Abstract
Patients diagnosed with neurological disorders exhibit a variety of physical and psychiatric symptoms, including muscle atrophy, general immobility, and depression. Patients who participate in physical rehabilitation at times show unexpected clinical improvement, which includes diminished depression and other stress-related behaviors. Regenerative medicine has advanced two major stem cell-based therapies for central nervous system (CNS) disorders, transplantation of exogenous stem cells, and enhancing the endogenous neurogenesis. The latter therapy utilizes a natural method of re-innervating the injured brain, which may mend neurological impairments. In this study, we examine how inactivity-induced atrophy, using the hindlimb suspension model, alters neurogenesis in rats. The hypothesis is that inactivity inhibits neurogenesis by decreasing circulation growth or trophic factors, such as vascular endothelial growth or neurotrophic factors. The restriction modifies neurogenesis and stem cell differentiation in the CNS, the stem cell microenvironment is examined by the trophic and growth factors, including stress-related proteins. Despite growing evidence revealing the benefits of “increased” exercise on neurogenesis, the opposing theory involving “physical inactivity,” which simulates pathological states, continues to be neglected. This novel theory will allow us to explore the effects on neurogenesis by an intransigent stem cell microenvironment likely generated by inactivity. 5-bromo-2-deoxyuridine labeling of proliferative cells, biochemical assays of serum, cerebrospinal fluid, and brain levels of trophic factors, growth factors, and stress-related proteins are suggested identifiers of neurogenesis, while evaluation of spontaneous movements will give insight into the psychomotor effects of inactivity. Investigations devised to show how in vivo stimulation, or lack thereof, affects the stem cell microenvironment are necessary to establish treatment methods to boost neurogenesis in bedridden patients.
Keywords: Immobilization, neurogenesis, neurological disorders, physical exercise, stem cells
A Call to Examine Stem Cell Effects with Physical Inactivity
Our encompassing hypothesis is absence of exercise impacts the neurogenic niche in the brain, thereby modifying the stem cell microenvironment. Until a short time ago, the nonregenerative ability of the adult injured brain was accepted as a scientific creed. Yet, growing evidence over the last decade reveals that neurons and astrocytes can be produced from isolated cells of the adult mammalian central nervous system (CNS).[1] Shortly after, several laboratory studies investigated stem cell therapy for treating numerous diseases in the CNS, including stroke, traumatic brain injury, and neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease. Stem cell therapy, nonetheless, continues to be considered an experimental treatment. Countless patients continue to deteriorate because of these diseases, and the number of bedridden cases is rising. Bedridden patients’ muscles begin to atrophy, their everyday activities are reduced, and some patients begin to present a depressive mood. In addition, patients who are able to attend rehabilitation in the clinic occasionally show remarkable clinical improvements, along with reduced depression and a diminishment of other stress-related behaviors. Unfortunately, there is a scarce amount of information available regarding the effects of disuse atrophy on the innate functions of the brain including neurogenesis.
Regenerative medicine is a new scientific field that has advanced stem cell therapy for the purpose of treating brain disorders, with a focus on either transplanting exogenous stem cells or amplifying endogenous stem cells through neurogenesis.[2,3,4,5,6,7,8,9,10,11,12,13] Our envisioned research project is directed toward the latter method, which utilizes an inherent technique of mending the injured brain and repairing the neurological deficiencies through physical rehabilitation. Within this study, we are interested in elucidating if inadequate exercise-induced disuse atrophy, via the hindlimb suspension (HS) model, will alter neurogenesis in adult rats. The relationship between inadequate exercise and neurogenesis stands to be identified; compared to the inverse model of elevated exercise levels.[14] Specifically, exercise has been demonstrated to activate neurogenesis.[14,15,16] Furthermore, various conditions, such as cerebral ischemia, have been shown to upregulate neurogenesis.[17] The theory of augmented neurogenesis achieved through exercise leads to the overarching concept of our thesis that inadequate exercise inhibits neurogenesis possibly by decreasing circulating factors, such as vascular endothelial growth factor (VEGF) or brain-derived neurotrophic factor (BDNF). Laboratory studies are justified to unveil the underlying biological mechanism of neurogenesis. The comprehensive observations associated with these studies will allow the development of treatments designed to further neurogenesis in patients that demonstrate a lack of mobility.
Adaptation of Hindlimb Suspension to Assess Stem Cell Therapy
The hindlimb suspension model
The HS model was originally suggested for analyzing spaceflight-associated phenomena,[18] due to the initial evidence which showed an inability for bone formation during spaceflight.[19] Following the initial use, the model has been subjected to different modifications,[20] along with bone formation studies,[21] analysis of muscle,[22] and vascular system of the hindlimbs[20]. Thus far, a majority of studies have been directed toward the peripheral response caused by the HS model.[23,24,25] There has only been a handful of studies which use the HS model to measure changes in the CNS, with a majority targeting depression.[26,27] In 2005, Dupont et al. determined that neuronal growth factor (NGF) and BDNF mRNA and also NGF protein are upregulated in the somatosensory cortex of animals placed in the HS model.[28] These documented changes in neurotrophic factor levels reinforce the idea that “exercise,”[14,15] or lack of, controls neurotrophic factor expression. Even though there has been no analysis of HS model, figure 1, in CNS disorders, there has been evidence of forelimb disuse and overuse models in stroke and Parkinson's disease.[29,30,31,32] Forced disuse (using one-sleeved casts) in stroke rats, but not overuse, of the afflicted forelimb during the early phases of recovery restricts the functional result of the healing.[33] Within rats that had somatosensory cortical lesions, involuntary overuse of the injured forelimb within the initial phases of repair was related to a decreased functional outcome from rehabilitation.[34] Led by Schallert and Jones, a group reported the aforementioned contrasting results and continued to explain that there are fundamental differences in the effects of forelimb disuse and overuse that may be resulting from the site-specific anatomical changes (e.g., subcortical in stroke versus cortical in the somatosensory cortical lesion model) that are produced by different injuries.[35] Subsequently, subcortical injury responded to the involuntary use of the affected forelimb, unlike the cortical injury. Involuntary use of the affected forelimb directly following unilateral 6-hydroxydopamine (6-OHDA) lesions in rats diminished behavioral deficits and the involuntary forelimb exercises preceding the 6-OHDA lesions boosted neurotrophic factor glial cell-derived neurotrophic factor levels from glial cells guarding against cognitive deficiencies.[36,37] The recognized reduction of behavioral deficits within stroke and Parkinson's disease animal models coincides with decreased neuroanatomical damage and fewer neurochemical deficiencies.[29,30,31,32,38,39,40] Through these studies, the practice of enforcing or denying a regimen of physical therapy reveals the effects on the CNS involving these diseased conditions. With these results, we can validate the effects that controlling physical therapy has on the CNS via the HS model.
Figure 1.
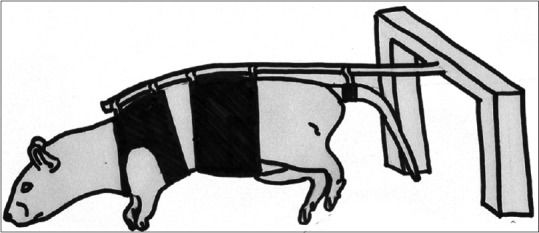
The hindlimb suspension model is aimed to withhold the movement of posterior extremities for periods of time by suspending the rear limbs. This allows for observations to be drawn between experimental groups with various allotted times in the hindlimb suspension model
Function of neurogenesis
Stroke can be the result of either a blockage located in an artery or a hemorrhage of a blood vessel within the cerebrovascular system.[41] Discreet brain areas shown to exhibit cell proliferation during adulthood are labeled as neurogenic niches.[41] Neurogenesis is defined as the proliferation and differentiation of neural stem cells into neurons.[41] The two main neurogenic niches are the sub-granular zone (SGZ) and sub-ventricular zone (SVZ) of the dentate gyrus (DG), where neural stem cells accumulate and develop into mature neurons.[41] However, neurogenesis is not limited to these locations, and recent research has shown neurogenesis in the vicinity of the peri-impact area, which can become an active sight of neural repair following a stroke.[41] The proposed mechanism of the HS model is to stimulate these neurogenesis niches to facilitate neural repair.[42] Following stroke, there is documented acute endogenous neurogenesis, allowing cells to proliferate in these neurogenic niches, thereafter facilitating the migration of neuroblasts toward the peri-impact area.[41] Because endogenous neurogenesis by itself is not sufficient to sustain neural repair, the HS model aims to increase the proliferation of the neural stem cells within neurogenesis niches, as well as enhance the migration of neuroblasts to the peri-impact area, altogether improving neural repair outcomes.
Neurogenesis, exercise, and growth factors
During innovative studies concerning exercise-induced neurogenesis, conducted by van Praag et al.,[14,43] rats were allowed a running wheel for 3 h during their active period and it resulted in substantial increases of 5-bromo-2-deoxyuridine (BrdU) labeled newly developed cells within the SGZ.[44] This optional use of exercise correlated with a diminished threshold for long-term potential (LTP) in the DG, concurrently increasing LTP, which conveys the theory of exercising improving memory.[43,45] Increased levels of VEGF[15,17] and BDNF[45,46,47,48] following exercise-induced neurogenesis give the appearance of these growth factors playing a role in exercise and neurogenesis. Within the SVZ and DG, the increase of neurotrophic factors reveals an association with the microenvironments of these familiar neurogenic sites. Due to the ability of diffusion for these growth factors, the effect of exercise-induced neurogenesis is not limited to specific neurogenic sites. In a recent study,[49] the posterior hypothalamic area (PHA) exhibited modified activity pertaining to in vitro and in vivo spontaneous firing rate of PHA was noticeably diminished from the rats that exercised in comparison to the nonexercising rats. To this end, exercise-induced neurogenesis has been recognized in neurogenic sites, and potentially may stimulate neurogenesis-like neuronal activity in other non-neurogenic brain areas. In light of these results, rats with the ability to exercise have been shown to have neurogenesis in neurogenic site, but these results may also prompt similar neurogenesis effects in non-neurogenic areas of the brain. The relationship between different levels of exercise and neurotrophic factor levels indicate that introducing a regimen of exercise should result in increased levels of neurotrophic factors as well as neurogenesis. Accompanying aging is a declining capability to exercise along with the advancement of several debilitating CNS diseases, which can provide a basis to investigate the effects of lack of exercise on neurotrophic factors along with neurogenesis. Although a lack of physical activity has been linked to a wide number of health issues (e.g., osteoporosis, obesity, and cardiovascular diseases),[50,51,52,53] there has not been an in-depth look at the effect of reduced exercise on neurotrophic growth factors and neurogenesis. In order to further the research involving the effects of lack of exercise and develop physical therapies to improve the behavioral deficits caused by an inability to exercise, making it vital to discern the essential growth factors that are available at sites of neurogenesis and how they are affected by the lack of exercise. Physical activity inhibited by a debilitated state triggers motor and cognitive functions, which intensifies behavioral deficiencies within the debilitated state.[54,55] By creating a scope of research that evaluates only specific conditions surrounding physical inactivity, it would allow preliminary rehabilitation techniques to be developed to not only attempt to prevent early brain degeneration, but also promote healing from brain injuries.
Neurogenesis and stress proteins
Neurotrophic growth factors along with stress proteins are the fundamental indicators of the effects of physical inactivity, which allows the perception of both ends of the spectrum. In theory, a decline of neurotrophic growth factors and elevated stress proteins should be the result of physical inactivity and lead to decreased neurogenesis. Through trials of the HS model, it revealed that chronic stress positively correlates with physical inactivity.[27] Increased signs of depression along with chronic stress are related to increases in glucocorticoids and reductions of serotonin.[56,57,58] Within the aging subjects, they displayed increased glucocorticoids and reduced insulin-like growth factor-1 (IGF-1).[59] Throughout the chronic stress and aging samples, both presented with reduced neurogenesis, drawing attention to the function of IGF-1 in neurogenesis.[58] Using a similar reference, the samples examining depression and chronic stress revealed that BDNF and serotonin play a part in neuronal plasticity.[60,61,62,63] Through these prior evaluations, the chronic stress often follows the use of the HS model should reveal key data that shed light on the function of stress on neurogenesis.
Neurological effects of deficient exercise
When patients with limited mobility are restricted in their regiment of physical activity, it hinders the ability of a full clinical recovery. Thorough research has presented evidence that consistent exercise encourages endogenous neurogenesis and may also have a preventive measure against CNS disorders. In the past, we have examined the effects of limited physical activity relating to neurogenesis using the HS model for a 2-week stint. The HS model procedure involves lifting the rat by the tail, therefore raising their hindlimbs and transfers the weight to the forelimbs. The exercise and recovery time for the rats that were returned to a normal caging environment following HS were assessed as well. Rats received an injection of BrdU (50 mg/kg, i.p.), which is a chemical used as a marker for proliferative cells, every 8 h for the remaining 4 days of each treatment group. Immunohistochemistry results revealed that HS dramatically reduced the levels of BrdU/doublecortin (Dcx) double-positive cells within the SVZ as well as the DG zone of the brain. Although atrophy of the soleus muscle was reduced through exercise and a recovery period, the reduced levels of BrdU/Dcx-positive cells did not restore to pre-HS levels. Another similar group of rats was given an identical HS treatment along with the addition of an enzyme-linked immunosorbent assay (ELISA) of neurotrophic factors that were administered on the brain tissue, which was collected following the completion of HS treatment. Furthermore, plasma from all animals treated was administered ELISA assays of neurotrophic factors. The results imply that the levels of natural BDNFs within the hippocampus as well as VEGF plasma levels were reduced by the treatment of the HS model. Through this experiment, it has been revealed that a reduced exercise regimen following brain injuries reduces neurogenesis due to lower levels of neurotrophic factors within the brain. By combining the HS model with the CNS disease models, the effects of various levels of physical activity on neurotrophic factors and neurogenesis can be evaluated further.
Rehabilitation and neurogenesis
Previous research studies have identified that reduced physical activity can lead to a variety of health complications (e.g., osteoporosis, obesity, and cardiovascular diseases).[50,51,52] The functions of neurogenesis and neurotrophic factors have yet to be assessed under these circumstances of reduced exercise. When limitations reduce the amount of physical activity, a recovering patient can participate in; it creates atypical cognitive and motor functions, which can affect the patient once they resume a normal healthy state of life. When a recuperating patient lacks physical activity, it exacerbates the behavioral deficits.[54,55] Within clinics, the positive effects of increased exercise are noted, although some therapy regimens are not proven and have shown few results. Likewise, an 18-day regimen of forced treadmill physical therapy showed no recovery progress involving memory and motor functions concerned with the upregulation of BDNF mRNA in CA1 and CA3, but not DG.[64] Similarly, the process of immobilizing a nonimpaired limb using a cast to further the use of the impaired limb following an injury of the sensorimotor cortex, the restraint, in fact, diminishes co-ordinated movement of both limbs.[35] However, there is substantial evidence that physical activity reduces the neuronal damage and motor function in rodent neurological disorder models.[65] The multitude of rehabilitation strategies that have various durations, regularity, and other intricacies along with the level of severity of the patient's condition can have an effect on the data. These variables can lead to difficulty replicating for future research. The standard recovery phase of 2-4 weeks and post-HS physical therapy for 2 weeks reduced the inflammation of the soleus muscle, so it neared to a normal level. On the contrary, when the HS-impaired neurogenesis was removed in the SVZ and DG, it was not improved by recovery and exercise, revealing the need for more development of the rehabilitation through physical therapy. Our study sheds light on the possible roles of neurotrophic factors that could help determine therapeutic candidates for reversing the physical inactivity due to behavioral deficits. Specifically, neurotrophic factors, BDNF and VEGF, show involvement with neurogenesis.
Conclusions
We have analyzed a novel paradigm - the HS model, to investigate the effects of physical inactivity on neurogenesis in the adult brain. The basis for concentrating on neurotrophic factors along with other stress proteins to monitor the effects of physical inactivity on neurogenesis is derived from the widely accepted theory of “increased exercise” or “enriched environment,” also from successful trials of the HS model in peripheral injury (bone and muscle). Besides this being the 1st time the HS model is incorporated into the CNS model (i.e., neurogenesis), the novel scientific development in this designed study is our aspiration to offer a more accurate approximation of aging and diseased brain states, where physical inactivity is a major characteristic. As a result, the physical inactivity paradigm will present new information regarding neurogenesis that would have been otherwise overlooked during the increased exercise and enriched environment models. Preclinical studies are a necessity to evaluate the possible modifications in neurogenesis within the models of immobilized rats. Furthermore, biomarkers for stem cell alterations have to be achieved in which they are essential to include growth factors and stress-related proteins, anticipating the alterations in neurogenesis caused by physical inactivity.
Financial support and sponsorship
Dr. Cesar V. Borlongan is funded by the National Institutes of Health (Grant 1R01NS071956-01A1), the Department of Defense (Grant W81XWH-11-1-0634), and the VA Merit Review.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
“The authors thank Kelly Kirby for technical assistance in the preparation of this manuscript.” and the Funding Source read “CVB is funded by NIH R01NS07195, NIH 21NS089851, NIH R21NS094087, DOD W81XWH-11-1-0634 and VA Merit Review I01 BX001407.
References
- 1.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 2.Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 4.Pan GJ, Chang ZY, Schöler HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12:321–9. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- 5.Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: Limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76:223–31. doi: 10.1002/jnr.20040. [DOI] [PubMed] [Google Scholar]
- 6.Yano A, Shingo T, Takeuchi A, Yasuhara T, Kobayashi K, Takahashi K, et al. Encapsulated vascular endothelial growth factor-secreting cell grafts have neuroprotective and angiogenic effects on focal cerebral ischemia. J Neurosurg. 2005;103:104–14. doi: 10.3171/jns.2005.103.1.0104. [DOI] [PubMed] [Google Scholar]
- 7.Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, et al. Transplantation of cryopreserved human bone marrow-derived multipotent adult progenitor cells for neonatal hypoxic-ischemic injury: Targeting the hippocampus. Rev Neurosci. 2006;17:215–25. doi: 10.1515/revneuro.2006.17.1-2.215. [DOI] [PubMed] [Google Scholar]
- 8.Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, et al. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson's disease. Eur J Neurosci. 2004;19:1494–504. doi: 10.1111/j.1460-9568.2004.03254.x. [DOI] [PubMed] [Google Scholar]
- 9.Yasuhara T, Shingo T, Muraoka K, Kameda M, Agari T, Wen Ji Y, et al. Neurorescue effects of VEGF on a rat model of Parkinson's disease. Brain Res. 2005;1053:10–8. doi: 10.1016/j.brainres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Yasuhara T, Shingo T, Muraoka K, Kameda M, Agari T, Wenji Y, et al. Toxicity of semaphorin3A for dopaminergic neurons. Neurosci Lett. 2005;382:61–5. doi: 10.1016/j.neulet.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 11.Yasuhara T, Shingo T, Muraoka K, Kobayashi K, Takeuchi A, Yano A, et al. Early transplantation of an encapsulated glial cell line-derived neurotrophic factor-producing cell demonstrating strong neuroprotective effects in a rat model of Parkinson disease. J Neurosurg. 2005;102:80–9. doi: 10.3171/jns.2005.102.1.0080. [DOI] [PubMed] [Google Scholar]
- 12.Yasuhara T, Shingo T, Muraoka K, Wen Ji Y, Kameda M, Takeuchi A, et al. The differences between high and low-dose administration of VEGF to dopaminergic neurons of in vitro and in vivo Parkinson's disease model. Brain Res. 2005;1038:1–10. doi: 10.1016/j.brainres.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 13.Yasuhara T, Hara K, Maki M, Matsukawa N, Fujino H, Date I, et al. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007;149:182–91. doi: 10.1016/j.neuroscience.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 14.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 15.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 16.Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wörtwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res. 2005;79:772–8. doi: 10.1002/jnr.20418. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morey ER. Spaceflight and bone turnover: Correlation with a new rat model of weightlessness. Bioscience. 1979;29:168–72. [Google Scholar]
- 19.Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science. 1978;201:1138–41. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- 20.Fujino H, Kohzuki H, Takeda I, Kiyooka T, Miyasaka T, Mohri S, et al. Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. J Appl Physiol (1985) 2005;98:1407–13. doi: 10.1152/japplphysiol.00961.2004. [DOI] [PubMed] [Google Scholar]
- 21.Barou O, Palle S, Vico L, Alexandre C, Lafage-Proust MH. Hindlimb unloading in rat decreases preosteoblast proliferation assessed in vivo with BrdU incorporation. Am J Physiol. 1998;274(1 Pt 1):E108–14. doi: 10.1152/ajpendo.1998.274.1.E108. [DOI] [PubMed] [Google Scholar]
- 22.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 23.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv Space Biol Med. 2005;10:7–40. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- 24.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: Technical aspects. J Appl Physiol (1985) 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 25.Picquet F, Falempin M. Compared effects of hindlimb unloading versus terrestrial deafferentation on muscular properties of the rat soleus. Exp Neurol. 2003;182:186–94. doi: 10.1016/s0014-4886(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 26.Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–64. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Dupont E, Canu MH, Stevens L, Falempin M. Effects of a 14-day period of hindpaw sensory restriction on mRNA and protein levels of NGF and BDNF in the hindpaw primary somatosensory cortex. Brain Res Mol Brain Res. 2005;133:78–86. doi: 10.1016/j.molbrainres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Borlongan CV, Sanberg PR. Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15(7 Pt 2):5372–8. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004;35:2206–10. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- 31.Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Neuroprotection by encapsulated choroid plexus in a rodent model of Huntington's disease. Neuroreport. 2004;15:2521–5. doi: 10.1097/00001756-200411150-00018. [DOI] [PubMed] [Google Scholar]
- 32.Borlongan CV, Stahl CE, Redei E, Wang Y. Prepro-thyrotropin -releasing hormone 178-199 exerts partial protection against cerebral ischemia in adult rats. Neuroreport. 1999;10:3501–5. doi: 10.1097/00001756-199911260-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bland ST, Pillai RN, Aronowski J, Grotta JC, Schallert T. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- 34.Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–92. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- 35.Schallert T, Jones TA. “Exuberant” neuronal growth after brain damage in adult rats: The essential role of behavioral experience. J Neural Transplant Plast. 1993;4:193–8. doi: 10.1155/NP.1993.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–35. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 38.Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain Res. 2005;1056:158–67. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Katsuragi S, Ikeda T, Date I, Shingo T, Yasuhara T, Ikenoue T. Grafting of glial cell line-derived neurotrophic factor secreting cells for hypoxic-ischemic encephalopathy in neonatal rats. Am J Obstet Gynecol. 2005;192:1137–45. doi: 10.1016/j.ajog.2004.10.619. [DOI] [PubMed] [Google Scholar]
- 40.Katsuragi S, Ikeda T, Date I, Shingo T, Yasuhara T, Mishima K, et al. Implantation of encapsulated glial cell line-derived neurotrophic factor-secreting cells prevents long-lasting learning impairment following neonatal hypoxic-ischemic brain insult in rats. Am J Obstet Gynecol. 2005;192:1028–37. doi: 10.1016/j.ajog.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Marlier Q, Verteneuil S, Vandenbosch R, Malgrange B. Mechanisms and functional significance of stroke-induced neurogenesis. Front Neurosci. 2015;9:458. doi: 10.3389/fnins.2015.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends Mol Med. 2015;21:543–8. doi: 10.1016/j.molmed.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 45.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo . Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- 48.Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 49.Beatty JA, Kramer JM, Plowey ED, Waldrop TG. Physical exercise decreases neuronal activity in the posterior hypothalamic area of spontaneously hypertensive rats. J Appl Physiol (1985) 2005;98:572–8. doi: 10.1152/japplphysiol.00184.2004. [DOI] [PubMed] [Google Scholar]
- 50.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–34. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 51.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 52.Renehan AG, Howell A. Preventing cancer, cardiovascular disease, and diabetes. Lancet. 2005;365:1449–51. doi: 10.1016/S0140-6736(05)66399-4. [DOI] [PubMed] [Google Scholar]
- 53.Wallis C. The evolution wars. Time. 2005;166:26. [PubMed] [Google Scholar]
- 54.Lewis MH. Environmental complexity and central nervous system development and function. Ment Retard Dev Disabil Res Rev. 2004;10:91–5. doi: 10.1002/mrdd.20017. [DOI] [PubMed] [Google Scholar]
- 55.Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: Relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog Neurobiol. 2004;72:167–82. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Fitch RH, McGivern RF, Redei E, Schrott LM, Cowell PE, Denenberg VH. Neonatal ovariectomy and pituitary-adrenal responsiveness in the adult rat. Acta Endocrinol (Copenh) 1992;126:44–8. doi: 10.1530/acta.0.1260044. [DOI] [PubMed] [Google Scholar]
- 57.Kawano S, Ohmori S, Kanda K, Ito T, Murata Y, Seo H. Adrenocortical response to tail-suspension in young and old rats. Environ Med. 1994;38:7–12. [PubMed] [Google Scholar]
- 58.Tamashiro KL, Nguyen MM, Sakai RR. Social stress: From rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–22. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 60.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–72. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: Insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–64. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Stahl CE, Redei E, Wang Y, Borlongan CV. Behavioral, hormonal and histological stress markers of anxiety-separation in postnatal rats are reduced by prepro-thyrotropin-releasing hormone 178-199. Neurosci Lett. 2002;321:85–9. doi: 10.1016/s0304-3940(01)02349-7. [DOI] [PubMed] [Google Scholar]
- 64.Hicks RR, Boggs A, Leider D, Kraemer P, Brown R, Scheff SW, et al. Effects of exercise following lateral fluid percussion brain injury in rats. Restor Neurol Neurosci. 1998;12:41–47. [PubMed] [Google Scholar]
- 65.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–6. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]


