NCCT findings in WUS patients and patients who presented with clear onset of symptoms.[3,33] This suggests that a favorable percentage of WUS patients might be eligible for thrombolytic benefits. Costa et al. recently observed similar clinical severity, neuroimaging findings, and clinical outcomes between WUS patients and those who arrive within therapeutic window of IV tPA.[34] However, NCCT provides limited details for EICs and is not a potent tool to determine symptom onset time.
Computed tomography perfusion
NCCT has recently evolved with the inclusion of new imaging modalities, especially perfusion studies. CTP has been utilized to assess cerebral ischemia in acute, subacute, and chronic phase of AIS [Figure 1]. CTP involves CBF, cerebral blood volume, and mean transit time (MTT) as hemodynamic parameters to recognize critical hypoperfused zone and differentiate from infarct core. Ischemic penumbra is usually interpreted as elevated MTT or time-to-maximum (Tmax) parameters.[35] DEFUSE and EPITHET studies defined hypoperfused tissue as Tmax >6 s, i.e., Tmax contrast arrival delay of more than 6 s, delineates penumbral tissue at risk of irreversible injury.[36]
Figure 1.
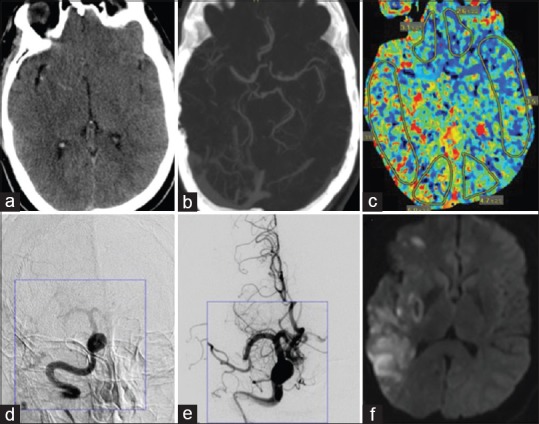
A 50-year-old male with a history of hypertension woke up with difficulty walking and left side weakness with a summated NIH stroke scale of 7. (a) Initial noncontrast computed tomography demonstrated hyperdense right middle cerebral artery vessel and early ischemic changes. (b) Computed tomography angiogram demonstrated mid-distal right M1 segment occlusion. (c) Computed tomography perfusion shows perfusion mismatch in right middle cerebral artery territory. (d and e) Digital subtraction angiogram showing right M1 segment occlusion in both pre- and post-endovascular procedure images. (f) Diffusion-weighted imaging sequence showing patchy infarcts in the right middle cerebral artery territory
Perfusion studies have been studied in small case series involving patients with unclear time of symptom onset and WUS patients. The efficacy and safety of these imaging modalities are being investigated in ongoing randomized controlled trials.[37,38] These modalities have extended the scope of off-label use of thrombolysis in WUS patients or patients with unclear symptom onset.[39] Despite the wide access and rapidity of NCCT and CTP, the final consensus is still debatable when compared with alternative perfusion imaging modalities.
Perfusion- and diffusion-weighted magnetic resonance imaging mismatch
Perfusion techniques using MRI similarly delineate salvageable tissue or penumbra and diffusion lesion correlating with infarct core volume. Similar to CT, assessment of optimal threshold values for MR-based ischemic penumbra and infarct core continues to be a challenge.[40] Cerebral ischemia is a dynamic process with interplay of various hemodynamic components that influence imaging parameters, especially perfusion studies. Perfusion-dependent imaging sequences reflect the volume of viable tissue at that specific time point of image acquisition. The risk of further expansion and evolution into infarct core continues to loom,[41] with a propensity for infarct evolution.
Perfusion-weighted imaging (PWI) and DWI compare the volume of ischemic injury and evaluate the presence of mismatch between these two measures of at-risk tissue versus infarct core, respectively. Clinicians are often faced with the challenge of delineating the size of the infarct core and ischemic penumbra or area at-risk to consider further therapies. Recently, various threshold values have been formulated using software tools that differentiate tissue at-risk from infarct core using color-coded maps.[42] Different authors have further refined threshold values of Tmax >6 s and perfusion-diffusion mismatch ratio >1.2 indicating a favorable penumbral pattern while DWI lesion volume >70 cc for infarct core correlating with poor clinical outcome.[43] Various pooled studies have shown improvement in clinical outcome for treating such MRI-defined ischemic penumbra with thrombolytics in an extended time window.[44] Perfusion-diffusion mismatch has been suggested as a reliable technique to consider thrombolytic decisions in WUS patients.[45]
Diffusion and fluid-attenuated inversion recovery-defined mismatch
MRI provides various imaging sequences that render exquisite details regarding discrete aspects of cerebral parenchyma. DWI and PWI have remained the primary sequences that differentiate perfusion-dependent tissue from infarct core. Recently, a new concept to assess tissue viability has evolved as a substitute to DWI-PWI comparative technique that involves comparison of DWI and fluid-attenuated inversion recovery (FLAIR) images [Figure 2]. DWI detects cytotoxic edema due to restriction of permeability of extracellular water within minutes of ischemic onset while FLAIR detects vasogenic edema that develops in the following hours. DWI-FLAIR mismatch estimation tends to assess cerebral tissue viability and thus, supplements PWI-DWI comparative sequences.[46]
Figure 2.
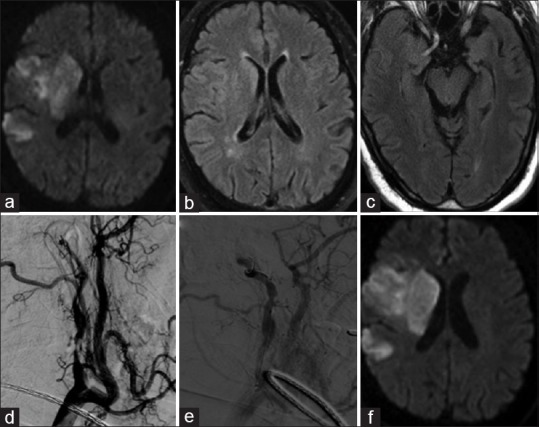
A 61-year-old male with a history of hypertension, diabetes, coronary artery disease woke up with left-sided weakness and numbness. (a) Diffusion-weighted imaging sequence showing acute to subacute infarct in the right middle cerebral artery territory involving caudate head, basal ganglia, and parietal lobe. (b and c) Fluid-attenuated inversion recovery sequence shows subtle hyperintensity suggesting diffusion weighted imaging-fluid attenuated inversion recovery mismatch, distal vascular hyperintensities secondary to slow flow and absence of flow void in proximal, middle cerebral artery segment. (d) Digital subtraction angiogram demonstrating critical stenosis of proximal right internal carotid artery with tandem occlusion of supraclinoid segment. (e) balloon angioplasty of proximal stenosis (f) final evolution of right MCA territory infarct
Ischemic lesions tend to evolve and become conspicuous beyond 3 h from symptom onset using DWI-FLAIR mismatch.[47] DWI-FLAIR mismatch estimates the age of an ischemic lesion and tends to identify WUS patients who could be safely administered reperfusion therapies.[48,49] FLAIR demonstrates chronological evolution of cerebral ischemia with initial sluggish flow due to large vessel occlusion (LVO) as hyperintense vessels followed by vasogenic edema, thus serves as an image surrogate for time from ischemia onset.[47] Clinicians tend to rely on intensity of FLAIR signals to guide them during thrombolytic decision-making. Hyperintense FLAIR signal has been associated with poor clinical outcome at 3 months although these results further need to be confirmed in randomized controlled trials.[50] Kufner et al. have shown an association of early observation of FLAIR signal hyperintensity with increased hemorrhage risk[51] while another group of authors did not find such association in their study.[52] DWI-FLAIR comparison has been of paramount significance to estimate the age of ischemic lesion although definite bleeding risk using these sequences remains yet to be determined.
Experience with Endovascular Reperfusion Therapy in Wake-up Stroke
Endovascular therapy (ET) has gained paramount attention after the recent success of five randomized controlled trials and is now the standard of care for AIS patients with clear time for symptom onset.[53,54,55,56,57] The superior clinical outcomes of these trials were fueled by rapid patient triage, incorporation of multimodal techniques, especially angiography and perfusion studies, and utilization of state-of-the-art stent retriever thrombectomy devices.[28] Patients with WUS or unclear symptom onset time were not included in these successful trials. The majority of these trials enrolled patients within 6–8 h of symptom onset, except the ESCAPE trial that enrolled patients within 12 h of symptom onset. However, the vast majority of enrolled subjects were in the earliest time epochs. The ESCAPE trialists set enrollment criteria for patients within 12 h of symptom onset though median time to randomization was 169 min and only small number of patients was enrolled beyond 6 h.[55]
Although ET has been investigated for patients with unclear onset of symptoms or WUS patients, its efficacy for this subgroup of patients is yet to be determined. A few case series used intra-arterial urokinase and the MERCI retrieval device for this subgroup of patients, but failed to show any benefit.[58,59] Two recent case series failed to convincingly prove better clinical outcomes despite the use of cutting edge stent retrievers in majority of their patients.[60,61] Their results also showed high rates of mortality (23% and 37%) and symptomatic intracerebral hemorrhage (sICH) (14% and 21%) in the respective studies. Stampfl et al. used stent retrievers in 19 WUS patients and found increased sICH rate with poor clinical outcome at 3 months.[61] Although the results of these ET studies have been majorly negative, few authors showed similar clinical outcomes using stent retrievers for patients with known and unknown time of symptom onset.[59]
Various neuroimaging modalities have emerged especially multimodal techniques to discern exquisite ischemic details for this subset of patients who might benefit from reperfusion therapies. Many authors have utilized perfusion techniques both CT and MR in patients with unclear time of symptom onset to assess safety and efficacy of mechanical thrombectomy in this subclass of stroke patients.[59,61] A few case series have used both CT[62] and MR[63] perfusion modalities to assess clinical outcomes after using either IV thrombolysis or ET. Cho et al. studied the safety and efficacy of thrombolysis and ET using penumbral and DWI-FLAIR mismatch.[64] This preliminary concept was further studied in a multicenter trial that included 19.3% of patients with unclear time of symptom who were treated in similar fashion with either IV thrombolysis or intra-arterial revascularization.[45] Various ongoing endovascular reperfusion trials are currently underway that will guide future therapeutic management in this subclass of stroke patients.
Clinical Implications in Wake-up Stroke
It has been well-studied in the literature that onset of hemodynamic changes or symptom onset in patients with unclear-onset stroke or WUS approximates with the time of patient awakening.[16] Patients with unclear onset of symptoms usually are a part of broad subgroup that involves WUS patients or daytime-unwitnessed stroke (DUS) patients. DUS patients are similar to WUS patients and are excluded from potential benefits of thrombolysis. A recent study comparing WUS and DUS patients observed more frequent diffusion-FLAIR and diffusion-perfusion mismatch patterns in DUS patients.[65] The authors also observed that DUS patients arrived earlier to seek medical attention and have higher likelihood to receive reperfusion therapy as compared to WUS patients. This analogy concluded time of symptom recognition as a more valuable tool than TLSW for patients with unclear time of symptom onset or WUS patients.
Reperfusion of a hypoperfused zone, either through IV tPA or through ET, has been shown to reduce final infarct core volume and is associated with better clinical outcomes.[66,67] Investigators have tried to extend the thrombolytic benefits beyond the narrow therapeutic window of 3–4.5 h, but majorly have been studded with risk/benefit assessment in these patients. Patients with indefinite or unclear onset of symptoms usually present with large volume of ischemic zone that is prone to increased hemorrhagic risk from thrombolysis and worse clinical outcomes.[68]
Various CT or MR sequences have been utilized to extend and study clinical implications with thrombolysis in patients with unclear time onset or presenting as WUS. Similar rates of sICH have been found in WUS or patients with clear symptom onset when treated with IV tPA.[62] On the contrary, AbESTT-II was a randomized controlled trial that used abciximab in WUS patients and found increased hemorrhagic transformation rate when compared to patients with definite time of symptom onset.[69] Similarly, many other studies have failed to show similar results and have been futile in their efforts. Despite these confounding results, physicians tend to arrive at a clinical equipoise by assessing multimodal images and determine risk/benefit ratio for each individual case separately.
Multimodal imaging techniques have emerged as a boon to stroke community that renders multifaceted refined data during emergent settings. Use of these novel imaging techniques has been a topic of diagnostic pursuit in WUS patients and those with unclear time of symptom onset. These techniques are being utilized in majority of the stroke centers to assess the size of salvageable penumbral tissue with a risk to evolve into infarct core. Although these imaging modalities seem to be an appealing concept in this subgroup of patients, interpreting subtle FLAIR and perfusion signals are studded with drawbacks of inter-observer variability and reliability. Recent trials have utilized automated softwares for perfusion and infarct volume analysis such as RAPID software,[42] but their validity is yet to be confirmed. However, certain issues including threshold values for FLAIR intensity, inter-observer discrepancies, and generalizability of automated softwares need to be figured out before its applicability in clinical practice for WUS patients.
Trials of Intravenous Thrombolysis using Penumbral Imaging
EXTEND
EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) is a randomized, double-blinded, multicenter, placebo-controlled, phase III trial.[37] It compares the efficacy of IV tPA (0.9 or 0.6 mg/kg) and placebo for AIS patients with penumbral mismatch pattern presenting at 3-4.5 h (depending on guidelines followed by participating center) up to 9 h post symptom onset or who wake up with symptoms. For WUS, the investigators selected midpoint from sleep onset and time of awakening to be ≤9 h. Diffusion-perfusion mismatch (MRI) or CTP is used to assess the penumbral pattern with Tmax >6 s for perfusion lesion and DWI-MRI or CBF-CT to define infarct core volume. Various neuroimaging threshold values used as inclusion criteria comprise infarct core volume ≤70 cc, ratio of hypoperfusion to infarct core volume >1.2, and an absolute mismatch difference >10 cc. The investigators intend to enroll 400 patients in different centers across Australia along with other concomitant international centers (EXTEND international). In addition, there is another European study in progress (ECASS-4: EXTEND) that intends to enroll patients based on the design of EXTEND trial, except patients will receive 0.9 mg/kg dose of IV tPA using MRI only.
Trials of Intravenous Thrombolysis using Magnetic Resonance Estimates of Lesion Age
WAKE-UP
Efficacy and safety of MRI-based thrombolysis in WUS (WAKE-UP) is a randomized, double-blinded controlled trial currently recruiting patients across ~60 European centers.[70] Investigators plan to enroll 800 AIS patients with unknown time of symptom onset including WUS patients. Investigators evaluate the safety and efficacy of MRI-based thrombolysis with 0.9 mg/kg dose of IV tPA utilizing the novel approach of diffusion-FLAIR mismatch as an indicator of lesion age <4.5 h. Primary efficacy end-point is favorable clinical outcome defined as modified Rankin scale (mRS) 0–1 at 3 months and mortality as a tool for primary safety end-point. The enrollment was started in October 2012 and is expected to complete by December 2016.
THAWS
THrombolysis for Acute Wake-up and unclear-onset Strokes with Alteplase at 0.6 mg/kg Trial (THAWS) is a randomized, single-blinded controlled trial conducted across Japan.[71] THAWS trial is the Asian counterpart of WAKE-UP trial[70] that intends to enroll 300 patients and study MRI-based thrombolysis in patients with unknown symptom onset time or who present as WUS. The investigators are using 0.6 mg/kg dose of IV tPA that has been shown to be safe, efficacious, and a licensed dose in Japanese AIS patient population. The trial includes patients with last well known >4.5 h, DWI-ASPECTS score ≥5, and absence of FLAIR signal hyperintensity. The enrollment started in May 2014 and the trial was expected to complete by March 2017. THAWS trial, if successful, could trigger more studies to test the efficacy of lower dose of IV tPA using MRI-based thrombolysis in AIS patients who present with unclear time of symptom onset.
NOR-TEST
The Norwegian tenecteplase stroke trial is an ongoing prospective, randomized, open-label, blinded, multicenter trial to establish the safety and efficacy of tenecteplase versus alteplase in AIS patients.[72] The trial compares 0.4 mg/kg tenecteplase (single bolus IV dose) with standard 0.9-mg/kg dose of alteplase (10% bolus followed by 90% of infusion over 60 min) in AIS patients. Investigators are enrolling three groups of patients: (a) Arriving with known symptom onset in ≤4.5 h, (b) undergoing ET within 6 h of symptoms onset, (c) WUS patients presenting within 4.5 h. For WUS subgroup, diffusion-FLAIR mismatch was used as imaging inclusion criteria. Primary efficacy end-point is mRS 0–1 at 3 months while secondary end-points include clinical improvement and bleeding complications. The trial began in September 2012 with a plan to enroll 954 patients and is expected to finish by March 2017.
Trials of Endovascular Stroke Treatment in Unknown Time Window
There are four endovascular intervention trials that target to observe clinical outcomes for patients with unknown symptom onset or presenting as WUS.
DAWN trial
DWI or computerized tomography perfusion Assessment with clinical mismatch in the triage of Wake-up and late presenting strokes undergoing Neurointervention (DAWN) trial was a randomized, multicenter, controlled trial.[38] The main objective was to assess the safety and efficacy of ET in WUS patients with stroke onset between 7 and 23 h (treatment to be initiated between 8 and 24 h). Neuroimaging inclusion criteria included vessel occlusion in internal carotid artery (ICA) through M1 segment, ASPECTS >7 on CT or MRI, and penumbral pattern on CT or MR perfusion. Investigators utilized stent retrievers including Solitaire and Trevo devices and assessed primary outcome of mRS >2 at 90 days. DAWN trial was further heralded by a similar trial that is a prospective, multicenter, phase II/III, adaptive, randomized controlled trial conducted across 50 centers in North America and Europe.[73] The objective is to demonstrate the efficacy of Trevo stent retriever with medical management as compared to standard management alone for patients with unknown time for symptom onset or who present as WUS. Investigators randomized AIS patients between 6 and 24 h from the time they were last seen well in either of the two therapeutic arms. Neuroimaging inclusion criteria included (a) <1/3 MCA territory involvement on CT or MRI, (b) presence of LVO in ICA or M1 segment on CT or MR angiograms, and (c) clinical imaging mismatch involving NIHSS and infarct core volumes using MR-DWI or CT-CBF thresholds. Primary outcome includes weighted mRS and stroke-related mortality at 3 months. The trial was initiated in July 2014 with a plan to enroll 500 patients. There are currently 92 cases enrolled so far and is expected to complete by July 2017.
POSITIVE
Perfusion Imaging Selection of Ischemic Stroke Patients for Endovascular Therapy (POSITIVE) is an open-label, randomized controlled clinical trial to assess the safety and efficacy of ET versus standard medical therapy for AIS patients.[74] Investigators included AIS patients with TLSW within 12 h with following neuroimaging criteria: (a) <1/3 MCA territory involvement on CT/MRI (b) LVO between distal ICA through M1 bifurcation, and (c) presence of ischemic penumbra on CT/MRI perfusion. Primary outcome was adjusted by the investigators as shift analysis with a goal mRS of 0–2 at 90 days. The trial was started in September 2013 with an estimate to enroll 750 patients and has enrolled 24 patients so far.
RESTORE
REperfusion therapy in unclear-onset Stroke Based on MRI Evaluation (RESTORE) was an observational, prospective, single-arm, multicenter study to assess the efficacy of ERT along with IV thrombolysis in patients with unclear-onset stroke patients arriving within 6 h of symptom detection.[45] For thrombolytic therapy, investigators selected any of the three reperfusion therapies: (a) 0.9 mg/kg IV tPA for patients arriving within 3 h of symptom detection without LVO, (b) 0.6 mg/kg of IV tPA + IA therapy for patients arriving within 3 h of symptom detection with LVO, and (c) IA therapy alone for patients arriving within 3–6 h of symptom detection with LVO. MRI-based inclusion criteria involved diffusion-perfusion mismatch >20% and negative-to-subtle FLAIR signal alterations. The study included 83 out of total 430 patients who received reperfusion therapy in the form of IV tPA alone, IV tPA followed by IA therapy or IA therapy alone. The clinical outcome determined by mRS 0-2 at 3 months was observed in 44.6% of patients while sICH-causing change in NIHSS by ≥4 points occurred in 3.6% of patients. MRI-based reperfusion therapy was found to be safe and feasible for patients with unclear onset of stroke symptoms using diffusion-perfusion and diffusion-FLAIR imaging mismatch criteria.
MR WITNESS
MR WITNESS: A Phase IIa Safety Study of IV Thrombolysis with Alteplase in MRI-Selected Patients (MR WITNESS) is an observational, open-label, single-arm, safety study conducted across 10 centers in the United States.[75] The study intends to assess the safety and efficacy of 0.9 mg/kg IV tPA administered to patients with TLSW within 24 h of triage. Neuroimaging inclusion criterion was diffusion-FLAIR mismatch to estimate the age of the lesion, while the presence of >10 microbleeds on gradient-recalled echo sequence was considered as exclusion criteria. Primary outcome was to assess the safety using IV tPA within 24 h of TLSW based on MR evidence of early ischemia and assess sICH rate in these patients. The study was started in January 2011 with an estimated enrollment of 100 patients, has finished the recruitment phase, and the study results are awaited with completion by December 2016. Another ongoing study called Imaging-WIndow Thrombolysis iN Emergent Stroke Syndromes intends to determine the safety profile of IV tPA for patients with TLSW within 24 h, using both CT or MR neuroimaging techniques to assess tissue viability.
Conclusion
Almost a quarter of AIS patients wake up with stroke symptoms and are excluded from thrombolysis based on the current guidelines. Various observational studies have laid immense relevance on patient selection in this subgroup based on novel imaging patterns that discern the tissue at risk from infarct core. Multimodal imaging techniques have played a crucial role in selecting WUS patients who might be ideal candidates for thrombolysis based on tissue-rather-time based guidelines. Ongoing prospective trials are investigating distinct penumbral imaging assays that might streamline optimal therapeutic strategies for this neglected group of patients. The results of various trials are expected to provide safety and efficacy for both IV thrombolysis and ET that might alter current treatment guidelines for patients with unknown time of symptom onset. Patient selection based on exquisite imaging protocols to determine thrombolysis is expected to become the standard of clinical practice in the near future.
Financial support and sponsorship
NIH funding: K24NS072272 to Dr. Liebeskind.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lazzaro MA, Malhotra K, Mohammad YM. The role of antithrombotics in secondary stroke prevention. Semin Neurol. 2010;30:492–500. doi: 10.1055/s-0030-1268860. [DOI] [PubMed] [Google Scholar]
- 2.Hackett CT, Ramanathan RS, Malhotra K, Quigley MR, Kelly KM, Tian M, et al. Safety of venous thromboembolism prophylaxis with fondaparinux in ischemic stroke. Thromb Res. 2015;135:249–54. doi: 10.1016/j.thromres.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–7. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moradiya Y, Janjua N. Presentation and outcomes of “wake-up strokes” in a large randomized stroke trial: Analysis of data from the international stroke trial. J Stroke Cerebrovasc Dis. 2013;22:e286–92. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649–55. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 7.IST-collaborative group, Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet. 2012;379:2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, et al. Wake-up stroke: Clinical and neuroimaging characteristics. Cerebrovasc Dis. 2010;29:336–42. doi: 10.1159/000278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 11.Buck D, Shaw LC, Price CI, Ford GA. Reperfusion therapies for wake-up stroke: Systematic review. Stroke. 2014;45:1869–75. doi: 10.1161/STROKEAHA.114.005126. [DOI] [PubMed] [Google Scholar]
- 12.Curfman D, Connor LT, Moy HP, Heitsch L, Panagos P, Lee JM, et al. Accuracy of emergency medical services-reported last known normal times in patients suspected with acute stroke. Stroke. 2014;45:1275–9. doi: 10.1161/STROKEAHA.113.003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford AL, Williams JA, Spencer M, McCammon C, Khoury N, Sampson TR, et al. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–8. doi: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh EE, 3rd, Biller J, Adams HP, Jr, Marler JR, Hulbert JR, Love BB, et al. Circadian variation in onset of acute ischemic stroke. Arch Neurol. 1990;47:1178–80. doi: 10.1001/archneur.1990.00530110032012. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Conde J, Ois A, Rodríguez-Campello A, Gomis M, Roquer J. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol. 2007;254:782–8. doi: 10.1007/s00415-006-0438-y. [DOI] [PubMed] [Google Scholar]
- 16.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–71. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 17.Roveri L, La Gioia S, Ghidinelli C, Anzalone N, De Filippis C, Comi G. Wake-up stroke within 3 hours of symptom awareness: Imaging and clinical features compared to standard recombinant tissue plasminogen activator treated stroke. J Stroke Cerebrovasc Dis. 2013;22:703–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Fink JN, Kumar S, Horkan C, Linfante I, Selim MH, Caplan LR, et al. The stroke patient who woke up: Clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–93. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 19.Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: A hospital-based study. Stroke. 1998;29:1873–5. doi: 10.1161/01.str.29.9.1873. [DOI] [PubMed] [Google Scholar]
- 20.Kim BJ, Lee SH, Shin CW, Ryu WS, Kim CK, Yoon BW. Ischemic stroke during sleep: Its association with worse early functional outcome. Stroke. 2011;42:1901–6. doi: 10.1161/STROKEAHA.110.602243. [DOI] [PubMed] [Google Scholar]
- 21.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 22.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73:1313–22. doi: 10.1212/WNL.0b013e3181bd137c. [DOI] [PubMed] [Google Scholar]
- 23.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 24.Batool-Anwar S, Goodwin JL, Kushida CA, Walsh JA, Simon RD, Nichols DA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA)? J Sleep Res. 2016 May 30; doi: 10.1111/jsr.12430. doi: 10.1111/jsr.12430. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra K, Conners JJ, Lee VH, Prabhakaran S. Relative changes in transcranial Doppler velocities are inferior to absolute thresholds in prediction of symptomatic vasospasm after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:31–6. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Beelke M, Angeli S, Del Sette M, Gandolfo C, Cabano ME, Canovaro P, et al. Prevalence of patent foramen ovale in subjects with obstructive sleep apnea: A transcranial Doppler ultrasound study. Sleep Med. 2003;4:219–23. doi: 10.1016/s1389-9457(02)00256-3. [DOI] [PubMed] [Google Scholar]
- 27.Shanoudy H, Soliman A, Raggi P, Liu JW, Russell DC, Jarmukli NF. Prevalence of patent foramen ovale and its contribution to hypoxemia in patients with obstructive sleep apnea. Chest. 1998;113:91–6. doi: 10.1378/chest.113.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra K, Liebeskind DS. Imaging in endovascular stroke trials. J Neuroimaging. 2015;25:517–27. doi: 10.1111/jon.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke. 2014;16:131–45. doi: 10.5853/jos.2014.16.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang DW, Kwon JY, Kwon SU, Kim JS. Wake-up or unclear-onset strokes: Are they waking up to the world of thrombolysis therapy? Int J Stroke. 2012;7:311–20. doi: 10.1111/j.1747-4949.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 31.Huisa BN, Raman R, Ernstrom K, Tafreshi G, Stemer A, Meyer BC, et al. Alberta stroke program early CT score (ASPECTS) in patients with wake-up stroke. J Stroke Cerebrovasc Dis. 2010;19:475–9. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta stroke programme early CT score. Lancet. 2000;355:1670–4. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 33.Reid JM, Dai D, Cheripelli B, Christian C, Reidy Y, Gubitz GJ, et al. Differences in wake-up and unknown onset stroke examined in a stroke registry. Int J Stroke. 2015;10:331–5. doi: 10.1111/ijs.12388. [DOI] [PubMed] [Google Scholar]
- 34.Costa R, Pinho J, Alves JN, Amorim JM, Ribeiro M, Ferreira C. Wake-up stroke and stroke within the therapeutic window for thrombolysis have similar clinical severity, imaging characteristics, and outcome. J Stroke Cerebrovasc Dis. 2016;25:511–4. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology. 2013;267:543–50. doi: 10.1148/radiol.12120971. [DOI] [PubMed] [Google Scholar]
- 36.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. RAPID automated patient selection for reperfusion therapy: A pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) Study. Stroke. 2011;42:1608–14. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma H, Parsons MW, Christensen S, Campbell BC, Churilov L, Connelly A, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for thrombolysis in emergency neurological deficits (EXTEND) Int J Stroke. 2012;7:74–80. doi: 10.1111/j.1747-4949.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 38.Noguiera R, Liebeskind D, Gupta R, Levy E, Rai A, Barreto A, et al., editors. Seattle, WA: AAN; 2009. Preliminary Data for the DAWN Trial (DWI/PWI and CTP Assessment in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention): Imaging based Endovascular Therapy for Proximal Anterior Circulation Occlusions Beyond Eight Hours from Last Ween Well in 193 Stroke Patients. [Google Scholar]
- 39.Natarajan SK, Snyder KV, Siddiqui AH, Ionita CC, Hopkins LN, Levy EI. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke. 2009;40:3269–74. doi: 10.1161/STROKEAHA.109.555102. [DOI] [PubMed] [Google Scholar]
- 40.Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute stroke imaging research roadmap II. Stroke. 2013;44:2628–39. doi: 10.1161/STROKEAHA.113.002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–52. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 42.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–37. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–75. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomalla G, Schwark C, Sobesky J, Bluhmki E, Fiebach JB, Fiehler J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: Comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke. 2006;37:852–8. doi: 10.1161/01.STR.0000204120.79399.72. [DOI] [PubMed] [Google Scholar]
- 45.Kang DW, Sohn SI, Hong KS, Yu KH, Hwang YH, Han MK, et al. Reperfusion therapy in unclear-onset stroke based on MRI evaluation (RESTORE): A prospective multicenter study. Stroke. 2012;43:3278–83. doi: 10.1161/STROKEAHA.112.675926. [DOI] [PubMed] [Google Scholar]
- 46.Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krützelmann A, Fiehler J, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 47.Petkova M, Rodrigo S, Lamy C, Oppenheim G, Touzé E, Mas JL, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: Implications for patients with unknown onset time. Radiology. 2010;257:782–92. doi: 10.1148/radiol.10100461. [DOI] [PubMed] [Google Scholar]
- 48.Bai Q, Zhao Z, Fu P, Sui H, Xie X, Chen J, et al. Clinical outcomes of fast MRI-based trombolysis in wake-up strokes compared to superacute ischemic strokes within 12 hours. Neurol Res. 2013;35:492–7. doi: 10.1179/1743132813Y.0000000208. [DOI] [PubMed] [Google Scholar]
- 49.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Watanabe M, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis. 2011;31:435–41. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 50.Ebinger M, Kufner A, Galinovic I, Brunecker P, Malzahn U, Nolte CH, et al. Fluid-attenuated inversion recovery images and stroke outcome after thrombolysis. Stroke. 2012;43:539–42. doi: 10.1161/STROKEAHA.111.632026. [DOI] [PubMed] [Google Scholar]
- 51.Kufner A, Galinovic I, Brunecker P, Cheng B, Thomalla G, Gerloff C, et al. Early infarct FLAIR hyperintensity is associated with increased hemorrhagic transformation after thrombolysis. Eur J Neurol. 2013;20:281–5. doi: 10.1111/j.1468-1331.2012.03841.x. [DOI] [PubMed] [Google Scholar]
- 52.Campbell BC, Costello C, Christensen S, Ebinger M, Parsons MW, Desmond PM, et al. Fluid-attenuated inversion recovery hyperintensity in acute ischemic stroke may not predict hemorrhagic transformation. Cerebrovasc Dis. 2011;32:401–5. doi: 10.1159/000331467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 54.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 55.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 56.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 57.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 58.Aghaebrahim A, Leiva-Salinas C, Jadhav AP, Jankowitz B, Zaidi S, Jumaa M, et al. Outcomes after endovascular treatment for anterior circulation stroke presenting as wake-up strokes are not different than those with witnessed onset beyond 8 hours. J Neurointerv Surg. 2015;7:875–80. doi: 10.1136/neurintsurg-2014-011316. [DOI] [PubMed] [Google Scholar]
- 59.Jung S, Gralla J, Fischer U, Mono ML, Weck A, Lüdi R, et al. Safety of endovascular treatment beyond the 6-h time window in 205 patients. Eur J Neurol. 2013;20:865–71. doi: 10.1111/ene.12069. [DOI] [PubMed] [Google Scholar]
- 60.Mokin M, Kan P, Sivakanthan S, Veznedaroglu E, Binning MJ, Liebman KM, et al. Endovascular therapy of wake-up strokes in the modern era of stent retriever thrombectomy. J Neurointerv Surg. 2016;8:240–3. doi: 10.1136/neurintsurg-2014-011586. [DOI] [PubMed] [Google Scholar]
- 61.Stampfl S, Ringleb PA, Haehnel S, Rocco A, Herweh C, Hametner C, et al. Recanalization with stent-retriever devices in patients with wake-up stroke. AJNR Am J Neuroradiol. 2013;34:1040–3. doi: 10.3174/ajnr.A3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–32. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JT, Park MS, Nam TS, Choi SM, Kim BC, Kim MK, et al. Thrombolysis as a factor associated with favorable outcomes in patients with unclear-onset stroke. Eur J Neurol. 2011;18:988–94. doi: 10.1111/j.1468-1331.2011.03351.x. [DOI] [PubMed] [Google Scholar]
- 64.Cho AH, Sohn SI, Han MK, Lee DH, Kim JS, Choi CG, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis. 2008;25:572–9. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 65.Kim YJ, Kim BJ, Kwon SU, Kim JS, Kang DW. Unclear-onset stroke: Daytime-unwitnessed stroke vs. wake-up stroke. Int J Stroke. 2016;11:212–20. doi: 10.1177/1747493015616513. [DOI] [PubMed] [Google Scholar]
- 66.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 67.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 68.Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38:2275–8. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams HP, Jr, Leira EC, Torner JC, Barnathan E, Padgett L, Effron MB, et al. Treating patients with ‘wake-up’ stroke: The experience of the AbESTT-II trial. Stroke. 2008;39:3277–82. doi: 10.1161/STROKEAHA.107.508853. [DOI] [PubMed] [Google Scholar]
- 70.Thomalla G, Fiebach JB, Østergaard L, Pedraza S, Thijs V, Nighoghossian N, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) Int J Stroke. 2014;9:829–36. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]
- 71.Koga M, Toyoda K, Kimura K, Yamamoto H, Sasaki M, Hamasaki T, et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0.6 mg/kg (THAWS) Trial. Int J Stroke. 2014;9:1117–24. doi: 10.1111/ijs.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logallo N, Kvistad CE, Nacu A, Naess H, Waje-Andreassen U, Asmuss J, et al. The Norwegian tenecteplase stroke trial (NOR-TEST): Randomised controlled trial of tenecteplase vs. alteplase in acute ischaemic stroke. BMC Neurol. 2014;14:106. doi: 10.1186/1471-2377-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trevo and Medical Management Versus Medical Management Alone in Wake Up and Late Presenting Strokes (DAWN) [Last updated 2016 Apr 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT02142283 .
- 74.POSITIVE: PerfusiOn Imaging Selection of Ischemic STroke PatIents for EndoVascular ThErapy. [Last updated 2015 Oct 01]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01852201 . [DOI] [PubMed]
- 75.MR WITNESS: A Phase IIa Safety Study of Intravenous Thrombolysis With Alteplase in MRI. Selected Patients. [Last updated 2016 Mar 21]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01282242?term=MR+WITNESS+%28NCT01282242%29 and rank=1 .


