Abstract
Congenital hypoplasia of bilateral internal carotid arteries (ICAs) is an extremely rare anomaly with less than 25 reported cases in literature till date. We present a case of a 30-year-old primigravida, who developed seizures and subsequently loss of consciousness just few minutes after the delivery of a healthy male child. To the best of our knowledge, this is the first case with bilateral ICA hypoplasia presenting in postpartum female who developed infarct in bilateral frontal region and subarachnoid hemorrhage (SAH). On a postpartum three-dimensional (3D) computed tomography (CT) angiography, bilateral ICA hypoplasia was confirmed and the manifestations of infarcts were probably the consequence of altered hemodynamics of pregnancy. In conclusion, a patient in her late pregnancy and postpartum period, having nonspecific cerebral symptoms or having suffered a cerebrovascular accident, should not only be evaluated for pregnancy or puerperium-related complications but also whenever possible a baseline screening with Doppler study of neck vessels and a noncontrast magnetic resonance imaging (MRI) angiography of neck and cerebral vessels should be performed to rule out congenital anomalies.
Keywords: Bilateral internal carotid artery hypoplasia, postpartum female, seizures
Introduction
Congenital hypoplasia of bilateral carotid arteries is an extremely rare anomaly with less than 25 reported cases in literature till date. The actual number is probably higher as most cases are asymptomatic.[1] This case report shows presentation of a primigravida in her postpartum period, presenting with seizures. She had severe headache for last 7 days that was managed conservatively by medications. No radiological investigations were done previously other than routine obstetric ultrasonography. Subsequent imaging revealed acute infarcts involving bilateral cerebral hemispheres with minimal perisylvian subarachnoid hemorrhage (SAH). Three-dimensional (3D) computed tomographic (CT) angiography confirmed the diagnosis of bilateral internal carotid artery (ICA) hypoplasia.
Case Report
We present a case of a 30-year-old primigravida who developed seizures and short-term loss of consciousness just few minutes after normal vaginal delivery of a healthy male child. She had although complained about on and off severe headaches since last 10 days, for which no specific cause related treatment was initiated. Her baseline hematological work-up and vitals including blood pressure were within the normal limits during the antenatal and natal periods.
There was no past as well as antenatal history of trauma, fever, fits, or blurring of vision. Her past medical history did not reveal any comorbid medical conditions and she had not had any similar episodes in the past.
A further detailed personal history revealed that there was no evidence of substance abuse or any sort of addictions. On examination, she had a pulse of 80 beats per minute, mildly elevated blood pressure of 140/90 mm of mercury, respiratory rate of 22 breaths per minute. Her blood parameters were within normal limits too, hemoglobin level was measured to be 14 gm/100 mL. A fundoscopic examination was within normal limits. Neurological examinations revealed that the patient had developed left-sided hemiparesis and aphasia and was managed with conservative medical therapy.
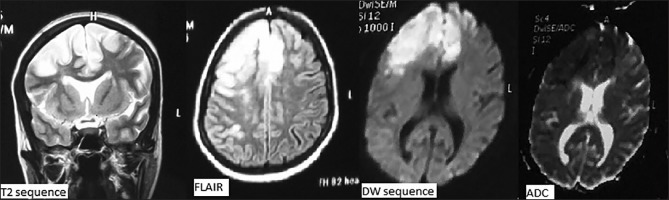
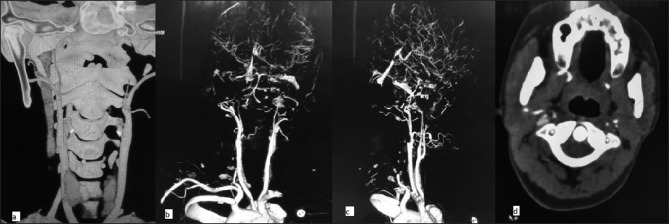
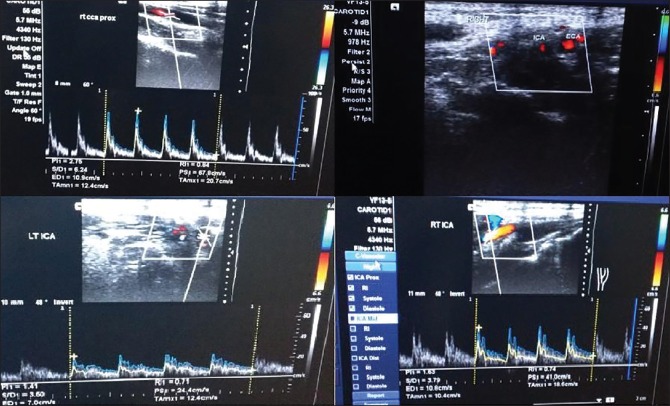
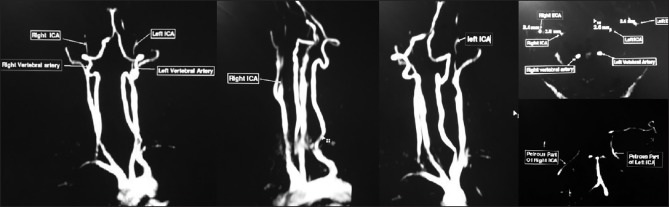
Following seizure and loss of consciousness, a preliminary baseline noncontrast CT [Figure 1] scan of head was performed, it showed changes of early infarct involving bilateral cerebral hemispheres, with the infarct being randomly interspersed and not belonging to any specific vascular territory. There was minimal subarachnoid hemorrhage in the right frontal and perisylvian region. These findings led to a thought of venous infarct and hence an magnetic resonance (MRI) scan was done to rule out any possibility of cerebral venous thrombosis. MRI findings corroborated the CT findings of acute infarct [Figure 2] and SAH [Figure 3]. It confirmed the infarct but the etiology still remained unclear as the MR venogram was absolutely normal [Figure 4]. With no renal derangement and the baby being bottle fed, she underwent a 3D CT angiography of the head and neck vessels to evaluate for any aneurysmal bleed. The observations from the CT angiography were remarkable as it showed severe narrowing of bilateral internal carotid arteries, just distal to bifurcation of both common carotid arteries [Figure 5]. With the patient being young and having no definite identifiable causes for severe narrowing of bilateral internal carotid arteries, we inferred it to be an extremely rare presentation of bilateral ICA hypoplasia. This was further confirmed by the measurement of the bony petrous part of bilateral internal carotid canals [Figure 6] measuring approximately 2.5 mm on right side and 2.8 mm on left side. The cerebral circulation comprised of multiple collaterals having been developed predominantly from bilateral external carotid arteries and the vertebrobasilar arteries. A retrospective color Doppler study [Figure 7] and noncontrast MR angiography [Figure 8] were done for corroborating our findings. On follow-up, the patient after 1 month showed no significant improvement in her clinical condition and she had residual neurological deficit as a result of previous vascular insult.
Figure 1.
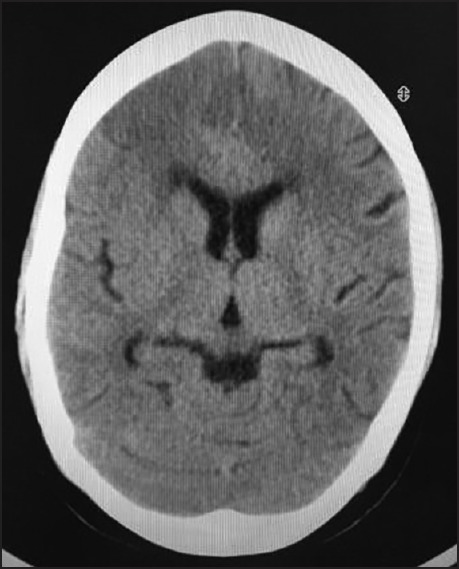
Noncontrast CT scan image done immediately after her presentation with seizures showing hypodensities in bilateral frontal lobes suggestive of acute infarct
Figure 2.
MRI images showing hyperintensity involving bilateral frontal lobes on T2 and FLAIR sequences and area of restriction on diffusion and ADC sequences, corroborating with acute infarcts on CT scan
Figure 3.
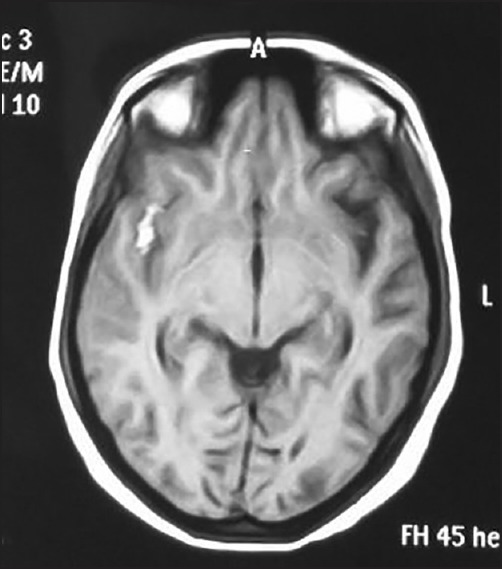
MRI image showing hyperintensity in right perisylvian region suggestive of subarachnoid hemorrhage
Figure 4.
MR venogram of the patient that was absolutely normal
Figure 5.
CT angiography: MPR image showing severe narrowing of bilateral internal carotid arteries, distal to bifurcation of both common carotid arteries (not involving its proximal 1-2 cm part), and axial section at the level of atlantoaxial joint showing decreased diameter of both internal carotid arteries
Figure 6.
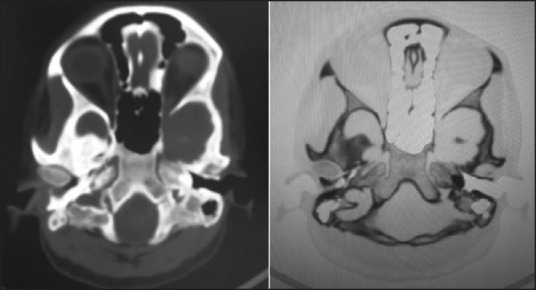
CT images showing bony petrous part of bilateral internal carotid canals measuring approximately 2.5 mm on right side and 2.8 mm on left side. This confirmed the diagnosis of bilateral hypoplasia
Figure 7.
Color Doppler images showing normal flow in CCA, decreased caliber of bilateral ICA as seen on transverse image, decreased flow in bilateral ICA with decreased PSV
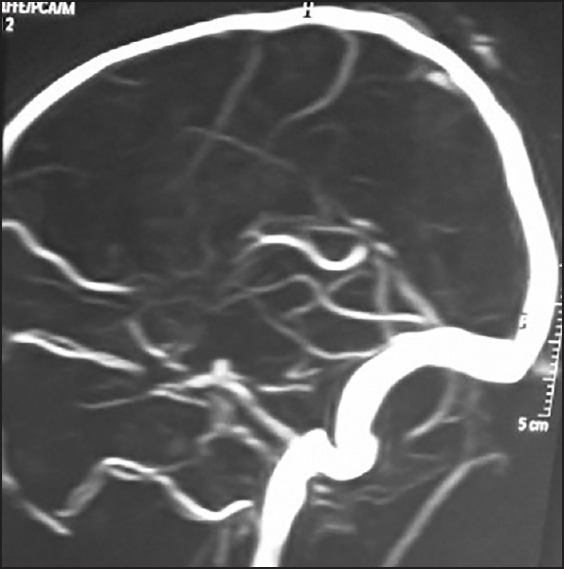
Figure 8.
MR angiogram showing hypoplastic bilateral ICA 2-3 cm distal to their origin
Treatment
There is no consensus about the optimal therapeutic strategy, perhaps due to the rarity of this clinical entity. Extreme search of literature did not reveal any appropriate management strategy. The role of lower segment cesarean section (LSCS) versus vaginal delivery is controversial and its outcome are not known.
Discussion
Hyrtle was the first to describe the presence of hypoplasia of the ICA in 1,836 in a postmortem series.[2] Since then, about 60 cases of ICA hypoplasia have been described in the literature. However, the first cases of bilateral ICA hypoplasia in living patients were reported by Hawkins and Scott in 1967[1] and there have since been a total of 24 reported cases. ICA hypoplasia is a rare congenital anomaly with an incidence rate below 0.01%.[3,4] Unilateral ICA hypoplasia has been reported to be more frequent on the left.[5,6,7,8] However, Lee[3] has reported hypoplasia mostly on the right in nine reported cases. Bilateral ICA hypoplasia is very rare.[3,9,10]
It has been hypothesized that hypoplasia of the ICA arises due to the incomplete development of the fetal dorsal aorta, which normally gives rise to the distal cervical segment of the ICA, up to the clinoidal segment.[3] This theory is also supported by the relatively constant location of the internal carotid artery hypoplasia (that is, 1 cm to 2 cm above the bifurcation to the cranium) and the fact that the first cervical segment, which develops from the third aortic arch, is normal. Collateral circulation is found in ICA hypoplasia cases. This collateral circulation may be in the form of a transcranial anastomosis with the external carotid artery (so called “rete mirabile”) is found in ICA hypoplasia cases. This collateral circulation may be in the osmotic channels in the circle of Willis.[3,11] The collateral circulation was predominantly from bilateral external carotid arteries and few collaterals from vertebrobasilar arteries. The patients with unilateral or bilateral ICA may be completely asymptomatic due to adequate collateral circulation. However, the majority of the reported cases have been diagnosed following a clinical presentation, such as headache, epilepsy, cerebral ischemia, hemiplegia, and SAH,[3,6,9,10,12,13,14] as were in our case. In a study done by Arata Watanabe and Tomohiro Omata[15] for Bony carotid canal hypoplasia, the normal diameter of bony carotid canal in adults was 5.27 mm (mean d In our case diameter of carotid canal were 2.5 mm of right side and 2.8 mm of left side that were much lower than normal values. This confirmed the diagnosis of congenital hypoplasia.
Furthermore, a 3D CT angiography showed blood supply to the anterior part of circle of Willis, including bilateral middle cerebral artery (MCA) and anterior cerebral artery (ACA) being contributed by collaterals arising predominantly from bilateral ECA and partly from vertebrobasilar arteries. Retrospective analysis with carotid Doppler study and MR angiography were done to assess their role for screening such type of patients. Color-coded carotid duplex sonography provides important clues for establishing a diagnosis of ICA hypoplasia. Direct findings are a long segmental small-caliber (52% diameter of the normal side) lumen associated with a markedly decreased flow volume (13% of the normal side) in the internal carotid artery but without substantial intraluminal atherosclerosis, a false lumen, or prominent wall thickening. Indirect findings include a markedly increased total flow volume in the bilateral vertebral artery (increased by 133% of the normal value), antegrade ipsilateral ophthalmic arterial flow, reduced vessel diameter, and increased flow resistance in the ipsilateral common carotid artery. Normal luminal diameters of CCA is 5.9 mm in women and 6.5 mm in men and of ICA is 4.0 mm in women and 4.4 mm in men. In our case carotid Doppler showed luminal diameter of ICAs were 2.0 mm on right side and 2.6 mm on left side that were significantly lower than normal values. This raised the suspicion of hypoplasia of bilateral ICA and MR Angio further confirmed the finding of hypoplasia. Hence, both of these modalities (carotid duplex color Doppler and MRI Brain with contrast angiography) can play an important role in screening of such patients presenting with severe headaches in their late third trimester or early labor and thus an aid in the management of these patients predisposed to development of cerebrovascular accidents.
In pregnant women important factors predisposing the risk of SAH include race particularly Caucasians, hypertensive disorders, coagulopathy, thrombocytopenia, tobacco abuse, drug abuse, intracranial venous, thrombosis, sickle-cell disease, hypercoagulability, alcohol abuse, and rare causes include congenital anomalous of cerebral circulation. Although the precise etiology of this increased risk is unknown, the marked hemodynamic, coagulation, and hormonal fluctuations accompanying the delivery/postpartum period may be important contributors. In pregnant women presenting with severe headache a proper clinical examination and investigation should be done to diagnose the risk of cerebrovascular accidents. Patient should undergo screening with carotid Doppler ultrasound and an MRI must be done, as these rule out many causes of cerebrovascular accidents (CVAs) such as aneurysm, congenital anomalous cerebral circulation, PRES, cerebral venous thrombosis, cavernous hemangioma.
If any cause is found, then the patient should be managed with proper medical and surgical care to decrease the risk of cerebrovascular accidents as compared to normal population. In our patient, her complaints of severe headache were neglected, no specific imaging to find the exact etiology of the headaches was done, she was managed by nonspecific medication for headache alone. This resulted in grave consequences of development of bilateral cerebral infarcts frontal lobes and subsequent left hemiparesis and aphasia.
Conclusion
In conclusion, a patient in her late pregnancy and postpartum period, having no specific cerebral symptoms or having suffered a cerebrovascular accident, should not only be evaluated for pregnancy or puerperium related complications leading to such an event, but also whenever possible a baseline screening with Doppler study of neck vessels and a noncontrast MRI angiography of neck and cerebral vessels should be performed to rule out such congenital anomalies.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to express our special thanks of gratitude to Dr. Sunil Gupta for his constant encouragement without which this assignment would not have been possible. We also take this opportunity to express a deep sense of gratitude to our friends Dr. Sunil Saini, Dr. Shruti Agarwal, and Dr. Sagar Satpute for their cordial support, valuable information, and guidance, which helped us in completing this task through various stages.
References
- 1.Hawkins TD, Scott WC. Bilateral rete carotidis in man. Clin Radiol. 1967;18:163–5. doi: 10.1016/s0009-9260(67)80011-4. [DOI] [PubMed] [Google Scholar]
- 2.Lie TA. Congenital Anomalies of the Carotid Arteries. Amsterdam: Excerpta Medica Foundation; 1968. pp. 35–51. [Google Scholar]
- 3.Lee JH, Oh CW, Lee SH, Han DH. Aplasia of the internal carotid artery. Acta Neurochir (Wien) 2003;145:117–25. doi: 10.1007/s00701-002-1046-y. [DOI] [PubMed] [Google Scholar]
- 4.Smith RR, Kees CJ, Hogg ID. Agenesis of the internal carotid artery with an unusual primitive collateral. Case report. J Neurosurg. 1972;37:460–2. doi: 10.3171/jns.1972.37.4.0460. [DOI] [PubMed] [Google Scholar]
- 5.Czarnecki EJ, Silbergleit R, Mehta BA, Sanders WP. Absence of the supraclinoid internal carotid artery in association with intracranial aneurysms. Neuroradiology. 1980;40:11–4. doi: 10.1007/s002340050529. [DOI] [PubMed] [Google Scholar]
- 6.Ide C, De Coene B, Mailleux P, Baudrez V, Ossemann M, Trigaux JP. Hypoplasia of the internal carotid artery: A noninvasive diagnosis. Eur Radiol. 2000;10:1865–70. doi: 10.1007/s003300000457. [DOI] [PubMed] [Google Scholar]
- 7.Orakdöğen M, Berkman Z, Erþahin M, Biber N, Somay H. Agenesis of the left internal carotid artery associated with anterior communicating artery aneurysm: Case report. Turk Neurosurg. 2007;17:273–6. [PubMed] [Google Scholar]
- 8.Florio F, Balzano S, Nardella M, Strizzi V, Cammisa M, Bozzini V, et al. Congenital absence of the internal carotid artery. Cardiovasc Intervent Radiol. 1999;22:74–8. doi: 10.1007/s002709900334. [DOI] [PubMed] [Google Scholar]
- 9.Briganti F, Maiuri F, Tortora F, Elefante A. Bilateral hypoplasia of the internal carotid arteries with basilar aneurysm. Neuroradiology. 2004;46:838–41. doi: 10.1007/s00234-002-0909-5. [DOI] [PubMed] [Google Scholar]
- 10.Okita R, Maeshima S, Ozaki F, Yamaga H, Moriwaki H. Cerebral aneurysm in a patient with aplasia of the internal carotid arteries. J Neurol. 2001;248:227–8. doi: 10.1007/s004150170231. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka K, Yonekawa Y, Matsuba K. Hypoplasia of the internal carotid artery--report of an unusual case. Neurol Med Chir (Tokyo) 1991;31:290–2. doi: 10.2176/nmc.31.290. [DOI] [PubMed] [Google Scholar]
- 12.Cali RL, Berg R, Rama K. Bilateral internal carotid artery agenesis: A case study and review of the literature. Surgery. 1993;113:227–33. [PubMed] [Google Scholar]
- 13.Kunishio K, Yamamoto Y, Sunami N, Asari S. Agenesis of the left internal carotid artery, common carotid artery, and main trunk of the external carotid artery associated with multiple cerebral aneurysms. Surg Neurol. 1987;27:177–81. doi: 10.1016/0090-3019(87)90292-8. [DOI] [PubMed] [Google Scholar]
- 14.Zink WE, Komotar RJ, Meyers PM. Internal carotid aplasia/hypoplasia and intracranial saccular aneurysms: Series of three new cases and systematic review of the literature. J Neuroimaging. 2007;17:141–7. doi: 10.1111/j.1552-6569.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe A, Omata T, Koizumi H, Nakano S, Takeuchi N, Kinouchi H. Bony carotid canal hypoplasia in patients with Moyamoya disease. J Neurosurg Pediatr. 2010;5:591–4. doi: 10.3171/2010.3.PEDS09417. [DOI] [PubMed] [Google Scholar]