Abstract
The effects of zerumbone on the proliferation and apoptosis of esophagus cancer cells and on the P53 and Bcl-2 expression levels were studied. The esophagus cancer EC-109 cells were cultured and inoculated. The effect of zerumbone on proliferation of EC-109 cells was detected via the Cell Counting Kit-8 (CCK-8) method. Cell apoptosis was detected via TdT-mediated dUTP nick end-labeling (TUNEL) staining. Moreover, the mRNA expression levels of P53 and Bcl-2 were detected via reverse transcription-polymerase chain reaction (RT-PCR), and the protein expression levels of P53 and Bcl-2 were evaluated via western blotting. CCK-8 detection results showed that compared with control group, zerumbone in different concentrations could inhibit the activity of EC-109, and the proliferation inhibition rate was significantly increased in a concentration-dependent manner with the increase of concentration. TUNEL staining showed that cell apoptosis gradually occurred in administration group, and the number of apoptotic cells was increased in a concentration-dependent manner with the increase of concentration. RT-PCR detection results showed that the mRNA expression level of P53 in administration group was significantly increased compared with that in control group, but that of Bcl-2 was significantly decreased. Western blotting showed that the protein expression level of Bcl-2 in administration group in different concentrations was significantly increased with the increase of zerumbone concentration, but that of Bcl-2 was significantly decreased in a concentration-dependent manner. Zerumbone can inhibit the proliferation and induce apoptosis of esophageal cancer EC-109 cells, and its induction of apoptosis may be realized through upregulating the mRNA expression of P53 and downregulating the mRNA expression of Bcl-2, and upregulating the protein expression of P53 and downregulating the protein expression of Bcl-2.
Keywords: zerumbone, esophageal cancer, EC-109, proliferation, apoptosis, P53, Bcl-2
Introduction
Esophageal cancer is a common malignant tumor that occurs frequently in China, the incidence rate ranks fifth and mortality rate fourth in malignant tumors (1), dominated by squamous carcinoma. At present, the main clinical treatment methods include surgery, radiotherapy and chemotherapy. Even so, patients with esophageal squamous cell carcinoma have not only a poor prognosis, but also a low 5-year survival rate of less than 15% (2), so study of effective therapeutic drugs is urgent.
The existing clinical treatment often cause great adverse reactions, which not only bring great pain to the patient's, but also seriously reduce their life quality. Traditional Chinese medicine combined with conventional medicine has many advantages, and the active ingredients have high efficiency and low toxicity; traditional Chinese medicine has attracted increasingly more attention of scientists in China and worldwide because of these irreplaceable advantages. It has been reported that the active ingredients in traditional Chinese medicine and its extracts play significant roles in inhibiting the proliferation or inducing apoptosis of cancer cells, and reducing adverse reactions to radiotherapy and chemotherapy (3). Zerumbone, extracted from Zingiber zerumbeta, has been proven by many studies to have antitumor, anti-inflammation and other pharmacological activities; in particular, the anticancer effect of zerumbone and its mechanism have been investigated by a large number of scientists (4,5).
This study aimed to assess the effects of zerumbone on the proliferation and apoptosis of esophageal cancer EC-109 cells and the possible relevant mechanism during the apoptosis process, so as to provide a basis for the clinical treatment of esophageal cancer with zerumbone.
Materials and methods
Materials and reagents
Esophageal cancer EC-109 cells (Cell Bank of Chinese Academy of Sciences, Shanghai, China); zerumbone (Sigma, New York, NY, USA); methyl thiazolyl tetrazolium (MTT) (Sigma, St. Louis, MO, USA); rabbit anti-human P53 and Bcl-2 poluclonal primary antibodies and goat anti-rabbit horseradish peroxidase (HRP)-labeled secondary polyclonal antibody (cat. nos. 10442-1-AP, 12789-1-AP and SA00001-2; Wuhan Sanying Biotechnology, Wuhan, China; Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA); TdT-mediated dUTP nick end-labeling (TUNEL) apoptosis kit, TRIzol and reverse transcription-polymerase chain reaction (RT-PCR) kits (Invitrogen; Thermo Fisher Scientific, Inc.); primer synthesis (Takara, Dalian, China).
Cell culture
The cells were cultured using DMEM cell culture fluid containing 10% fetal bovine serum, amino acid and double antibodies (100 kU/l penicillin and 0.1% streptomycin) in an incubator at 37°C under 5% CO2 and saturated humidity for continuous subculture. The culture fluid was regularly replaced, and the cells were digested with trypsin for follow-up experiments when they grew until covering 80% of the bottom of bottle.
Detection of cell proliferation inhibition rate via Cell Counting Kit-8 (CCK-8) method
The esophageal cancer EC-109 cells in logarithmic growth phase was taken and counted using the cell counter after digestive treatment. Single-cell suspension (100 µl) (1×105) was added into the 96-well plate. After the cells adhered to the wall, the original culture fluid was removed and the drugs with corresponding concentration were added according to the experimental grouping, and the volume was 100 µl. After 24, 48 and 72 h, 10 µl CCK-8 solution was added into the 96-well plate. After 4 h, the optical density (OD) value at the wavelength of 450 nm of each well was measured using the microplate reader. Inhibition rate = (1 - OD value in experimental group/OD value in blank control group) × 100%.
Detection of apoptosis of EC-109 via TUNEL method
EC-109 cells were inoculated in a laser confocal culture dish and fixed with 4% paraformaldehyde for 15 min after drug treatment. After being washed with phosphate buffered saline (PBS) for 5 min ×2 times, tissues were treated with 100 µl 20 µg/ml proteinase K at room temperature for 10–30 min, and then washed with PBS for 3 min. Cells in treatment group were mixed evenly with 1 µl rTdT + 1 µl biotin-labeled dUTP + 98 µl equilibrium liquid; rTdT was replaced with tri-distilled water in negative control group; 100 µl DNase I buffer was added into the positive control group for incubation for 5 min, and 100 µl DNase I (10 U/ml) was added for enzyme digestion for 10 min after the liquid was removed; finally, cells were rinsed with deionized water 4 times and washed with PBS for 5 min; 100 µl TUNEL reaction mixture was added onto the specimen, and the specimen was covered with cover glass or sealed with sealing film for reaction in the dark wet box at 37°C × 1 h; enzyme-labeled reaction: 100 µl streptavidin-labeled HRP (diluted by 1:500 PBS) was added for 30 min. Finally, cells were observed under a laser confocal microscope (Olympus Corporation, Tokyo, Japan), and photographed in the randomly-selected visual field.
Detection of the mRNA expression of P53 and Bcl-2 via RT-PCR
The cultured cells were inoculated into the 6-well plate with 104 cells per well. After 24 h, the supernatant was discarded and zerumbone in the concentration of 30, 40 and 50 µg/ml was administered, respectively. After the reaction for 48 h, the EC-109 cells in each group were collected and used for the total RNA extraction with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). Then cDNA was synthesized via reverse transcription with the qualified total RNA as the template. The specific reaction conditions were as follows: incubation at 42°C for 15 min and incubation at 95°C for 3 min. Then, cDNA was cooled on ice and stored at −80°C for later use. The qPCR amplification system is 25 µl: SYBR Premix Ex Taq II (2×) 12.5 µl, PCR Forward Prime (10 µM) 1 µl, DNA Templates 2 µl, DDW 8.52 µl. The reaction conditions are as follows: denatured at 95°C for 30 sec and annealed at 55°C for 60 sec, extension at 72°C, 30 cycles. Routine amplification was performed according to the primer sequences in Table I.
Table I.
RT-PCR primer sequences of Fas and FasL mRNA.
| Gene name | Primer sequence |
|---|---|
| P53 | Forward: 5′-GGAAATCTCACCCCATCCCA-3′ |
| Reverse: 5′-CAGTAAGCCAAGATCACGCC-3′ | |
| Bcl-2 | Forward: 5′-GGCCTGTGTCTCCTTGTGAT-3′ |
| Reverse: 5′-TGCCAGCTCCTTCTGAAGTA-3′ |
Detection of the protein expression of P53 and Bcl-2 via western blotting
The cultured cells were inoculated into the 6-well plate with 104 cells per well. After 24 h, the supernatant was discarded and zerumbone in the concentration of 30, 40 and 50 µg/ml was administered, respectively. After the reaction for 48 h, the cells in each group were collected and the total protein was extracted and the protein concentration was determined. After the samples were treated, 50 µg protein was isolated via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the protein isolated was electronically transferred onto the polyvinylidene fluoride (PVDF) membrane. The membrane was sealed using the blocking solution at room temperature for 1 h and incubated with primary antibodies (1:500) at 4°C overnight. After the membrane was fully washed with Tween Tris base buffer solution (TTBS), the secondary antibody (1:2,000) was added for incubation at room temperature for 1 h, followed by washing with TTBS, color development and photography.
Statistical analysis
The data were presented as mean ± standard deviation, and processed with SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used for the statistical analysis of data and the post hoc test was Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of zerumbone on the inhibition of EC-109 proliferation
Compared with that in control group, the activity of EC-109 cells could be inhibited in administration groups with the zerumbone concentration of 30, 40 and 50 µmol/l. With the increase of concentration and time, the inhibition rate of proliferation was significantly increased in a significant concentration-dependent manner. The results in this study showed that the inhibition rate of EC-109 growth and proliferation was 49.82% under the action of zerumbone in the concentration of 40 µmol/l for 48 h. Therefore, 40 µmol/l and 48 h were selected as the administration concentration and dosage in this experiment (Table II).
Table II.
Effects of zerumbone in different concentrations on the inhibition of EC-109 proliferation (mean ± SD, %).
| Proliferation inhibition rate (%) | |||
|---|---|---|---|
| Concentration (µmol/l) | 24 h | 48 h | 72 h |
| Control group | 0 | 0 | 0 |
| 30 | 3.35±0.31a | 16.21±2.31a | 28.25±3.43a |
| 40 | 6.68±1.52a | 49.82±3.58a | 59.43±4.25a |
| 50 | 15.54±1.12a | 59.77±3.56a | 70.53±4.79a |
P<0.01, compared with control group.
Effect of zerumbone on apoptosis of EC-109 cells
Compared with that in control group, the yellow green-stained cells in nucleus were apoptotic cells under the action of zerumbone in the concentration of 30, 40 and 50 µmol/l for 48 h. The results revealed that the number of apoptotic cells increased gradually with the increase of administration concentration (Fig. 1).
Figure 1.
Effect of zerumbone on apoptosis of EC-109 cells.
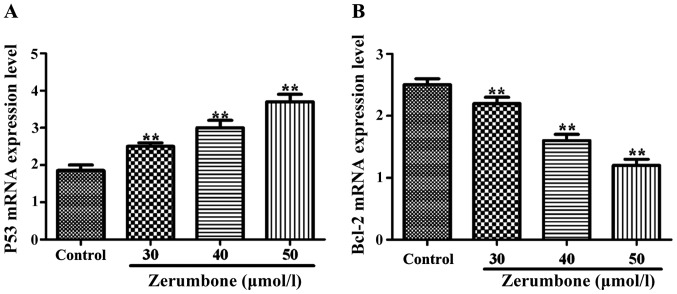
Effects of zerumbone on mRNA levels of P53 and Bcl-2
Compared with those in control group, under the action of zerumbone in the concentration of 30, 40 and 50 µmol/l for 48 h, the mRNA expression level of P53 in each group was obviously increased (P<0.05), but the mRNA expression level of Bcl-2 was significantly decreased (P<0.01) in a significant concentration-dependent manner (Fig. 2).
Figure 2.
Detection of the effect of zerumbone on mRNA expression levels of P53 and Bcl-2 in EC-109 cells via RT-PCR. (A) mRNA expression of P53; (B) mRNA expression of Bcl-2. **P<0.01, compared with control group.
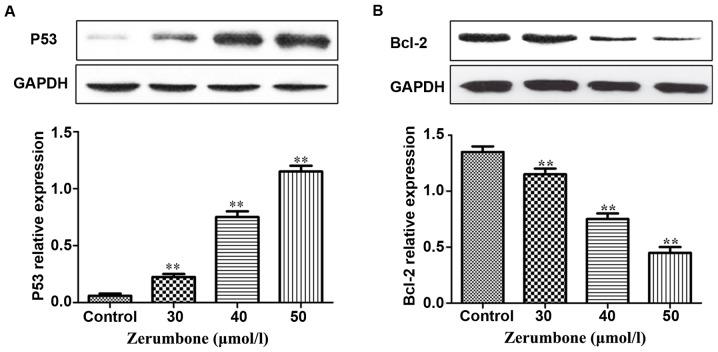
Effects of zerumbone on protein expression levels of P53 and Bcl-2
Compared with those in control group, under the action of zerumbone in the concentration of 30, 40 and 50 µmol/l for 48 h, the protein expression level of P53 in each group was remarkably increased with the increase of zerumbone concentration, but the protein expression level of Bcl-2 was remarkably decreased in a significant concentration-dependent manner (Fig. 3).
Figure 3.
Detection of the effect of zerumbone on protein expression levels of P53 and Bcl-2 in EC-109 cells via western blotting. (A) Protein expression of P53; (B) protein expression of Bcl-2. **P<0.01, compared with the control group.
Discussion
Esophageal cancer has a high incidence rate in certain regions in China, and the clinical surgical treatment, combined with radiotherapy and chemotherapy, is still the preferred choice for the treatment of esophageal cancer, but its curative effect still needs improvement. Zerumbone is a kind of sesquiterpene substance extracted from the rhizome of wild Zingiber zerumbeta with antitumor, anti-inflammation and other pharmacological activities. It has been reported that zerumbone is effective for many cancer cells, such as colon cancer, lung cancer (6), leukemia (7) and liver cancer (8), but there is little research on the biological activity of zerumbone on esophageal cancer. In this experiment, the effects of zerumbone on the proliferation and apoptosis of esophageal cancer EC-109 cells and the possible mechanism in apoptosis process were studied preliminarily.
The wild-type P53 gene can monitor the abnormalities of cell genome and play a negative regulatory role in the cell growth process. When the DNA in cells or cells tend to be cancerous, the P53 gene can remove them in time, thus playing an anticancer effect (9–11); but if the P53 gene mutation occurs, it will lose such a regulatory effect and mutate from the cancer cell-removing gene into the oncogene, thus being transformed from normal cells into cancer cells. Studies have shown that P53 gene locus mutation occurs in more than 60% of gastric cancer; the wild-type P53 protein is unstable in the normal physiological conditions, whose half-life is very short, so it is difficult to be detected via ordinary immunological detection. On the contrary, after the P53 protein mutation, its half-life is extended, and its stability is also increased significantly, so that the immunological detection can detect the mutant P53 protein (12–14). A large number of studies have found that the P53 protein is overexpressed in colorectal cancer, lung cancer, gastric cancer, liver cancer and other tumor cells; moreover, the P53 gene is also widely used in the gene therapy at present (15–18). Bcl-2 gene has the biological function of inhibiting apoptosis (19), and plays an important role in the mechanism of apoptosis, which can protect cells from various forms of death and improve cell survival, thus increasing the number of cells. In some tumor cells, when the expression of Bcl-2 gene is inhibited, it will cause tumor cell apoptosis (20), indicating that Bcl-2 gene has a very close relationship with tumors.
The results of CCK-8 showed that different concentrations of zerumbone could inhibit the activity of EC-109 compared with that in control group, and the proliferation inhibition rate was significantly increased with the increase of concentration in a concentration-independent manner. TUNEL staining revealed that the cell apoptosis began to occur gradually in the administration group, and the number of apoptotic cells was increased with the increase of concentration in a concentration-independent manner. The results of RT-PCR detection showed that with the increase of zerumbone concentration, the mRNA expression level of P53 was gradually increased, but that of Bcl-2 was gradually decreased. Moreover, the results of western blotting showed that with the increase of zerumbone concentration, the protein expression level of P53 was gradually increased, but that of Bcl-2 was gradually decreased, suggesting that zerumbone can upregulate the protein expression of P53 and downregulate the protein expression of Bcl-2, thus inducing apoptosis. Similar to this study, antitumor drugs significantly increase the expression of P53 in lung cancer cells (21). Xi et al (22) studied and revealed that some antitumor drugs can induce tumor cell apoptosis through downregulating the Bcl-2 expression. For example, carnosol can decrease the Bcl-2 protein expression in leukemia cells by 34–53%.
In conclusion, it was proved in this study that zerumbone can inhibit the proliferation of esophageal cancer EC-109 cells and induce the occurrence of apoptosis. Moreover, its induction of apoptosis may be realized by upregulating the expression of P53 and downregulating the expression of Bcl-2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SM was a major contributor in writing the manuscript and CCK-8 test. YL made contributions to cell culture. LZ helped with data analysis. JW performed TUNEL method. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, He Y, Zheng R, Zhang S, Zeng H, Zou X, He J. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis. 2013;5:19–26. doi: 10.3978/j.issn.2072-1439.2013.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Olaku O, White JD. Herbal therapy use by cancer patients: A literature review on case reports. Eur J Cancer. 2011;47:508–514. doi: 10.1016/j.ejca.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami A, Tanaka T, Lee JY, Surh YJ, Kim HW, Kawabata K, Nakamura Y, Jiwajinda S, Ohigashi H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int J Cancer. 2004;110:481–490. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]
- 5.Abdul AB, Abdelwahab SI, Bin Jalinas J, Al-Zubairi AS, Taha MM. Combination of zerumbone and cisplatin to treat cervical intraepithelial neoplasia in female BALB/c mice. Int J Gynecol Cancer. 2009;19:1004–1010. doi: 10.1111/IGC.0b013e3181a83b51. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 7.Xian M, Ito K, Nakazato T, Shimizu T, Chen CK, Yamato K, Murakami A, Ohigashi H, Ikeda Y, Kizaki M. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98:118–126. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakinah SA, Handayani ST, Hawariah LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiman KG. Pharmacological reactivation of mutant p53: From protein structure to the cancer patient. Oncogene. 2010;29:4245–4252. doi: 10.1038/onc.2010.188. [DOI] [PubMed] [Google Scholar]
- 10.Farnebo M, Bykov VJ, Wiman KG. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun. 2010;396:85–89. doi: 10.1016/j.bbrc.2010.02.152. [DOI] [PubMed] [Google Scholar]
- 11.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado-Silva A, Perrier S, Bourdon JC. p53 family members in cancer diagnosis and treatment. Semin Cancer Biol. 2010;20:57–62. doi: 10.1016/j.semcancer.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Chari NS, Pinaire NL, Thorpe L, Medeiros LJ, Routbort MJ, McDonnell TJ. The p53 tumor suppressor network in cancer and the therapeutic modulation of cell death. Apoptosis. 2009;14:336–347. doi: 10.1007/s10495-009-0327-9. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 15.Tang NP, Wu YM, Wang B, Ma J. Systematic review and meta-analysis of the association between P53 codon 72 polymorphism and colorectal cancer. Eur J Surg Oncol. 2010;36:431–438. doi: 10.1016/j.ejso.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Tam CW, Liu VW, Leung WY, Yao KM, Shiu SY. The autocrine human secreted PDZ domain-containing protein 2 (sPDZD2) induces senescence or quiescence of prostate, breast and liver cancer cells via transcriptional activation of p53. Cancer Lett. 2008;271:64–80. doi: 10.1016/j.canlet.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Oguztüzun S, Aydin M, Demirag F, Yazici U, Ozhavzali M, Kiliç M, Işcan M. The expression of GST isoenzymes and p53 in non-small cell lung cancer. Folia Histochem Cytobiol. 2010;48:122–127. doi: 10.2478/v10042-008-0084-6. [DOI] [PubMed] [Google Scholar]
- 18.Baker L, Quinlan PR, Patten N, Ashfield A, Birse-Stewart-Bell LJ, McCowan C, Bourdon JC, Purdie CA, Jordan LB, Dewar JA, et al. p53 mutation, deprivation and poor prognosis in primary breast cancer. Br J Cancer. 2010;102:719–726. doi: 10.1038/sj.bjc.6605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cory S, Huang DCS, Adams JM. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 20.Packham G, Cleveland JL. c-Myc and apoptosis. Biochim Biophys Acta. 1995;1242:11–28. doi: 10.1016/0304-419x(94)00015-t. [DOI] [PubMed] [Google Scholar]
- 21.Dworakowska D, Jassem E, Jassem J, Boltze C, Wiedorn KH, Dworakowski R, Skokowski J, Jaśkiewicz K, Czestochowska E. Prognostic value of cyclin D1 overexpression in correlation with pRb and p53 status in non-small cell lung cancer (NSCLC) J Cancer Res Clin Oncol. 2005;131:479–485. doi: 10.1007/s00432-005-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi S, Dyer KF, Kimak M, Zhang Q, Gooding WE, Chaillet JR, Chai RL, Ferrell RE, Zamboni B, Hunt J, et al. Decreased STAT1 expression by promoter methylation in squamous cell carcinogenesis. J Natl Cancer Inst. 2006;98:181–189. doi: 10.1093/jnci/djj020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.