Abstract
Long non-coding RNAs (lncRNAs) have been demonstrated to be involved in different types of cancer, including gastric cancer. Although altered lncRNAs profiles have been observed in or around gastric cancer tissues, the diagnostic value of circulating lncRNAs in gastric cancer remains unclear. In the present study, a number of highly expressed lncRNAs, including uc001lsz, GACAT2, ABHD11-AS1, GACAT3, SUMP1P3, CHET1, TUG1, SNHG12, GAS5, PVT1, LINC00152, HOTAIR, CCAT1, H19, HULC and ZNFX1-AS1, were investigated as potential minimally invasive biomarkers for this tumor. Preliminary screening experiments revealed that ZNFX1-AS1 and HULC were differentially expressed in the plasma of gastric cancer patients and healthy control subjects. The study further examined the relative expression of ZNFX1-AS1 and HULC in the plasma of 50 matching preoperative and postoperative patients, 50 gastrointestinal stromal tumor (GIST) patients, 50 gastritis/peptic ulcer patients and 50 healthy control subjects through reverse transcription-quantitative polymerase chain reaction. The correlation of lncRNA relative expression with the general characteristics and clinicopathological factors was analyzed. It was observed that the levels of ZNFX1-AS1 and HULC in the plasma of preoperative patients were markedly higher compared with those in the plasma of GIST patients, gastritis/peptic ulcer patients and healthy control subjects, while no significant difference was detected among these three groups. Receiver operating characteristic curve analysis was also conducted to distinguish gastric cancer patients from healthy control subjects. The area under the curve was 0.85 and 0.65 for ZNFX1-AS1 and HULC, respectively. In conclusion, the results indicated that the lncRNAs ZNFX1-AS1 and HULC are promising in the clinical diagnosis of gastric cancer.
Keywords: gastric cancer, long non-coding RNA, HULC, ZNFX1-AS1, tumor marker
Introduction
Gastric cancer is the third leading cause of mortality associated with cancer and the fifth most common neoplasm worldwide, constituting a serious public health problem. Annually, ~1,000,000 new gastric cancer cases are diagnosed and ~700,000 patients succumb to this disease, accounting for approximately 10% of the cancer-associated mortalities worldwide (1). The progression-free survival and prognosis of gastric cancer are highly dependent on the disease stage at diagnosis. Its high mortality rate is correlated with a lack of standard screening programs and the absence of clear symptoms at early stages (1). Therefore, the identification of gastric cancer biomarkers is vital for early diagnosis and early treatment (2).
Long non-coding RNAs (lncRNAs) are a cohort of non-coding-protein RNA molecules with a length of >200 nucleotides (2). In recent years, several studies have reported that lncRNAs are involved in gene expression and protein modification (3,4), as well as contribute to a variety of genetic diseases (5,6). Studies have also indicated that lncRNAs demonstrate distinct regulation of transcriptional patterns in malignant tumors (3), certain of which are involved in tumor metastasis, cell invasion and poor prognosis (6,7). It has been demonstrated that specific lncRNAs, such as H19 and HOTAIR, serve an important role in gastric cancer (8,9). For instance, through the tumor suppressor runt-related transcription factor 1, H19 induced production of microRNA-675 regulated gastric cancer cell proliferation (9). In addition, the overexpression of HOTAIR may be involved in tumor escape mechanisms (8). These observations strongly revealed that lncRNAs are a molecular etiology of gastric cancer. Notably, the detection of circulating lncRNAs provided a novel type of plasma biomarkers for gastric cancer (10), which are promising for the monitoring and screening of gastric cancer patients.
In the present study, the levels of lncRNAs that are highly expressed in the tissues of gastric cancer (11,12) were analyzed in the plasma of gastric cancer patients and age-matched healthy control subjects. Subsequently, the expression levels of these lncRNAs were compared in preoperative, postoperative, gastrointestinal stromal tumor (GIST) and gastritis/peptic ulcer patients, and healthy control subjects. The study also investigated the potential association between plasma lncRNAs levels and the clinicopathological features of gastric cancer patients, including tumor site, tumor size, pathological differentiation, TNM classification and lymphatic invasion. The results revealed that the circulating lncRNAs HULC and ZNFX1-AS1 are potential biomarkers for gastric cancer patients.
Materials and methods
Study design
Patients were excluded if they had previous or coexisting cancer or had undergone gastrectomy for benign tumors. Tumor site was assessed by NCCN Clinical Practice Guidelines in Oncology: Version I 2013 (13). Pathological differentiation and histological type were determined by WHO Classification of Tumors of the Digestive System: 4th edition (14). TNM classification and Lymphatic invasion were evaluated by AJCC Staging Manual: 7th edition (15). Subtotal gastrectomy or tumor resection was performed for the gastric cancer patients. The current study consisted of two sequential phases. In the initial phase, the levels of several lncRNAs highly expressed in tissues, including uc001lsz, GACAT2, ABHD11-AS1, GACAT3, SUMP1P3, CHET1, TUG1, SNHG12, GAS5, PVT1, LINC00152, HOTAIR, CCAT1, H19, HULC and ZNFX1-AS1, were assessed in the plasma of 10 patients with gastric cancer and 10 healthy control subjects preoperatively and postoperatively. All the plasma samples were collected from inpatients at the Peking University People's Hospital (Beijing, China) between July 1, 2015 and September 1, 2015. The clinical characteristics for the gastric cancer patients and healthy control subjects were collected. For healthy control subjects, there was no evidence of disease and were age- and gender-matched to the gastric cancer patients. The aim of this analysis was to screen and identify lncRNAs that may have a potential clinical diagnostic value for gastric cancer.
In the subsequent phase of the study, the sample size was expanded to 50 patients with gastric cancer, 50 patients with GIST, 50 patients with gastritis/peptic ulcer and 50 healthy control subjects preoperatively and postoperatively. Plasma and serum samples were simultaneously collected from each patient. All the samples were collected from inpatients at the Peking University People's Hospital (Beijing, China) between September 2, 2015 and November 1, 2015. The levels of two lncRNAs that were considered to be potential biomarkers, including HULC and ZNFX1-AS1, as well as the levels of four traditional biomarkers, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), CYFRA 21-1 (CY211) and neuron-specific enolase (NSE), were respectively assessed in the plasma and serum. In addition, the general characteristics of all patients, including gender, age and clinicopathological factors (tumor site, tumor size, histological type, pathological differentiation, TNM classification and lymphatic invasion) were recorded and are listed in Table I. The aim of this assessment was to confirm the diagnostic value of circulating lncRNAs HULC and ZNFX1-AS1 compared with that of traditional serum biomarkers, and to investigate the potential association between the plasma lncRNA levels and the clinicopathological features of gastric cancer.
Table I.
General characteristics and clinicopathological factors of gastric cancer patients and healthy control subjects.
| Variables | Healthy control (n=50) | Gastritis/peptic ulcer (n=50) | GIST (n=50) | Gastric cancer (n=50) |
|---|---|---|---|---|
| Gender | ||||
| Male | 38 | 35 | 32 | 39 |
| Female | 12 | 15 | 18 | 11 |
| Age (years) | ||||
| Mean | 61 | 60 | 60 | 61 |
| Range | 47–80 | 37–89 | 30–85 | 37–91 |
| Tumor sitea | ||||
| Upper third | – | – | – | 10 |
| Middle third | – | – | – | 12 |
| Lower third | – | – | – | 28 |
| Tumor size (cm) | ||||
| ≥5 | – | – | – | 24 |
| <5 | – | – | – | 26 |
| Histological typeb | ||||
| Adenocarcinoma | – | – | – | 47 |
| Other | – | – | – | 3 |
| Pathological differentiationb | ||||
| Undifferentiated | – | – | – | 1 |
| High | – | – | – | 1 |
| Moderate | – | – | – | 2 |
| Between moderate and poor | – | – | – | 10 |
| Poor | – | – | – | 34 |
| No Result | – | – | – | 2 |
| TNM classificationc | ||||
| I + II | – | – | – | 17 |
| III + IV | – | – | – | 33 |
| Lymphatic invasionc | ||||
| Positive | – | – | – | 35 |
| Negative | – | – | – | 15 |
National Comprehensive Cancer Network guidelines
World Health Organization classification of tumors of the digestive system, and the
American Joint Committee on Cancer staging system.
The current study was approved by the Research Ethics Committee of Peking University People's Hospital. Patient data and samples were treated according to the ethical and legal standards adopted by the Declaration of Helsinki 2013. Written informed consent regarding ethics approval and patient consent was obtained from all participants.
Collection and stock of samples
The plasma and serum samples were collected in BD Vacutainer EDTA tubes and BD Vacutainer SST tubes (BD Biosciences, Franklin Lakes, NJ, USA), respectively. Preoperative samples from patients with gastric cancer were obtained prior to surgery, or at least 2 months after radiotherapy or chemotherapy. Postoperative samples from these patients were collected 7–10 days following surgery. The samples from patients with GIST or gastritis/peptic ulcer were collected prior to any treatment. Heathy control samples were collected randomly from normal subjects. For plasma samples, a special protocol of centrifugation (2,348 × g for 30 min at 4°C; 4,696 × g for 5 min at 4°C; 10,733 × g for 5 min at 4°C) was conducted to prevent the contamination of cellular nucleic acids. The plasma samples were stored in a 3-fold volume of TRIzol® reagent (Qiagen, Inc., Valencia, CA, USA) at −80°C for further analyses. Serum samples were detected directly.
Total RNA extraction and reverse transcription (RT)
According to the manufacturer's protocol, total RNA was extracted from the plasma using the miRNeasy Serum/Plasma kit (Qiagen, Inc.). The concentration and purity of the total RNA were detected with a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNase-free water was then used to dissolve the total RNA, and RT was performed immediately using the PrimeScript RT Master Mix (Takara Bio, Inc., Kyushu, Japan) following the manufacturer's protocol.
Quantitative polymerase chain reaction (qPCR) analysis
Subsequent to RT, qPCR was performed using the TransStart Green qPCR SuperMix (TransGen, Beijing, China) on a LightCycler 480 Instrument II (Roche Diagnostics, Basel, Switzerland) following the manufacturer's protocol. The primers used in qPCR were designed by Primer Premier software, version 4.0 (Premier Biosoft International, Palo Alto, CA, USA) and synthesized by Beijing Sunbiotech Co. Ltd. (Beijing, China). The sequence of all primers, including GAPDH, are listed in Table II. The solution was incubated for 3 min at 93°C, followed by 40 cycles of 1 min at 95°C and 1 min at 60°C. Given that the level of GAPDH lncRNA was observed to be relatively stable in the plasma, GAPDH was selected as the endogenous control for data normalization. For expression calculation, the formula ΔCq=Cqselected lncRNA-CqGAPDH lncRNA was used, where ΔCq was defined as the difference in quantification cycle (Cq) values (13). Each sample was analyzed in triplicate and repeated three times.
Table II.
List of primers used in quantitative polymerase chain reaction.
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GAPDH | ACCCACTCCTCCACCTTTGAC | TGTTGCTGTAGCCAAATTCGTT |
| H19 | TACAACCACTGCACTACCTG | TGGAATGCTTGAAGGCTGCT |
| CCAT1 | CATTGGGAAAGGTGCCGAGA | ACGCTTAGCCATACAGAGCC |
| HOTAIR | GGTAGAAAAAGCAACCACGAAGC | ACATAAACCTCTGTCTGTGAGTGCC |
| HULC | ACTCTGAAGTAAAGGCCGGA | TGCCAGGAAACTTCTTGCTTG |
| LINC00152 | CTCCAGCACCTCTACCTGTTG | GGACAAGGGATTAAGACACACA |
| ZNFX1-AS1 | CCAGTTCCACAAGGTTAC | GCAGGTAGGCAGTTAGAA |
| PVT1 | CTTGAGAACTGTCCTTACG | CAGATGAACCAGGTGAAC |
| GAS5 | CACAGGCATTAGACAGAA | AGGAGCAGAACCATTAAG |
| SNHG12 | GACTTCCGGGGTAATGACAG | GCCTTCTGCTTCCCATAGAG |
| TUG1 | TAGCAGTTCCCCAATCCTTG | CACAAATTCCCATCATTCCC |
| CHET1 | CCCCACAAATGAAGACACT | TTCCCAACACCCTATAAGAT |
| SUMP1P3 | ACTGGGAATGGAGGAAGA | TGAGAAAGGATTGAGGGAAAAG |
| GACAT3 | GGGGGCTTGTTTCTTTGTGTAG | CATTCGGCTCTGACCTCTCAC |
| ABHD11-AS1 | GAACGGGATGAAGCCATTG | GCTGATTCTGGACCTGCTG |
| GACAT2 | TGGATGCTTACAAAGGACTGG | CTGCAATTACGGAAAGAGCTG |
| uc001lsz | GACGGCACCTACTACACCTT | GCTGACCACCTTGTTGTTGAA |
Serum traditional biomarker assay
The levels of traditional biomarkers were assessed by an electrochemiluminescence immunoassay using Combas e601 analyzer (Roche Diagnostics) according to the previous study (16). The normal reference values for these biomarkers were CEA≤4.7 ng/ml, CA19-9≤39.0 U/ml, NSE≤16.3 ng/ml and CY211≤3.3 ng/ml. When the serum levels were identified to be greater than the reference values, the patients were considered to be positive for CEA, CA19-9, NSE or CY211.
Statistical analysis
The relative lncRNA expression levels in the plasma were calculated using the 2−ΔΔCq formula (17). All the relative lncRNA expression levels in the plasma were assessed according to this formula. Statistical differences in lncRNA expression levels in the postoperative, preoperative, GIST, gastritis/peptic ulcer and healthy control groups were analyzed by Student's t-test. Correlations between lncRNA expression levels and clinicopathological factors were analyzed by non-parametric tests. Furthermore, receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic value of circulating lncRNAs and traditional serum biomarkers in differentiating between gastric cancer patients and healthy subjects. SPSS software, version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Differences with a P<0.05 were considered as statistically significant.
Results
Relative expression of a selected subset of plasma lncRNAs in 10 pairs of gastric cancer patients and 10 healthy control subjects
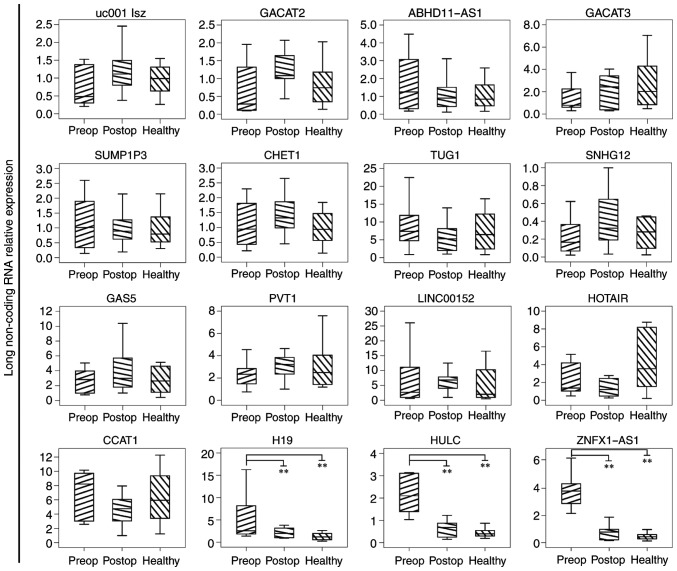
A total of 10 pairs of preoperative and postoperative gastric cancer patients, as well as 10 healthy control subjects, were enrolled into the present study. The general characteristics and clinicopathological factors of all subjects are provided in Table III. There is no significance between the different groups. The relative expression levels of 16 lncRNAs, including uc001lsz, GACAT2, ABHD11-AS1, GACAT3, SUMP1P3, CHET1, TUG1, SNHG12, GAS5, PVT1, LINC00152, HOTAIR, CCAT1, H19, HULC and ZNFX1-AS1, were assessed in the plasma of all these subjects. As shown in Fig. 1, the levels of the lncRNAs H19, HULC and ZNFX1-AS1 in the plasma of preoperative gastric cancer patients was significant higher compared with that in postoperative patients and healthy control subjects (P<0.01). In the present article, as circulating H19 has been demonstrated to be a potential biomarker in a previous study in 2013 (10), the current study focused on plasma HULC and ZNFX1-AS1.
Table III.
General characteristics and clinicopathological factors of gastric cancer patients and healthy control subjects.
| Variables | Gastric cancer (n=10) | Healthy control (n=10) |
|---|---|---|
| Sex | ||
| Male | 7 | 7 |
| Female | 3 | 3 |
| Age (years) | ||
| Mean | 61 | 62 |
| Range | 48–70 | 47–80 |
| Tumor sitea | ||
| Upper third | 1 | – |
| Middle third | 5 | – |
| Lower third | 4 | – |
| Tumor size (cm) | ||
| ≥6 | 4 | – |
| <6 | 6 | – |
| Histological typeb | ||
| Adenocarcinoma | 10 | – |
| Other | 0 | – |
| Pathological differentiationb | ||
| High and moderate | 2 | – |
| Poor | 8 | – |
| TNM classificationc | ||
| I + II | 2 | – |
| III + IV | 8 | – |
| Lymphatic invasionc | ||
| Positive | 8 | – |
| Negative | 2 | – |
National Comprehensive Cancer Network guidelines
World Health Organization classification of tumors of the digestive system, and the
American Joint Committee on Cancer staging system.
Figure 1.
Relative expression levels of a selected subset of lncRNAs in the plasma of 10 patients with gastric cancer and 10 healthy control subjects preoperatively and postoperatively, which assessed by quantitative polymerase chain reaction. Box-plot diagrams of the lncRNA relative expression are shown. The upper limits of the boxes, lower limits and the line inside the boxes indicate the 75th percentiles, 25th percentiles and median value, respectively. Bars represent the minimum and maximum values. P-value was determined using the t-test. **P<0.01. lncRNA, long non-coding RNA.
Relative expression of the lncRNAs HULC and ZNFX1-AS1 in the plasma of preoperative gastric cancer patients, GIST patients, gastritis/peptic ulcer patients and healthy control subjects
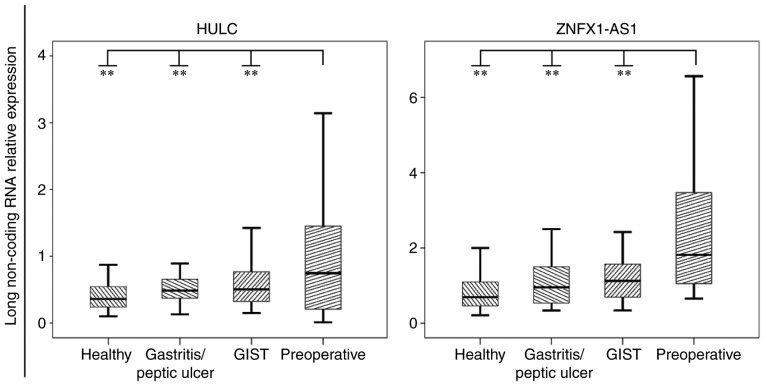
The general characteristics and clinicopathological factors of 50 preoperative gastric cancer, 50 GIST, 50 gastritis/peptic ulcer and 50 healthy control subjects are summarized in Table I. The relative expression levels of HULC and ZNFX1-AS1 were assessed by qPCR in the plasma of all subjects. As shown in Fig. 2, the levels of the lncRNAs HULC and ZNFX1-AS1 in the plasma of preoperative patients were significant higher compared with those detected in the other three groups (P<0.01).
Figure 2.
Relative expression levels of the lncRNAs HULC and ZNFX1-AS1 in the plasma of preoperative gastric cancer (n=50), GIST (n=50) and gastritis/peptic ulcer (n=50) patients, as well as in healthy control subjects (n=50), assessed by quantitative polymerase chain reaction. Box-plot diagrams of the relative lncRNA expression levels are shown. The upper limits of the boxes, lower limits and the line inside the boxes indicate the 75th percentiles, 25th percentiles and median value, respectively. Bars represent the minimum and maximum values. P-value was determined using the t-test. **P<0.01. lncRNA, long non-coding RNA; GIST, gastrointestinal stromal tumor.
Relative expression of the lncRNAs HULC and ZNFX1-AS1 in the plasma of preoperative gastric cancer patients with different TNM stages
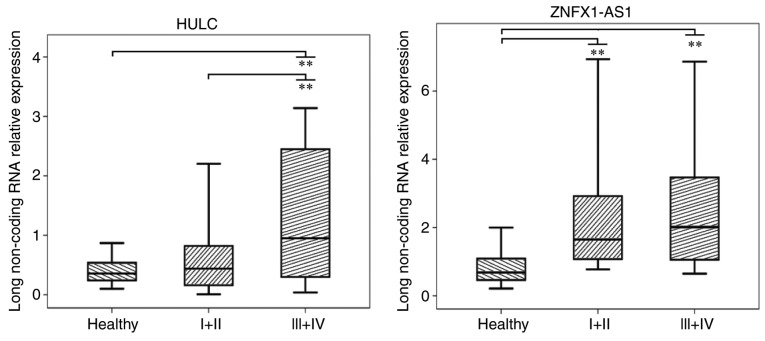
The 2−∆∆Cq method was used to assess the relative expression levels of the lncRNAs HULC and ZNFX1-AS1 in the plasma of patients with different TNM stages, including Stage I/II (n=17) and Stage III/IV (n=33) patients. As shown in Fig. 3, in healthy controls, Stage I/II patients and Stage III/IV patients, the median values of the relative expression of HULC were 0.36, 0.44 and 0.95, respectively, while these values were 0.69, 1.65 and 2.01 for ZNFX1-AS1, respectively. For lncRNA HULC, the relative expression in patients with Stage III/IV disease was significantly higher when compared with that in Stage I/II patients and healthy controls (P<0.01; Fig. 3). For lncRNA ZNFX1-AS1, the relative expression in patients with Stage I/II or Stage III/IV disease was significantly higher as compared with the healthy controls (P<0.01; Fig. 3).
Figure 3.
Relative expression of lncRNAs HULC and ZNFX1-AS1 in the plasma of patients with different TNM stages, including Stage I+II (n=17) and Stage III+IV (n=33) preoperative patients, as well as in healthy controls (n=50). Box-plot diagrams of the relative lncRNA expression levels are shown. The upper limits of the boxes, lower limits and the line inside the boxes indicate the 75th percentiles, 25th percentiles and median value, respectively. Bars represent the minimum and maximum values. P-value was determined using the t-test. **P<0.01. lncRNA, long non-coding RNA.
Relative expression of the lncRNAs HULC and ZNFX1-AS1 in 50 matching of preoperative and postoperative gastric cancer patients
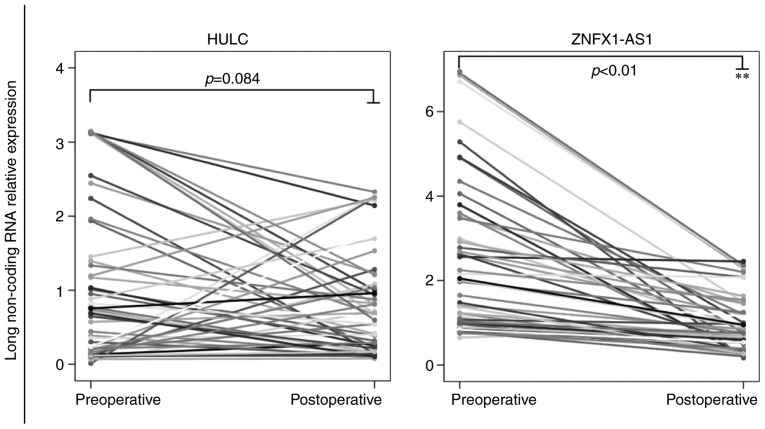
A total of 50 matching preoperative and postoperative plasma samples were included in the present study. It was observed that the relative expression levels of HULC in the plasma exhibited no difference between preoperative and postoperative gastric cancer patients, as assessed by paired t-test (P=0.084). However, the ZNFX1-AS1 levels decreased in 45/50 gastric cancer patients (90%) after surgery in comparison with the preoperative levels (P<0.01; Fig. 4).
Figure 4.
Expression levels of lncRNAs HULC and ZNFX1-AS1 in 50 matching preoperative and postoperative plasma samples. Each endpoint represents the preoperative or postoperative relative expression of lncRNAs HULC and ZNFX1-AS1 in the plasma of patients, while the line connecting the two endpoints indicates the up- or downregulation tendency of the lncRNA relative expression. P-value was determined using the paired t-test. **P<0.01. lncRNA, long non-coding RNA.
Correlation between plasma lncRNA (HULC and ZNFX1-AS1) levels and the clinicopathological characteristics of patients with gastric cancer
As shown in Table IV, no statistical correlation was detected between the plasma HULC levels of gastric cancer patients and clinicopathological features, with the exception of the TNM classification. The relative expression of HULC in advanced gastric cancer patients was significantly higher in comparison with that in patients at an early stage of the disease (P<0.01). For the lncRNA ZNFX1-AS1, gastric cancer patients with positive lymphatic invasion had a much higher relative expression in their plasma as compared with those with negative lymphatic invasion (P<0.01). Although a statistically significant difference was not detected for tumor size, the data demonstrated a tendency toward higher ZNFX1-AS1 levels in patients with a larger tumor size (P=0.051).
Table IV.
Correlation of the relative expression levels of the long non-coding RNAs HULC and ZNFX1-AS1 with the clinicopathological factors of patients.
| HULC relative expression | ZNFX1-AS1 relative expression | ||||
|---|---|---|---|---|---|
| Variables | No. of cases | Mean ± SD | P-value | Mean ± SD | P-value |
| Sex | |||||
| Male | 39 | 1.082±1.071 | 0.797 | 2.397±1.757 | 0.993 |
| Female | 11 | 1.053±1.012 | 2.478±1.813 | ||
| Age (years) | |||||
| ≤61 | 28 | 0.963±1.007 | 0.545 | 2.719±1.984 | 0.087 |
| ≥62 | 22 | 1.219±1.106 | 2.027±1.348 | ||
| Tumor sitea | |||||
| Upper third | 10 | 1.157±1.248 | 0.301 | 1.953±1.482 | 0.572 |
| Middle third | 12 | 0.666±0.465 | 2.305±1.417 | ||
| Lower third | 28 | 1.223±1.135 | 2.626±1.971 | ||
| Tumor size | |||||
| ≥5 cm | 24 | 1.277±1.097 | 0.448 | 2.586±2.009 | 0.051 |
| <5 cm | 26 | 0.890±0.986 | 2.256±1.498 | ||
| Pathological differentiationb | |||||
| Undifferentiated or poor | 35 | 1.142±1.096 | 0.931 | 2.628±1.870 | 0.100 |
| High and moderate | 15 | 0.922±0.945 | 1.917±1.365 | ||
| TNM classificationc | |||||
| I + II | 17 | 0.638±0.669 | 0.003 | 2.516±2.004 | 0.709 |
| III + IV | 33 | 1.301±1.143 | 2.366±1.638 | ||
| Distant metastasisc | |||||
| Positive | 4 | 1.396±1.045 | 0.987 | 2.344±1.890 | 0.968 |
| Negative | 46 | 1.048±1.056 | 2.421±1.761 | ||
| Lymphatic invasionc | |||||
| Positive | 35 | 1.137±1.093 | 0.42 | 2.812±1.917 | 0.001 |
| Negative | 15 | 0.933±0.958 | 1.486±0.697 | ||
National Comprehensive Cancer Network guidelines
World Health Organization classification of tumors of the digestive system, and the
American Joint Committee on Cancer staging system.
Diagnostic accuracy of plasma HULC, ZNFX1-AS1 and traditional serum biomarkers
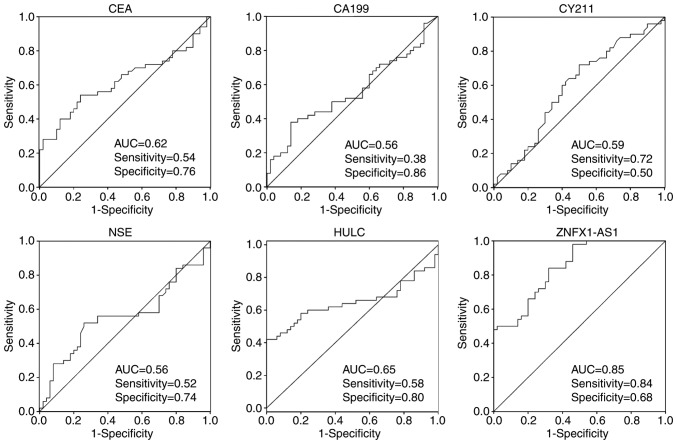
Regarding the potential of markers for discriminating gastric cancer patients from healthy control subjects, the ROC analyses revealed that the area under the curve (AUC) values of plasma HULC and ZNFX1-AS1 were 0.65 and 0.85, respectively (Fig. 5). The highest AUC value of traditional serum biomarkers for this discrimination was observed for CEA (AUC=0.62), while the AUC value of the remaining three traditional biomarkers were lower. The highest accuracy was at a cut-off expression value of 0.63 for HULC and 0.97 for ZNFX1-AS1, where the sensitivity and specificity to identify a patient with gastric cancer were 0.58 and 0.80 for HULC, respectively, and 0.84 and 0.68 for ZNFX1-AS1, respectively (Fig. 5).
Figure 5.
Receiver operating characteristic curves of the lncRNAs HULC and ZNFX1-AS1, and of traditional serum biomarkers (CEA, CA19-9, CY211 and NSE), attempting to discriminate gastric cancer patients from healthy control subjects. The curves were plotted to examine the diagnostic potential and discriminatory accuracy of HULC, ZNFX1-AS1, CEA, CA19-9, CY211 and NSE. The corresponding AUC, sensitivity and specificity values are reported. lncRNA, long non-coding RNA; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CY211, CYFRA 21-1; NSE, neuron-specific enolase; AUC, area under the curve.
Discussion
Gastric cancer is one of the most malignant types of cancer and tends to be asymptomatic in the early stages of the disease, frequently resulting in detection when the tumor has progressed to an advanced stage (18). Thus, improving the early discovery, diagnosis and treatment is essential to increase the survival rate of gastric cancer patients. Certain serum biomarkers, such as CA-125 and CEA, are known to have a potential capacity to detect specific types of cancer at an early stage (19). However, the sensitivity and specificity of such traditional serum biomarkers for gastric cancer is low and does not satisfy the clinical diagnostic requirements (20). Therefore, more sensitive and specific biomarkers for gastric cancer are required, particularly in China, where a high prevalence of gastric cancer is reported (21).
Increasing evidence has indicated that lncRNAs serve an important role in gastric cancer occurrence, invasion and distant metastasis by regulating gene expression or signaling pathways (18). However, the association between the expression levels of circulating lncRNAs and the clinicopathological characteristics has been fully clarified to date. The present study, to the best of our knowledge, is the first to focus on the comparison of circulating HULC and ZNFX1-AS1 with the clinicopathological characteristics of gastric cancer. As circulating lncRNAs originate from apoptotic tumor cells and the absolute content is low, several lncRNAs that are highly expressed in tissues were select for qPCR assessment. The lncRNAs HULC and ZNFX1-AS1 in gastric cancer cells were released into peripheral blood through exosomes, which resulted in elevated HULC and ZNFX1-AS1 levels in the peripheral blood (22). Highly expressed HULC and ZNFX1-AS1 will further activate the Wnt/β-catenin signaling pathway and then promote gastric cancer cell proliferation (19,23). The data of the present study revealed that circulating H19, HULC and ZNFX1-AS1 may be potential biomarkers for the diagnosis of gastric cancer at an early stage of the disease. As circulating H19 has been demonstrated to be a potential biomarker in a previous study in 2013 (10), the present study focused on plasma HULC and ZNFX1-AS1.
To confirm the clinical diagnostic value of HULC and ZNFX1-AS1 in gastric cancer, the sample size of cancer and non-malignant disease patients was expanded in subsequent analyses. The data demonstrated that the relative expression levels of HULC and ZNFX1-AS1 in the plasma of gastric cancer patients were significantly higher when compared with those in the plasma of GIST, gastritis/peptic ulcer and healthy control subjects, as shown in Fig. 2. Furthermore, the diagnostic value of traditional serum biomarkers, including CEA, CA19-9, CY211 and NSE, was examined in the present study, and their specificity and sensitivity values were observed to be similar to those reported previously in the literature (24). ROC curve analysis was subsequently performed in the current study to compare the diagnostic value of circulating HULC and ZNFX1-AS1 with that of traditional tumor markers. The results indicated that the diagnostic value of HULC and ZNFX1-AS1 was much superior in comparison with that of traditional biomarkers. To further examine the diagnostic value for early stage gastric cancer, the relative expression levels in the plasma of TNM stage I/II and III/IV patients were analyzed. As shown in Fig. 3, no significant difference in ZNFX1-AS1 level was detected between different stages. However, for HULC, the relative expression in advanced gastric cancer patients was much higher as compared with that in early stage patients. These findings suggested that the expression of circulating ZNFX1-AS1 is stable even in the early stage of gastric cancer, whereas the relative expression of HULC was increased in the advanced stage of the disease. Therefore, circulating ZNFX1-AS1 and HULC may be the novel biomarkers of early and advanced stage gastric cancer, respectively.
To clarify the effect of surgical treatment on the circulating HULC and ZNFX1-AS1 levels, the relative expression levels of in the plasma of 50 matching preoperative and postoperative gastric cancer patients were analyzed using paired t-test in the present study. The relative expression of circulating ZNFX1-AS1 was evidently declined in postoperative patients, while no significant difference was detected for HULC. More specifically, the circulating ZNFX1-AS1 expression in the plasma significantly decreased in 45/50 pairs on day 10 after surgery, whereas the level was not markedly reduced in the remaining 5 patients. The medical history of this patients was assessed, among these 5, 2 patients suffered from a hepatic cyst, 1 patient suffered from drug-induced hepatitis, 1 patient had a history of radical mastectomy and 1 patient presented renal insufficiency. Therefore, it can be deduced that these complicated diseases may affect the circulating ZNFX1-AS1 levels of postoperative patients. However, the association between renal insufficiency and ZNFX1-AS1 remains clear, and further investigations are required to identify the underlying mechanisms and involvement.
To investigate the correlation between the general characteristics and clinicopathological factors of patients with the relative expression of lncRNAs HULC and ZNFX1-AS1, the medical information of patients was collected in the present study. It has been reported that ZNFX1-AS1 is associated with the metastatic progression of hepatocellular carcinoma and dysregulation of breast cancer (25). In addition, it has been indicated that a high level of lncRNA ZNFX1-AS1 in the tissue may function as an unfavorable prognostic biomarker in gastric cancer patients (26). In the present study, it was verified that a high expression level of circulating ZNFX1-AS1 was significantly associated with lymphatic invasion (P<0.01). In addition, there was a tendency for patients with larger tumors to express higher ZNFX1-AS1 levels (P=0.051). By contrast, the lncRNA HULC expression was not correlated with any general characteristics or clinicopathological factors of the patients. It has been reported that HULC was significantly overexpressed in gastric cancer cell lines and tissues (11,12), and that this overexpression was associated with distant metastasis, advanced tumor node metastasis stages and lymph node metastasis. The result of the present study demonstrated that the expression of HULC is not correlated with the patients' clinical characteristics. This may be due to the insufficient release of this lncRNA from tissues into blood or due to the limited sample size.
In conclusion, the present study investigated the expression levels of the circulating lncRNAs HULC and ZNFX1-AS1 in gastric cancer. The relative expression levels of HULC and ZNFX1-AS1 in preoperative patients were significant higher as compared with those in patients with non-malignant disease and healthy control subjects. In addition, the plasma ZNFX1-AS1 levels in patients with gastric cancer were reduced on day 10 after surgery as compared with the preoperative levels. Thus, it is suggested that circulating HULC and ZNFX1-AS1 may be potential biomarkers for the diagnosis of gastric cancer. Furthermore, the plasma ZNFX1-AS1 level may serve as a novel biomarker for prognosis evaluation following surgical treatment, since it was observed to be associated with lymphatic invasion. Further studies are required to confirm the diagnostic value of circulating lncRNAs in gastric cancer and the associated regulating mechanism.
Acknowledgements
The authors would like to thank Peking University People's Hospital Center Laboratory for the experimental process support.
Funding
This study was supported by a grant from the Beijing Municipal Science and Technology Project (grant no. Z141107002514156).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HPX analyzed the experimental data and completed the draft of manuscript. ZLZ completed all of the experiments. YJS collected all of the clinical data. BL and XTZ are responsible for designing of the work and final approval of the version to be published.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Peking University People's Hospital. Written informed consent regarding ethics approval and patient consent was obtained from all participants.
Patient consent for publication
Written informed consent for publication was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Song Y, Chen X, Zhao J, Gao P, Huang X, Xu H, Wang Z. Novel long non-coding RNA RP11-119F7.4 as a potential biomarker for the development and progression of gastric cancer. Oncol Lett. 2015;10:115–120. doi: 10.3892/ol.2015.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauptman N, Glavač D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14:4655–4669. doi: 10.3390/ijms14034655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vance KW, Sansom SN, Lee S, Chalei V, Kong L, Cooper SE, Oliver PL, Ponting CP. The long non-coding RNA Paupar regulates the expression of both local and distal genes. EMBO J. 2014;33:296–311. doi: 10.1002/embj.201386225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C, Zhou XY, Du X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J Transl Med. 2013;11:122. doi: 10.1186/1479-5876-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei D, Song H, Wang K, Lou Y, Sun W, Liu Z, Ding X, Guo J. Up-regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association. Med Oncol. 2013;30:709. doi: 10.1007/s12032-013-0709-2. [DOI] [PubMed] [Google Scholar]
- 8.Song B, Guan Z, Liu F, Sun D, Wang K, Qu H. Long non-coding RNA HOTAIR promotes HLA-G expression via inhibiting miR-152 in gastric cancer cells. Biochem Biophys Res Commun. 2015;464:807–813. doi: 10.1016/j.bbrc.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- 10.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 11.Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: Novel insights into gastric cancer. Cancer Lett. 2015;356:357–366. doi: 10.1016/j.canlet.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang E, Li W, Yin D, De W, Zhu L, Sun S, Han L. c-Myc-regulated long non-coding RNA H19 indicates a poor prognosis and affects cell proliferation in non-small-cell lung cancer. Tumor Biol. 2016;37:4007–4015. doi: 10.1007/s13277-015-4185-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim HL, Puymon MR, Qin M, et al. NCCN Clinical Practice Guidelines in Oncology. 2013 [Google Scholar]
- 14.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Fourth Edition. World Health Organization. 2010;3:417. [Google Scholar]
- 15.Washington K. 7th edition of the AJCC cancer staging manual: Stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 16.Xia CS, Fan CH, Yang TS. Establishing reference intervals for serum alpha-fetoprotein and carcinoembryonic antigen in the normal population. Chin J Lab Diagnos. 2013;448:31–35. (In Chinese) [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Resende C, Thiel A, Machado JC, Ristimäki A. Gastric cancer: Basic aspects. Helicobacter. 2011;16(Suppl 1):S38–S44. doi: 10.1111/j.1523-5378.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- 19.Timms JF, Arslan-Low E, Kabir M, Worthington J, Camuzeaux S, Sinclair J, Szaub J, Afrough B, Podust VN, Fourkala EO, et al. Discovery of serum biomarkers of ovarian cancer using complementary proteomic profiling strategies. Proteomics Clin Appl. 2014;8:982–993. doi: 10.1002/prca.201400063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis) Cancer Biol Med. 2013;10:148–157. doi: 10.7497/j.issn.2095-3941.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.1186/s40880-015-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 23.Liu X, Chen Z, Zhao X, Huang M, Wang C, Peng W, Yin J, Li J, He G, Li X, Zhu X. Effects of IGF2BP2, KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic gastric cancer treated with EOF regimen. Pharmacogenomics. 2015;16:959–970. doi: 10.2217/pgs.15.49. [DOI] [PubMed] [Google Scholar]
- 24.Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: A systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. doi: 10.1007/s10120-013-0259-5. [DOI] [PubMed] [Google Scholar]
- 25.Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani SR, et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA. 2011;17:878–891. doi: 10.1261/rna.2528811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JJ, Chen JT, Yao KH, Hua L, Wang CY, Hu JH. Up-regulated expression of long non-coding RNA ZFAS1 associates with aggressive tumor progression and poor prognosis in gastric cancer patients. Int J Clin Exp Pathol. 2016;9:2059–2063. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.