Abstract
In this paper, which is the first in a three-part series that reviews cerebrovascular anatomy, pathogenesis, and stroke, we lay the anatomical foundation for the rest of the series. Beginning with its origin in the branches of the aorta, we start by describing the arterial system. This system is partitioned into two major divisions (anterior and posterior circulations) that differ significantly in features and pathogenic potential. The systems, and the major branches that comprise them, are described. Description of the arterial system proceeds to the point of the fulfillment of its function. This function, the exchange of gases and nutrients with the cerebral parenchyma, is the subject of a subsequent section on the microcirculation and blood–brain barrier. Finally, the cerebral venous system, which is composed of cerebral veins and dural venous sinuses, is described. Thus, an anatomical context is supplied for the discussion of cerebrovascular disease pathogenesis provided by our second paper.
Keywords: Cerebral arteries, cerebral circulation, cerebral microcirculation, blood brain barrier, cerebral venous system, cerebrovascular anatomy, cerebrovascular disease
Origin of the Cerebral Circulation
While the brain is 2% of the total body mass, it uses nearly 50% of the human body's glucose. This makes it the most energy-intensive organ of the human body.[1] Thus, it follows straightforwardly that the brain ought also to be one of the most perfused organs in the body, which, indeed, it is. Two major sources of arterial blood provide this perfusion: the anterior circulation that originates in the internal carotid arteries [Figure 1] and the posterior (or vertebrobasilar) circulation that originates in the vertebral arteries [Figure 2]. Once these arteries enter the cranium, they branch exuberantly, eventually supplying blood to all deep and superficial regions of the brain. Perturbation of any portion of this blood supply, whether intracranially or extracranially, promotes the development of cerebrovascular diseases, the most common and notorious of which is stroke.
Figure 1.
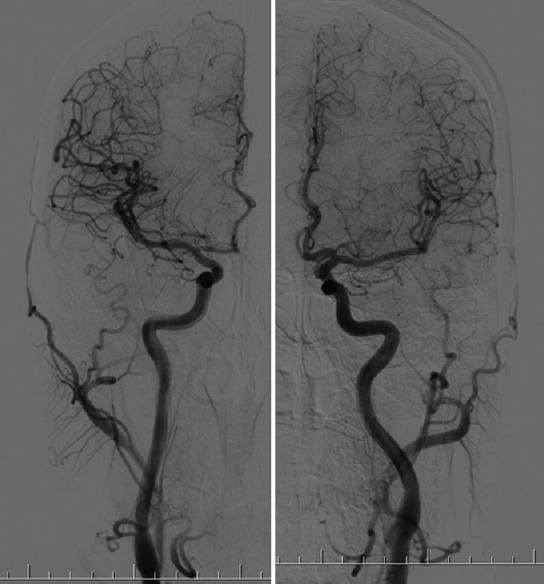
Anterior circulation: Left and right internal carotid arteries as seen with angiography
Figure 2.
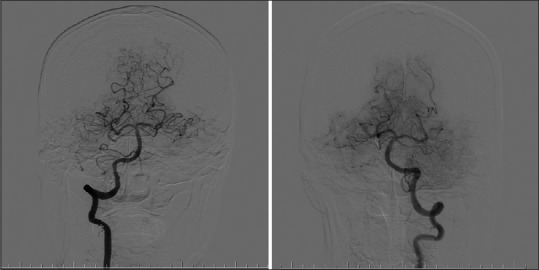
Posterior circulation: Left and right vertebrobasilar artery system as seen with angiography
Just like the circulation to the rest of the body, the cerebral circulation originates in the left heart and is conducted by the aorta. The conduction of blood begins during systole, with the left ventricular ejection of oxygenated blood into the aorta. As the aorta ascends, it becomes the ascending aorta and subsequently forms the aortic arch, which gives rise to three branches.[2] The first and largest branch is the brachiocephalic trunk, which originates behind the manubrium; the second branch is the left common carotid artery, which originates to the left of the brachiocephalic trunk; and the third is the left subclavian artery, which ascends with the left common carotid artery through the superior mediastinum and along the left side of the trachea.[2]
The vertebral arteries arise as the most proximal ascending branch from the subclavian arteries on each side of the body and enter deep into the cervical vertebral transverse processes, typically at the level of the 6th cervical vertebra, but about 7.5% of the time at the level of C7.[3] Other variations in the vertebral arterial route have also been noted, including anomalies in intraforaminal position,[4] and in length of the looped segment between C2 and the dura mater pierce point. Although uncommon, some of these variations may be of considerable surgical or pathophysiological relevance. It is thought, for example, that a sufficiently short loop segment can predispose patients to fatal traumatic basal subarachnoid hemorrhage.[5] Typically positioned or otherwise, the arteries in any case proceed superiorly in the transverse foramen of each cervical vertebra, ultimately passing through the transverse foramen of the atlas (C1). Once here, they make a sharp posterior bend, traveling across the posterior arch of C1 and through the suboccipital triangle, piercing the dura mater on their way toward the foramen magnum.[2] Passage through the foramen magnum marks the beginning of the arteries’ intracranial course, which constitutes the incipient segment of the posterior cerebral circulation and will be detailed in a subsequent section of this paper.
Whereas the vertebral arteries are bilaterally symmetrical in course, marked lateral variation in the origin of the common carotid arteries is characteristic of human anatomy. While the left common carotid artery arises directly from the aortic arch, the right internal carotid artery (ICA) arises from the brachiocephalic trunk.[6] The first and largest branch of the arch, the brachiocephalic trunk, originates on the right side of the chest near the trachea, and bifurcates posterior to the sternoclavicular joint into the right subclavian artery and right common carotid artery as it moves rightward within the superior mediastinum. On the left side of the body, however, there is no brachiocephalic trunk: on this side, the common carotid artery comes directly from the aortic arch as its second branch.[7] Despite this variation in origin, the common carotid arteries then pursue a symmetrical ascent in the chest, ending as they split into the internal and external carotid arteries at the superior border of the thyroid cartilage, at around the level of fourth cervical vertebra.[2] Unlike the external carotid arteries, the internal carotid arteries do not give off any branches in the neck. The only task of these vessels is to supply the brain's anterior circulatory system, which they begin to do after entering the cranial cavity just anteriorly to the jugular foramen, through the carotid canal.
Anatomy of the Cerebral Circulation
To begin with its simplest anatomical classificatory scheme, the cerebral circulation is composed of a supplying arterial circulation and a draining venous circulation. The arterial system can then itself be subdivided according to anatomical position, into anterior and posterior cerebral circulations, as has been discussed. One can also divide the arterial circulation by size. According to this scheme, the macrocirculation may be considered to comprise the gross branches of the cerebral vascular responsible for the regional perfusion of the cerebrum. The microcirculation is then derivatively defined as the microscopic site of oxygen and nutrient exchange within the vasculature, as well as of the blood–brain barrier (BBB).[8] Continuing with the anatomical scheme, the microcirculation is terminally productive of the brain's venous circulation: a freely communicating, interconnected system of dural venous sinuses and cerebral veins.[9,10] Although the venous system is often given less attention than its arterial counterpart (likely due to the relevance of the latter to the topic of ischemic stroke), it should not be forgotten that it, too, can serve as a significant focus of cerebral pathology, as is described below. Beginning with the arterial supply, we will discuss each of these circulatory systems in detail.
The Arterial System
Following their entry into the cranial cavity, the internal carotid and vertebral arteries fulfill the formidable role of exclusive suppliers of the blood necessary to maintain the brain, and all its many crucial functions, in working order (note that this is not the only function served by these enormously important vessels; the arterial networks also drain interstitial fluid and protein from the brain).[11] It has already been mentioned that the carotids are the major conduit for blood,[12] but the vertebral supply should not therefore be discounted. Before and after anastomosing to form the single, midline basilar artery, the vertebral vessels represent the primary supply of blood to the brainstem; compromise of this system can thus entail catastrophic consequences related to disruption in the critical brainstem autonomic centers. By contrast, both circulatory divisions provide major branches to the diencephalic and telencephalic regions of the brain proper. To do so, the circulations first meet as the Circle of Willis [Figure 3]: an arterial wreath, located approximately within the brain's interpeduncular fossa, and surrounding the optic chiasm. Initially described by Thomas Willis in 1664, the circle is a salient anatomical landmark of considerable anatomic variability. Variability notwithstanding, it is in any case the critical anastomotic junction between the internal carotid and vertebral artery supply to the brain, from which the rest of the major branches stem. It therefore occupies a very important position in the collateral pathway of cerebral arteries. This becomes especially important in ischemic conditions, under which variation can predispose patients to pathology. For example, a study of 976 atherosclerotic patients found that an incomplete anterior Circle of Willis, present in 23% of the study population, carries a hazard ratio of 2.8 for future anterior circulation ischemic strokes.[13] In addition, it has been proposed that demographic variation in this arterial wreath may partially explain the different incidence of some varieties of cerebrovascular diseases in different racial groups.[14]
Figure 3.
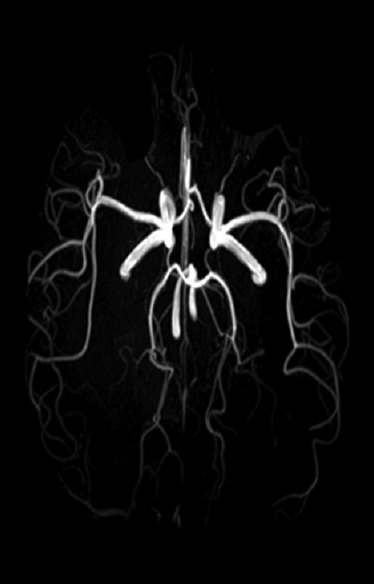
Circle of Willis as seen with magnetic resonance angiography
The branches of the circle are as follows: the anterior cerebral artery (ACA), anterior communicating artery (ACoA), ICA, posterior cerebral artery (PCA), posterior communicating artery (PCoA), and basilar artery.[15] From their origin within the interpeduncular fossa, these branches course centrifugally toward their divergent cerebral targets, becoming the cerebral arteries that are grossly visible covering the cortical surface (also known as leptomeningeal arteries), or ending as perforating or choroidal branches to deeper structures.[16] We will not treat these tiny and numerous terminal branches in any detail, but, since it is indispensable to a working appreciation of normal neurovascular function, we will discuss each of the larger branches in detail. Moreover, an understanding of the course of (and structures supplied by) these vessels is also the foundation necessary to localize and treat stroke, which will be the subject of our follow-up paper.[17] In this paper, they will be presented according to the anatomical classificatory scheme, beginning with the anterior circulation.
The Anterior Cerebral Circulation
The anterior cerebral circulation is composed of branches from the ICA. There are many such branches, but the ACA, middle cerebral artery (MCA), and the anterior choroidal artery (AChA) are highly prominent and pathophysiologically significant. Overall, the function of the anterior division of the cerebral circulation is to supply blood to a large proportion of the forebrain, including the frontal, temporal, and parietal lobes, as well as parts of the diencephalon and internal capsule. The contribution of the anterior circulation to total cerebral blood flow has been measured by phase-contrast magnetic resonance imaging (MRI) at 72%.[18]
Anterior cerebral artery
The ACA primarily supplies blood to the most medial aspect of the cerebral cortical surface, located along the longitudinal fissure that divides the brain's two hemispheres. This area includes portions of the frontal lobes, as well as the superomedial parietal lobes; because it ends rostral to the parieto-occipital sulcus, this artery does not supply the medial occipital lobe. Its course is as follows: after arising from the anterior clinoid portion of the ICA, it courses anteromedially over the superior surface of the optic chiasm, toward the longitudinal fissure. Shortly after arrival in the fissure, it forms an anastomosis with the contralateral ACA. This anastomosis is called the anterior communicating artery. While small, this artery is nevertheless the most common location of (36%) of cerebral aneurysms and is thus of enormous pathological importance.[19] It also marks the first segmental division of the ACA, which is divided regionally into five segments along its course: A1–A5. Table 1 describes these segments, their major branches, the structures they supply, and the significance of any anatomical variation associated with them. As the ACA proceeds, then, beyond A1, it begins its posterior course toward the parieto-occipital sulcus, following the contour of the callosal sulcus between the two cerebral hemispheres. Throughout this whole course, the ACA provides deep and cortical branches; these arise from the proximal and distal portions of ACA, respectively.[30,31]
Table 1.
Anterior cerebral artery segments and their blood supply
| Segment | Boundaries | Branches | Regions supplied | Important variants |
|---|---|---|---|---|
| A1 | Between ICA and ACoA | Medial lenticulostriate artery; ACoA; small arterial branches to perforated substance, subfrontal area, dorsal surface of optic chiasm, hypothalamus, and suprachiasmatic nucleus[20] | Caudate nucleus and anterior limb of the internal capsule, anterior hypothalamus, septum pellucidum, anterior commissure, fornix, and the anterior striatum[21] | Fenestrated A1: Rare, it is associated with aneurysms[22,23,24] |
| A2 | ACoA to the bifurcation forming the pericallosal and supramarginal arteries | Recurrent artery of Heubner (may also arrive from A1, rarely),[25] orbitofrontal artery and frontopolar artery, small arterial branches to perforated substance, subfrontal area, dorsal surface of optic chiasm, hypothalamus, and suprachiasmatic nucleus[3] | Anterior portion of caudate nucleus, Internal capsule, inferior and inferomedial surfaces of the frontal lobe including gyri recti[9] | Superior anterior CoA: An anomalous communicating vessel between the ACAs near the corpus callosum has been associated with aneurysms (book) (ii) Azygos ACA: Associated with terminal aneurysms[26,27] and holoprosencephaly[28] |
| A3 (pericallosal artery) | Pericallosal sulcus, extends around genu of corpus callosum | Superior and inferior internal parietal arteries, precuneal artery, callosal marginal artery (present only in 60% of cases)[25] | Corpus callosum, superior frontal gyrus, precuneus, and medial aspect of hemisphere above corpus callosum[29] | Contralateral hemisphere supply: In about 64% of people, A3 has branches supplying the contralateral hemisphere[30] |
| A4 and A5[5,6,7,8] | Smaller branches that go over corpus callosum | Callosal arteries (smaller arteries) | Corpus callosum |
ACoA: Anterior communicating artery, ICA: Internal carotid artery, ACAs: Anterior cerebral arteries
Anterior choroidal artery
AChA is a branch of the ICA that typically arises from the supraclinoid portion, just before the bifurcation of the middle and anterior cerebral arteries.[32] However, many anatomical variations have been found: AChA has also been found to originate from the MCA or even from the PCoA.[33,34] Although rare, still other variations have also been observed, including complete absence, as well as duplication, of AChA.[35,36] As should hardly be surprising given this variety of possible courses, the vascular territory of the AChA has been a matter of debate in recent years. Whatever its course, however, the AChA gives off both deep and superficial branches. The vascular territory of the deep branches includes the posterior two-thirds of the internal capsule, adjacent optic and auditory radiations, medial portion of the globus pallidus, and tail of the caudate nucleus; the territory of the superficial branches includes piriform cortex and uncus, hippocampal head, amygdala, and most lateral portion of the thalamic lateral geniculate nucleus.[32,37,38,39,40]
Middle cerebral artery
MCA is the largest and most complexly distributed of the cerebral vessels, supplying many critical cerebral structures along its sinuous course.[41] Neither that the MCA is the most common site of stroke,[42] nor that large-territory MCA strokes often carry a very poor prognosis,[43] should therefore come as a surprise. The artery has a relatively consistent route: variations have been found in 3.8% of patients, and have not been determined to be of clinical significance.[44] It originates from the bifurcation of the ICA, just lateral to the optic chiasm at the medial end of the Sylvian fissure,[3,33] and passes laterally from there along the ventral surface of the frontal lobe, entering the Sylvian fissure between the temporal lobe and insular cortex. Within this region, the artery typically bifurcates or trifurcates, giving rise to two or three principal trunks. These, in turn, extend superiorly and inferiorly over the insular surface, supplying, by means of an arterial arborization that ultimately extends over most of the lateral surface of the cerebral hemisphere, the following cortical territories: all of the insular cortex and opercular surface, the superior and middle temporal gyri, a parietal territory that comprehends the inferior parietal lobule and much of the postcentral gyrus, and a frontal territory that comprehends inferior and middle frontal gyri, much of the precentral gyrus, and the lateral part of the orbital surface. Just as has been seen above for the sake of rendering a large and sinuous vascular route more comprehensible in the case of the ACA, these many MCA territories have been partitioned into segments. The names of these segments, their paths, branches, and the structures they supply are shown in [Table 2].
Table 2.
Middle cerebral artery segments and their supply
| Segment | Anatomical path | Branches | Areas supplied |
|---|---|---|---|
| M1 (horizontal) | Originates at carotid bifurcation, becomes middle cerebral artery, and branches turn superiorly into the area between temporal lobe and insula | Medial and lateral lenticulostriate arteries (15-17 in number) and anterior temporal artery | Head and body of the caudate nucleus, the upper part of the anterior limb, the genu and anterior portion of the posterior limb of internal capsule, the putamen and the lateral pallidum and anterior temporal lobe |
| M2 (insular) | Entry point at temporal lobe and insula, ascends along the insular cleft and makes a hairpin turn at the insular sulcus | Terminal branches: 2-3 main trunks Superior division: Orbitofrontal artery, prefrontal artery, precentral artery, and central arteries Inferior division: Temporopolar artery, temporo-occipital artery, angular artery and anterior, middle and posterior temporal arteries | Superior division: Orbitofrontal area to the posterior parietal love Inferior division: Temporal pole to the angular area of parietal lobe |
| M3 (opercular) | Begins at the apex of the hairpin turn in the insular sulcus and terminates as the branches reach the lateral convexity of the cerebral hemisphere | Terminal branches/2-3 main trunks: Upper and lower trunks | Frontal, parietal, and temporal operculae |
| M4 (cortical) | Begins at the surface of the Sylvian fissure, extends over cerebral hemisphere and arises between frontal, parietal and temporal lobes[3,35] | Cortical branches | Hemispheric surface of frontal and parietal lobes[45] |
The Posterior Cerebral Circulation
Although it provides only about one-third of the total flow perfusing the brain, the posterior cerebral circulation still maintains many of the nervous system's most critical functions. Also known as the vertebrobasilar circulation, it is comprised of the vertebral arteries, the basilar artery into which the vertebrals fuse, and several branches from these main conduits to be detailed below.[36] This circulation may be broadly considered to supply blood to the posterior portion of the brain that includes the occipital lobe, most of the anterior and posterior portions of the brainstem, and all of the cerebellum.[3,36] Beginning with its origin in the vertebral arteries, we will now provide an anatomically organized overview of this circulatory division. Because it is a less frequent focus of cerebrovascular pathology, accounting for only 20% of ischemic strokes,[48] our survey will be brisker than what was presented of the anterior circulation. Nevertheless, there are many features of pathological interest in this circulation, and they will be described below.
Vertebral arteries
As introduced above, these are paired, bilaterally symmetrical arteries that arise from the subclavian vessels on each side of the body. Like the MCA, they have been partitioned, for the sake of organizational clarity, into four segments. Unlike MCA, the first three of these segments are extracranial. The most proximal segment, V1, extends from the vessels’ subclavian origin to the vertebral transverse foramen, and is, along with the distal V4, the most common site of vertebral artery infarction.[49] The succeeding V2 segment then courses through the transverse foramen. V3, which has already been discussed for the predisposition to hemorrhage possessed by its shortened variations, loops from approximately the level of C2 around the atlas and then enters the dura. Once through the dura, the arteries become V4 – this is the intracranial segment of the vertebral arteries. Running from its dural entrance to the rostral medulla or caudal pons, this segment has many vascular tasks. Immediately after entering the brain through the foramen magnum and prior to its anastomosis at the caudal pontine level, it gives rise to two important branches. The first of these is the posterior inferior cerebellar artery (PICA).[36] Second, and immediately proximal to the anastomosis, each V4 gives rise to a small branch that joins its contralateral counterpart to form the descending, midline anterior spinal artery. There is also a set of posterior spinal arteries. These may arise either from PICA or from the vertebral arteries, but will not be discussed further, due to the relatively small brainstem territory they supply, as well as the rarity and variability characteristic of lesions in these vessels.[50] There are, by contrast, several structural and functional features of PICA and the anterior spinal artery that bear discussion. These are now summarized.
Posterior inferior cerebellar artery
PICA is the largest branch of the vertebral artery. After splitting off from its vertebral origin, it winds posteriorly around the upper medulla, passes between the origins of the vagus and accessory nerves, and then proceeds along the inferior cerebellar peduncle to reach the ventral surface of the cerebellum, where it divides into medial and lateral branches. The artery provides blood to the part of the medulla and cerebellum corresponding to its course: the dorsolateral region of the medulla and a region of the ventral surface of the cerebellar hemispheres that includes the inferior vermis.[3,36] PICA lesions produce the symptoms consistent with this distribution, including ipsilateral loss of facial pain and temperature sensation, contralateral loss of the same in the body, cerebellar ataxia, and bulbar palsy. This collection of symptoms composes the classical neurological syndrome known as Wallenberg's syndrome.[51]
Anterior spinal artery
As already discussed, the anterior spinal artery arises as a midline anastomosis of two small branches from the vertebral arteries. From this anastomosis, the artery proceeds within the anterior median fissure down the whole length of the spinal cord. Before doing so, however, it rests on and supplies a region in the anterior medulla. Consequently, like PICA, anterior spinal artery lesions are associated with a characteristic neurological syndrome, with symptoms consistent with hypoperfusion of the medullary regions normally supplied by this vessel.[52]
Basilar artery
After its anastomosis at the pontomedullary junction, the paired V4 segments give way to a single, massive basilar artery that travels along the midline anterior pons, and terminates near the pontomesencephalic junction. During its course, the artery branches prolifically, providing several perforating arteries (classified by distribution as either paramedian or circumferential) to the pons, the anterior inferior cerebellar and superior cerebellar arteries (AICAs and SCAs, respectively) to a broad cerebellar territory, and then supplies much of the posterior cortical surface through its terminal split into the posterior cerebral arteries (PCAs).[3,36,39] As is readily appreciable due to the vastness of this vascular area, lesions of the basilar artery are, although uncommon, very grave in prognosis: a mortality rate of 85% has been reported of basilar artery occlusions.[53] The symptomology of survivors, moreover, can also be quite grave. A condition known as locked-in syndrome can occur, in which patients are quadriplegic and incapable of either speech or horizontal eye movements, but are nevertheless conscious.[54] Lesions of individual basilar branches can also, of course, occur, giving rise to a clinical presentation circumscribed to the function of the affected region. We briefly discuss the distribution of these branches.
Perforating branches of the basilar artery (pontine branches)
These arteries are small, numerous, basilar branches that collectively provide a substantial contribution to the complex vascular territory of the pontine brainstem. When categorized according to distribution, they fall into two classes. The first class, referred to as paramedian, extends immediately from the basilar artery into the substance of the pons and supplies structures located relatively close to midline, such as the corticospinal tract. The second class is called circumflex, due to its more lateral distribution, which extends its longest branches all the way around to the dorsal aspect of the pons as posterior pontine arteries.[55,56]
Anterior inferior cerebellar artery
This artery is the first large branch of the basilar artery and has an analogous direction of course to the more caudally positioned PICA. It generally proceeds from the caudal one-third of the basilar artery, traveling laterally along the middle cerebellar peduncle to reach the cerebellum. In addition to typically supplying the petrosal surface of the cerebellum,[57] it also supplies a subregion of the pontine brainstem that includes the middle cerebellar peduncle,[56] and the central portions of several sensory pathways, as evidenced by the ability of AICA lesions to mimic schwannomas of the vestibular, facial, and trigeminal nerves. AICA also usually gives rise to the labyrinthine artery of the inner ear, which perhaps helps account for the deafness lesions in this artery can also produce.[58,59]
Superior cerebellar artery
This artery may be thought of as, like AICA, another PICA analog. The final, nonterminal branch of the basilar artery, SCA runs laterally over the superior cerebellar peduncle, supplying the peduncle itself and, by way of spreading medial and lateral branches, much of the superior surface of the cerebellum. It also supplies the deep nuclei embedded within the cerebellar white matter, and a brainstem region adjacent to the rostral pontine tegmentum.[56,59]
Posterior cerebral artery
The final posterior circulation contribution to consider is that provided by the posterior cerebral arteries. These arteries usually arise bilaterally from the terminal bifurcation of the basilar arteries but have been found to originate unilaterally at the ICA in between 11% and 29% of cases examined. This is a clinically consequential variant: it can mimic cerebrovascular pathology on perfusion MRI due to an asymmetric signature.[60] In any case, after splitting from the basilar artery or otherwise, the PCA encircles the midbrain at the pontomesencephalic junction, immediately rostral to the root of the third (oculomotor) cranial nerve. Along its posterior passage, it travels over the cerebral peduncles, and thence to the ventromedial surface of the cortex, thus through an elaborate pattern of branches constituting a major source of blood to the occipital lobe, the inferior and medial parts of the temporal lobe, and a posterior portion of the inferior parietal lobe. As has been the case in every example mentioned above, the cortical symptoms produced by PCA infarcts are consistent with the functions of the artery's territory: most commonly (84%–100%), such infarcts produce visual field deficits, but alexia and agnosias can also occur, such as, owing to fusiform gyrus dysfunction, prosopagnosia.[61,62] PCA also has a substantial subcortical territory that covers the thalamus, midbrain, and choroid plexus; deep areas have been found to be involved in almost 30% of pure PCA infarcts.[63] Like the ACA and MCA of the anterior circulation, the PCA has been divided into segments by location along its extent, but we will not discuss these further. The interested reader may refer to our sources.[40,41,59]
The Microcirculation and Blood–brain Barrier
Having broadly overviewed the macrocirculatory components of the cerebrovascular system, we proceed now to discuss the cerebral microcirculation. Recall that the microcirculation was defined above as the endpoint interface of the macrocirculation with the cerebrum: the site of the exchange of gases and nutrients that accomplishes the formidable task of maintaining the totality of cerebral function in working order. In this section, we will first discuss the main structural components that make this exchange possible. This requires the detailed description of the BBB presented, which includes presentations of the mechanisms by which the transport of water and other molecules to the cerebrum is regulated. In Part II of our review series, we will draw upon the microcirculatory anatomy presented here for an account of the paradigmatic consequence of microcirculatory dysfunction: cerebral edema.[64]
Microcirculatory Function
Neuronal activity is, of course, intimately linked with cellular metabolism and cerebral blood flow: even minor interruptions in flow through the cerebrovascular system can adversely affect cognitive function.[65] The whole cerebrovascular system is thus subjected to the tight regulation necessary to ensure concordance between neuronal metabolic needs, on the one hand, and the blood flow that underwrites these needs, on the other.
The brain's microcirculatory system is at the core of its regulatory apparatus. This system spans the arterial and venous circulations and consists of three components: parenchymal arterioles (diameter 30 μm; wall thickness 6 μm), capillaries (diameter 8 μm; wall thickness 0.5 μm), and venules (diameter 20 μm; wall thickness 1 μm). The capillary bed that intervenes between the system's two ends consists of the dense network of miniscule vessels that represents the primary site of oxygen, nutrient, and metabolite exchange. Flow through this central region is regulated by muscular actions at its arterial and venous ends: contraction and dilation of precapillary arterioles and postcapillary venules. By this mechanism, flow through the capillaries is made highly dynamic, capable of velocities ranging from 0.3 mm/s to 3.2 mm/s.[66,67] The resultant heterogeneity in capillary flow of which the system is capable reflects the importance of local regulation. Unfortunately, this tightly regulated system is prone to injury, especially during cerebral ischemia.
Due to its role in several cerebral diseases (including cerebral ischemia), the BBB is arguably the most important component of the microcirculatory system. It is certainly the most studied. The BBB is present throughout the microcirculation's regulatory system, in the arterioles, capillaries, and venules described above. It is both a selective barrier and a transport apparatus, formed chiefly by endothelial cells that are interlocked by tight junctions and buttressed by pericytes, astrocytes, and a basal lamina; most of the transport it facilitates occurs by the way of a transcellular route through its endothelial cells. Small gaseous molecules (such as O2 and CO2) and small lipophilic agents (such as barbiturates and ethanol) simply diffuse through the lipid membrane of these cells.[68] Transport of hydrophilic molecules, on the other hand, is controlled as it is peripherally: by specific endothelial transporters [Figure 4]. In contrast to the endothelia of other organ systems, however, the cerebral endothelium has a much lower degree of endocytotic and transcytotic activity.[69] It also expresses lower levels of leukocyte adhesion molecules than are seen in peripheral endothelia. This helps inhibit immune cell infiltration into healthy central nervous system parenchyma.[70]
Figure 4.
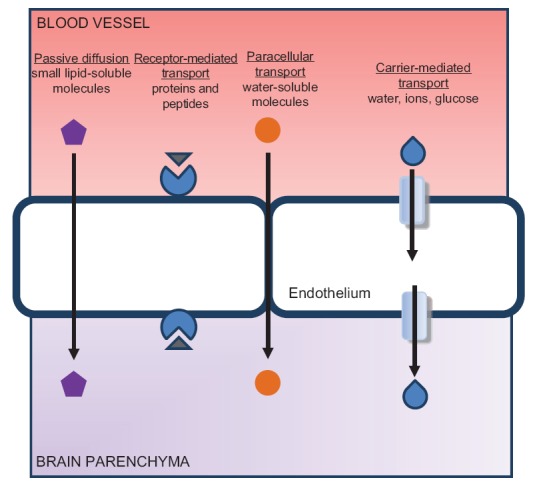
The regulation of transport across the blood–brain barrier
In the context of selective transport, it is important to highlight the role of tight junctions. Tight junctions confer low paracellular permeability and are vulnerable to damage during primary cerebral ischemia, and also during the secondary inflammatory response that follows primary ischemia. Tight junctions are composed of adhesion molecules-1,[71] occludins,[72,73,74] claudins,[75] and membrane-associated guanylate kinase-like proteins (ZO-1, ZO-2, and ZO-3).[76] ZO-1, because it links transmembrane proteins of the tight junction to the endothelial cytoskeleton, plays an especially important role in the pathogenesis of cerebral edema. The dissociation of ZO-1 is associated with increased BBB permeability.[77,78] In addition to tight junctions, pericytes, astrocytes, and a basal lamina also serve to stabilize the BBB, as noted above. Pericytes regulate BBB permeability, angiogenesis, metabolite clearance, and capillary blood flow.[79,80] The extracellular matrix, comprised fibronectin, laminin, and type IV collagen, serves as an anchor for the endothelium, playing a key role in BBB integrity.[81,82,83] Astrocytes stabilize the BBB, closing the gaps between tight junctions.[84,85] Furthermore, astrocytic end feet contain high concentrations of surface transporters and receptors that regulate the brain's extracellular solute concentration and water content. This is critical to the formation and resolution of cerebral edema.
The Cerebral Venous System
Once it proceeds through the capillary beds of the cerebral microcirculation, and is modified according to the demand set by cerebral metabolism (that is, stripped of O2 and nutrients and loaded with CO2 and metabolites), cerebral blood enters the venous circulation. Continuing along our anatomically organized tour of the cerebral circulation, we will now therefore discuss this circulation. The structures that compose this circulation show greater anatomical variations than the brain's arterial structures. Cerebral veins often differ not only between individuals but also between the two hemispheres in the brain of a single individual. This high degree of variability makes the classification of the cerebral veins difficult. Nevertheless, some general patterns can be described, and understanding these patterns is crucial for determining and treating intracranial pathologies (e.g., cerebral venous thrombosis [CVT] and brain tumor) related to these structures. Thus, they are reviewed below, and central venous thrombosis is briefly presented as a pathological process that illustrates the distinct physiological properties of the venous system.
The cerebral venous system can be divided into two anastomosing networks according to position with respect to the cortical surface: the more superficially located dural venous sinuses, and the deeper cerebral veins.[86] Dural venous sinuses are endothelially lined channels, the general function of which may be considered the collection of venous blood from the cerebral veins, and the delivery of this blood to the systemic venous circulation.[59,87] They are usually formed between the outer (periosteal) and inner (meningeal) layers of dura mater, located adjacent to the osseous surfaces inside the cranium. The confluence of sinuses (CS) is one of the major sinuses, and may be thought of as a pooling point for venous blood destined, by the way of carotid bulb, for the systemic circulation. It is located at the occipital pole of the cranial cavity and is formed by the junction of several of the other sinuses. These include the superior sagittal sinus that runs along the calvaria through the falx cerebri; the straight sinus that represents the continuation of the inferior sagittal sinus and great cerebral vein of Galen;[9] the occipital sinus just inferior to the CS; and the transverse sinuses that, by way of the sigmoid sinuses, traverse the base of the occipital bone to connect the CS with the jugular blub.[87] The inferior sagittal sinus runs in the inferior concave free border of the cerebral falx, thus constituting an exception to the general rule that the sinuses are composed of both meningeal and periosteal dura. As mentioned, this sinus continues as the straight sinus, running between the falx cerebri and tentorium cerebelli to join the CS.
Another important component of the dural sinus system is composed by the bilateral cavernous sinuses. Located superior to the body of the sphenoid bone and demarcated by the superior orbital fissure anteriorly, the temporal bone posterity, and the sella turcica medially, these sinuses receive blood from the superior and inferior ophthalmic veins, superficial middle cerebral veins, sphenoparietal sinuses, and inferior cerebral veins.[88] Because they also serve as major pooling points and are also in communication with the jugular vein, these sinuses may be conceived as the anterior equivalents of the occipitally located CS. Two intercavernous sinuses (an anterior and a posterior) connect the two cavernous sinuses across the midline. Two sets of petrosal sinuses then drain the cavernous sinuses: the superior petrosal sinuses exit posteriorly, and travel superiorly along the petrous part of the temporal bone to connect with the sigmoid/transverse sinuses, while the inferior petrosal sinuses exit inferiorly, and traverse the petrobasilar suture to drain directly into the internal jugular veins.[87] The inferior petrosal sinuses are interconnected by the basilar venous plexus. The sigmoid sinus is a continuation of the transverse sinuses that passes inferiorly in an “S”-shaped groove posteromedial to the jugular foramen, and serves to channel cerebral venous blood to the internal jugular vein.
Despite the impression that the preceding description may have given, however, the internal jugular vein is not the only manner by which the dural sinus system communicates with the extracranial venous system. There are many emissary veins that serve this purpose, permitting anastomosis between the extracranial veins of the face and scalp. These anastomoses are clinically significant: because the emissary veins lack valves, they permit the retrograde flow of blood from superficial structures into the brain, thus permitting superficial infections to cause infections of the brain.[89] Other channels exist as well. The vertebral venous plexus, for example, receives blood from both the inferior petrosal sinus and the occipital sinus.[9,87]
While the dural sinus system may be broadly understood as the outlet for cerebral venous blood, it is the cerebral veins that directly drain the brain's parenchyma. Once they have done so, they then pierce the meninges and continue as bridging veins into the cranial venous sinuses to exit the cranial cavity as described above. The cerebral veins can be divided into superficial (cortical) cerebral veins, and deep (cerebral) veins. The superficial cerebral veins course along the cortical sulci and drain the cortex of the brain. Most cortical veins are unnamed; exceptions are made, however, for a few large cortical veins that anastomose directly with the sinus system, such as superficial middle cerebral veins, superior and inferior anastomotic veins, and superior and inferior cerebral veins.
The deep cerebral veins, which are responsible for draining deep white matter and gray matter structures surrounding the lateral ventricles, third ventricle, and interpeduncular cistern, are distinguished from the superficial veins both by position and by drainage polarity: while the superficial veins drain centrifugally toward the lateral parts of the sinus system, the deep veins drain centripetally, converging at midline as the great cerebral vein of Galen.[9] These veins have several named vessels among their number, including the internal cerebral veins, the basal veins of Rosenthal, and the great cerebral vein of Galen just mentioned. The regionally named choroid veins, septal veins, and thalamostriate veins join to form the internal cerebral veins. The choroid veins receive blood from the hippocampus, fornix, and corpus callosum. The septal veins receive blood from the septum pellucidum and corpus callosum. The thalamostriate veins receive blood from the longitudinal caudate veins. The two internal cerebral veins then run beneath the splenium of the corpus callosum until joining in this region to form the great cerebral vein of Galen. The basal veins of Rosenthal are formed by the union of the anterior cerebral veins, deep middle cerebral veins (deep Sylvian vein), and striate veins. These veins then travel posteriorly around the cerebral peduncle to drain into the great cerebral vein of Galen, which, in turn, curves posteriorly to drain into the straight sinus.
Both the cerebral veins and sinuses have neither valves nor a tunica muscularis layer, and the cerebral veins are linked by numerous anastomoses. These qualities give the venous system a substantial compensatory capacity in the event of a major occlusion. The result of this compensatory capacity is that CVT is an uncommon cause of cerebral infarction relative to arterial disease: it is responsible for only 0.5%–1% of strokes.[90] Still, it is an important consideration both because of its potential for morbidity and its effect on the younger population (age <50 years). Based on a study of 624 patients, oral contraceptives (54.3%), hereditary prothrombotic conditions (e.g., antithrombin III deficiency, protein C and S deficiencies) (34.1%), pregnancy (21%), parameningeal infections (12.3%), cancer (7.4%), and antiphospholipid antibodies (5.9%) account for the majority of the risk factors of CVT.[91] The use of oral contraception contributes to the predominance of females affected by the disease. Amoozegar et al. found that third-generation oral contraceptives (desogestrel-, gestodene-, and norgestimate-containing drugs) have the greatest capacity to induce CVT.[92,93] Furthermore, the combination of oral contraceptive use with a prothrombotic condition dramatically increases the risk of CVT.[93,94] The main cerebral venous sinuses affected by CVT are the superior sagittal sinus (72%) and the transverse sinuses (70%).[95] The current treatment of choice includes intravenous heparin,[95,96] thrombolysis,[97] and oral anticoagulation.[46,47,95]
Conclusion
In this review of the cerebral circulatory system and cerebrovascular disease, we sought to recapitulate the complex cerebral circulation. In doing so, we attempted to interweave several of the more salient pathological consequences of disruptions in this system. This involved discussing some of the classical stroke syndromes along with the arterial anatomy, a discussion of BBB components that may produce edema when disturbed along with our presentation of microcirculatory anatomy, and a discussion of the compensatory capacity possessed by the venous anatomy against central venous thrombosis.
With the anatomical context thus established, we will proceed, in Part II of our series,[64] with an introduction to the major categories of cerebrovascular disease mechanisms: those that are intrinsic to the vessel, those that are extrinsic, cerebral hypoperfusion, and cerebral hemorrhage. By combining this information with the anatomical context established above, we hope to supply the physiological background essential to a working understanding of the vascular pathology of the brain. In Part III of our review series,[17] we will seek to enrich this understanding through a detailed presentation of its most common and most (rightfully) reviled instantiation: stroke.
Financial support and sponsorship
This work was partially supported by the American Heart Association Grant-in-Aid (14GRNT20460246) (YD), Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service (YD), the National Natural Science Foundation of China (81501141) (XG), and Beijing NOVA program (xx2016061) (XG)
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fehm HL, Kern W, Peters A. The selfish brain: Competition for energy resources. Prog Brain Res. 2006;153:129–40. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 2.Drake R, Vogl AW, Mitchell AW. Gray's Anatomy for Students. 3rd ed. xxv. Philadelphia, PA: Churchill Livingstone/Elsevier; 2015. p. 1161. [Google Scholar]
- 3.Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. London, United Kingdom: Elsevier Health Sciences; 2015. [Google Scholar]
- 4.Eskander MS, Drew JM, Aubin ME, Marvin J, Franklin PD, Eck JC, et al. Vertebral artery anatomy: A review of two hundred fifty magnetic resonance imaging scans. Spine (Phila Pa 1976) 2010;35:2035–40. doi: 10.1097/BRS.0b013e3181c9f3d4. [DOI] [PubMed] [Google Scholar]
- 5.Medcalf JE, Paul Johnson C, Taktak A, Grabherr S. Variations in the anatomy of the vertebral artery cervical loop segment – A potential predisposing factor for traumatic basal subarachnoid hemorrhage? Forensic Sci Med Pathol. 2016;12:444–50. doi: 10.1007/s12024-016-9819-4. [DOI] [PubMed] [Google Scholar]
- 6.Manbachi A, Hoi Y, Wasserman BA, Lakatta EG, Steinman DA. On the shape of the common carotid artery with implications for blood velocity profiles. Physiol Meas. 2011;32:1885–97. doi: 10.1088/0967-3334/32/12/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta B, Yadav R, Singhal M, Kadam N, Gehlot KB, Singh R. A rare case report of bilateral internal carotid artery hypoplasia in postpartum female: Clinical spectrum and role of various modalities in diagnosis. Brain Circ. 2016;2:99–103. doi: 10.4103/2394-8108.186286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li WA, Geng X, Ding Y. Stroke is a global epidemic: New developments in clinical and translational cerebrovascular diseases research. Neurol Res. 2017;39:475–6. doi: 10.1080/01616412.2017.1330307. [DOI] [PubMed] [Google Scholar]
- 9.Schaller B. Physiology of cerebral venous blood flow: From experimental data in animals to normal function in humans. Brain Res Brain Res Rev. 2004;46:243–60. doi: 10.1016/j.brainresrev.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Kiliç T, Akakin A. Anatomy of cerebral veins and sinuses. Front Neurol Neurosci. 2008;23:4–15. doi: 10.1159/000111256. [DOI] [PubMed] [Google Scholar]
- 11.Weller RO, Preston SD. The spectrum of vascular disease in dementia. From ischaemia to amyloid angiopathy. Adv Exp Med Biol. 2001;487:111–22. doi: 10.1007/978-1-4615-1249-3_9. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran S. Imaging markers of stroke risk in asymptomatic carotid artery stenosis. Brain Circ. 2015;1:38–46. [Google Scholar]
- 13.van Seeters T, Hendrikse J, Biessels GJ, Velthuis BK, Mali WP, Kappelle LJ, et al. Completeness of the circle of Willis and risk of ischemic stroke in patients without cerebrovascular disease. Neuroradiology. 2015;57:1247–51. doi: 10.1007/s00234-015-1589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty ML, Woo D, Haverbusch M, Sekar P, Khoury J, Sauerbeck L, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36:934–7. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 15.Purves D, et al. Neuroscience. Sunderland, Massachusetts: Sinauer Associates; 2008. [Google Scholar]
- 16.Djulejic V, Marinkovic S, Milic V, Georgievski B, Rašic M, Aksic M, et al. Common features of the cerebral perforating arteries and their clinical significance. Acta Neurochir (Wien) 2015;157:743–54. doi: 10.1007/s00701-015-2378-8. [DOI] [PubMed] [Google Scholar]
- 17.Chandra A, Stone CR, Du X, Li WA, Huber M, Bremer R, et al. The cerebral circulation and cerebrovascular disease III: Stroke. Brain Circ. 2017;3:66–77. doi: 10.4103/bc.bc_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015;35:648–54. doi: 10.1038/jcbfm.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Kang M, Choi JH, Kim DW. Safety of coil occlusion of the parent artery for endovascular treatment of anterior communicating artery aneurysm. Neuroradiol J. 2016;29:201–7. doi: 10.1177/1971400916639604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Freitas GR, Christoph DDeH, Bogousslavsky J. Topographic classification of ischemic stroke. Handb Clin Neurol. 2009;93:425–52. doi: 10.1016/S0072-9752(08)93022-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoksbergen AW, Legemate DA, Csiba L, Csáti G, Síró P, Fülesdi B. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis. 2003;16:191–8. doi: 10.1159/000071115. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. A study of 38 cases. J Neurosurg. 1992;76:455–8. doi: 10.3171/jns.1992.76.3.0455. [DOI] [PubMed] [Google Scholar]
- 23.Minakawa T, Kawamata M, Hayano M, Kawakami K. Aneurysms associated with fenestrated anterior cerebral arteries. Report of four cases and review of the literature. Surg Neurol. 1985;24:284–8. doi: 10.1016/0090-3019(85)90040-0. [DOI] [PubMed] [Google Scholar]
- 24.Choudhari KA. Fenestrated anterior cerebral artery. Br J Neurosurg. 2002;16:525–9. doi: 10.1080/0268869021000030979. [DOI] [PubMed] [Google Scholar]
- 25.Stefani MA, Schneider FL, Marrone AC, Severino AG, Jackowski AP, Wallace MC. Anatomic variations of anterior cerebral artery cortical branches. Clin Anat. 2000;13:231–6. doi: 10.1002/1098-2353(2000)13:4<231::AID-CA1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.Cinnamon J, Zito J, Chalif DJ, Gorey MT, Black KS, Scuderi DM, et al. Aneurysm of the azygos pericallosal artery: Diagnosis by MR imaging and MR angiography. AJNR Am J Neuroradiol. 1992;13:280–2. [PMC free article] [PubMed] [Google Scholar]
- 27.Huber P, Braun J, Hirschmann D, Agyeman JF. Incidence of berry aneurysms of the unpaired pericallosal artery: Angiographic study. Neuroradiology. 1980;19:143–7. doi: 10.1007/BF00342389. [DOI] [PubMed] [Google Scholar]
- 28.Osaka K, Matsumoto S. Holoprosencephaly in neurosurgical practice. J Neurosurg. 1978;48:787–803. doi: 10.3171/jns.1978.48.5.0787. [DOI] [PubMed] [Google Scholar]
- 29.Harrigan MR, Deveikis JP. Handbook of Cerebrovascular Disease and Neurointerventional Technique. 2nd ed. xvii. Dordecht: Humana Press; 2013. Contemporary medical imaging; p. 850. [Google Scholar]
- 30.Perlmutter D, Rhoton AL., Jr Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204–28. doi: 10.3171/jns.1978.49.2.0204. [DOI] [PubMed] [Google Scholar]
- 31.Marino R., Jr The anterior cerebral artery: I. Anatomo-radiological study of its cortical territories. Surg Neurol. 1976;5:81–7. [PubMed] [Google Scholar]
- 32.Palomeras E, Fossas P, Cano AT, Sanz P, Floriach M. Anterior choroidal artery infarction: A clinical, etiologic and prognostic study. Acta Neurol Scand. 2008;118:42–7. doi: 10.1111/j.1600-0404.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 33.Rhoton AL, Jr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171–87. [PubMed] [Google Scholar]
- 34.Herman LH, Fernando OU, Gurdjian ES. The anterior choroidal artery: An anatomical study of its area of distribution. Anat Rec. 1966;154:95–101. doi: 10.1002/ar.1091540109. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter MB, Noback CR, Moss ML. The anterior choroidal artery; its origins course, distribution, and variations. AMA Arch Neurol Psychiatry. 1954;71:714–22. doi: 10.1001/archneurpsyc.1954.02320420042005. [DOI] [PubMed] [Google Scholar]
- 36.Hussein S, Renella RR, Dietz H. Microsurgical anatomy of the anterior choroidal artery. Acta Neurochir (Wien) 1988;92:19–28. doi: 10.1007/BF01401968. [DOI] [PubMed] [Google Scholar]
- 37.Ghika JA, Bogousslavsky J, Regli F. Deep perforators from the carotid system. Template of the vascular territories. Arch Neurol. 1990;47:1097–100. doi: 10.1001/archneur.1990.00530100063014. [DOI] [PubMed] [Google Scholar]
- 38.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: Cerebral hemispheres. Neurology. 1998;50:1699–708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 39.Hamoir XL, Grandin CB, Peeters A, Robert A, Cosnard G, Duprez T. MRI of hyperacute stroke in the AChA territory. Eur Radiol. 2004;14:417–24. doi: 10.1007/s00330-003-2220-1. [DOI] [PubMed] [Google Scholar]
- 40.Feekes JA, Hsu SW, Chaloupka JC, Cassell MD. Tertiary microvascular territories define lacunar infarcts in the basal ganglia. Ann Neurol. 2005;58:18–30. doi: 10.1002/ana.20505. [DOI] [PubMed] [Google Scholar]
- 41.Figueroa B, Clark J, Ellens N. The use of barbiturate-induced coma during cerebrovascular neurosurgery procedures: A review of the literature. Brain Circ. 2015;1:140–5. [Google Scholar]
- 42.Sada S, Reddy Y, Rao S, Alladi S, Kaul S. Prevalence of middle cerebral artery stenosis in asymptomatic subjects of more than 40 years age group: A transcranial doppler study. Neurol India. 2014;62:510–5. doi: 10.4103/0028-3886.144443. [DOI] [PubMed] [Google Scholar]
- 43.Walcott BP, Miller JC, Kwon CS, Sheth SA, Hiller M, Cronin CA, et al. Outcomes in severe middle cerebral artery ischemic stroke. Neurocrit Care. 2014;21:20–6. doi: 10.1007/s12028-013-9838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchino A, Kato A, Takase Y, Kudo S. Middle cerebral artery variations detected by magnetic resonance angiography. Eur Radiol. 2000;10:560–3. doi: 10.1007/s003300050960. [DOI] [PubMed] [Google Scholar]
- 45.Bradley WG. Neurology in Clinical Practice. 3rd ed. Boston: Butterworth-Heinemann; 2000. [Google Scholar]
- 46.Ferro JM, Canhão P. Cerebral venous sinus thrombosis: Update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. doi: 10.1007/s11886-014-0523-2. [DOI] [PubMed] [Google Scholar]
- 47.Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5:332–41. [PubMed] [Google Scholar]
- 48.Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: A review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. doi: 10.3389/fneur.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–24. doi: 10.1161/01.cir.43.5.711. [DOI] [PubMed] [Google Scholar]
- 50.Sakurai T, Wakida K, Nishida H. Cervical posterior spinal artery syndrome: A case report and literature review. J Stroke Cerebrovasc Dis. 2016;25:1552–6. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Kato S, Takikawa M, Ishihara S, Yokoyama A, Kato M. Pathologic reappraisal of wallenberg syndrome: A pathologic distribution study and analysis of literature. Yonago Acta Med. 2014;57:1–14. [PMC free article] [PubMed] [Google Scholar]
- 52.Kiloh LG. The syndromes of the arteries of the brain and spinal cord. II. Postgrad Med J. 1953;29:119–27. doi: 10.1136/pgmj.29.329.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mak CH, Ho JW, Chan KY, Poon WS, Wong GK. Intra-arterial revascularization therapy for basilar artery occlusion-a systematic review and analysis. Neurosurg Rev. 2016;39:575–80. doi: 10.1007/s10143-015-0693-4. [DOI] [PubMed] [Google Scholar]
- 54.Pearce JM. The locked in syndrome. Br Med J (Clin Res Ed) 1987;294:198–9. doi: 10.1136/bmj.294.6566.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stopford JS. The arteries of the pons and medulla oblongata. J Anat Physiol. 1916;50(Pt 2):131–64. [PMC free article] [PubMed] [Google Scholar]
- 56.Bassetti C, Bogousslavsky J, Barth A, Regli F. Isolated infarcts of the pons. Neurology. 1996;46:165–75. doi: 10.1212/wnl.46.1.165. [DOI] [PubMed] [Google Scholar]
- 57.Savoiardo M, Bracchi M, Passerini A, Visciani A. The vascular territories in the cerebellum and brainstem: CT and MR study. AJNR Am J Neuroradiol. 1987;8:199–209. [PMC free article] [PubMed] [Google Scholar]
- 58.Marchini AK, Mosimann PJ, Guichard JP, Boukobza M, Houdart E. Anterior inferior cerebellar artery aneurysms mimicking vestibular schwannomas. J Neuroimaging. 2014;24:404–6. doi: 10.1111/j.1552-6569.2012.00752.x. [DOI] [PubMed] [Google Scholar]
- 59.Haines DE, Ard MD. Clinical key Flex. Fundamental Neuroscience for Basic and Clinical Applications. xi. Philadelphia, PA: Elsevier/Saunders; 2013. p. 492. [Google Scholar]
- 60.Wentland AL, Rowley HA, Vigen KK, Field AS. Fetal origin of the posterior cerebral artery produces left-right asymmetry on perfusion imaging. AJNR Am J Neuroradiol. 2010;31:448–53. doi: 10.3174/ajnr.A1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinaud O, Pouliquen D, Gérardin E, Loubeyre M, Hirsbein D, Hannequin D, et al. Visual agnosia and posterior cerebral artery infarcts: An anatomical-clinical study. PLoS One. 2012;7:e30433. doi: 10.1371/journal.pone.0030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sacks O. The man who mistook his wife for a hat. Br J Psychiatry. 1995;166:130–1. doi: 10.1192/bjp.166.1.130. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto Y, Georgiadis AL, Chang HM, Caplan LR. Posterior cerebral artery territory infarcts in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1999;56:824–32. doi: 10.1001/archneur.56.7.824. [DOI] [PubMed] [Google Scholar]
- 64.Chandra A, Stone CR, Li WA, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease II: Pathogenesis of cerebrovascular disease. Brain Circ. 2017;3:57–65. doi: 10.4103/bc.bc_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen Q, Duong TQ. Magnetic Resonance Imaging of Cerebral Blood Flow in Animal Stroke Models. Brain Circ. 2016;2:20–7. doi: 10.4103/2394-8108.178544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: An engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab. 2012;32:1167–76. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardridge WM. Blood-brain barrier drug targeting: The future of brain drug development. Mol Interv. 2003;3:90–105. doi: 10.1124/mi.3.2.90. 51. [DOI] [PubMed] [Google Scholar]
- 70.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 71.Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int J Dev Biol. 2000;44:743–8. [PubMed] [Google Scholar]
- 72.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–9. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 73.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, et al. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 74.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: Structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–24. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 77.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 78.Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91:2525–38. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- 79.Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–9. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- 80.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 81.Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: Mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52:1572–9. doi: 10.1007/s12035-014-8952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neuwelt EA. Mechanisms of disease: The blood-brain barrier. Neurosurgery. 2004;54:131–40. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 83.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 84.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–35. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dehouck MP, Méresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro . J Neurochem. 1990;54:1798–801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 86.Bell R. Neurovascular Anatomy: A Practical Guide. Philadelphia: W.B. Saunders; 2009. pp. 233–48. [DOI] [PubMed] [Google Scholar]
- 87.Tatu L, Vuillier F, Moulin T. Chapter 13 Anatomy of the circulation of the brain and spinal cord. Handb Clin Neurol. 2009;92:247–81. doi: 10.1016/S0072-9752(08)01913-1. [DOI] [PubMed] [Google Scholar]
- 88.Yasuda A, Campero A, Martins C, Rhoton AL, Jr, de Oliveira E, Ribas GC. Microsurgical anatomy and approaches to the cavernous sinus. Neurosurgery. 2008;62(6 Suppl 3):1240–63. doi: 10.1227/01.neu.0000333790.90972.59. [DOI] [PubMed] [Google Scholar]
- 89.Kawamata T, Takeshita M, Ishizuka N, Hori T. Patent foramen ovale as a possible risk factor for cryptogenic brain abscess: Report of two cases. Neurosurgery. 2001;49:204–6. doi: 10.1097/00006123-200107000-00032. [DOI] [PubMed] [Google Scholar]
- 90.Perry JM, McCabe KK. Recognition and initial management of acute ischemic stroke. Emerg Med Clin North Am. 2012;30:637–57. doi: 10.1016/j.emc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–92. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 92.Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: Reviewing the evidence and balancing the risks. Hum Reprod Update. 1999;5:721–35. doi: 10.1093/humupd/5.6.721. [DOI] [PubMed] [Google Scholar]
- 93.Amoozegar F, Ronksley PE, Sauve R, Menon BK. Hormonal contraceptives and cerebral venous thrombosis risk: A systematic review and meta-analysis. Front Neurol. 2015;6:7. doi: 10.3389/fneur.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Bruijn SF, Stam J, Koopman MM, Vandenbroucke JP. Case-control study of risk of cerebral sinus thrombosis in oral contraceptive users and in [correction of who are] carriers of hereditary prothrombotic conditions. The Cerebral Venous Sinus Thrombosis Study Group. BMJ. 1998;316:589–92. doi: 10.1136/bmj.316.7131.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992;10:87–111. [PubMed] [Google Scholar]
- 96.Bousser MG, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis – A review of 38 cases. Stroke. 1985;16:199–213. doi: 10.1161/01.str.16.2.199. [DOI] [PubMed] [Google Scholar]
- 97.Chow K, Gobin YP, Saver J, Kidwell C, Dong P, Viñuela F. Endovascular treatment of dural sinus thrombosis with rheolytic thrombectomy and intra-arterial thrombolysis. Stroke. 2000;31:1420–5. doi: 10.1161/01.str.31.6.1420. [DOI] [PubMed] [Google Scholar]


