Abstract
In this paper, our review series on cerebrovascular disease anatomy, physiology, and pathology ends with a thorough discussion of the most significant cerebrovascular pathology: stroke. This discussion proceeds through two layers of organization. First, stroke is divided up into its main etiologic categories (ischemic stroke/transient ischemic attack, hemorrhagic stroke, and ischemic to hemorrhagic transformation). Then, the epidemiological, pathophysiological, clinical, and therapeutic (employed currently as well as emerging) aspects of each etiology are explored; emphasis is placed upon the therapeutic aspects. Finally, once we have covered all aspects of each etiologic category, we end our review with a defense of the thesis that there is much hope for the future of stroke treatment to be derived from familiarity with the literature on emerging therapies.
Keywords: Cerebral circulation, cerebrovascular disease, hemorrhagic stroke, ischemic stroke, ischemic to hemorrhagic transformation, stroke therapies
Introduction
Stroke is a ubiquitous killer of almost unmatched proportions. The World Health Organization estimates that around 17.5 million people succumb annually to cardiovascular diseases, making this the world's most deadly category of disease. Of cardiovascular diseases, stroke, which is responsible for 6.7 million of those deaths, is second only to coronary heart disease.[1] The majority of these deaths take place in low- and middle-income countries; however, even in the United States, where highly sophisticated treatment modalities [Table 1] are available to ameliorate the disease, stroke claims enough lives to rank as the fifth leading cause of death.[2] To gain a more visceral appreciation for this figure, consider that it translates to one stroke death every 4 minutes.
Table 1.
Summary of current pharmacological and procedural interventions, categorized by stroke type
| CVD | Pharmacological interventions | Procedural interventions |
|---|---|---|
| Ischemic stroke/transient ischemic attack | Thrombolytics (tPA) Anticoagulants (heparin) Antiplatelets (aspirin) |
Mechanical clot removal Decompressive craniotomy Carotid endarterectomy |
| Hemorrhagic stroke | Calcium channel blockers (nimodipine) | Decompressive craniotomy Surgical clipping of aneurysms |
| IHT | None specific to IHT | None specific to IHT |
TPA=Tissue plasminogen activator, IHT=Ischemic to hemorrhagic transformation, CVD=Cardiovascular disease
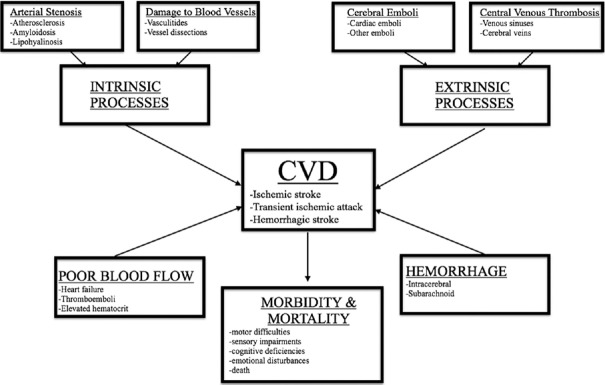
Even so, mortality alone hardly accounts for the total suffering caused by stroke [Figure 1]. Stroke is the leading cause of disability in the US[3] because of the severity and frequency of impairment left in its wake; in fact, only 10% of stroke patients ever recover completely.[4] For many survivors, of whom there are approximately 4.5 million in the United States, the life that remains after stroke barely resembles the one that preceded it. According to the National Institutes of Neurological Disorders and Stroke, five kinds of disability can result after initial stabilization: paralysis or other motor difficulties, sensory disturbances, cognitive impairment, and emotional disturbances.[5] Patients can also experience urinary incontinence and sexual impairment. They can be victims of a number of chronic pain disorders. Twenty-five percent have some types of aphasia, which can disconnect them from the social engagement they enjoyed before disease. Perhaps worse, their total cognitive capacity can be so compromised as to render even nonlinguistic social relations impossible. Unsurprisingly, emotional disorders are noted in this population. Depression, anxiety, and other maladies can be caused among them either directly, by stroke-induced brain damage itself, or also, of course, in reaction to the consequences of that damage. The following findings, from a study that analyzed quality of life in a population of first-ever stroke victims, put these things quantitatively: on a scale measuring health-related quality of life from 0 to 1, almost a quarter were rated ≤0.1, and 8% received a rating equivalent to death.[6]
Figure 1.
Flowchart summarizing the causes and consequences of cerebrovascular diseases
It must be emphasized, however, that the consequences of stroke vary tremendously according to the category. Despite what the data just presented may appear to suggest, prognosis exists on a continuum from totally reversible to fatal. Happily, the most fatal subcategory is also the least common.
We will now discuss the major stroke categories in greater depth, illustrating the breadth of this complex disease, as well as the corresponding variety of methods, both in practice and in development, with which it may be managed.
Ischemic Stroke and Transient Ischemic Attack
Epidemiology
Ischemic stroke is a major type of stroke, defined broadly as a neurological deficit caused by impaired blood flow to a focal area of the brain. It is responsible, at about 87%,[4] for the majority of all strokes. Within this large proportion, however, many different subtypes are concealed. Ischemic stroke can be classified relatively coarsely according to the clot that precipitates it. In this scheme, strokes are either thrombotic or embolic. More precise etiologic classification is also possible. A population-based study by Petty et al., for example, recognizes five separate causative categories:[7] large-vessel cervical or intracranial atherosclerosis with >50% stenosis (16%), cardioembolic (29%), lacunar (16%), uncertain cause (36%), and other (3%). In addition, ischemic strokes can be subdivided according to the infarct position; due to its centrality to the discussion of clinical presentation, this classification will be addressed below. Since some areas of the cerebrovascular tree are more essential to survival than others, mortality varies substantially across these subtypes. Overall, in-hospital mortality for ischemic stroke has been reported to be 5%–10%.[4]
As is to be expected of a disease with such varying etiologies, the risk factors of stroke are also highly heterogeneous. But, they may be generally clumped into two camps: modifiable and nonmodifiable.[8] Major nonmodifiable factors include age, gender, ethnicity, and genetic predisposition. Of these, age is considered most important; the mean age of onset for ischemic stroke is 70.5 years.[9] Genetic factors, by contrast, are currently considered to be relatively minor.[10] Of the modifiable risk factors, the most important are hypertension, dyslipidemia, diabetes mellitus, and smoking.[3] Others include obesity, heavy alcohol consumption, and renal insufficiency.[11] The importance of prevention by risk factor modification cannot be understated.
Pathophysiology
As stated above, the primary mechanism by which stroke causes injury is the focal deprivation of blood flow to the cerebral parenchyma. While a variety of phenomena can result in such ischemia, large-arterial atherosclerosis is the most prevalent. In atherosclerosis, as was discussed more extensively in Part II of this review,[12] accumulations of fatty material in the arterial subintima amass platelet clumps. These clumps then attract thrombin, fibrin, and erythrocyte debris that can ultimately coagulate to a size that poses stenotic risk to the cerebral vasculature.[13] Local blood flow stagnation due to low wall shear stress is thought to predispose certain areas of the vasculature, such as the carotid bulb, to atherosclerotic plaque development.[11] In any case, the resulting thrombus deprives cells of the cerebral parenchyma of the oxygen they need to function, causing pathology. Plaque development and succeeding stenosis are not necessarily in situ, however. Plaques can also travel to the cerebral circulation from another location, in which case they are called emboli. The heart, by way of atrial fibrillation, is the most common source of these, but they can come from other diseased parts of the arterial system, as well.[4,14]
There are many other pathogenic routes to cerebral ischemia. In addition to the large-vessel infarcts just discussed, which involve the carotid, vertebral, and basilar arteries, as well as major branches of the circle of Willis, small-vessel (or lacunar) infarcts are also a major etiology. Commonly by lipohyalinosis or micro-atheroma, but also occasionally by the same mechanism by which larger arteries are blocked, the blockage of these small, penetrating arteries running at right angles to the major branches produces the focal deficits characteristic of stroke. Some less frequently observed causes include acute arterial dissection secondary to fibromuscular dysplasia, hematologic disorders such as sickle cell anemia, and recreational use of cocaine or amphetamines.[9] For a more thorough discussion of several of these etiologies, we refer the reader to Part II of our review.[12]
No matter the precipitating event, the result of prolonged ischemia is cerebral cell death. The normal amount of flow, sufficient to meet the significant energy demands of cerebral tissue, is 60 mL/100 g/min. If the tissue experiences perfusion under 10 mL/100 g/min, cell membrane failure results in brain damage of a degree of severity and irreversibility that is in proportion to the duration of ischemia.[4]
Presentation
The clinical manifestation of an ischemic event depends entirely on its placement within the vasculature. In other words, a patient's observed deficit is a direct consequence of which and what proportion of the brain's 100 billion neurons are affected by infarction. The broadest distinction to appreciate in this connection is between the anterior and posterior cerebral circulations. To recapitulate the anatomical details of these divisions presented in Part I of our review,[15] the anterior circulation, which consists of the internal carotid artery's distribution and accounts for about 80% of perfusion to the brain, produces symptomology consistent with that distribution. To take a readily appreciable example, consider the ophthalmic artery. Unilateral ischemia of this artery's distribution produces symptoms consistent with the function of the neurons it perfuses: monocular blindness. Similarly, ipsilateral ischemia of the dominant hemisphere's middle cerebral artery produces marked contralateral sensory and motor disturbances, ipsilateral agnosia, and aphasia.[4] Regarding the likelihood of cognitive symptoms, it is important to note that ischemia of either distribution can be either cortical or subcortical.[16] Posterior/vertebrobasilar circulation strokes constitute the second major division of ischemic strokes. Because this is the distribution that supplies the brainstem, it is more frequently associated with loss of consciousness than anterior circulation strokes. These strokes can cause a wide variety of deficits, including impairment of cerebellar functions, vomiting, dysphagia, and third nerve palsies.[4]
As important as localization is to clinical prognostication, one cannot predict stroke severity on this basis alone. Another physiological variable that is critical to determining outcome is the degree of collateral circulation possessed by stroke victims.[17] The more perfusion pathways possessed by a given area of the brain, the greater the degree and duration of ischemia necessary to produce damage.
Therapies
As exciting as it is to be possessed of the sophisticated diagnostic capacity that can be supplied for stroke with modern neuroimaging strategies, this erudition does not, unfortunately, translate into a wealth of currently available therapies. In fact, there is currently only one Food and Drug Administration (FDA)-approved treatment for ischemic stroke:[4] intravenous (IV)-administered tissue plasminogen activator (tPA) for patients who meet the criteria.[18] Even worse, since one of these criteria is temporal (IV thrombolysis must in most cases be administered within 3 h of symptom onset), only a minority of victims receive it. A Centers for Disease Control and Prevention-sponsored study of thrombolysis rates showed that only 4.3% of stroke patients received this therapy.[19] One alternative to medical thrombolysis is endovascular clot retrieval, also known as revascularization, using devices such as Mechanical Embolus Removal in Cerebral Ischemia. Until recently, randomized clinical trials productive of evidence for the efficacy of such devices were lacking. In the last few years, however, several trials have produced such evidence, the cumulative result of which is that, for large vessel, anterior circulation strokes, endovascular therapy performed within 6 h has become part of the standard of care.[20,21,22,23] Further data and analysis of outcomes are necessary before this development can be expanded to treatment beyond 6 h and to strokes of the posterior cerebral circulation; there are multiple ongoing trials designed to furnish this data.[24] Surgery, too, can be indicated in some situations, such as when cerebral edema demands decompression therapy,[25,26] or carotid endarterectomies for patients with over 70% occlusion.[9]
Because of this paucity of available approved treatments, combined with the relatively small set of circumstances under which they are applicable, stroke treatment is generally supportive, consisting of stabilization in a stroke unit, treatment of comorbidities, and prevention of future ischemic events.[11,27] This also accounts for the enormous importance of risk factor modification in stroke prevention already mentioned. According to the INTERSTROKE[28] study of stroke risk factors across 22 different countries, just five modifiable factors – hypertension, current smoking, abdominal obesity, poor diet, and inadequate physical activity – account for 80% of the global risk of ischemic stroke. Just slight population-wide modifications in these factors could produce reductions of stroke-related suffering of invaluable magnitude. Other prevention strategies address specific etiologies. For example, because it is the source of 90% of cardioembolic strokes, surgeons have recommended the removal of the left atrial appendage in patients with atrial fibrillation whenever it is surgically exposed.[29]
The subject of prevention is also the heading under which consideration of transient ischemic attack (TIA) is appropriate. Also known as mini-strokes, the difference between TIAs and ischemic strokes is one of degree. For this reason, TIA is often discussed in its capacity as a warning sign of more severe infarcts to come. This accounts for the American Heart Association's emphasis,[30] in its definition of TIA, on noninfarction: “a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.” The absence of infarction is confirmed with magnetic resonance imaging, and symptoms typically resolve within minutes.[11] Nevertheless, the relative mildness and reversibility of TIA should not be mistaken for clinical insignificance: 10% of TIA patients will go on to have a full stroke within the next 90 days, and 25%–50% of such strokes will occur within the first 2 days.[9] For this reason, it is crucial in TIA patients to employ prophylaxis such as antiplatelet medicine, anticoagulation therapy, and surgery, against the possible onset of stroke.
Although it is true that the current state of stroke therapy is hampered by a relatively small arsenal of available interventions, this should not be considered a cause for despair. Stroke therapy is a very young field: It was not until 1996 that tPA received its nod from the FDA. Moreover, an age in which increased insight into the cellular and molecular neuropathology of stroke yields new therapies seems to be approaching. A review of emerging therapies published recently in the journal Neuron[31] gives a sense of this approach. One well-studied cellular avenue to damage is excitotoxicity, in which insufficient glucose and oxygen lead to ionic gradient collapse and glutamate release.[32] This, in turn, causes a calcium influx that initiates multiple cell death pathways, including mitochondrial injury, reactive oxygen species (ROS) production, and protein misfolding.[33] The immune response launched in the wake of ischemia also plays a role in damage through the release of pro-inflammatory cytokines. Once understood, mechanisms like these yield means of forestallment. From understanding of the excitatory-inhibitory imbalance initiated by ischemia comes cortical stimulation therapy to rectify such imbalances. A similar mechanism-to-treatment pathway is evident in the case of stem cell therapy,[34] which is thought by some to mitigate ischemic damage by immunoregulatory means.
Yet, another example is provided by the case of the blood–brain barrier (BBB), in which interest as a therapeutic target has begun to grow. This interest is derivative of the role the BBB has been found to play, partially through the administration of tPA therapy, in the facilitation of ischemic injury. tPA has been shown to be clinically effective in reducing neurological deficits and improving functional outcomes in patients with ischemic stroke – hence its FDA approval. It achieves these benefits through promoting fibrinolytic dissolution (or thrombolysis) of the clot responsible for ischemia,[35] which causes reperfusion of the affected tissue. This results in salvage of tissue that might otherwise have undergone ischemic necrosis. Unfortunately, however, salvage from ischemia is not the only consequence of clot dissolution: The therapeutic profile of tPA is vastly complicated by the fact that, for metabolic reasons that exceed the scope of this review, ROS and pro-inflammatory enzymes are produced by reperfused tissue, precipitating the pathologic entity known in the literature as reperfusion injury. In addition to these effects, thrombolysis promotes matrix degradation in the ischemic brain through activation of matrix metalloproteinase-9 (MMP-9).[36] Thus, tPA is considered a double-edged sword: Although it promotes the adaptive reestablishment of blood flow, it also increases BBB permeability, which can exacerbate cerebral edema (the reasons for this, including the role played by MMP, are described at length in Part II of this review in the context of microcirculatory dysfunction of the cerebral vasculature).
In deference to the benefit unquestionably produced by tPA administration, therapeutic research has attempted, rather than searching for alternative compounds, to discover complimentary treatments for coadministration with tPA to reduce reperfusion-associated injury. One such therapy is the induction of hypothermia, which has been suggested as a means of inhibiting hemorrhagic transformation (the role of tPA in promoting hemorrhagic transformation is discussed in more detail below). In two large, randomized trials from Europe and Australia, it was shown that therapeutic induction of hypothermia (at 34°C) substantially improved neurological outcome after cardiac arrest.[37,38] In addition, in animal studies, systemic hypothermia after reperfusion was effective in reducing infarct volume by 50%. It also reduced mortality.[39,40] Mechanistically, hypothermia reduces ROS production, tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), adhesion molecules, as well as MMP-9,[41] and thus reduces inflammatory cell infiltration, preserving BBB integrity. Furthermore, it reduces AQP4 expression on the astrocytic membrane during the acute phase of ischemia, attenuating cytotoxic edema[42,43] (see our Part II). Thus, this therapy is thought to be neuroprotective because of its ability to modulate several mechanisms involved in reperfusion injuries.
Neuroprotective properties in stroke have been elicited by other methods as well, such as ethanol treatment. In both in vivo and in vitro models, ethanol treatments have been shown to reduce MMP-2 and MMP-9 and thereby to preserve zonula occludens-1 and the basal lamina components of the BBB.[44,45,46] When combined with the significant decrease in AQP4 expression it also appears to cause, ethanol thus decreases cerebral edema and improves BBB integrity following ischemia/reperfusion.[44,46] It also mitigates the excess glucose utilization and attendant lactic acidosis and ROS production caused by the ischemia/reperfusion process by attenuating hyperglycolysis and associated nicotinamide adenine dinucleotide phosphate oxidase activation. This results in improved salvation of the region surrounding infarcted cells that is vulnerable to infraction itself if ischemia is prolonged, known as the ischemic penumbra.[47]
Other experimental therapies are also under active investigation.[48] Several studies have demonstrated that hyperbaric oxygen intervention can increase oxygen supply, reduce ischemia/reperfusion injury, and alleviate the extent of irreversible neurological impairment in stroke. It achieves these effects through the promotion a variety of adaptive mechanisms, such as increase of antioxidant enzymes, alleviation of oxidative stress, reduction in the formation of free radicals, enhancement of superoxide dismutase and catalase activity, and modulation of inflammation by increasing anti-inflammatory cytokines and reducing pro-inflammatory cytokines.[49,50,51]
Thus, hyperbaric oxygen, ethanol, and hypothermia are united by their ability to intervene in multiple pathological pathways associated with increasing BBB permeability and consequent cerebral edema. It is impossible to predict if or when either these therapies, or others that target different mechanisms, such as cortical stimulation and stem cell therapies, will fulfill their potential clinically. We expect, however, that our readers will agree that there is ample reason to hope that the answer is soon.
Hemorrhagic Stroke
Epidemiology
Returning to the prognostic continuum with which we introduced the categories of stroke is one way of understanding hemorrhagic stroke in comparison with its ischemic counterpart. Ravages of ischemic stroke notwithstanding, hemorrhagic stroke is a much graver disease. At 40%–60%, the in-hospital mortality rate for hemorrhagic stroke is greater than ischemic stroke by up to a factor of 12. Within 30 days of onset, the mortality rate is 50%; for survivors, drastic disability prevents a full 80% from living independently within 6 months.[4] Thus, even though hemorrhagic stroke accounts for only 13% of total stroke incidence, the profundity of its pathologic effect justifies its classification as a major type of stroke. Data from France's Dijon Stroke Registry offer little hope for future improvement of this situation. It may seem superficially encouraging that incidence among the part of the population under 60 years of age has decreased in recent years by 50%. After all, hemorrhagic stroke is the most common type among those under 40.[52] Unfortunately, this decrease has been accompanied by an increase of 80% in the population ≥75 years old.[53] This increase in rate among the aged population, considered in the context of the overall older population to be expected over the next century, led Stein et al. to project that, by 2050, in-hospital mortality for intracerebral haemorrhage (ICH) will rise to 60.2%, and the number of ICHs patients in the United States will double.[54] Assuming no breakthroughs in treatment occur over the next 30 years, then, it appears that hemorrhagic stroke is here to stay.
Risk factors for hemorrhagic stroke resemble those for ischemic stroke and can be categorized analogously according to modifiability. Age and race are among the nonmodifiable factors identified so far and appear to interact in a manner that is not yet fully understood.[55] Interethnic differences, based on differential exposure to genetic and environmental aggravators, are also being studied but have not yet produced definitive data.[56] Finally, cerebral amyloid angiopathy (discussed in Part II) has received attention as a contributor to ICH pathophysiology, especially when present in the elderly.[4] Of the modifiable factors, hypertension, which is present in 72%–81% of ICH cases, is the most significant. Taken together, the modifiable risk factors for ICH, which also include cigarette smoking, alcohol consumption, hypocholesterolemia, elevated waist-to-hip ratio, psychosocial stress, and an unhealthy diet, are thought to account for 90.8% of the population-attributable ICH risk.[53]
Pathophysiology
Much like ischemic stroke, hemorrhagic stroke is a heterogeneous entity. In every case, the rupture of a cerebral blood vessel initiates the pathology. There is an important distinction to be made, however, between different possible loci of rupture. The majority of hemorrhagic strokes are classified as ICH and involve arterial bleeding into the cerebral parenchyma.[4] Among Western populations, these account for 5%–10% of total strokes, but this proportion can rise as high as 22%–34% in Southeast Asian, South American, and African populations.[53] This type of stroke can be further subdivided by etiological factors. If onset cannot be accounted for by identification of an underlying structural or pathological factor, it is considered primary ICH. If, on the other hand, it is preceded by such a factor, it is considered secondary ICH. Factors that can cause secondary ICH include bleeding from tumors, hemorrhagic conversion of ischemic stroke, dural venous sinus thrombosis, and vasculitis.[57] In addition to ICH, there is another type of hemorrhagic stroke to consider. A smaller but nonnegligible proportion of these strokes (2%–7% of total stroke incidence[58]) are subarachnoid hemorrhages (SAHs), the majority of which involve the spontaneous rupture of cerebral aneurysms into the cerebrospinal fluid.[4]
Rupture usually occurs at the end of a hypertension-induced, progressive process of vascular weakening. Subsequently, this initial rupture precipitates further rupture of other vessels by mechanical impingement. Finally, these ruptures result in even more rupturing by the same mechanism, and so on, as the hematoma expands. The location of this cascade in the case of ICH is usually the small penetrating arteries of the cerebral parenchyma.[53] In the case of SAH, in which there is an aneurysmal precursor, a variety of insults, such as endothelial cell injury and damage to the arterial tunica media, are thought to interact with an inflammatory response to induce rupture. Aneurysms predominately arise from anterior circulation arterial branches from the circle of Willis,[58] especially from the anterior communicating artery.
While hematoma expansion is associated on its own with exacerbating neurological deficit and poor prognosis,[4] halting it would not entirely forestall ICH-mediated cerebral injury. This is because, in addition to the primary injury mediated by mechanical disturbance, there are a variety of secondary mechanisms in concomitant operation. One such mechanism involves the immune response to the escaped blood elements. These elements cause the activation of adjacent microglia and astrocytes. These cells serve an adaptive purpose, secreting neurotrophic factors, and phagocytizing hematoma-associated infiltrates. Unfortunately, however, they also promote a cytokine-mediated pro-inflammatory response that damages brain tissue. Another mechanism of secondary injury is the generation of iron-associated ROS from lysed red blood cells. Although these secondary injury pathways are currently an active area of investigation, there are not currently any interventions available to preclude them.[53]
Presentation
While hemorrhagic stroke presents classically as increased intracranial pressure, sudden-onset headache, severe hypertension, and vomiting,[53] as well as rapidly progressing neurologic deficits, neuroimaging is necessary to differentiate it from ischemic stroke.[4] Noncontrast computed tomography (CT) of the head is used for this purpose because it is highly sensitive for the presence of hemorrhage. To predict hematoma expansion, CT angiogram can also be used.[59] In aneurysmal cases, once lumbar puncture has established the presence of aneurysm, catheter angiography is employed to localize it. Exactly as is the case with ischemic stroke, neurologic clinical features vary according to the location of injury. There are, however, several manifestations common to all locations. In addition to those enumerated above (headache, hypertension, vomiting, and the progressive nature of neurologic deficit), declining alertness is present in three-fifths or more of cases; in two-thirds of such cases, the decline reaches the level of coma.[51]
Therapies
As is unsurprising given its catastrophic nature, there is currently no treatment available for ICH beyond supportive medical care.[60] There are some scenarios in which emergent neurosurgery is necessary. Cerebellar hemorrhage, owing to confinement in the infratentorial space and the proximity of brainstem centers essential to life, is one such scenario. In some patients with large lobar hemorrhages close to the cortical surface, surgical drainage may be beneficial.[4] SAH also requires emergent attention toward securing the aneurysm, which can be done either surgically or by endovascular techniques. For neuroprotection, the calcium antagonist nimodipine is also administered in cases of SAH.
Otherwise, the treatment of both SAH and ICH is confined to medical support and the prevention of complications. In ICH, hypertension and intracranial pressure elevation should be managed aggressively (craniotomy may be necessary if more conservative methods fail). Precipitating coagulopathy should be corrected. Hyperglycemia, which is associated with worse outcome, should be lowered to <200 mg/dl, and antipyretics should be administered when fever is present.[59] SAH is managed similarly and also according to its many possible complications, including aneurysmal rebleeding, hydrocephalus, seizures, delayed cerebral ischemia, and cerebral edema.[58]
Readers may have noticed that there is, regardless of the type under discussion, much more known about how stroke occurs than there is about how to reverse its consequences. Lamentable as this undoubtedly is, maintaining a historical perspective on stroke science is conducive to optimism. As recently as the latter third of the 20th century, stroke patients were still considered undesirable patients with hopeless outcomes.[61] It must be acknowledged that, today, this is far from the case. Substantial advances in our cellular, genetic, and molecular understanding of stroke pathophysiology have ushered in an era of rapid discovery and a frenetic search for treatments. In the case of ICH, investigation of the established inflammatory route to secondary injury has yielded promising therapeutic possibilities.[62] The same is true for iron-mediated oxidative stress.[63] For SAH, research foci include optimization of aneurysmal management, factors that predict rupture, complication management, and neuroprotective strategies.
Again, it is not possible to predict when this research will begin to make a meaningful prognostic difference in the lives of those afflicted with stroke. Until it does, population-level management of the myriad modifiable risk factors remains essential. If one thinks of risk factors as a cliff, and stroke as what happens when one falls from the top, one can then think of the development of acute interventions as elaborate attempts to catch the falling patient. As successful as some catches may turn out to be, it is much better to avoid climbing the cliff in the first place.
Ischemic to Hemorrhagic Transformation
Epidemiology
While a significant effort has been invested into ischemic and hemorrhagic stroke therapies in isolation, there has been much less work put into the risk minimization and treatment of the occurrence of intracranial bleeding associated with an ischemic stroke.[64] This occurrence is known as ischemic to hemorrhagic transformation (IHT). IHT has been estimated to occur in a substantial 30%–40%[65] of all ischemic strokes, but estimates range from as low as 18% to as high as 71%,[64] depending on whether the information was derived from CT scans or autopsy. Either way, IHT is a major cause of early mortality in acute ischemic stroke patients, having been found responsible for 26–154 extra deaths per 1000 ischemic stroke patients.[64]
The risk factors for IHT largely mirror those of ischemic and hemorrhagic strokes in isolation. Modifiable risk factors include an anticoagulative state, hyperglycemia, and low total cholesterol and low-density lipoprotein (LDL); the anticoagulative state, however precipitated, is considered the chief risk factor for IHT. This has given rise to concern over the use of thrombolytics in the treatment of ischemic stroke: it appears that the very antithrombotic mechanism necessary to rid vessels of ischemia-inducing clots can render those same vessels susceptible to hemorrhage.
With good reason, this potential to induce ICH is the most feared complication of thrombolytic therapy.[66] In a recent study, treatment with tPA resulted in a 10-fold increase in mortality associated with symptomatic hemorrhage (47%) as compared to placebo (4.7%). However, this study also found that mortality was ultimately decreased by tPA administration (17% compared to 21%) due to a decrease in death from nonhemorrhagic strokes,[67] which highlights the importance of a thorough understanding of IHT risk factors when selecting candidates for thrombolysis. Long-term warfarin use also encourages IHT events. In a recent study by Pfeilschifter et al., every mouse (n = 21) pretreated with warfarin developed IHT after induction of ischemic stroke, compared with two out of 20 in the control (no anticoagulation) group.[64,68] In addition, low levels of coated platelets (that is, platelets activated by exposure to collagen and thrombin) have been associated with early IHT in patients with nonlacunar ischemic strokes.[69]
Hyperglycemia has been consistently found to encourage IHT by increasing oxidative stress, upregulating MMP-9 expression, and generally exacerbating BBB disruption.[65,70] Rates of symptomatic IHT were increased in patients with diabetes mellitus.[64] The neuroprotective agent isoflurane has been shown, paradoxically, to exacerbate infarct volume, brain edema, and neurobehavioral deficits in hyperglycemic rats. This underscores the complex interaction that evidently occurs between the variety of factors in IHT pathophysiology:[71] the case of failed neuroprotection with isoflurane in the context of hyperglycemia shows that these factors are not merely additive. Clearly, there is still much to unravel about the nature of IHT.
In addition, low levels of total cholesterol and LDL have been correlated with an increased IHT risk. Although the precise pathophysiology of this phenomenon has yet to be elucidated, it has been proposed that cholesterol is necessary to maintain the integrity of small vessels.[72] This finding, as one can imagine, is vexing to researchers in the field of stroke prevention. Hypercholesterolemia is a major modifiable risk factor for ischemic stroke; therefore, a large proportion of patients at high risk are encouraged to make concerted efforts to lower their cholesterol levels. In thus lowering their risk for ischemic stroke, are they thereby put at greater risk for IHT? Evidence exists that suggests that they are: while high doses of atorvastatin decrease risk of ischemic stroke by 22%, they increase the risk of hemorrhagic stroke by 66%.[73]
Clearly, this represents a clinical dilemma. The prevention of ischemic stroke cannot be rationally or ethically pursued at the expense of exposing a patient to IHT. As such, a strong understanding of the interplay between potential risk factors is essential to providing the highest level of care possible.
Pathophysiology
IHT can either occur spontaneously or after treatment with tPA. Once again, the pathophysiology of IHT is complex and still not completely understood, but it is thought that IHT arises primarily as an iatrogenic consequence of ischemic stroke therapy: tPA administration, in addition to the deleterious effects already reviewed, may also dissolve clots that are essential to the hemostatic integrity of the cerebral vasculature. Unfortunately, the current therapeutic guidelines do not appear to be sufficiently protective against this possibility. In a 1999 study, 61% (96 out of 156) of patients with acute ischemic stroke developed IHT when treated with streptokinase (a tPA analog), as compared to 31% (61 out of 154) who were treated with placebo.[64]
IHT can also develop secondary to the structural deterioration caused by ischemic stroke. Neurons are depleted of their adenosine triphosphate stores within minutes of the onset of hypoxia, which leads to a breakdown in numerous homeostatic pathways, including those involved in maintenance of BBB. This culminates in a degradation of the BBB integrity.[74] In addition, the inflammation caused by ischemia stimulates macrophages to release IL-1β and TNF-α, both of which increase production of MMPs that further facilitate BBB disruption.[68,75] Inflammation also impairs the autoregulatory properties of cerebral vessels, rendering them less able to compensate for hemostatic disturbances once the infarct area is reperfused. Ultimately, BBB disruption, when coupled with this diminished autoregulatory capacity, predisposes the vicinity of the infarct to blood extravasation into the brain parenchyma upon reperfusion.[74]
Presentation
The clinical presentation of IHT depends entirely on two factors: the timing of the transformation and location of the ischemia and hemorrhage. Before transformation, the stroke is purely ischemic and will present as such. Once IHT has begun, a symptomatic hemorrhage will present with a combination of ischemic and hemorrhagic symptoms. Symptomatic hemorrhage was defined in the PROACT II study as “an increase of four or more points in the NIHSS score in comparison with the preangiography score, within 36 h from treatment initiation associated with any intracranial blood on CT.”[76] However, the majority of hemorrhages in IHT are asymptomatic; in a 1999 study, IHT was noted in 159 of 310 participants, just 36 of which were symptomatic.[77] In this case, the disease will continue to present as an ischemic stroke clinically, with the attendant hemorrhage manifesting only through the assistance of radiological studies.
Regarding the type of hemorrhage, IHT can be categorized as a parenchymal hematoma (PH) or a hemorrhagic infarction (HI), both of which may be further split into two subcategories based on radiological findings.[66] PH refers to a dense, homogeneous intracranial hematoma with mass effect. Within this category, PH 1 describes a homogeneous hyperdensity that occupies <30% of the infarcted zone with some mass effect, while PH 2 describes a homogeneous hyperdensity that occupies >30% of the infarcted zone with a significant mass effect. There is broad agreement that PH has a significant impact on 3-month patient outcomes.[66] HI describes a heterogeneous hyperdensity that occupies a portion of the ischemic region without mass effect. Within this group, HI1 involves small, hyperdense petechiae, while HI2 describes a more confluent hyperdensity that spans across the infarcted area. HI has not been found to have a significant effect on 3-month patient outcomes.[66] HI occurs more frequently than PH; a recent study found that HI occurred in 9% of acute ischemic stroke patients, while PH was only found in about 3%.[70]
Therapies
Given the iatrogenic potential already described, IHT presents a therapeutic challenge. As such, there are no widely used therapies to effectively prevent IHT. However, in light of the gravity of IHT, efforts are underway to develop such therapy. There are some data indicating that treating IHT patients with MMP inhibitors decreases the incidence of IHT. Thus, it is possible that therapeutic hypothermia induction could provide benefit to these patients since it has been shown to decrease MMP expression after stroke.[78,79] A few other medications have also shown some efficacy in protecting against IHT. Early administration of deferoxamine is neuroprotective against cerebral hemorrhage and SAH, so it would likely have a similar effect in IHT.[80,81] Estrogen has also shown some promise in combatting the unwanted effects of tPA. It is known to decrease BBB permeability, MMP-9 activity, and incidence of IHT, and is therefore productive of anti-IHT effects when given before tPA.[82] In a rat study, coadministration of tPA and E2 estrogen resulted in an attenuation in BBB permeability back to the levels of the control group (ischemia followed by reperfusion without tPA).[82] Interestingly, the phosphodiesterase-3 inhibitor cilostazol has also been found to prevent IHT when given before tPA. Evidence suggests that cilostazol minimizes endothelial damage by preventing lipopolysaccharide-induced apoptosis and inhibiting neutrophil adhesion, and it is believed that these mechanisms are also responsible for the observed IHT prevention.[83]
Nevertheless, despite this profusion of possible future therapies, prevention of IHT before it starts through the prevention of preceding ischemic stroke remains, far and away, the ideal approach. The damage wrought on the brain by stroke is in the very best case attenuable through therapy, and, in many cases, therapeutic attenuation still leaves patients with permanently transformative deficits. Thus, while we must continue to work vigorously toward the discovery of new treatments for both IHT and the other varieties of stroke from which millions continue to suffer, we must also work, and with equal vigor, to mitigate the need to apply such treatment in the first place.
Summary and Future Direction
In this paper, our three-part review of cerebrovascular anatomy, physiology, and disease culminated in a detailed discussion of stroke.[12,15] For each major stroke modality, we outlined the epidemiology, pathophysiology, presentation, and methods, both in use and emerging, by which treatment proceeds. As we have emphasized persistently, risk factor management is an indispensable part of stroke treatment. This is especially true within the context of the demographic trends displayed by many major stroke risk factors among many populations worldwide: The population is aging, hypertension and diabetes are on the rise, and obesity is, at best, leveling off.[84,85,86] These trends have led researchers to project that, the many advances in understanding the disease made in recent years notwithstanding, the incidence and prevalence of stroke are likely to increase in the United States during the coming decades.[87,88]
Clearly, the treatment of stroke requires a concerted commitment to discovering ways to maintain health at the population level. Concomitantly, however, the extremity of suffering produced by stroke at the level of individual patients, families, and caregivers must not be neglected. As has been reviewed above, the pharmacological approach remains a robustly researched way of treating stroke at the individual level, but is currently limited in scope, and, moreover, carries substantial iatrogenic potential. This is true both of the thrombolytic agents employed to treat stroke acutely, as well as the anticoagulants and antiplatelet agents used to prevent its recurrence.[89,90] – carries substantial iatrogenic potential. Clinicians must therefore exercise vigilance when selecting therapeutic and prophylactic approaches for patients with cerebrovascular disease.
They must also be vigilant in their attention to the progress currently underway both toward the improvement of current therapies and toward the development of novel ones. An example of the former category is afforded by the clinical dilemma of choice between the newer intra-arterial thrombolysis (IAT) and the original IV thrombolysis (IVT). Theoretically, IAT seems to be superior. While in IVT, the diffuse spread of the thrombolytic agent may produce unwanted effects elsewhere within the vasculature, IAT is targeted by catheter directly to the pathologic focus and should therefore avoid these unwanted effects.[91] This theoretical superiority has not, however, invariably produced better clinical outcomes. One recent meta-analysis found that IAT was associated with increased recanalization and lower mortality, while others found no difference in mortality.[92] Further research and methodological standardization across investigations are needed to consolidate the evidence in favor of IAT.
As has been highlighted in the previous sections, there are also many novel treatment methods under development in the field of stroke therapy. Neuroprotection, including therapeutic hypothermia, hyperbaric oxygen therapy, and the administration of pharmacologic agents such as ethanol, is a method that has enjoyed especially vigorous interest among researchers in recent years. This method, because it is utilizable alongside traditional thrombolytic therapy, is regarded as attractive for the ease with which it could be incorporated into clinical practice. It is true that there have not yet been large clinical trials that support such a contention;[93] however, preliminarily encouraging results have already been produced in both animal and human studies. In the case of hypothermia, for instance, a recent study of thirty patients with anterior circulation ischemic strokes demonstrated that better prognosis and lower incidence of hemorrhagic transformation resulted when mild hypothermia was induced in addition to IAT.[94] Large, well-designed trials are warranted to investigate the generalizability of these results.
Thus, the field of cerebrovascular disease therapeutics may excite multiple, conflictual impressions among its researches, its physicians, and the patients they serve. On the one hand, the severity of disability, mortality, and other kinds of suffering imposed by stroke still vastly overwhelm the interventions currently at our disposal to forestall this suffering. Moreover, the specter of iatrogenic harm looms large over the clinical management of this disease. On the other hand, however, each day brings the many investigators working to change this situation closer to realizing their goal. The hundreds of published experiments with neuroprotection, including nonpharmacologic approaches such as exercise for ischemic conditioning,[95] as well as the advent of promising methods for the chronic phase of stroke management such as repetitive transcranial magnetic stimulation,[96] provide ample justification of hope for a brighter future in this field.
As stated above, there was a time when stroke sufferers were often considered undesirable patients with hopeless outcomes.[97] Happily, this is no longer the case. As severe an affliction as stroke often undoubtedly still is, to describe it as hopeless now would be both hyperbolic and empirically inaccurate: although the incidence and prevalence of stroke are expected to increase, the decline in mortality arising from the disease is substantial enough to be regarded as one of the most significant public health successes of the past 50 years.[98]
It is our earnest hope that as stroke risk factors are addressed, as stroke therapies emerge, and as the admirable work undertaken by physicians, patients, and caregivers to manage stroke proceeds, this trend of declining morality will also characterize the 50 years to come.
Financial support and sponsorship
This work was partially supported by American Heart Association Grant-in-Aid (14GRNT20460246) (YD), Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service (YD), National Natural Science Foundation of China (81501141) (XG), and Beijing NOVA program (xx2016061) (XG).
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Cardiovascular Diseases (CVDs), Fact Sheet. 2016. [Last cited on 2017 May 24]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Centers for Disease Control and Prevention. Stroke Fact Sheet. 2016. [Last cited on 2017 Jun 01]. Available from: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_stroke.htm .
- 3.Krishnamohan P. Ferri's Clinical Advisor 2017. Philadelphia: Elsevier; 2017. Stroke, acute ischemic; pp. 1209–12. [Google Scholar]
- 4.Crocco TJ, Goldstein JN. Rosen's Emergency Medicine. Philadelphia: Elsevier; 2014. Stroke; pp. 1363–74. [Google Scholar]
- 5.National Institutes of Neurological Disorders and Stroke. Post-Stroke Rehabilitation. 2016. [Last cited on 2017 Jun 01]. Available from: https://stroke.nih.gov/materials/rehabilitation.htm .
- 6.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, et al. Quality of life after stroke: The North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2004;35:2340–5. doi: 10.1161/01.STR.0000141977.18520.3b. [DOI] [PubMed] [Google Scholar]
- 7.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke. 1999;30:2513–6. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke Risk factors. Stroke. 1997;28:1507–17. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 9.Ledyard H. Emergency Medicine: Clinical Essentials. Philadelphia: Elsevier; 2013. Transient ischemic attack and acute ischemic stroke; pp. 870–80. [Google Scholar]
- 10.Rockman CB, Maldonado TS. Emery and Rimoin's Principles and Practice of Medical Genetics. Amsterdam, Netherlands: Elsevier; 2013. Genetics of stroke; pp. 1–20. [Google Scholar]
- 11.Rockman CB, Maldonado TS. Rutherford's Vascular Surgery. Amsterdam, Netherlands: Elsevier; 2014. Cerebrovascular disease: General considerations; pp. 1456–72. [Google Scholar]
- 12.Chandra A, Stone CR, Li WA, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease II: Pathogenesis of cerebrovascular disease. Brain Circ. 2017;3:57–65. doi: 10.4103/bc.bc_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis R, Caplan MD. Caplan's Stroke. Philadelphia: Elsevier; 2009. Basic pathology, anatomy, and pathophysiology of stroke; pp. 22–63. [Google Scholar]
- 14.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 15.Chandra A, Li WA, Stone CR, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease I: Anatomy. Brain Circ. 2017;3:45–56. doi: 10.4103/bc.bc_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemming KD, Brown RD, Jr, Petty GW, Huston J, 3rd, Kallmes DF, Piepgras DG. Evaluation and management of transient ischemic attack and minor cerebral infarction. Mayo Clin Proc. 2004;79:1071–86. doi: 10.4065/79.8.1071. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y, Chen Q, Li ZY. A 3D numerical study of the collateral capacity of the circle of willis with anatomical variation in the posterior circulation. Biomed Eng Online. 2015;14(Suppl 1):S11. doi: 10.1186/1475-925X-14-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Wu X, Zhao H, Min L, Tao Z, Ji X, et al. Effects of erythropoietin combined with tissue plasminogen activator on the rats following cerebral ischemia and reperfusion. Brain Circ. 2016;2:54–60. doi: 10.4103/2394-8108.178552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–9. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 20.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 21.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 22.Rajah GB, Ding Y. Experimental neuroprotection in ischemic stroke: A concise review. Neurosurg Focus. 2017;42:E2. doi: 10.3171/2017.1.FOCUS16497. [DOI] [PubMed] [Google Scholar]
- 23.Carcora Y, Hussain M, Geng X, Ding Y. A review of current clinical studies leading to improved outcomes in patients treated with newer-generation thrombectomy devices. Brain Circ. 2015;1:9–13. [Google Scholar]
- 24.Jadhav A, Jovin T. Endovascular therapy for acute ischemic stroke: The standard of care. Brain Circ. 2016;2:178–82. doi: 10.4103/2394-8108.195283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bope ET, Kellerman RD. Conn's Current Therapy. Amsterdam, Netherlands: Elsevier; 2017. The nervous system; pp. 637–714. [Google Scholar]
- 26.Figueroa B, Clark J, Ellens N. The use of barbiturate-induced coma during cerebrovascular neurosurgery procedures: A review of the literature. Brain Circ. 2015;1:140–5. [Google Scholar]
- 27.Ji X. Forward thinking in stroke treatment: Advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circ. 2015;1:1–2. [Google Scholar]
- 28.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 29.Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: Our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg. 2000;17:718–22. doi: 10.1016/s1010-7940(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 30.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 31.George PM, Steinberg GK. Novel stroke therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87:297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey B, Wang Y. Reducing excitoxicity with glutamate transporter-1 to treat stroke. Brain Circ. 2016;2:118–20. doi: 10.4103/2394-8108.192523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang S, Li W. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016;2:153–63. doi: 10.4103/2394-8108.195279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suárez-Meade P, Carvajal HG, Yasuhara T, Tajiri N, Date I, Borlongan CV. Regenerative medicine for central nervous system disorders: Role of therapeutic molecules in stem cell therapy. Brain Circ. 2015;1:125–32. [Google Scholar]
- 35.Gravanis I, Tsirka SE. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin Ther Targets. 2008;12:159–70. doi: 10.1517/14728222.12.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 37.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 38.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 39.Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: Neurological outcome and infarct size. J Neurosurg. 2001;94:90–6. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 40.Huh PW, Belayev L, Zhao W, Koch S, Busto R, Ginsberg MD. Comparative neuroprotective efficacy of prolonged moderate intraischemic and postischemic hypothermia in focal cerebral ischemia. J Neurosurg. 2000;92:91–9. doi: 10.3171/jns.2000.92.1.0091. [DOI] [PubMed] [Google Scholar]
- 41.Tahir RA, Pabaney AH. Therapeutic hypothermia and ischemic stroke: A literature review. Surg Neurol Int. 2016;7(Suppl 14):S381–6. doi: 10.4103/2152-7806.183492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, Li CS, Gong P, Tang ZR, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83:913–20. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Kurisu K, Abumiya T, Nakamura H, Shimbo D, Shichinohe H, Nakayama N, et al. Transarterial regional brain hypothermia inhibits acute aquaporin-4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery. 2016;79:125–34. doi: 10.1227/NEU.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 44.Zeng X, Asmaro K, Ren C, Gao M, Peng C, Ding JY, et al. Acute ethanol treatment reduces blood-brain barrier dysfunction following ischemia/reperfusion injury. Brain Res. 2012;1437:127–33. doi: 10.1016/j.brainres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Chou DY, Ding JY, Fredrickson V, Peng C, Schafer S, et al. Reduction of brain edema and expression of aquaporins with acute ethanol treatment after traumatic brain injury. J Neurosurg. 2013;118:390–6. doi: 10.3171/2012.8.JNS12736. [DOI] [PubMed] [Google Scholar]
- 46.Peng C, Li WA, Fu P, Chakraborty T, Hussain M, Guthikonda M, et al. At low doses ethanol maintains blood-brain barrier (BBB) integrity after hypoxia and reoxygenation: A brain slice study. Neurol Res. 2013;35:790–7. doi: 10.1179/1743132813Y.0000000198. [DOI] [PubMed] [Google Scholar]
- 47.Kochanski R, Peng C, Higashida T, Geng X, Hüttemann M, Guthikonda M, et al. Neuroprotection conferred by post-ischemia ethanol therapy in experimental stroke: An inhibitory effect on hyperglycolysis and NADPH oxidase activation. J Neurochem. 2013;126:113–21. doi: 10.1111/jnc.12169. [DOI] [PubMed] [Google Scholar]
- 48.Goel D, Mittal S. Mortality in ischemic stroke score: A predictive score of mortality for acute ischemic stroke. Brain Circ. 2017;3:29–34. doi: 10.4103/2394-8108.203256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, et al. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–9. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Korkmaz A, Oter S, Sadir S, Topal T, Uysal B, Ozler M, et al. Exposure time related oxidative action of hyperbaric oxygen in rat brain. Neurochem Res. 2008;33:160–6. doi: 10.1007/s11064-007-9428-4. [DOI] [PubMed] [Google Scholar]
- 51.Chen LF, Tian YF, Lin CH, Huang LY, Niu KC, Lin MT. Repetitive hyperbaric oxygen therapy provides better effects on brain inflammation and oxidative damage in rats with focal cerebral ischemia. J Formos Med Assoc. 2014;113:620–8. doi: 10.1016/j.jfma.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Louis R, Caplan MD. Caplan's Stroke. Philadelphia: Elsevier; 2009. Intracerebral hemorrhage; pp. 487–522. [Google Scholar]
- 53.Kase CS, Shoamanesh A, Greenberg SM, Caplan LR. Stroke: Pathophysiology, Diagnosis, and Management. Amsterdam, Netherlands: Elsevier; 2016. Intracerebral hemorrhage; pp. 466–515. [Google Scholar]
- 54.Stein M, Misselwitz B, Hamann GF, Scharbrodt W, Schummer DI, Oertel MF. Intracerebral hemorrhage in the very old: Future demographic trends of an aging population. Stroke. 2012;43:1126–8. doi: 10.1161/STROKEAHA.111.644716. [DOI] [PubMed] [Google Scholar]
- 55.Howard G, Cushman M, Howard VJ, Kissela BM, Kleindorfer DO, Moy CS, et al. Risk factors for intracerebral hemorrhage: The REasons for geographic and racial differences in stroke (REGARDS) study. Stroke. 2013;44:1282–7. doi: 10.1161/STROKEAHA.111.000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai CF, Anderson N, Thomas B, Sudlow CL. Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and white populations: Systematic review and meta-analysis. PLoS One. 2016;11:e0151743. doi: 10.1371/journal.pone.0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, et al. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med. 2001;29:152–7. doi: 10.1097/00003246-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 58.José I. Suarez, Bershad EM. Stroke: Pathophysiology, Diagnosis, and Management. Amsterdam, Netherlands: Elsevier; 2016. Aneurysmal subar hemorrhage; pp. 516–36. [Google Scholar]
- 59.Krishnamohan P. Ferri's Clinical Advisor 2017. Philadelphia: Elsevier; 2017. Stroke, hemorrhagic; pp. 1213–4. [Google Scholar]
- 60.Chaudhary N, Gemmete JJ, Thompson BG, Xi G, Pandey AS. Iron – Potential therapeutic target in hemorrhagic stroke. World Neurosurg. 2013;79:7–9. doi: 10.1016/j.wneu.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 61.Louis R, Caplan MD. Caplan's Stroke: A Clinical Approach. Philadelphia: Elsevier; 2009. Introduction and perspetive; pp. 3–21. [Google Scholar]
- 62.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–77. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke. 2011;42:1781–6. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, et al. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999;30:1326–32. doi: 10.1161/01.str.30.7.1326. [DOI] [PubMed] [Google Scholar]
- 65.Fisher M, Vasilevko V, Cribbs DH. Mixed cerebrovascular disease and the future of stroke prevention. Transl Stroke Res. 2012;3(Suppl 1):39–51. doi: 10.1007/s12975-012-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37:556–61. doi: 10.1161/01.STR.0000196942.84707.71. [DOI] [PubMed] [Google Scholar]
- 67.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28:2109–18. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 68.Pfeilschifter W, Spitzer D, Czech-Zechmeister B, Steinmetz H, Foerch C. Increased risk of hemorrhagic transformation in ischemic stroke occurring during warfarin anticoagulation: An experimental study in mice. Stroke. 2011;42:1116–21. doi: 10.1161/STROKEAHA.110.604652. [DOI] [PubMed] [Google Scholar]
- 69.Prodan CI, Stoner JA, Cowan LD, Dale GL. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction. J Thromb Haemost. 2010;8:1185–90. doi: 10.1111/j.1538-7836.2010.03851.x. [DOI] [PubMed] [Google Scholar]
- 70.Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: Rate, predictive factors, and influence on clinical outcome: Results of a prospective multicenter study. Stroke. 2008;39:2249–56. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 71.Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, et al. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–6. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nardi K, Leys D, Eusebi P, Cordonnier C, Gautier S, Hénon H, et al. Influence of lipid profiles on the risk of hemorrhagic transformation after ischemic stroke: Systematic review. Cerebrovasc Dis Extra. 2011;1:130–41. doi: 10.1159/000335014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stroke Prevetion by Aggressive Reduction in Cholestrol Levels (SPARCL) Investigators. Karam JG, Loney-Hutchinson L, McFarlane SI. High-dose atorvastatin after stroke or transient ischemic attack: The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J Cardiometab Syndr. 2008;3:68–9. doi: 10.1111/j.1559-4572.2008.07967.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Yang Y, Sun H, Xing Y. Hemorrhagic transformation after cerebral infarction: Current concepts and challenges. Ann Transl Med. 2014;2:81. doi: 10.3978/j.issn.2305-5839.2014.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huber M, Duan H, Chandra A, Li F, Wu L, Geng X, et al. Hypothermia in stroke therapy: Systemic versus local application. In: Zheng J, Zhou C, editors. Hypoxia and Human Disease. Rijeka, Croatia: InTech; 2017. [Google Scholar]
- 76.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 77.Multicenter Acute Stroke Trial – Europe Study Group. Hommel M, Cornu C, Boutitie F, Boissel JP. Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med. 1996;335:145–50. doi: 10.1056/NEJM199607183350301. [DOI] [PubMed] [Google Scholar]
- 78.Baumann E, Preston E, Slinn J, Stanimirovic D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 2009;1269:185–97. doi: 10.1016/j.brainres.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 79.Burk J, Burggraf D, Vosko M, Dichgans M, Hamann GF. Protection of cerebral microvasculature after moderate hypothermia following experimental focal cerebral ischemia in mice. Brain Res. 2008;1226:248–55. doi: 10.1016/j.brainres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 80.Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A. Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–9. doi: 10.1016/j.neuroscience.2012.03.036. doi:10.1016/j.neuroscience.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xing Y, Hua Y, Keep RF, Xi G. Effects of deferoxamine on brain injury after transient focal cerebral ischemia in rats with hyperglycemia. Brain Res. 2009;1291:113–21. doi: 10.1016/j.brainres.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, Zhang Z, Sun W, Koehler RC, Huang J. 17ß-estradiol attenuates breakdown of blood-brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44:277–83. doi: 10.1016/j.nbd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishiguro M, Mishiro K, Fujiwara Y, Chen H, Izuta H, Tsuruma K, et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS One. 2010;5:e15178. doi: 10.1371/journal.pone.0015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finkelstein EA1, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 85.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 87.Howard G, Goff DC. Population shifts and the future of stroke: Forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20. doi: 10.1111/j.1749-6632.2012.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–75. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 89.Bansal S, Sangha KS, Khatri P. Drug treatment of acute ischemic stroke. Am J Cardiovasc Drugs. 2013;13:57–69. doi: 10.1007/s40256-013-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: A meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–5. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 91.Saver JL. Intra-arterial thrombolysis. Neurology. 2001;57(5 Suppl 2):S58–60. doi: 10.1212/wnl.57.suppl_2.s58. [DOI] [PubMed] [Google Scholar]
- 92.Ma QF, Chu CB, Song HQ. Intravenous versus intra-arterial thrombolysis in ischemic stroke: A systematic review and meta-analysis. PLoS One. 2015;10:e0116120. doi: 10.1371/journal.pone.0116120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel RA, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. 2017 doi: 10.1016/j.pcad.2017.04.005. pii: S0033-062030057-9. [DOI] [PubMed] [Google Scholar]
- 94.Ma LL, Song L, Yu XD, Yu TX, Liang H, Qiu JX. The clinical study on the treatment for acute cerebral infarction by intra-arterial thrombolysis combined with mild hypothermia. Eur Rev Med Pharmacol Sci. 2017;21:1999–2006. [PubMed] [Google Scholar]
- 95.Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–9. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 96.Chen F, Qi Z, Luo Y, Hinchliffe T, Ding G, Xia Y, et al. Non-pharmaceutical therapies for stroke: Mechanisms and clinical implications. Prog Neurobiol. 2014;115:246–69. doi: 10.1016/j.pneurobio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caplan LR. Caplan's Stroke a Clinical Approach. Philadelphia: Elsevier, Saunders; 2009. Clinical key flex; p. ix. (656). [Google Scholar]
- 98.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: A statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–53. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]