Abstract
Background and Objectives:
Despite advance in treatment of status epilepticus (SE), a major neurological emergency, it is still associated with mortality and morbidity. The objective of our study was to estimate sociodemographic profile, semiology, and etiology in the children with SE admitted in pediatric intensive care.
Materials and Methods:
Children between 1 month and 18 years with continuous seizure activity of >5 min or two or more sequential seizures without full recovery of consciousness between seizures, admitted in the Pediatric Intensive Care Unit of the Department of Pediatrics, Government Medical College, Srinagar, were included in the study. A semi-structured tool was designed to record the sociodemographic details. Detailed history, clinical examination, and investigations (including neuroimaging as and when needed) were used to determine the type of seizure and etiology.
Results:
A total of 51 patients were included in our study. Most of the patients were <5 years with 47.10% in 1–5 years’ age group and 43.10% in >1-year age group. Males (60.80%) outnumbered females. Thirty-nine patients (76.47%) belonged to “known” or “symptomatic” group with 35 (68.60%) of them presenting with SE as their fresh seizure. Thirty-nine (76.47%) of our patients had generalized tonic–clonic seizure (GTCS) type of SE.
Conclusion:
Most of the children (90%) were below the age of 5 years with male predominance. Most of the patients had SE as their first seizure without prior history of seizures with GTCSs was the most frequent seizure type.
KEYWORDS: Children, generalized tonic clonic, status epilepticus
INTRODUCTION
Status epilepticus (SE) is a common pediatric neurological emergency which, if not managed promptly, may result in significant neuromorbidity and mortality and thus requires immediate and vigorous management.[1] The longer it takes to gain control over seizures, worse is the neurological outcomes for the child, and the harder it is to terminate the seizures.[2] The original International League Against Epilepsy (ILAE) definition of SE, by Gastaut: “an epileptic seizure that is sufficiently prolonged or repeated at sufficiently brief intervals so as to produce an unvarying and enduring epileptic condition” although vague, has allowed a dynamic interpretation.[3] Subsequent definition of SE specifically defined it as continuous seizure activity or recurrent seizures without recovery of consciousness for >30 min.[4] Later on, operational definition for treatment purposes defined SE as >5 min of either continuous seizure activity or recurrent seizures without recovery in between.[5]
The ILAE Task Force on Classification of SE has updated the definition and classification of SE to include four axes.[6] SE is now defined as “a condition resulting from either the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms which lead to abnormally prolonged seizures (after time point t1).[6] It is a condition which can have long-term consequences (after time point t2), including neuronal death, neuronal injury, and alteration of neural networks, depending on the type and duration of seizures.”[6] This definition is conceptual, with two operational dimensions: the first is the length of the seizure and the time point (t1) at which the seizure should be regarded as an “abnormally prolonged seizure.” The second time point (t2) is the time of ongoing seizure activity beyond which there is a risk of long-term consequences.[6] Tonic–clonic SE t1 equals 5 min and t2 equals 30 min. For focal SE with impaired consciousness, t1 equals 10 min while t2 is >60 min.[6] Absent SE has t1 equal to 10–15 min with unknown t2.[6] The new classification puts SE on four axes with Axis 1 as semiology; Axis 2 as etiology; Axis 3 as electroencephalography correlates, and Axis 4 as age.[6] Under the semiologic axis, SE is now divided into SE with prominent motor symptoms and without prominent motor symptoms (non-convulsive SE [CSE]).[6] In this classification, etiology is now included on Axis 2 with known (symptomatic) or unknown (cryptogenic) etiologies.[6] The term “known” or “symptomatic” is used – consistent with the common neurologic terminology – for SE caused by a known disorder, which can be structural, metabolic, inflammatory, infectious, toxic, or genetic.[6] Based on its temporal relationship, the subdivisions acute, remote, and progressive can be applied. The term “unknown” or “cryptogenic” is used in its strict original meaning: unknown cause.[6]
The causes, risk factors, and prognosis of SE vary from country to country and from region to region. In developed countries, predominant etiology is cerebrovascular disease whereas central nervous system (CNS) infections account for the majority in developing countries, varying between 20% and 67% in various studies.[7,8,9] Studies in developed resource-equipped settings have reported various trigger factors for SE in children most common of which include fever (presumed infection) in 36% of cases, change in medication (20%), no clear cause (9%), metabolic derangement (8%), congenital malformations (7%), anoxic events (5%), and a diverse array of other factors (trauma, vascular, infection, tumor, and drugs),[10,11] but these figures may not be true reflections of the proportions in resource-limited settings, such as India, where neuroinfections are so prevalent. The incidence of SE in children is in the range of 18–23 children per 100,000 child population per year.[12] This is much greater than the adult incidence of around 4–6 per 100,000 per year.[13] In our study, we aimed to find the sociodemographics, semiology, and etiology of the SE patients who were admitted in pediatric intensive care of our hospital.
MATERIALS AND METHODS
The present study was conducted in the Postgraduate Department of Pediatrics, Government Medical College, Srinagar. This was a cross-sectional study conducted over a period of 1 year from April 2015 to March 2016. The study was approved by the Ethical Committee of Government Medical College, Srinagar. Informed consent was taken from the parents/guardian. All the patients between 1 month and 18 years with continuous seizure activity of >5 min or two or more sequential seizures without full recovery of consciousness between seizures, admitted in pediatric intensive care of our hospital during the study period constituted the study population. A semi-structured tool was designed to record the sociodemographic details. Detailed history, clinical examination, and investigations (including neuroimaging as and when needed) were used to determine the type of seizure and etiology. The type of seizure and etiology was established based on updated definition of the ILAE Task Force on Classification of SE.[6] The duration of SE was ascertained from a reliable patient's relative or attendant or the referring physician's note. The time to travel from the place of patients’ home place to the hospital was also taken into consideration while determining the duration of SE. Data were entered in a Microsoft Excel spreadsheet. Statistical analysis was carried out with the Results thus generated were presented as frequencies and percentages.
RESULTS
Among 51 patients admitted with SE, 22 patients (43.10%) were <1 year of age, 24 patients (47.10%) were between 1 and 5 years, and 5 patients (9.80%) were above 5 years of age [Table 1].
Table 1.
Age distribution

Males predominate the study population with 31 males (60.80%) and 21 females (39.20%) [Table 2].
Table 2.
Sex distribution

None of our patients had non-CSE. Most common type of seizure observed in our patients was generalized tonic–clonic seizures (GTCSs) comprising 39 of 51 patients (76.40%). Only 4 patients (7.8%) have had focal motor type of SE while 6 patients (11.80%) comprised the tonic status. Two patients (3.90%) had myoclonic SE [Table 3].
Table 3.
Semiology

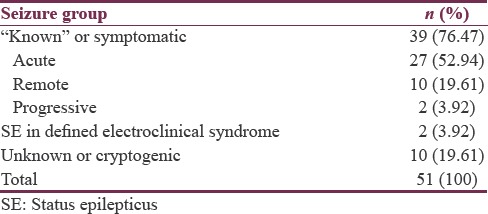
With regard to etiology, 39 patients (76.47%) belonged to “known” or “symptomatic” group with 27 patients (52.94%), 10 patients (19.61%), and 2 patients (3.92%) belonging to acute, remote, and progressive subgroups, respectively. Two patients (3.92%) belonged to “SE in defined electroclinical syndrome” with both of them having myoclonic SE. “Cryptogenic” or “unknown” SE occurred in 10 patients (19.61%) [Table 4].
Table 4.
Etiology
Thirty-five patients (68.60%) in our study were having this episode of SE as their first episode of seizure activity while 16 patients (31.40%) have had seizures in the past as well [Table 5].
Table 5.
Previous or fresh seizure episode

Thirty-seven patients (72.50%) in our study had seizure activity lasting anywhere between 5 min and 3 h. Nine patients (17.60%) had the seizure activity lasting between 3 and 6 h duration while 4 patients (7.80%) had seizures lasting for 6–24 h. Only one patient (20%) has had the seizure activity lasting for >24 h.
DISCUSSION
In our study, 22 patients (43.1%) were <1 year of age and 24 patients (47.1%) belonged to 1–5 years’ age group. Only 5 patients (9.8%) were 5 years old or above. This is in coherence with the study of Jan et al.[14] who found about 70% of their patients below 6 years of age. Our results are also consistent with many Indian studies which have shown predominant involvement of younger age group.[9,15] With regard to the studies from the middle east, Saz et al.[16] found SE occurring more frequently in younger age groups with a mean age of 3.8 years. Chin et al.[17] reported the incidence of SE highest in infants. Singh and Gaillard[18] in their study on SE in children found that it is most commonly encountered among children younger than 2 years of age (particularly among those <1 year of age).
Our results are in contradiction with Ahmed et al.[19] who found that only 18% of patients were infants, but they stated that their results are contrary to other studies which have shown predominant involvement of younger age group. The reason for this predominance of SE in younger children is not known. Probably, mechanisms for control of seizure activity are fragile in younger children and may get disrupted with minimal abnormalities in neurofunction.[16] In our study, there were 31 (60.8%) males and 20 (39.2%) females with a male-to-female ratio of 1.55:1. A study of Jan et al.[14] supports our finding with a male-to-female ratio of 1.35:1 in their study. In addition, our results are consistent with many of the Indian studies which have found male preponderance for SE.[9,15] Studies outside India have also found male predominance for SE in children.[2,20,21] However, to the best of our knowledge, no definite causal relationship was found in literature for this male preponderance and warrants further investigation in future studies. However, there have been few studies which have found female preponderance for SE in children.[22,23] All patients in our study were having CSE. Cherian and Thomas[24] in “Status Epilepticus in the Resource Poor Countries” states that most studies on SE in resource-poor countries describe CSE since nonconvulsive epilepticus is rarely detected in these regions. In our study, GTCS was the most common type of seizure accounting for 39 cases (76.4%), followed by tonic SE in 6 cases (11.8%) and focal motor type SE in 4 cases (7.8%) and myoclonic SE in 2 patients (3.9%). Jan et al.[14] also found GTCS as the most common type of seizure with 85% of their patients having this type of seizure but found a different profile for other types of seizure than our study with 10% of their patients having myoclonic seizures, 4% with tonic type and 0.83% with CPS type. Although there is some variation, majority of patients in both these studies were having the same type of seizure. Our results are in coherence with other studies from India and outside which have shown GTCS as the most common type of seizure.[15,20,25,26] Although the majority of patients with SE have GTCSs, some studies indicate that partial seizures also represent a major type of SE in children which is contradictory to our results. Besli et al.[20] in their study found 27% of SE as partial seizure type and 22% as secondarily generalized type. DeLorenzo et al.[26] in their study found that the onset seizure type for SE was partial in 64% of the patients and GTCS in 36% of SE cases and 35% of partial type progressed to generalized seizure activity, and thus, the final seizure activity in SE was generalized in 71% and partial in 29% of SE cases. Chin et al.[2] also reported that about one-third of motor seizures start partially but also reported that the rate of secondary generalization is high in seizures that start partially and those seizures most frequently end in the generalized tonic–clonic type. Less number of patients with partial type in our study could be explained as our hospital being a tertiary care referral center; patients here are referred from peripheral centers where their initial presentation might have been partial which has undergone secondary generalization in the time course of referral from these centers to our hospital. DeLorenzo et al.[27] mentioned in their study that partial seizure with secondary generalization of SE is still significantly under diagnosed because it is not possible to obtain witnessed information at the initiation of all SE cases. In our study, 39 patients (76.47%) belonged to “known” or “symptomatic” group with 27 (52.94%) patients belonging to the acute symptomatic group, 10 patients (19.61%) belonging to remote symptomatic group, and 2 patients (3.92%) belonging to progressive symptomatic (previously called as progressive encephalopathy) group. “Cryptogenic” or “unknown” SE occurred in 10 patients (19.61%) while 2 patients (3.92%) had “SE in defined electroclinical syndrome” with both of them having myoclonic SE. Studies from India and other developing countries have found similar results.[7,8,9,15]
Jan et al.[14] found remote symptomatic cause in 30% and prolonged febrile convulsion in 23.3% while 18% of their patients had acute symptomatic etiology. Their results are inconsistent with our study, but they stated in their study that their results are in discordance with most of the previous studies. The high prevalence of acute symptomatic group in our study may be because our pediatric emergency unit encounters high number of cases diagnosed with CNS infection. In our study, we found fresh seizure in 35 cases (68.6%) while 16 patients (31.4%) had previous history of seizure episodes. Our results are in accordance with other studies from India and outside which have shown almost similar figures.[15,28] As most of our patients were of younger age, our results could be explained by the fact that in young children, the majority of cases of SE occurred in patients with no history of seizures, rather than as part of an established seizure disorder, and there are a higher proportion of older children with prior seizures or epilepsy. This is significantly attributed to the change of underlying causes based on age.[2,27] Our results are in contradiction with the study of Besli et al.[21] who found 22 cases (39.3%) to be first seizure attack whereas 34 cases (55.4%) were with prior seizures or epilepsy. When their results were evaluated based on age, only 33.3% of their cases younger than 2 years of age had previously experienced seizure which is similar to our results. A study from Pakistan by Ahmed et al.[19] reported 50% of cases as being newly diagnosed and rest 50% as having previous episodes of seizure activity. A study from the west by Dunn[25] have shown 36% of SE to be fresh episodes, while 64% of SE to be having previous seizures. Their contradictory result could be explained as most of the episodes of SE in their study (60%) occurred in children older than 3 years, and studies have shown that a higher proportion of older children with SE have established seizure disorder.[27] In our study, we found that the seizure lasted for a time duration between 5 min and 3 h in 37 patients (72.5%) and for 3–6 h in 9 patients (17.6%), while in 4 (7.8%) patients, it lasted between 6 and 24 h and only one patient had seizure for >24 h. Our study results are in accordance with other studies which have reported most of patients having seizure activity lasting for ≤3 h.[15,20,21,26]
CONCLUSION
SE being a neurological emergency needs urgent management to prevent morbidity and mortality. In our study, we found generalized tonic–clonic status most common type and most of these patients had “known” or symptomatic etiology. Further studies need to be conducted in our setting to find the outcome in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hanhan UA, Fiallos MR, Orlowski JP. Status epilepticus. Pediatr Clin North Am. 2001;48:683–94. doi: 10.1016/s0031-3955(05)70334-5. [DOI] [PubMed] [Google Scholar]
- 2.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC, et al. Treatment of community-onset, childhood convulsive status epilepticus: A prospective, population-based study. Lancet Neurol. 2008;7:696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastaut H. Classification of status epilepticus. Adv Neurol. 1983;34:15–35. [PubMed] [Google Scholar]
- 4.Dodson WE. The treatment of convulsive status epilepticus: Recommendations of the Epilepsy Foundation of America's Working Group on status epilepticus. J Am Med Assoc. 1993:270–7. [PubMed] [Google Scholar]
- 5.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–2. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 6.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus – Report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 7.Aminoff MJ, Simon RP. Status epilepticus. Causes, clinical features and consequences in 98 patients. Am J Med. 1980;69:657–66. doi: 10.1016/0002-9343(80)90415-5. [DOI] [PubMed] [Google Scholar]
- 8.Mbodj I, Ndiaye M, Sene F, Salif Sow P, Sow HD, Diagana M, et al. Treatment of status epilepticus in a developing country. Neurophysiol Clin. 2000;30:165–9. doi: 10.1016/s0987-7053(00)00203-3. [DOI] [PubMed] [Google Scholar]
- 9.Murthy JM, Jayalaxmi SS, Kanikannan MA. Convulsive status epilepticus: Clinical profile in a developing country. Epilepsia. 2007;48:2217–23. doi: 10.1111/j.1528-1167.2007.01214.x. [DOI] [PubMed] [Google Scholar]
- 10.Haafiz A, Kissoon N. Status epilepticus: Current concepts. Pediatr Emerg Care. 1999;15:119–29. doi: 10.1097/00006565-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Singh RK, Stephens S, Berl MM, Chang T, Brown K, Vezina LG, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology. 2010;74:636–42. doi: 10.1212/WNL.0b013e3181d0cca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tasker RC. Status epilepticus in children. Curr Opin Pediatr. 2014;26:653–4. doi: 10.1097/MOP.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 13.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50:735–41. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- 14.Jan M, Naik S, Ali S, Rafiq W, Kachroo A, Maqbool M. Frequency, etiology and immediate outcome of children admitted to Pediatric Intensive Care Unit (PICU) with convulsive status epilepticus in Kashmir North India. J Evol Med Dent Sci. 2015;4:10887–96. [Google Scholar]
- 15.Kumar M, Kumari R, Narain NP. Clinical profile of status epilepticus (SE) in children in a tertiary care hospital in Bihar. J Clin Diagn Res. 2014;8:PC14–7. doi: 10.7860/JCDR/2014/9288.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saz EU, Karapinar B, Ozcetin M, Polat M, Tosun A, Serdaroglu G, et al. Convulsive status epilepticus in children: Etiology, treatment protocol and outcome. Seizure. 2011;20:115–8. doi: 10.1016/j.seizure.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: Prospective population-based study. Lancet. 2006;368:222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 18.Singh RK, Gaillard WD. Status epilepticus in children. Curr Neurol Neurosci Rep. 2009;9:137–44. doi: 10.1007/s11910-009-0022-9. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed K, Kaleem Jafr S, Bhatti F, Rafique A. Clinical profile and outcome of children admitted with status epileptics in PICU of a developing country. Pak J Neurol Sci. 2013;8:1–6. [Google Scholar]
- 20.Besli GE, Saltik S, Erguven M, Bulut O, Abul MH. Status epilepticus in children: Causes, clinical features and short-term outcome. Pediatr Int. 2010;52:749–53. doi: 10.1111/j.1442-200X.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- 21.Scott RC, Surtees RA, Neville BG. Status epilepticus: Pathophysiology, epidemiology, and outcomes. Arch Dis Child. 1998;79:73–7. doi: 10.1136/adc.79.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim SH, Yezdan MA, Nizami SQ. Status epilepticus in children: A five-year experience at Aga Khan university hospital. J Pak Med Assoc. 2003;53:597–9. [PubMed] [Google Scholar]
- 23.Newton CR. Status epilepticus in resource-poor countries. Epilepsia. 2009;50(Suppl 12):54–5. doi: 10.1111/j.1528-1167.2009.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherian A, Thomas SV. Status epilepticus. Ann Indian Acad Neurol. 2009;12:140–53. doi: 10.4103/0972-2327.56312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn DW. Status epilepticus in children: Etiology, clinical features, and outcome. J Child Neurol. 1988;3:167–73. doi: 10.1177/088307388800300303. [DOI] [PubMed] [Google Scholar]
- 26.DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 27.Asadi-Pooya AA, Poordast A. Etiologies and outcomes of status epilepticus in children. Epilepsy Behav. 2005;7:502–5. doi: 10.1016/j.yebeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Kang DC, Lee YM, Lee J, Kim HD, Coe C. Prognostic factors of status epilepticus in children. Yonsei Med J. 2005;46:27–33. doi: 10.3349/ymj.2005.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


