Abstract
In this paper, we review the cerebral circulation and cerebrovascular disease (CVD) with an overview of the major types of CVD pathogenesis. These, as categorized here, are as follows: occlusive injury intrinsic to blood vessels, occlusive injury extrinsic to blood vessels, cerebral hypoperfusion, and cerebral hemorrhage. Following an overview of each of these categories, we conclude with a discussion of cerebral edema to illustrate how the pathological origins we covered can progress clinically. The content of this paper sets the stage for the detailed, clinically oriented discussion of stroke with which our series culminates in its subsequent Part III.
Keywords: Atherosclerosis, cerebal hemorrhage, cerebral circulation, cerebral edema, cerebral emboli, cerebral hypoperfusion, cerebral vessel damage, cerebrovascular disease
Introduction
Having provided a broad, anatomical overview of the cerebral circulation in Part I of this series[1] we now resume our review with a discussion of the various pathological processes that lead to cerebrovascular disease (CVD). We hope that our reader, by drawing upon the anatomical picture already presented, begins to assemble a narrative sense of why a localized cerebrovascular insult might produce a characteristic profile of symptoms. If it is possible after reading, for example, to understand why dissection, aneurysm, or atherosclerosis of a certain branch of the anterior cerebral circulation could all produce visual deficits,[2] this review shall have served its purpose.
This part of the review will proceed according to an organizational scheme we have devised to render this complex subject maximally approachable. The CVD process will be divided into four pathogenic categories. Although this division is somewhat arbitrary, and may thus be transgressed by certain types of disease (the hemorrhagic transformation discussed later in our review series is a prominent example of such transgression), it provides a useful conceptual foundation over which to build an explanation of the clinical presentation of CVD. The four categories are as follows: occlusive pathology intrinsic to blood vessels, such as stenosis and arterial wall damage; occlusive pathology due to extrinsic causes, such as venous thrombosis and extracranial emboli; inadequate cerebral blood flow; and cerebral hemorrhage. After becoming familiar with these categories, our reader will possess the background necessary to fully appreciate the clinical presentations and treatments of stroke that are presented in Part III of this series.[3] Thereafter, we will end with a discussion of cerebral edema. This will serve as an illustration of how the pathogenic categories may proceed to produce disease.
Cerebrovascular Disease Pathogenesis: Processes Intrinsic to the Blood Vessel
Blood vessels are the conduits through which oxygen and nutrients reach all tissues of the body. Disruption of the flow of blood by intrinsic processes therefore causes disruption of the flow of oxygen and nutrients to the receiving tissues. This results in hypoxia and, if prolonged, ischemic death of the affected tissue. In the brain, there are several intrinsic processes that can cause hypoxic disease. Much in the same manner by which the major cerebral arteries are organized segmentally to facilitate organization of their function, these intrinsic processes are divided into two broad categories. These categories, arterial stenosis and damage to the arterial wall, are now introduced.
Arterial stenosis
Arterial stenosis of both the intracranial and certain extracranial arteries plays a major role in the pathogenesis of CVD.[4] Stenosis of all etiologies leads to reduced luminal surface area within these vessels, and thus to reduced blood flow to the brain. Numerous case studies and research data have demonstrated that internal carotid artery stenosis is the most common extracranial pathological process implicated in CVD.[5,6,7] Extracranial vessels from the vertebrobasilar system may also be involved in the generation of CVD; strokes of this circulation cause, as noted in Part I, around 20% of ischemic strokes worldwide.[8,9,10,11,12] In proportion of the overall CVD incidence, however, it is intracranial arterial stenosis that predominates: this has been shown to be the most common cause of all CVDs worldwide.[13,14,15] Any artery within the cranium may undergo stenosis; when large arteries (e.g., the middle cerebral artery) do so, this causes CVD, such as stroke or transient ischemic attack (TIA).[13] While there are many factors that promote the onset of such disease-producing arterial stenosis, we will highlight the three most common and significant of these: atherosclerosis, amyloidosis, and lipohyalinosis.
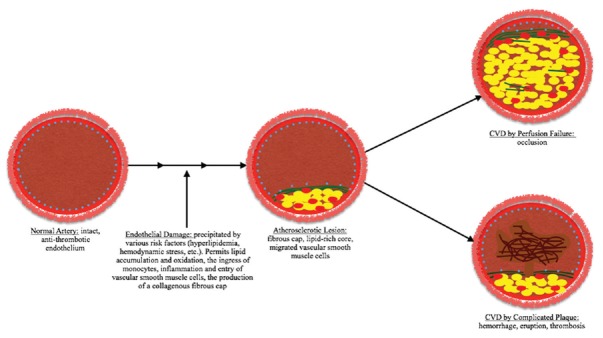
Atherosclerosis [Figure 1] is the leading cause of luminal stenosis in intracranial arteries, and is thus the leading cause of stroke worldwide.[14] There are many risk factors for the development of the atherosclerotic lesions that lead to stroke and these have been detailed elsewhere; the curious reader may review our sources.[16,17,18,19] Here, we focus on the pathogenesis of atherosclerosis. Broadly, disease is initiated by endothelial damage and dysfunction, which paves the way for the chronic, progressive narrowing process that results in stenosis of the arterial wall.[20] The precipitating endothelial damage is followed, due to its high affinity for glycoprotein molecules, by the entry and sequestration of low-density lipoprotein (LDL) particles into the subendothelial space.[21,22,23] Once within this space, LDL particles become oxidized by the action of mediators such as nitric oxide, myeloperoxidase, and 15-lipooxygenase.[21,22] This then leads to upregulation of cell adhesion molecules on the surface of the affected endothelial cells. While this upregulation serves the adaptive purpose of bringing monocytes and T-cells into the subendothelial space to clear the oxidized LDL particles, it also initiates deleterious local inflammation, whereby cytokine release attracts smooth muscle cells from the tunica media layer into the intima.
Figure 1.
The main features of atherosclerotic pathogenesis. Under nonpathogenic circumstances, an intact arterial endothelium (represented here as dots in light blue) prevents the ingress of atherosclerotic mediators. When normal endothelial function is compromised by factors such as hemodynamic stress and hyperlipidemia, however, the entry of low-density lipoprotein is facilitated. This begins the pathogenic cascade that culminates in the formation of an atherosclerotic lesion. Once formed, these lesions may continue to expand, resulting in cerebrovascular disease by occlusion. Alternatively, if possessed of certain predisposing histological features,[25] they may produce cerebrovascular disease through thrombosis or hemorrhage
A concomitant deleterious process occurs once invading monocytes become macrophages, and, through their scavenger receptors, phagocytose the oxidized LDL. First, this leads to the formation of foam cells,[21,22,24,25] which then continue to phagocytize the oxidized LDL until they die. Upon necrotic death, the foam cells release their fatty load in the extracellular space. Repeated many times, this death-and-release mechanism eventually results in the accumulation of a necrotic, lipid-rich fatty core within the vessel wall. This necrotic core is surrounded on the luminal side by the migrated smooth muscle cells and macrophages, and embedded in a matrix of Type I and Type III collagen, leading to the formation of a fibrous cap.
Thus, from the vessel lumen to the tunica adventitia of the vessel, the atherosclerotic plaque comprises a fibrous cap, a necrotic, lipid-rich core, and then the media from which smooth muscle cells have migrated.[22,24] Over time, such plaques may, as additional cholesterol accumulates within them, enlarge to protrude into the lumen. One of the two pathological consequences may then succeed protrusion. On the one hand, simple, in situ occlusion of the lumen may occur, causing CVD through perfusion failure. On the other hand, the formation of a complicated plaque (characterized by thrombosis, hemorrhage, erosion, or rupture) may occur, also leading to CVD.[21,22,26] Studies have shown that American Heart Association (AHA) Type V and Type VI plaques, which are classified as such using histological criteria developed by the AHA Committee on Vascular Lesions,[27] are typically associated with the highest risk of CVD development.[19,28]
Amyloidosis is another major etiology of stenosis leading to stroke. This group of disorders is characterized by a common cause: misfolded proteins undergo a conformational change (becoming β-pleated sheet structures) that triggers the formation, either locally or systemically, of amyloid fibrils in organs and tissues.[29] These disorders lead to CVD through the deposition of amyloid fibrils in the cerebral leptomeningeal and intracortical arteries, arterioles, and capillaries.[30,31]
Cerebral amyloid angiopathy (CAA) is one type of amyloidosis that is seen in the elderly and may be accompanied by Alzheimer's disease. Both inherited and sporadic forms of this disease exist: the sporadic form is due to the deposition of Aβ amyloid and the inherited form is due to various aberrant gene products that foster such deposition.[32,33] CAA predominantly affects persons older than 65 years, and increases in observed frequency until the eighth and ninth decades of life. Pathogenesis involves, following the deposition for Aβ amyloid, the acellular thickening of the tunica media that results in narrowing of the lumen. This represents the early, stenotic stage of the disease. Thereafter, with progressive deposition of Aβ amyloid, the smooth muscle in the tunica media degenerates, leading to thinning and weakening of the tunica media that is accompanied by dilatation of the lumen.[30,32] Thus, CAA (and the other vascular amyloidoses) can lead to cerebral infarcts by several mechanisms, including hypoperfusion or hemorrhage of the affected cerebral region:[32] it has been shown that CAA is an important risk factor for spontaneous nontraumatic intracranial hemorrhage (ICH), accounting for 5%–20% of all ICHs in elderly patients, with disease localized to the lobar regions in CAA-associated ICH.[31,34,35] However, studies by Attems et al. and Vonsattel et al. have found that ICH thought to be due to CAA alone may in fact involve other factors or comorbidities associated with CAA.[31,32]
Lipohyalinosis is the final stenotic etiology we will discuss. This etiology was first characterized in 1968. Through analysis of the arterial pathology manifested in serial sections of patients with small deep-brain infarcts, it was found that the small vessels supplying regions affected by these lacunar infarcts possessed a series of abnormalities. This series included focal enlargements, small hemorrhagic extravasations through the arterial wall, subintimal foam cells, and eosinophilic fibrinoid material in the vessel wall that often led to obliteration of the vessel lumen,[36] and was referred to by its discoverer as segmental arterial disorganization and lipohyalinosis.[36] Studies have shown that lipohyalinosis is strongly correlated with the presence of severe hypertension in patients and subsequently causes lacunar stroke in these patients due to the gradual occlusion of small perforating vessels, as described above for atherosclerosis.[37,38]
Damage to the arterial wall without stenosis
A separate category of cerebrovascular pathology can be delineated by differentiating damage to the arterial wall that results in stenosis from damage that is not stenotic in origin (stenosis may result from such damage, however, as is shown below). Having reviewed the former category above, the following discussion will concern the latter. This category is caused when the thin layer of endothelial cells that lines the lumen of all arteries is disrupted. A normal endothelial layer functions to guarantee smooth blood flow; when disrupted, loss of this function may lead to turbulent flow, obstruction of flow, or thrombosis, all of which may ultimately lead to stroke.[39,40,41] We describe some mechanisms by which disruption is initiated in the following section.
Vasculitides represent one such mechanism. These are a group of inflammatory diseases that are characterized by inflammation and necrosis of the blood vessel wall.[39,42] Thought to be autoimmune in pathogenesis, this is a heterogeneous group of diseases that may affect both the peripheral and central vasculatures,[43] and may be partitioned according to the size of the vessel they predominately affect: large, medium, or small.[44] Of all the primary vasculitides, giant cell arteritis, polyarteritis nodosa, and primary angiitis of the central nervous system (PACNS) all may have central nervous system (CNS) involvement productive of CVD, and are thus discussed.[39,40,45,46] Giant cell arteritis is the most common form of vasculitis among those over 50 years[47] and primarily affects large- and medium-sized blood vessels. This disease involves the granulomatous inflammation of affected vessels, which may lead to stroke.[48,49] A high incidence of such strokes in the vertebrobasilar circulation has been reported in the literature.[48] Polyarteritis nodosa is also capable of producing stroke: the 20% of cases with cranial involvement can cause stroke of both the ischemic and hemorrhagic varieties. Pathologically, this disease features a systemic necrotizing vasculitis typically targeted to medium-sized blood vessels.[39,50] Finally, PACNS is a rare disorder that exclusively targets the CNS vasculature. It is known that small-to-medium-sized vessels are principally affected, especially in the leptomeningeal and subcortical areas, but the complete pathogenesis of this disorder is not currently known. Experimental models have suggested, however, that its progression may be due to Th1-related cytokine inflammation, which is supported by the granulomatous nature of this vasculitis.[51] In any case, the disease produces lesion-specific symptoms, such as stroke with significant occlusion of the affected vessels.[40,51]
Dissection, or tearing, usually of the extracranial carotid and vertebral arteries (it has been reported, however, that intracranial artery dissection is more common among children and Asian populations studied[2]), is another vessel-damaging mechanism commonly implicated in causing “ischemic strokes” and stroke. This pathology usually succeeds trauma, which often involves sudden movements of the neck. Predisposing vascular abnormalities, either genetic or acquired, may also be involved.[41,52,53] Although arterial dissection of all causes accounts for only 2% of all ischemic strokes, it is still a highly significant cause of CVD among those under 45 years, causing 20% of all strokes in this population.[54]
The manner whereby dissection results in stroke may proceed in multiple fashions: one involves the formation of thromboemboli at the site of injury, and the other involves hemodynamic insufficiency due to occlusion or severe stenosis.[41] In either case, the initiating event is a tear in the tunica media, through which blood is admitted into the arterial wall. Once within the wall, this blood may impinge on the lumen to produce hemodynamic insufficiency; endothelial impingement, because it activates the coagulation cascade, may also result in thromboemboli. In addition, dissecting blood may make its way between the tunica media and adventitia, resulting in aneurysmal expansion that can be productive of subarachnoid hemorrhage.[55]
Vessel Blockage Due to Extrinsic Factors
In addition to causes that are intrinsic to the affected blood vessels, there are also extrinsic causes of vessel blockage that may lead to CVD. Of these, the most important are cerebral venous thrombosis (CVT) and cerebral emboli.
Cerebral venous thrombosis
CVT is a rare cause of stroke, accounting for only about 0.5% of all cases.[56] A major risk factor for CVT is the presence of a constellation of factors that is known as Virchow's triad (a hypercoagulable state characterized by stasis of blood, turbulent flow, and endothelial cell damage); other causes of CVT include acquired risk factors such as trauma, surgery, puerperium, pregnancy, oral contraceptives, and hypercoagulable states associated with malignancies and antiphospholipid syndrome.[56,57,58,59,60] Antithrombin III, protein S and C deficiency, prothrombin G20210A mutation, and factor V Leiden are among the genetic hypercoagulability conditions that can cause CVT.[58,60]
These risk factors can lead to CVD by two mechanisms, differentiated according to whether the occlusion is present in the cerebral veins or in the dural sinuses. If it is in the cerebral veins, occlusion causes local edema of the cerebral parenchyma (of both vasogenic and cytotoxic types, to be discussed below), focal venous infarction, and petechial hemorrhages that may coalesce into large intracranial hematomas.[61,62] If, on the other hand, it occurs in the venous sinuses, occlusion leads to increased intracranial and venous pressure and decreased capillary pressure and blood volume. These events then have several CVD-causative consequences, such as disruption of the blood–brain barrier (BBB), edema, extension of venous thrombosis due to obstructive stasis of blood, venous rupturing and subsequent hemorrhage, cerebrospinal fluid flow disruption, and stroke-like events.[62,63] Thus, when patients present with subarachnoid hemorrhage, it has been reported that CVT should be considered as an alternative etiology and investigated using venous imaging.[64]
As was discussed in Part I,[1] however, the cerebral venous circulation possesses a substantial intrinsic compensatory capacity against the development of CVT. This capacity is a direct consequence of several anatomic features possessed by the venous circulation, including the absence of valves, the lack of a tunica muscularis layer, and the possession of a rich, anastomotic network encompassing both veins and sinuses. It is only once these compensatory features are overwhelmed that central venous pathology is initiated.
Cerebral emboli
Cerebral emboli are blood clots that form at sites distal to the brain, but which then travel to the brain and lodge themselves in the cerebral vasculature. They are the most common cause of TIA and stroke, which they produce in exactly the same manner as a local occlusion. Thus, emboli are identical in both composition and pathologic consequence to the atherosclerotic thrombi discussed above. They are not, however, distinguished from thrombi exclusively according to their distinct origin.[65] In addition to this, certain clinical features, such as a rapid progression to peak symptomology, and the concomitant presence of an emboligenic heart disease, suggest an embolic etiology.[66]
While emboli can travel to the brain from virtually any site in the body, the most common source is the heart, along with the large vessels by which the heart supplies the brain. Taken together, these sources are implicated in about two-thirds of all ischemic strokes,[67,68,69,70] while emboli from the heart alone are thought to cause between 14% and 30% of ischemic strokes. Such emboli are of special concern in patients with atrial fibrillation, since this rhythm carries an increased risk of recurrent embolism production due to stasis, especially in the left atrial appendage.[68,71] Atrial myxomas, patent foramen ovale, and arteriovenous shunts also contribute to cerebral embolism production; in such cases, tumor removal and surgical patch-up of cardiac shunts is curative.[67] As other sources, such as complicated carotid plaques and aortic mural thrombi, also exist, it may be reasonably said, to summarize, that cerebral embolism is a highly heterogeneous pathology.[67,68,69]
Inadequate Cerebral Blood Flow
Since the brain is so heavily dependent on constant perfusion for the delivery of oxygen and glucose, any disruption in flow increases the risk of infarction. As was discussed in Part I of our review within the context of the cerebral microcirculation, disruptions in flow are normally avoided by the autoregulatory capacity possessed by this circulation. Cerebral autoregulation is not wholly understood, but several expository efforts have yielded plausible accounts. One of these accounts is referred to as the myogenic hypothesis, according to which the musculature of the cerebral vessels contracts in response to higher pressures, and dilates in the absence of pressure sufficient to initiate contraction, thus modulating resistance to preserve a constant rate of flow. Another account is known as the metabolic hypothesis: decreases in flow initiate the production of vasodilatory substances; dilation then decreases resistance, increasing flow.[72] Regardless of how it is achieved, this autoregulatory capacity is normally sufficient to supply the needs of the brain. However, when perfusion pressure is so severely compromised that it falls below the range over which autoregulation is effective, as can happen, for example, during heart failure,[73] cerebral blood flow compromise may ensue. Such drops can be productive of the so-called watershed patterns of infarction, in which distal regions of the cerebral vasculature are poorly perfused, producing infarction between arterial territories.[74]
Studies have also shown that increases in hematocrit, especially when they exceed 46%, significantly increase the incidence of cerebral infarcts and other CVD in younger patients, while an increase in hematocrit over 41% increases the risk of cerebral infarct among older patients (>78 years).[75,76,77] Moreover, the presence of cerebral atherosclerosis in addition to elevated hematocrit has been shown to have an additive effect on the incidence of infarction. Analysis of such cases has shown the severity of atherosclerosis to be directly proportional to increasing infarction incidence, especially in deep subcortical structures.[75] This may be due to the fact that, while the hemoglobin content of blood rises with an increase in hematocrit, there is also a sharp increase in blood viscosity. Thus, despite an increase in oxygen carriage capacity per unit time in affected blood, the highly viscous character of this blood causes a drop in flow rate to the brain, increasing the risk of infarction.[75]
Besides elevated hematocrit and atherosclerosis, occlusion of vessels due to thrombosis and emboli may also lead to inadequate cerebral blood flow, as discussed above.
Hemorrhage
Cerebral hemorrhage, which is classified according to location as either intracranial or subarachnoid, leads to the development of a pathology known as hemorrhagic stroke. Such strokes involve bleeding of vessels into the surrounding area (either subarachnoid or parenchymal) and subsequent damage to tissue contacted by the blood. While these strokes are less common than ischemic strokes, they still, due to high mortality rates, account for a significant number of deaths globally. More about hemorrhagic strokes, including the relevant epidemiology, presenting features, pathogenesis, and treatment, is discussed in Part III of our review series;[3,78] it is mentioned here merely to point out that the hemorrhaging of cerebral blood vessels represents a distinct avenue by which cerebrovascular pathology may emerge.
Microcirculatory Dysfunction: Cerebral Edema
Cerebral edema is one of the major secondary complications of stroke. Since stroke is a consequence of every modality of CVD pathogenesis presented above, edema is therefore an illustration of all these modalities. Due to this illustrative potential, cerebral edema is discussed here.
Although edema occurs among many other complications of stroke, it is comprehensible independently of any aspect of stroke pathophysiology apart from the cerebral microvascular anatomy. For the purposes of this discussion, it is sufficient to understand that the common origin of all stroke complications is disruption in the flow of blood. Disruption may be provoked by any of the pathogenic categories mentioned above. Consider, for example, the case of dural venous sinus thrombosis. When a clot develops in the lumen of a dural sinus, the drainage of deoxygenated blood from the brain is impaired.[79] The resulting raise in venous pressure then decreases cerebral blood flow, producing hypoxia. Hypoxia at the level of the BBB then produces edema, as described below. A similar causal chain leading from disruption to edema may be given for the other modalities of CVD pathogenesis.
After disruption, the brain can then experience two types of cerebral edema: vasogenic and cytotoxic.[80] In cytotoxic edema, the BBB remains intact, but ischemia-induced disruptions in cellular metabolism cause a decrease in adenosine triphosphate (ATP) production. ATP depletion then leads to Na/Ca channel dysfunction and an intracellular buildup of cations, causing a rapid influx of water into the cell. In the end, the result is a degree of cellular swelling that overwhelms homeostasis, becoming toxic. Astrocytes are more prone to pathological swelling than neurons, because they are involved in ion and metabolite clearance, which causes osmotic overload that in turn promotes water influx.
The vasogenic variety of edema, on the other hand, is due to physical breakdown of the BBB. This occurs at the end of the ischemic cascade, as follows: ischemia leads to energy depletion, causing neuronal injury and death. Reactive oxygen species released by injured cells then trigger microglial activation, cytokine and chemokine secretion, induction of adhesion molecules, and increased BBB permeability. This permits the invasion of peripheral immune cells into the brain parenchyma. Once inside the parenchyma, the invaders then secrete a variety of pro-inflammatory cytokines and matrix metalloproteinases that exacerbate BBB permeability, ultimately causing vasogenic edema.
Aquaporin 4 (AQP4) is a water transporter in the brain that plays multiple important roles in the pathogenesis and resolution of both types of edema. It is highly concentrated on the surface of astrocytic end-feet that surrounds the BBB[81] and functions as a water-specific bidirectional transporter. Under normal conditions, AQP4 is critical for water homeostasis in the brain. In models of vasogenic edema (e.g., BBB disruption secondary to permanent ischemia), brain swelling is exacerbated in AQP4 knockdown mice. In contrast, models of cytotoxic edema (e.g., acute transient ischemia, water intoxication) using AQP4 knockout mice demonstrate significant reduction in cerebral edema and improved neurological outcome.
To understand these findings, it is important to understand that cytotoxic edema does not cause brain swelling by itself. Swelling in this case is secondary to astrocytic dysfunction, which causes both BBB disruption, as well as the establishment of a positive osmotic gradient, due to the cessation of normal astrocytic clearance functions. AQP4 seems to play a detrimental, pro-swelling role under this circumstance by facilitating water movement into the brain. Cellular swelling begins within 30 minutes of infarction and persists for up to 24 h after reperfusion. Thus, since it results in the inhibition of cytotoxic edema-induced swelling, blockading AQP4 channels has been found to be neuroprotective when performed during the acute phase of ischemic stroke.[82,83,84]
In the contrasting case of vasogenic edema, AQP4 has been found to clear water from the brain parenchyma. It does so by providing a path of low resistance for the return of water from the parenchyma, back into the vascular compartment.[85] Vasogenic edema peaks at 10–20 days in humans following a stroke and subsides in 2–4 weeks.[86] Thus, an upregulation of AQP4 during the resolution phase may reduce infarct size and improve functional outcome.[87,88]
Summary
In this paper, we have presented a broad account of the various ways in which CVD can be initiated. First, we discussed initiating mechanisms intrinsic to the diseased vessel, such as atherosclerosis and arterial dissection. Then, we discussed factors that are extrinsic to the diseased vessel, such as cardiogenic emboli. Third, we described how inadequate blood flow to the brain may occur, and the various ways in which this inadequacy can then cause pathology. Finally, we introduced cerebral hemorrhage as a fourth pathological category. To illustrate how these four categories may proceed to produce clinically significant pathology, we then detailed the pathogenic events that culminate in cerebral edema.
Throughout our account, we have attempted not only to be succinct, but also sufficiently thorough to promote understanding of how pathogenesis is ultimately predictive of clinical course. The difference in clinical manifestation between thrombotic and embolic strokes described above is a good example of this pathogenesis–course correlation. Of course, there are many variables relevant to the science of clinical prognosis that are not discussed in this paper. Our goal was merely to sketch the physiological scaffold on which this prognostic science is constructed.
Financial support and sponsorship
This work was partially supported by American Heart Association Grant-in-Aid (14GRNT20460246) (YD), Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service (YD), National Natural Science Foundation of China (81501141) (XG), and Beijing NOVA program (xx2016061) (XG).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chandra A, Li WA, Stone CR, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease I: Anatomy. Brain Circ. 2017;3:45–56. doi: 10.4103/bc.bc_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30:359–94. doi: 10.1016/j.preteyeres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra A, Stone CR, Du X, Li WA, Huber M, Bremer R, et al. The cerebral circulation and cerebrovascular disease III: Stroke. Brain Circ. 2017;3:66–77. doi: 10.4103/bc.bc_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebeskind D. The collaterome: A novel conceptual framework of systems biology in cerebrovascular disorders. Brain Circ. 2015;1:3–8. [Google Scholar]
- 5.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 6.Sobieszczyk P, Beckman J. Carotid artery disease. Circulation. 2006;114:e244–7. doi: 10.1161/CIRCULATIONAHA.105.542860. [DOI] [PubMed] [Google Scholar]
- 7.Klijn CJ, Kappelle LJ, Tulleken CA, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997;28:2084–93. doi: 10.1161/01.str.28.10.2084. [DOI] [PubMed] [Google Scholar]
- 8.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 9.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: Analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 10.Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal PA, Belkin M, et al. Embolism from vertebral artery origin occlusive disease. Neurology. 1992;42:1505–12. doi: 10.1212/wnl.42.8.1505. [DOI] [PubMed] [Google Scholar]
- 11.Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27–54. doi: 10.1093/qjmed/hcg003. [DOI] [PubMed] [Google Scholar]
- 12.Cloud GC, Markus HS. Vertebral artery stenosis. Curr Treat Options Cardiovasc Med. 2004;6:121–7. doi: 10.1007/s11936-004-0040-5. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho M, Oliveira A, Azevedo E, Bastos-Leite AJ. Intracranial arterial stenosis. J Stroke Cerebrovasc Dis. 2014;23:599–609. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–9. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 15.De Silva DA, Woon FP, Lee MP, Chen CP, Chang HM, Wong MC. South Asian patients with ischemic stroke: Intracranial large arteries are the predominant site of disease. Stroke. 2007;38:2592–4. doi: 10.1161/STROKEAHA.107.484584. [DOI] [PubMed] [Google Scholar]
- 16.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 Suppl 1):III15–9. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 17.Khan M, Naqvi I, Bansari A, Kamal AK. Intracranial atherosclerotic disease. Stroke Res Treat. 2011;2011:282845. doi: 10.4061/2011/282845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathiesen EB, Joakimsen O, Bønaa KH. Prevalence of and risk factors associated with carotid artery stenosis: The Tromsø Study. Cerebrovasc Dis. 2001;12:44–51. doi: 10.1159/000047680. [DOI] [PubMed] [Google Scholar]
- 19.Mughal MM, Khan MK, DeMarco JK, Majid A, Shamoun F, Abela GS. Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther. 2011;9:1315–30. doi: 10.1586/erc.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della-Morte D, Rundek T. The role of shear stress and arteriogenesis in maintaining vascular homeostasis and preventing cerebral atherosclerosis. Brain Circ. 2015;1:53–62. doi: 10.4103/2394-8108.186288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 22.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Kishi M, Atsumi T, Bertolaccini ML, Makino H, Sakairi N, et al. Circulating oxidized LDL forms complexes with beta2-glycoprotein I: Implication as an atherogenic autoantigen. J Lipid Res. 2003;44:716–26. doi: 10.1194/jlr.M200329-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 25.Guyton JR, Klemp KF. Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks. Am J Pathol. 1993;143:1444–57. [PMC free article] [PubMed] [Google Scholar]
- 26.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 27.Stary HC. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler Thromb Vasc Biol. 2000;20:1177–8. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 28.Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. doi: 10.1016/j.jcmg.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Blancas-Mejía LM, Ramirez-Alvarado M. Systemic amyloidoses. Annu Rev Biochem. 2013;82:745–74. doi: 10.1146/annurev-biochem-072611-130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–24. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 31.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann Neurol. 1991;30:637–49. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 32.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 33.Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, et al. Cerebral amyloid angiopathies: A pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62:885–98. doi: 10.1093/jnen/62.9.885. [DOI] [PubMed] [Google Scholar]
- 34.Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: The CERAD experience, Part XV. Neurology. 1996;46:1592–6. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 35.Jellinger KA. Alzheimer disease and cerebrovascular pathology: An update. J Neural Transm (Vienna) 2002;109:813–36. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 36.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 37.Boiten J, Lodder J, Kessels F. Two clinically distinct lacunar infarct entities? A hypothesis. Stroke. 1993;24:652–6. doi: 10.1161/01.str.24.5.652. [DOI] [PubMed] [Google Scholar]
- 38.de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts: Further arguments from a study on prognosis. Stroke. 2002;33:2072–6. doi: 10.1161/01.str.0000022807.06923.a3. [DOI] [PubMed] [Google Scholar]
- 39.Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. 2010;3:29–42. doi: 10.1177/1756285609347123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alba MA, Espígol-Frigolé G, Prieto-González S, Tavera-Bahillo I, García-Martínez A, Butjosa M, et al. Central nervous system vasculitis: Still more questions than answers. Curr Neuropharmacol. 2011;9:437–48. doi: 10.2174/157015911796557920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redekop GJ. Extracranial carotid and vertebral artery dissection: A review. Can J Neurol Sci. 2008;35:146–52. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- 42.Arenillas J. Intracranial atherosclerosis and inflammation: Lessons from the East and the West. Brain Circ. 2015;1:47–52. [Google Scholar]
- 43.Hoffman GS, Calabrese LH. Vasculitis: Determinants of disease patterns. Nat Rev Rheumatol. 2014;10:454–62. doi: 10.1038/nrrheum.2014.89. [DOI] [PubMed] [Google Scholar]
- 44.Jennette JC, Falk RJ. Nosology of primary vasculitis. Curr Opin Rheumatol. 2007;19:10–6. doi: 10.1097/BOR.0b013e3280119877. [DOI] [PubMed] [Google Scholar]
- 45.Engel DG, Gospe SM, Jr, Tracy KA, Ellis WG, Lie JT. Fatal infantile polyarteritis nodosa with predominant central nervous system involvement. Stroke. 1995;26:699–701. doi: 10.1161/01.str.26.4.699. [DOI] [PubMed] [Google Scholar]
- 46.Tabarki B, Mahdhaoui A, Selmi H, Yacoub M, Essoussi AS. Kawasaki disease with predominant central nervous system involvement. Pediatr Neurol. 2001;25:239–41. doi: 10.1016/s0887-8994(01)00290-9. [DOI] [PubMed] [Google Scholar]
- 47.Ness T, Bley TA, Schmidt WA, Lamprecht P. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int. 2013;110:376–85. doi: 10.3238/arztebl.2013.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samson M, Jacquin A, Audia S, Daubail B, Devilliers H, Petrella T, et al. Stroke associated with giant cell arteritis: A population-based study. J Neurol Neurosurg Psychiatry. 2015;86:216–21. doi: 10.1136/jnnp-2014-307614. [DOI] [PubMed] [Google Scholar]
- 49.de Boysson H, Liozon E, Larivière D, Samson M, Parienti JJ, Boutemy J, et al. Giant cell arteritis-related stroke: A retrospective multicenter case-control study. J Rheumatol. 2017;44:297–303. doi: 10.3899/jrheum.161033. [DOI] [PubMed] [Google Scholar]
- 50.Schmidley JW. 10 questions on central nervous system vasculitis. Neurologist. 2008;14:138–9. doi: 10.1097/NRL.0b013e31815bdc2b. [DOI] [PubMed] [Google Scholar]
- 51.Lie JT. Classification and histopathologic spectrum of central nervous system vasculitis. Neurol Clin. 1997;15:805–19. doi: 10.1016/s0733-8619(05)70348-0. [DOI] [PubMed] [Google Scholar]
- 52.Lee VH, Brown RD, Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: A population-based study. Neurology. 2006;67:1809–12. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 53.Caplan LR, Biousse V. Cervicocranial arterial dissections. J Neuroophthalmol. 2004;24:299–305. doi: 10.1097/00041327-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 55.Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4:34–42. doi: 10.1038/ncpneuro0683. [DOI] [PubMed] [Google Scholar]
- 56.Bousser MG, Ferro JM. Cerebral venous thrombosis: An update. Lancet Neurol. 2007;6:162–70. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 57.Bombeli T, Basic A, Fehr J. Prevalence of hereditary thrombophilia in patients with thrombosis in different venous systems. Am J Hematol. 2002;70:126–32. doi: 10.1002/ajh.10103. [DOI] [PubMed] [Google Scholar]
- 58.Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–92. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 59.Martinelli I, Battaglioli T, Pedotti P, Cattaneo M, Mannucci PM. Hyperhomocysteinemia in cerebral vein thrombosis. Blood. 2003;102:1363–6. doi: 10.1182/blood-2003-02-0443. [DOI] [PubMed] [Google Scholar]
- 60.Martinelli I. Cerebral vein thrombosis. Thromb Res. 2013;131(Suppl 1):S51–4. doi: 10.1016/S0049-3848(13)70022-7. [DOI] [PubMed] [Google Scholar]
- 61.Itrat A, Shoukat S, Kamal AK. Pathophysiology of cerebral venous thrombosis – An overview. J Pak Med Assoc. 2006;56:506–8. [PubMed] [Google Scholar]
- 62.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–8. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 63.Aminoff M, Boller F, Swaab D. Stroke. Part II: Clinical Manifestations and Pathogenesis, Handbook of Clinical Neurology. Elsevier; 2009. [Google Scholar]
- 64.Konakondla S, Schirmer CM, Li F, Geng X, Ding Y. New developments in the pathophysiology, workup, and diagnosis of dural venous sinus thrombosis (DVST) and a systematic review of endovascular treatments. Aging Dis. 2017;8:136–48. doi: 10.14336/AD.2016.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carcora Y, Hussain M, Geng X, Ding Y. A review of current clinical studies leading to improved outcomes in patients treated with newer-generation thrombectomy devices. Brain Circu. 2015;1:9–13. [Google Scholar]
- 66.Arboix A, Alio J. Acute cardioembolic cerebral infarction: Answers to clinical questions. Curr Cardiol Rev. 2012;8:54–67. doi: 10.2174/157340312801215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stirling J, Muramatsu K, Shirai T. Cerebral embolism as a cause of stroke and transient ischemic attack. Echocardiography. 1996;13:513–8. doi: 10.1111/j.1540-8175.1996.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 68.Meyer JS, Charney JZ, Rivera VM, Mathew NT. Cerebral embolization: Prospective clinical analysis of 42 cases. Stroke. 1971;2:541–54. doi: 10.1161/01.str.2.6.541. [DOI] [PubMed] [Google Scholar]
- 69.Boughner DR, Barnett HJ. The enigma of the risk of stroke in mitral valve prolapse. Stroke. 1985;16:175–7. doi: 10.1161/01.str.16.2.175. [DOI] [PubMed] [Google Scholar]
- 70.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 71.Arboix A, Alió J. Cardioembolic stroke: Clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6:150–61. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75:353–61. doi: 10.1136/jnnp.2003.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke. 1984;15:221–3. doi: 10.1161/01.str.15.2.221. [DOI] [PubMed] [Google Scholar]
- 75.Tohgi H, Yamanouchi H, Murakami M, Kameyama M. Importance of the hematocrit as a risk factor in cerebral infarction. Stroke. 1978;9:369–74. doi: 10.1161/01.str.9.4.369. [DOI] [PubMed] [Google Scholar]
- 76.Hosoya H, Levine JJ, Henry DH, Goldberg S. Double the trouble: Acute coronary syndrome and ischemic stroke in polycythemia vera. Am J Med. 2017;130:e237–40. doi: 10.1016/j.amjmed.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Zoraster RM, Rison RA. Acute embolic cerebral ischemia as an initial presentation of polycythemia vera: A case report. J Med Case Rep. 2013;7:131. doi: 10.1186/1752-1947-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torpy JM, Burke AE, Glass RM. JAMA patient page. Hemorrhagic stroke. JAMA. 2010;303:2312. doi: 10.1001/jama.303.22.2312. [DOI] [PubMed] [Google Scholar]
- 79.Rafols J. Control of the brain microcirculation following traumatic brain injury and stroke. Brain Circu. 2015;1:146–58. [Google Scholar]
- 80.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–68. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009;190:159–70. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci. 2015;9:108. doi: 10.3389/fncel.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–63. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 85.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–93. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 87.Tourdias T, Mori N, Dragonu I, Cassagno N, Boiziau C, Aussudre J, et al. Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J Neuroinflammation. 2011;8:143. doi: 10.1186/1742-2094-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuda AM, Badaut J. Aquaporin 4: A player in cerebral edema and neuroinflammation. J Neuroinflammation. 2012;9:279. doi: 10.1186/1742-2094-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]