Abstract
Triclosan (TCS) is an antimicrobial used so ubiquitously that 75% of the U.S.A. population is likely exposed to this compound via consumer goods and personal care products. In September 2016, TCS was banned from soap products following the risk assessment by the U.S.A. Food and Drug Administration (FDA). However, TCS still remains, at high concentrations, in other personal care products such as toothpaste, mouthwash, hand sanitizer, and surgical soaps. Triclosan is readily absorbed into human skin and oral mucosa and found in various human tissues and fluids. The aim of this review was to describe TCS exposure routes and levels as well as metabolism and transformation processes. The burgeoning literature on human health effects associated with TCS exposure, such as reproductive problems was also summarized.
Introduction
Since the invention of antimicrobial chemicals in the mid 1900’s, their incorporation into a multitude of consumer products has significantly increased. Most of these chemicals are added to consumer products in the absence of a fully encompassed toxicological profile. Triclosan (TCS) is an antimicrobial that, since its original use in hospital settings in 1972, has been incorporated into a variety of consumer products including soaps, hand sanitizers, toothpaste, and mouthwash. In 1977, TCS production (covered by the United States Toxic Substances Control Act) was between 0.5 and 1 million pounds per year (Fang et al. 2010). This production increased to 1 to 10 million pounds in 1998 (Fang et al. 2010, FDA 2013). Estimated global production of TCS in 2011 was 14 million pounds, which decreased to 10.5 million pounds in 2015. Between 1999 and 2000, 75% of 178 liquid soaps sampled contained TCS and 30% of over 300 samples of bar soaps contained TCS (Perencevich et al 2001). In the late 2000’s (2008–2010), TCS as an active ingredient was found in 93% of liquid, gel, or foam soaps (FDA 2013). Consumer products containing antimicrobial active ingredients totaled $886 million in total sales (FDA 2013). TCS-containing products at concentrations ranging from 3.5 to 17 mM were sold at the rate of 278 million 16 oz units between September 2008 and 2009, resulting in annual consumption of 132 million liters (FDA 2013).
Interestingly, TCS-containing soap products were not found to provide any additional skin-sanitizing benefits compared to soap not containing TCS (Kim et al. 2015). In addition, TCS was found to produce bacterial resistance via target site modification which decreased the inhibitory effect of this chemical. Bacteria in the environment may also become TCS resistant following environmentally relevant exposure levels (Drury et al. 2013, Nietch et al. 2013). Bacteria in the microbiome might be altered due to TCS. TCS exposure might also result in bacterial resistance to other antimicrobials (Braoudaki and Hilton 2004, Chuanchuen et al. 2001). Increased TCS resistance is also associated with an elevation in resistance to multiple other antibiotics in clinical settings (Chen et al. 2009, Suller and Russell 2000). Narrowe et al (2015) demonstrated in fathead minnows that low nanomolar doses of TCS changed the fish microbiome which recovered after removal of TCS. However, Lawrence et al (2015) using rotating annular reactors to cultivate river biofilms found no recovery after removal of TCS. Recently, in zebrafish, Tal (2017) noted that TCS did not reduce the overall number of microbes but decreases the variety of microbial species. Originally, it was postulated that TCS-mediated inhibition of fatty acid synthase (Levy et al. 1999) did not occur in humans, which was later found to be incorrect (Liu et al. 2002). The fatty acid synthase enzyme enoyl-acyl carrier protein reductase (FabI) is target site for bacterial TCS (Levy et al. 1999), and mutations in this gene lead to resistance mechanisms (Brenwald and Fraise 2003, Yu et al 2010, Ciusa et al. 2012).
In September 2016, TCS was banned by the FDA in soap products (liquid, gel, foam, bar); however, TCS still remains allowed in toothpaste, hand sanitizer, and mouthwash (Kux 2016). The European Union (EU) banned TCS from all human hygiene biocidal products starting January 2017 (Juncker 2016). TCS is widely utilized in toothpaste as this agent helps fight gingivitis (Riley and Lamont 2013; Al Habashneh et al 2017; Muller et al. 2006). Colgate Total toothpaste, containing 10 mM TCS, was the number two leading toothpaste in sales in 2016 (Statista 2016b), with an 11.8% increase in sales from 2015 to 2016 (Statista 2016a). TCS also remains in use in hand sanitizer products within the health care field, postulated to be beneficial due to hand sanitizers’ enhancement of hand washing compliance (Jones et al. 2000). Based upon these statistics, consumers are clearly being exposed to TCS at high concentrations. However, TCS effects on human and environmental health are still currently under debate. Our aim was to provide an overview of recently published epidemiological studies on TCS human health effects. Data are also presented on comparisons of TCS levels in tissues which are relevant because TCS-containing products are typically utilized by consumers by direct application to human tissues. This review summarizes the literature on TCS exposure levels in humans and animals, TCS metabolism, occurrence of TCS, its breakdown products, and the health effects of this chemical.
Triclosan Exposure Levels in Humans
TCS exposure likely occurs primarily via consumer products that contain TCS. Environmental exposure may also occur through water and/or animal/food products contaminated with TCS. Measurable levels of TCS are present in milk and blood of nursing mothers (Allmyr et al. 2006) and human urine (Calafat et al. 2008). TCS was detected in 75% of the U.S. population between 2003 and 2004 with urine concentrations ranging from 7.9 nM - 13.1μM (Calafat et al. 2008). A 2014 study in Quebec City identified TCS in 44 of the 46 human urine samples (Provencher et al. 2014). A recent study in China showed that 80% of 209 participants tested displayed detectable levels of TCS in their urine (Yin et al. 2016). In Queensland, Australia, Heffernan et al (2015) conducted between 2012 and 2013 and found that TCS was detected in all 2,400 urine samples with concentrations ranging from 0.08 – 0.71 µM.
In many of these investigations, females tended to exhibit higher TCS concentrations than males, and the age group with the highest TCS concentrations tended to be in the 20’s (Yin et al. 2016; Heffernan et al. 2015). Weiss et al (2015)in Canada noted TCS in 87% of urine samples from 80 healthy pregnant women. Between 2007 and 2009, a study of expecting mothers in New York found TCS in 100% of the 181 samples and TCS in cord blood from the neonate in 51% of the samples (Pycke et al. 2014). These results indicate that consumers around the world are exposed to TCS, and TCS is readily absorbed into the body.
Body burdens of TCS differ greatly depending upon the (1) site being measured such as skin, blood or urine, (2) concentration of exposure, and (3) type of exposure. After use of mouth rinses or dentifrices that contain TCS, plasma levels of TCS increase rapidly (Sandborgh-Englund et al. 2006; Lin 2000; Bagley and Lin 2000; DeSalva et al 1989). Upon swallowing approximately 1 tablespoon of a mouthwash containing TCS which is analogous to an accidental ingestion of mouthwash, subject plasma TCS levels reached approximately 1 μM within 1 to 3 hr (Sandborgh-Englund et al. 2006). Oral exposure, via 15 ml mouth rinse used for 30 sec containing 0.03% (1 mM) TCS twice daily resulted in total mean plasma levels of 0.26–0.33 μM (Lin 2000). Studies demonstrated that retention of TCS in the oral mucosa is 4–13% when using a mouth rinse containing 0.03% (1 mM) TCS (Lin 2000) and 25% when brushing teeth with 0.2% (7 mM) TCS-containing toothpaste (Gilbert 1987). Today, some orally applied consumer products contain 0.3% (10 mM) TCS, possibly leading to even higher oral retention. Kanetoshi (1992) estimated that tissue levels, after dermal application of TCS, are between 14–67% of the applied TCS dose while human skin TCS concentrations might reach the millimolar levels (Queckenberg et al. 2010; Moss et al 2000).
The above studies demonstrated absorption and retention of TCS following dermal and oral absorption and retention, while other studies reviewed below illustrate the ability of TCS to disrupt biological processes. However, no apparent review article to our knowledge to date has directly compared levels of TCS within body tissues following topical application with antimicrobial cell/tissue levels (reported in the same units) that were found to lead to adverse effects. Most in vivo and epidemiological studies did not report TCS concentrations in the tissue after exposure. Epidemiological studies demonstrated that exposure to TCS may exert adverse health outcomes by presenting correlations between elevated TCS concentrations in urine or blood and detrimental effects. These studies measured TCS concentrations in body fluids rather than in directly-exposed tissues. Presumably, it is not the TCS concentration in urine that is attributed to the toxicity within tissues which manifests as organismal health effects but, rather, it is particular TCS doses within the affected tissues that are responsible for the adverse physiological outcomes. It is noteworthy that the urine concentration is an indirect indicator of TCS levels in the tissue. The TCS concentrations detected within exposed tissue samples which may be expressed as moles of TCS/mg tissue protein may be more relevant for toxicological comparisons than TCS levels present in human fluids because products like toothpaste, mouthwash, soaps, and sanitizers come into direct contact with skin and oral mucosa for min-long exposures of approximately10 mM TCS (Moss et al 2000; Lin 2000; Gilbert et al 1987; Sandborgh-Englund et al. 2006), and up to 25% of the applied TCS is quickly absorbed (Lin 2000; Gilbert 1987).
In order to conduct this comparison, initially data was used from a study that utilized human skin biopsies exposed to triclosan for one hr (Moss et al 2000). The skin biopsies were of height 230 μm and surface area 6.4 × 107 μm2—thus, 1.5 × 1010 μm3 in volume. Assuming a typical mammalian cell radius of 7.5 μm (Barrandon and Green 1985), the volume of a single cell would be 4/3*π*r3 = 1766 μm3. Thus, dividing total test tissue volume of 1.5 × 1010 by individual cell volume of 1766 μm3 results in the finding of approximately 8.3 million human skin cells per test patch. The skin tissue was exposed to 7 μl 64.5 mM TCS, and 3% of the applied dose of parent TCS compound was absorbed and remained in the parent form (not metabolized) in the skin after a one hr exposure (Moss et al 2000); this would be equivalent to approximately 2 mM (0.03*64.5 mM) exposure fully absorbed into the cells. The exposure had been provided via a 7 μl aliquot of TCS applied to the skin patch, such that (7 × 10−6 L)*(2 mmol/L) = 1.4 × 10−8 mol of TCS were absorbed into the skin patch. Since the patch contained about 8.3×106 human skin cells, 1.4 × 10−8 mol of TCS/(8.3×106 cells) = 1.7 × 10−15 mol TCS/cell. Thus, each cell was exposed to 1.7 × 10−15 mol TCS. A cell is 20% protein (by mass), and an average cell mass is 3.5 × 10−6 mg (Lodish et al 2000). Multiplying this quantity (1.7 × 10−15 mol TCS/cell) by (1 mg cell/0.2 mg protein) and also by (cell/3.5×10−6 mg), results in the finding that 2.4 nmol of TCS/mg protein was absorbed into these skin patches. Next, extrapolating down to a 10 mM TCS exposure (which is more likely found in consumer products, compared to 64.5 mM), it was estimated 10/64.5*2.4 nmol TCS/mg protein = approximately 0.4 nmol TCS/ mg protein. Therefore, from Moss et al (2000) data TCS tissue exposure levels from an antimicrobial-containing consumer product is approximately 0.4 nmol TCS/ mg tissue protein.
Manevski et al (2015) exposed human skin explants to 10 μM TCS dissolved in aqueous buffer for one hr and found that 72% of the absorbed TCS remained in the explant as the parent TCS compound, while 28% was metabolized--glucuronidated or sulfated. It was determined that 0.064 nmol of non-metabolized TCS was absorbed into and remained in these skin explants at the end of a one hr exposure per mg human protein within the explant (Manevski et al. 2015). These concentrations were determined based on the activity of TCS metabolite production and the percent of TCS still in parent form in the skin. Subsequently, extrapolating up to a 10 mM TCS (which is more likely found compared to 10 μM, in TCS-containing products), it was estimated 10 mM/10 μM*0.064 nmol TCSC/mg protein = 64 nmol TCS/mg protein. Therefore, roughly 64 nmol TCS/mg protein would be expected to be present in human skin following a one-hr application of a typical TCS product. Thus, these exposure investigations led us to conclude that following exposure to a typical TCS-containing product (10 mM TCS) for one hr, tissue concentrations might range between 0.4 to 64 nmol TCS/mg tissue protein.
Exposure of cells in culture to media containing 10 μM TCS produced adverse cellular effects, including mitochondrial uncoupling, in multiple cell types, within 1 hr (or less) of exposure (Weatherly et al. 2013; 2016; Palmer et al. 2012). In order to compare the 0.4–64 nmol/mg value to in vitro cellular studies of TCS effects, one needs to consider the 200 μl 10 μM TCS that is commonly utilized in an aqueous buffer for 100,000 cells. Multiplying this volume by concentration, dividing by 100,000 cells, and assuming 10% absorption (Lin 2000; Gilbert et al 1987; Moss et al 2000) yields 2 × 10−15 mol TCS per cell, within a one hr treatment period. Multiplying this result by the factors noted above, (1 mg cell/0.2 mg protein) and (cell/3.5×10−6 mg), yields approximately 2.8 nmol of TCS is absorbed per mg of mammalian protein within the cells. Thus, approximately 2.8 nmol TCS exposure/mg cellular protein induced mitotoxicity in rat basophilic leukemia (RBL) mast cells (Weatherly et al. 2016). Specifically using primary human keratinocytes, a significant decrease was noted in mitochondrial ATP production due to an exposure of 3 μM TCS (Weatherly et al. 2016), which corresponds to approximately 0.8 nmol/mg protein. These levels may be overestimates of the amount of TCS that permeates cells because absorption may be less than 10% of the total amount since 200 μl is a large volume compared to the volume of 100,000 cells. Further, organic solvents were not employed to dissolve TCS, which might further suppress the level of absorption into cells. Similar results were found by Newton et al (2005) using direct measurement of TCS absorbed into isolated rat liver mitochondria, where 2.5 nmol of TCS/mg of protein was shown to produce mitochondrial uncoupling. Escarrone et al (2016) detected TCS at 0.49 nmol TCS/mg of protein in fish brain again demonstrating tissue antimicrobial concentrations at levels known to produce adverse effects. Thus, data show that 0.8–2.8 nmol TCS/mg protein doses within cells/tissues induce adverse effects, and these concentrations lie within the 0.4–64 nmol TCS/mg tissue protein levels that result from human dermal exposure to TCS-containing products (Table 1). Evidence thus indicates that human exposure to TCS through consumer products is sufficient to produce adverse effects in cell types such as keratinocytes and oral mucosal cells that are directly exposed to consumer products.
Table 1.
Summary of TCS concentrations in tissues
| Study | Concentration (nmol TCS/mg tissue protein) | Citation |
|---|---|---|
| Human skin biopsies exposed to TCS product for 1 hr (extrapolated to 10 mM) | 0.4 | Moss et al 2000 |
| Human skin explants exposed to TCS product for 1 hr (extrapolated to 10 mM) | 64 | Manevski et al 2015 |
| In vitro cell culture; level which induced mitotoxicity | 0.8 – 2.8 | Weatherly et al 2016 |
| Isolated rat mitochondria; level which produced mitotoxicity | 2.5 | Newton et al 2005 |
| Fish brain | 0.49 | Escarrone et al 2016 |
Overall, TCS was detected in humans globally in an assortment of tissue samples such as blood, urine, and variety of different tissue types. TCS was also retained in the body with a half-life of 21 hr showing that even a brief use of a TCS containing consumer product may result in a prolonged exposure of multiple hr. Due to TCS ability to penetrate and remain in tissues, the tissue antimicrobial concentration is sufficiently high to induce harmful effects to humans.
Metabolism
Absorption
TCS may be absorbed and reach systemic circulation through the mucous membrane of the oral cavity (Lin 2000), by dermal exposure through the skin (Chedgzoy et al 2002; Moss et al 2000), and, after oral exposure, through the gastrointestinal tract (GIT) (Sandborgh-Englund et al. 2006; Lin 2000; Bagley and Lin 2000). Triclosan is readily absorbed through the skin due to its lipophilic properties (Moss et al 2000). TCS is absorbed through dermally in guinea pig (Black et al 1975), mouse (Kanetoshi et al. 1992), rat, and human skin (Moss et al 2000). Absorption of TCS into human skin might occur in the millimolar range with the current level of TCS found in consumer products (Queckenberg et al. 2010). When 1.6 mg TCS is applied directly to the mouse skin, TCS is readily absorbed with peak concentrations occurring between 12 and 18 hr after exposure (Kanetoshi 1992). Moss et al (2000) showed that TCS penetrates through rat skin more quickly than human skin in vitro indicating that antimicrobial exposure to the skin is more prolonged in human than rats. Moss et al (2000) also noted that around 12% of TCS dose is present in human skin in vitro after 24 hr, and 26% of the dose was detected in the stratum corneum in rats in vivo. Triclosan may also undergo buccal absorption, leading to oral and plaque retention and TCS metabolite detection in plasma (Lin 2000). TCS is also absorbed via the GIT after oral exposure reaching a peak concentration in plasma after 1 to 3 hr (Sandborgh-Englund et al. 2006).
Distribution
Human samples that underwent TCS exposure to consumer products were collected and analyzed during 2002 and Geens et al ( 2012) found that liver was the organ with the highest TCS concentration. Adipose tissue had the next highest concentration, followed by the brain with the lowest TCS concentration. Upon TCS application to skin of mice, this compound was detected in all 15 tissues tested 12 hr post application with the highest amounts in gall bladder, bladder, and skin application-site and lowest levels in the testes, thymus, and brain (Fang et al. 2016). In male rats, TCS does not accumulate in the prostate but high levels were present in the epididymis (Lan et al. 2015). In mice undergoing percutaneous absorption, the gall bladder and liver exhibited highest accumulations with 402 and 10.5 μg/g tissue, respectively (Kanetoshi 1992).
Metabolism
Metabolism of a chemical occurs via phase I or phase II; phase I renders the parent compound more polar and increases reactivity for phase II metabolism. Phase II involves conjugation of the chemical with a polar molecule to enhance water solubility. Glucuronidation and sulfonation are involved in phase II reactions where xenobiotics are conjugated with glucuronic acid and sulfate, which elevates their molecular weight and makes the metabolites less active. This process also detoxifies and produces more polar metabolites. TCS undergoes both phase I and II metabolism. TCS undergoes phase I hydroxylation metabolism (Wu et al 2010) and the major phase II pathways for TCS metabolism are glucuronidation and sulfonation (Wu et al 2010; James et al 2012). Glucuronic acid and sulfate are each added to hydroxyl group of TCS (Wu et al. 2016), thereby destroying the proton ionophore nature of TCS and adding a highly charged/polar moiety onto TCS. In a study with 46 participants, both TCS-sulfate and TCS-glucuronide were detected in urine samples (Provencher et al. 2014). The main metabolite found was TCS-glucuronide (Provencher et al. 2014). Lin (2000) in human participants showed after 21 days of using an oral rinse containing 4.5 mg TCS, the mean plasma concentration for TCS-glucuronide was 220–300 μM and for TCS-sulfate was 0.028–0.062 μM. In human HepG2 cells, TCS-glucuronide and TCS-sulfate were detected with a corresponding decrease of parent TCS (Wu et al. 2016). A decrease in TCS cytotoxicity with an rise in glucuronidation and sulfonation metabolism of TCS was reported (Wu et al. 2016). Wang et al (2004) demonstrated that the TCS concentration affects the main route of metabolism. In human liver fractions, following exposure to 1–5 μM TCS for 30 min both TCS-sulfate and TCS-glucuronide were produced equally. However, following dosages above 20 μM TCS, glucuronidation predominated while dosages below 1 μM led to sulfonation (Wang et al 2004).
The liver is the main source of TCS metabolism; however a small amount of TCS was metabolized in skin. When TCS is applied to human skin, TCS-sulfate is the only metabolite in the skin up to 8 hr after application, and both TCS-sulfate and TCS-glucuronide are present at 24 hr post application (Moss et al 2000). Interestingly, 24 hr after exposure only approximately3% of the TCS dose was detected as the TCS metabolites in the skin while approximately 12.5% the dose was present as the parent compound and the remaining TCS distributed elsewhere throughout the body (Moss et al 2000). Approximately 3.5% of the TCS dose was metabolized to TCS-glucuronide and passes through the skin by 24 hr after treatment (Moss et al 2000). At all time points tested, unchanged TCS was the predominate form found in skin at greater levels than any of its metabolites (Moss et al 2000). When TCS was applied dermally to mice, all tissues tested contained the parent TCS compound as the highest detected chemical 12 hr post application (Fang et al. 2016). In contrast, plasma contained only a small amount of TCS parent compound (Fang et al. 2016). TCS-sulfate and TCS-glucuronide metabolites were also present in these tissues but to a lesser extent than parent compound with TCS-sulfate being the major metabolite detected (Fang et al. 2016). The product 2,4-dichlorophenol was also a major metabolite found at the application site, plasma, liver, kidneys, and feces (Fang et al. 2016).
TCS may also undergo metabolism by cytochrome P450 (CYP). In human HepG2-derived cell lines, 7 CYP isoforms were able to metabolize TCS. These include CYP12A, CYP2B6, CYP2C19, CYP2D6, CYP1B1, CYP2C18, and CYP1A1 (Wu et al. 2016). Metabolism via CYP was also shown to diminish TCS cytotoxicity (Wu et al. 2016). In rats, TCS increased the activities of P450-dependent monooxygenases (Kanetoshi et al. 1992) and induced CYP2B subfamily isoforms (Hanioka et al. 1997). All tested doses of TCS (0.2, 0.4, and 0.8 mmol/kg) induced 7-benzyloxyresorufin O-debenzylase and 7-pentoxyresorufin O-depentylase activity (CYP2B1-dependent enzymes mediated by 2B1) (Hanioka et al. 1997). TCS also elevated protein levels of CYP2B in rat liver microsomes (Hanioka et al. 1997). In human cells, CYPs may catalyze the cleavage of the diphenyl ether bond of TCS, leading to the formation of three metabolites: 4-chlorocatechol, 5’-hydroxytriclosan and 2,4-dichlorophenol (Wu et al. 2016). The two metabolites, 2,4-dichlorophenol and 4-chlorocatechol, were found to be less toxic than TCS (5’-hydroxytriclosan was not further assessed for toxicity) (Wu et al. 2016). Tulp et al.(1979) showed that after oral administration in rats, TCS was excreted in feces and urine unchanged but also underwent both aromatic hydroxylation and cleavage of the ether bond of TCS which produced mono-hydroxylated TCS, 2,4-dichlorophenol, and 4-chlorocatechol in the urine and feces.
Elimination
Triclosan main route of elimination in humans is via the urine, and fecal elimination is the secondary route (Sandborgh-Englund et al. 2006; Bagley and Lin 2000; DeSalva et al 1989). The antimicrobial is excreted primarily via its conjugates (Bagley and Lin 2000; Moss et al 2000; DeSalva et al 1989; Black et al 1975). Following oral exposure, urinary excretion increases in humans within 24 hr. During the first 4 days after exposure, between 24 and 83% of the consumed TCS is excreted, and after 8 days excretion approaches baseline levels (Sandborgh-Englund et al. 2006). The half-life of TCS after oral exposure was found to be 21 hr (Sandborgh-Englund et al. 2006). After topical exposure in rats, 12% of the antimicrobial dose was excreted via feces and 1% via urine (Moss et al 2000). Siddiqui (1979) reported a rapid transfer of TCS from blood to tissues and that the compound did not sequester into long-term storage compartments, like fat, in the body (Lin 2000; Bagley and Lin 2000; DeSalva et al 1989).
TCS as a metabolic inhibitor
TCS is a selective inhibitor of the phase II enzymes during glucuronidation and sulfonation of phenolic xenobiotics (Wang et al 2004). TCS inhibits the sulfonation and glucuronidation of p-nitrophenol, acetaminophen, and 3-hydroxybenzo(a)pyrene (3-OH-BaP) at μM concentrations (Wang et al 2004). Of chemicals tested, the most sensitive compound to TCS sulfonation inhibition was 3-OH-BaP with an IC50 (inhibitor concentration) of 2.87 μM, and the least sensitive was acetaminophen with an IC50 of 17.8 μM (Wang et al 2004). TCS was less effective at glucuronidation inhibition with IC50 values for TCS using 3-OH-BaP and acetaminophen as substrates being 4.55 and 297 μM, respectively (Wang et al 2004). The mode of TCS inhibition for sulfonation is postulated to be non-competitive while glucuronidation inhibition is competitive (Wang et al 2004).
Triclosan Exposure Levels in Animals
TCS was also detected in the environment and animals. TCS was present in fish samples from rivers in Germany (Rudel et al. 2013) with mean concentrations in fish muscle tissue ranging from 4.6 to 18.6 ng/g (lipid weight); showing that TCS accumulates in fish tissue (Rudel et al. 2013). Investigators in the U.S. found 25 ng/g (wet weight) TCS in salmon tissue (Yeh et al. 2017, Meador et al. 2016). TCS was also detected in earthworms exposed to biosolid (treated sludge from sewage treatment plants) application for 4 years at concentrations from 15.7–51.4 ng/g (dry weight) (Macherius et al. 2014). Dolphins from both South Carolina and Florida exhibited levels of TCS in plasma (Fair et al. 2009). In South Carolina, 4 of the 13 samples with detectable levels of TCS noted a mean concentration of 0.18 ng/g (wet weight) and dolphins in Florida presented detectable levels in 3 of the 13 samples with a mean concentration of 0.072 ng/g (wet weight) (Fair et al. 2009). Environmental concentrations are discussed below in the “Occurrence” sub-section.
TCS exposure was also shown to produce a variety of adverse ecotoxicological effects on multiple species. TCS decreased the rate of population increase in zooplankton at concentrations above 3.5 nM and modulated hatching rate from 0.35 nM to 0.7 μM TCS (Zhang et al 2016). In earthworms, the compound induced a reduction in juvenile counts, generated DNA strand breaks, and increased heat shock protein (hsp) 70 gene expression (Lin et al. 2014). Sheepshead minnows displayed a delay in both hatching time development and altered thyroid hormone levels following TCS treatment (Schnitzler et al. 2016). TCS induced immunosuppression in clams at low nanomolar concentrations via disruption of hemocytes and induction of DNA fragmentation (Matozzo et al 2012). TCS also produced a change in bioenergetic state in mussels exhibited as a decrease in animal activity via movement but an elevation in protein level detoxification markers (Goodchild et al 2016). At concentrations between 0.2 and 0.5 μM antimicrobial, histological alterations in liver and genes involved in lipid metabolism and detoxification were noted in toad tadpoles (Chai et al. 2017).
Occurrence of Triclosan and Triclosan-methyl
Bodies of Water
Triclosan has been detected in multiple bodies of water around the world. In a study sampling 139 streams in 20 states in the US between 1999 and 2000, TCS was found in 57.6% of the streams sampled (Kolpin et al. 2002). A 41 mile long stream in Indiana showed a similar frequency with TCS detection in 57% of samples taken along the entire stream (mean concentration 0.24 nM, maximal level 0.8 nM) (Bernot et al 2013). This antimicrobial was present at the highest mean concentration of the 17 sampled compounds and at the second highest maximal quantity after acetaminophen (Bernot et al 2013). In a river in Washington, DC, TCS was detected with an average amount of 0.08 nM and maximal concentration of 0.35 nM (Shala and Foster 2010). In Switzerland Lindstrom et al (2002) reported TCS detection in lakes and river samples at concentrations up to 0.48 nM, with lower levels found in the summer probably due to increased photolysis. TCS-methyl, if present at all, was found at lower quantities up to 0.03 nM (Lindstrom et al. 2002), possibly because TCS-methyl is less water-soluble than TCS. The antimicrobial was not detected in water from a mountain lake or in “fossil” groundwater in Switzerland (Lindstrom et al. 2002). In Germany Bester (2005) found 0.01–0.03 nM TCS in surface water. TCS was detected at concentrations up to 0.26 nM in Australian rivers (Ying and Kookana 2007). The antimicrobial was present in higher concentrations in rivers in China ranging from 0.09 to 1.7 nM (Zhao et al. 2010). The half-life of TCS in surface freshwater was estimated to be 11 days (Bester 2005). TCS was also reported to be present in freshwater sediment at concentrations from 800–53,000 μg/kg (Singer et al. 2002).
Wastewater Treatment Plants
Due to triclosan use in such a wide array of products and subsequent disposal via residential drains, this agent undergoes treatment at waste water treatment plants (WWTP). In a conventional WWTP, half of TCS accumulates in the sludge while the remainder is transformed to products such as mTCS (Heidler and Halden 2007). In Ohio, WWTP influent TCS concentrations ranged from 13.1–57.3 nM (McAvoy et al. 2002). The final effluent levels ranged from 0.7–9.3 nM (McAvoy et al. 2002). In Switzerland, TCS was found at concentrations between 2.1–4.5 nM in the influents to a WWTP, while the corresponding effluents contained TCS concentrations of 0.4–2.2 nM (Lindstrom et al. 2002). Triclosan-methyl was detected at levels of ≤ 0.014 nM in the influent (Lindstrom et al. 2002) but was present in higher amounts in the effluent, up to 0.4 nM (Lindstrom et al. 2002), indicating that this antimicrobial is transformed to mTCS during WWTP treatment. In Germany, Bester (2005) noted TCS concentrations in WWTP effluent to range from 1 to 2 nM and mTCS levels were up to 0.07 nM. In Australia, TCS was detected in all 19 WWTP effluent samples (Ying and Kookana 2007). The TCS influent concentrations ranged from 2 to 3 nM but decreased to 0.21 to 0.55 nM in the effluent (Ying and Kookana 2007). In a WWTP in the US, Heidler and Halden (2007) reported TCS levels in biosolids averaged 30,000 μg/kg (dry weight). However, other investigators found lower TCS concentrations in biosolids with an average of 5,880 μg/kg (dry weight) (Davis et al 2012) and approximately 8,000 μg/kg (dry weight) (McAvoy et al. 2002).
The application of biosolids is a common farming practice and, therefore, a potential source of environmental exposure to TCS and mTCS. Factors that might influence the fate of organic chemicals in soil include soil temperature, moisture content, soil texture, and pH (Butler et al. 2012). TCS-methyl is the predominant transformation product of TCS found in soil (Al-Rajab et al. 2009; Wu et al 2009; Butler et al. 2012). In an experiment to measure the degradation and translocation of TCS in three different soil types including loamy sand, sandy clay loam, and clay following sludge incorporation, Butler et al (2012) found that antimicrobial concentrations decrease slowly until the summer months where dissipation increased, further supporting the fact that of TCS undergoes photodegradation. The fall in TCS levels occurred in parallel with a rise in mTCS, suggesting the reduction of TCS was due to biotransformation of TCS to mTCS. Evidence indicated that mTCS is the most significant metabolite in agricultural soils receiving sludge as fertilizer (Butler et al. 2012). Ying et al (2007) TCS noted a half-life of 18 days when the compound was added directly to soil and incubated under aerobic conditions. In soil, TCS was degraded by microbial processes under aerobic conditions but, under anaerobic conditions, resistance to biodegradation was observed (Ying et al 2007). Wu et al (2009) demonstrated that TCS displayed a strong affinity to both silt clay and sandy loam soils and that degradation of TCS was faster in silt clay soil compared to sandy loam soil. Increasing the soil pH from 4 to 8 enabled TCS to dissociate, leading to chemical protonation decreasing from 100 to 39% and diminishing antimicrobial sorption to soil. Based upon a first-order kinetic model, it was estimated the half-life of TCS ranged between 20 and 58 days. Wu et al (2009) indicated that under normal field conditions TCS might be even more stable due to the possibility of anaerobic conditions. Biosolid application containing 10,900 ng/g (dry weight) TCS still contained detectable antimicrobial concentrations 4 years later but at lower concentrations (2.5 ng/g dry weight) (Macherius et al. 2014). Triclosan-methyl was also detected 4 years after application at 9.1 ng/g dry weight (Macherius et al. 2014).
Triclosan and Triclosan-methyl Uptake into Plants from Soil
Treated wastewater is increasingly becoming a source of water for agricultural irrigation, specifically in arid regions (Kinney et al. 2006, Navon et al. 2011). However, personal care products, including TCS and mTCS, are present in the effluent from these treatment plants. Wu et al (2013) found that TCS was detected in both roots of common vegetables (lettuce, spinach, cucumber, and pepper) that were grown for 21 days in a solution containing personal care products. The compound accumulated to a higher degree in roots of lettuce and spinach than in those of cucumber or pepper (Wu et al. 2013). Mathews et al (2014)) showed TCS accumulation in roots and shoots of 11 food crops with higher concentrations found in roots. Here, the highest concentrations in the roots were present in pepper, cucumber, and onion, and the highest translocation factor from root to shoot was noted in asparagus, cabbage, and broccoli. Based upon these data, the highest exposure from crops were pepper, cabbage, and onion with an estimated mean exposure (to the whole population) of 35.2, 76.1, and 320.9 ng TCS/kg/day respectively. Macherius et al. (2012) found that when carrot cell culture was exposed to 3.5 μM TCS, dissolved in culture medium, after the first two hr treatment a maximal amount of 17.2 μg TCS in cell material suspended in 20 ml was reached. After 5 days exposure, TCS levels decreased to a final amount of 1.3 μg in cell material suspended in 20 ml, 8 metabolites were detected in cell extracts, and 6 found in culture medium. Macherius et al. (2012) confirmed that these metabolites were also present in whole plants by exposing potted carrot plants to TCS. TCS-methyl was also rapidly taken up into the cells from the medium but, in contrast to TCS, the concentrations remained relatively constant over 5 day treatment. Data indicate that mTCS cannot be metabolized in the carrot cell due to lack of a phenolic group. Macherius et al. (2012) suggested that it is important to not disregard metabolites on plant uptake of TCS when examining the potential harm of human exposure of antimicrobial agent through food and plant.
Triclosan Breakdown Products
Triclosan may be degraded via both biotic and abiotic transformation. Triclosan-methyl (mTCS) is a degradation product of TCS and generated through biodegradation. TCS-methyl is produced in the environment under aerobic conditions (Al-Rajab et al. 2009; Lindstrom et al. 2002; Butler et al. 2012; Chen et al. 2011) and is formed by O-methylation, which occurs when a methyl group is attached to the hydroxyl group on the TCS molecule. This process is known to occur via Rhodococcus, Acinetobacter, and Mycobacterium in other chlorophenolic compounds (Allard et al 1987; Haggblom et al 1988). Triclosan-methyl was reported to be present in treated WWTP effluent (Bester 2003), river water (Coogan et al. 2007; Lindstrom et al. 2002), and soil (Butler et al. 2012). In addition, degradation of TCS to mTCS occurs via WWTP (Lozano et al. 2013). WWTP limit the extent of total TCS removal by transforming the parent compound to mTCS which is more resistant to photolysis (Balmer et al. 2004; Lindstrom et al. 2002). Biotic transformation of TCS also produced 2,4-dichlorophenol (Mulla et al. 2016; Kim et al. 2011; Gangadharan et al. 2012; Lee and Chu 2013), 4-chlorophenol (Kim et al. 2011), 2-chlorohydroquinone (Mulla et al. 2016; Lee and Chu 2013), catechol (Gangadharan et al. 2012), and hydroquinone, (Mulla et al. 2016) depending upon the microbes present. Mulla et al. (2016) found the optimal biotransformation conditions to be at pH of 7 and 30°C.
Due to environmental transformation of TCS to mTCS, multiple species are exposed to the metabolite. Triclosan-methyl is more lipophilic than TCS and exhibits a higher bioaccumulation potential in aquatic organisms (Balmer et al. 2004, Rudel et al. 2013, Macherius et al. 2014). TCS-methyl was detected in fish samples from rivers in Germany (Rudel et al. 2013) with mean levels in fish muscle tissue ranging from 70.8 to 378 ng/g (lipid weight). Rudel et al (2013) showed that mTCS concentrations were higher than TCS indicating that mTCS accumulated in fish tissue more readily than TCS. TCS-methyl concentrations were also present in earthworms exposed to biosolid application for 4 years at 25.9 to 113.7 ng/g (dry weight) (Macherius et al. 2014). These observations reaffim that mTCS bioaccumulated more readily than TCS.
Due to the lack of a hydroxyl group, mTCS does not possess antimicrobial properties (Clayborn et al 2011). Triclosan-methyl was found to be less toxic than equal TCS concentrations in a variety of cell types (Gaume et al. 2012, Sadowski et al. 2014), but is more persistent in the environment (Latch et al. 2003, Lozano et al. 2013). Bioaccumulation of mTCS was noted in fish (Balmer et al. 2004; Rudel et al. 2013), in algae (Coogan et al. 2007), turtles (Basile et al. 2011), and snails (Coogan and La Point 2008). Interestingly, the sample site for the turtle study was not subject to WWTP effluent, such that the exposure route is not known (Basile et al. 2011). Few apparent studies exist on mTCS effects on animals and humans.
In abalone hemocytes, 4 μM mTCS induced cytotoxicity following a 24 hr treatment; no marked toxicity was found in gill cells (Gaume et al. 2012). In 144 hpf zebrafish, 3.3 μM mTCS induced tail and yolk-sac abnormalities along with muscular involuntary contractions (Macedo et al 2017). Sea urchin larval length was also decreased following 3.3 nM mTCS exposure (Macedo et al 2017). In tadpoles, thyroid hormone receptor β (TRβ) mRNA level was increased following 10 nM mTCS and TH-repressed Rana larval keratin type I (RLKI) mRNA levels were elevated following 1 nM mTCS (Hinther et al. 2011). In the rat pituitary cell line GH3, 1,000 nM mTCS reduced growth hormone mRNA levels, 100 nM mTCS decreased prolactin and 1 nM mTCS increased heat shock protein (Hsp) 70 mRNA (Hinther et al. 2011). These data indicate that mTCS induced hormone disruption and enhanced stress responses. However, these results were questioned due to concerns regarding validation of the assay (DeLeo et al. 2011).
Even fewer human studies exist for mTCS. In human liver carcinoma cells (HepG2), long term administration of low nanomolar TCS and mTCS induced changes in gene expression via effects on methylation status (Zeng et al. 2016). Interestingly, mTCS and TCS seemed to exert opposite effects on methylation of p16 tumor suppressor gene, an effectnot seen in higher micromolar TCS concentrations (Zeng et al. 2016). Triclosan-methyl also undergoes hydrophobic interactions with human serum albumin (HSA), leading to spontaneous binding and conformation changes to HSA (Lv et al. 2013).
TCS undergoes photodegradation rapidly at pH 8 while mTCS does not (Lindstrom et al. 2002). Lindstrom et al (2002) noted that TCS dissociate effects on photolysis rate were due to a shift in its UV absorbance spectrum. When TCS was not dissociated, there was little absorbance at wavelengths above 300 nm, indicating there is little overlap with the spectrum of tropospheric sunlight. However, dissociated TCS absorbs strongly at wavelengths between 300 and 320 nm (Lindstrom et al. 2002). Based on this characteristic, evidence indicated that TCS underwent photolysis faster in the dissociated than in non-dissociated state. Latch et al. (2003) also suggested that the ionized form of TCS was the photoreactive species, while the protonated form of TCS and mTCS were photostable. This photostability of mTCS may also indicate that mTCS is more environmentally persistent than TCS (Coogan et al. 2007; Latch et al. 2003; Lozano et al. 2013).
Due to abiotic photodegradation of the dissociated form of TCS, there are multiple additional breakdown products of TCS, including dioxins. Rapid abiotic photodegradation of TCS at high pH results in conversion to 2,8-dichlorodibenzo-p-dioxin (DCDD) (Lores et al. 2005). Photodegradation conditions for TCS were optimal at basic pH with a ferric ion concentration of 2 mg/L in the presence of no humic acid, leads to degradation half-life of 5.4 hr for 7 μM TCS under these most favorable conditions (Martinez-Zapata et al 2013). Qiao et al (2014) reported that the half-life of 3.5 μM TCS at pH 7 using sunlight as the light source was 2.55 hr. Aranami and Readman (2007) showed that 33 μM TCS in pure water with an initial pH of 7 exhibited a photodegradation half-life of longer than 8 days. The TCS half-life was shorter in fresh water (taken from an estuary) compared to pure water (around 8 days) and the shortest in sea water (around 4 days). The decreased half-life in fresh water may be due to the presence of microbes, since TCS is known to undergo biodegradation. After 3 days of light exposure, DCDD was detected in both fresh and sea water, while negligible amounts were found in pure water and in the dark controls (Aranami and Readman 2007). Approximately 1% of TCS was found to be photodegraded to DCDD. Conversions of TCS to DCDD vary based upon pH and light wavelength, but range from 1–12%, suggesting that DCDD is a significant by-product of TCS but not the predominant one (Latch et al. 2003). Photodegradation of TCS was also observed in surface water (Tixier et al. 2002, Latch et al. 2003). Latch et al (2003) confirmed the environmental significance of lab findings by using Mississippi River water with TCS added and irradiated with UV light to simulate sunlight exposure. These results were comparable to those found using sterile lab buffer conditions, suggesting that TCS likely is converted to DCDD in surface water under sunlight irradiation. Under sterile buffer conditions, TCS underwent photodegradation to 2,7/2,8-dibenzodichloro-p-dioxin (2,7/2,8-DCDD) at pH 7 (not at pH 5–6) (Mezcua et al. 2004). TCS underwent photodegradation to 2,7/2,8-DCDD more quickly when dissolved in wastewater compared to sterile water (Mezcua et al. 2004) indicating that biotic degradation of TCS might enhance dioxin production under UV light. Investigators demonstrated that 2,7-DCDD produced morphological and functional changes in vitro in human bronchial epithelial cells (Willey et al. 1984) and altered glucose transport activity in human granulosa cells (Enan et al. 1996).
Abiotic transformation of TCS also generates chlorophenols, which are a major class of by-products of TCS undergoing chemical treatments (Bedoux et al. 2012). TCS may be degraded when exposed to chlorine, into 4,5 dichloro-2-(2,4-dichlorophenoxy)phenol (4-Cl-TCS), 5,6 dichloro-2-(2,4-dichlorophenoxy)phenol (6-Cl-TCS), and 4,5,6 trichloro2–2(2,4-dichlorophenoxy)phenol (4,6-Cl-TCS) (Rule et al 2005); structures and pictorial review illustrated in (Dhillon et al. 2015). Canosa et al (2005) showed that with 0.17 μM TCS and 1 mg/L chlorine at neutral pH, 5 by-products were detected identified as 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP), tetraclosan I and II, and pentaclosan. The by-products 2,4-DCP and 2,4,6-TCP were found to have higher stability. This result is important because chlorination treatment by WWTP might contribute to the burden of these by-products in the environment. Kanetoshi et al (1992) found the toxicity of TCS derivatives rose as the number of chlorines increased. When 6 nM TCS was treated with 35 μM permanganate used to treat taste and odor at the intake of water treatment plants, the major degradation product found was 2,4-DCP (Wu et al. 2012). The 2,4-DCP degradation product was also generated via abiotic means through manganese oxide oxidation (Zhang and Huang 2003), free chlorine oxidation (Rule et al 2005), ferrate oxidation (Yang et al. 2011), and ozonation treatment (Chen et al. 2012) of antimicrobial.
The by-products 2,4-DCP and 2,4,6-TCP are known to exert endocrine disruptor activity (Ma et al. 2011; Fang et al. 2014). Along with endocrine disruption activity, 2,4-DCP was reported to induce toxicity in human (Bukowska 2003) and mouse cells (Chen et al. 2004) in vitro. There is also evidence of toxicity occurring in vivo along with mitochondrial uncoupling as reviewed by Tessier et al (2000). 2,4-DCP also produced hepatotoxicity in vivo and in vitro through mitochondrial and ER stress (Li et al. 2013, Fu et al. 2016).
Of further concern is the transformation of TCS into chlorodioxins upon incineration and under the influence of sunlight (Kanetoshi et al. 1988). Polychlorodibenzo-p-dioxins (PCDD) and polychlorodibenzofurans are also known by-products of TCS degradation (Aranami and Readman 2007; Mezcua et al. 2004; Latch et al. 2005;Yu et al. 2006; Sanchez-Prado et al. 2006). After TCS undergoes chlorination to produce 6-Cl-TCS, 4,6-Cl-TCS, and 4-Cl-TCS, photolysis might generate these products to form PCDD (Buth et al. 2009). The dioxin products include 2,8-dichlorodibenzo-p-dioxin (2,8-DCDD), 2,3,7-trichlorodibenzo-p-dioxin (2,3,7-TCDD), 1,2,8-trichlorodibenzo-p-dioxin (1,2,8-TCDD), and 1,2,3,8-tetrachlorodibenzo-p-dioxin (1,2,3,8-TeCDD) (Buth et al. 2009); structures and pictorial review illustriated in (Dhillon et al. 2015). Therefore, WWTP effluents that contain TCS or its chlorinated derivatives might undergo environmental photolysis producing the more harmful PCDD (Tavakoly Sany et al. 2015).
Dioxin-like compounds (DLC) are known to affect the aryl hydrocarbon receptor (AhR), which leads to dysregulation of certain genes (Sorg 2014). DLC are also associated with development of multiple types of cancer, developmental and reproductive defects, as well as immunotoxicity (Tavakoly Sany et al. 2015). Even though TCS is photodegraded into dioxins by UV light, no apparent studies investigated formation of dioxins from TCS breakdown/metabolism in human skin/tissues (Fang et al. 2010) or in other eukaryotic organisms. However, Alvarez-Rivera et al (2016) noted that using artificial skin two dioxin like products were detected upon TCS exposure and UV-irradiation indicating that TCS might be undergoing phototransfomation into DLC on human skin.
Triclosan-mediated Adverse Health Effects
Within just the last few years, the literature on TCS-mediated adverse health effects has expanded from virtually non-existent to quite robust. Figure 1 summarizes several adverse human health effects now been documented for TCS. This section discusses these effects along with related studies in animals and in cell culture which lend support to these epidemiological findings. TCS also induced a multitude of effects on cellular function and molecular pathways. This topic was recently reviewed by Ruszkiewicz et al (2017). Briefly, TCS was found to affect mitochondrial function (Weatherly et al. 2016; Shim et al. 2016; Newton et al. 2005; Ajao et al. 2015), disrupt proper calcium signaling (Cherednichenko et al. 2012), alter zinc homeostasis (Tamura et al. 2012), and affect immunological parameters (Anderson et al. 2013; Yueh et al. 2014; Udoji et al. 2010; Weatherly et al. 2013).
Figure 1.
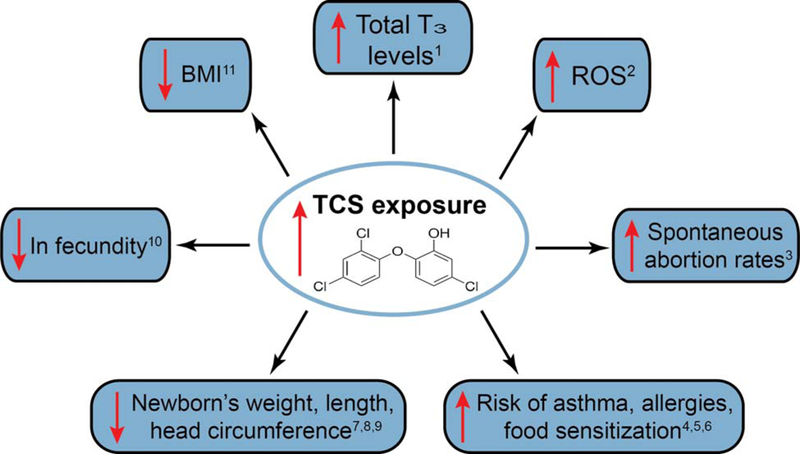
Recent human epidemiology studies show an association of an increase in urinary triclosan concentrations with a variety of detrimental endpoints. 1 Koeppe et al 2013; 2 Lv et al 2016; 3 Wang et al 2015; 4 Clayton et al 2011; 5 Spanier et al 2014; 6 Hong et al 2014; 7 Lassen et al 2016; 8 Philippat et al 2014; 9 Etzel et al 2017; 10 Velez et al 2015; 11 Li et al 2015.
Several investigators reported that TCS is an endocrine disrupting chemical (EDC) in multiple species (Wang et al. 2015; Ishibashi et al. 2004; Raut and Angus 2010; Axelstad et al. 2013; Louis et al, 2017), including humans (Koeppe et al. 2013). TCS effects on endocrine disruption were reviewed previously (Wang and Tian 2015; Ruszkiewicz et al. 2017; Olaniyan et al 2016; Witorsch 2014), this review focuses on more recent in vivo and human studies.
Exposure of various fish species to nanomolar levels of TCS elevated levels of hepatic vitellogenin (Vtg), an egg yolk precursor used as a biomarker for endocrine disruption (Ishibashi et al. 2004; Raut and Angus 2010). A decrease in sperm count was also found with TCS exposure in fish (Raut and Angus 2010). A reduction in hormone levels including progesterone, estradiol, and testosterone in rat serum occurred after oral gavage of TCS (Feng et al. 2016). This antimicrobial lowered thyroxine (T4) hormone levels in female rats after oral TCS administration (Louis et al. 2017, Paul et al. 2010; 2012); a decrease in T4 levels also occurred in pups treated orally with TCS (Axelstad et al. 2013). A reduction in T4 was also seen in male juvenile rats exposed to TCS (Zorrilla et al. 2009). A fall in T4 and triiodothyronin (T3) levels after TCS exposure was noted in mice (Wang et al. 2015). Multiple investigators proposed that the mechanism underlying the decrease in T4 levels may be due to an upregulation of phase I CYP450 and phase II glucuronyltransferase and sulfonyltransferase enzymes (Paul et al. 2010; 2012; Zorrilla et al. 2009). In humans, epidemiological studies found an association between a decrease in T4 levels in the mother and cognitive impairment in the child (Ghassabian et al. 2011; Hollowell et al; 1999; Li et al. 2010); although, to date, no apparent study directly assessing TCS effects on cognitive function has been published. Interestingly, in a human epidemiology study, Koeppe et al (2013) reported a rise in TCS urine concentrations was associated with an elevation in total T3 levels in adolescents ages 12 to19.
TCS is also associated with cancer development. In mouse liver, TCS enhanced hepatocyte proliferation and reactive oxygen species (ROS) production thereby acting as a liver tumor promoter (Yueh et al. 2014). A correlation between increased ROS and elevated TCS concentrations in human urine samples also indicates that TCS enhanced ROS production in humans (Lv et al. 2016). TCS elevated proinflammatory cytokine expression of TNF-α and IL-6 in mouse liver (Yueh et al. 2014). Many other in vitro studies suggested that TCS promotes cancer (Kim et al. 2014; Lee et al. 2014; Winitthana et al 2014; Wu et al. 2015). Conversely, in other studies, investigators provided data suggesting that TCS may inhibit cancer development (Sadowski et al. 2014; Deepa et al. 2012; Schmid et al. 2005). Sadowski et al (2014) showed that TCS is more cytotoxic to prostate cancer compared to non-malignant cells.
TCS was reported to decrease cardiovascular functions in mice with 12.5 and 25 mg/kg concentrations (Cherednichenko et al. 2012). This antimicrobial also impairs muscle function in both mice and fathead minnows assessed via grip strength and swimming performance, respectively (Cherednichenko et al. 2012). The molecular mechanism underlying this impairment is postulated to be related to TCS effects on calcium signaling in cardiac and skeletal muscle cells (Cherednichenko et al. 2012). In addition, TCS exposure increased cytosolic calcium levels in resting myotubes (Ahn et al. 2008). [3H]ryanodine binding was also enhanced with TCS (Cherednichenko et al. 2012) suggesting that this is a possible molecular mechanism may be involved in muscle function changes due to the compound directly targeting type 1 ryanodine receptor.
Recent human epidemiological studies correlated TCS with reproductive and developmental defects (Wang et al. 2015; Velez et al 2015; Etzel et al. 2017). Wang et al. ( 2015) demonstrated an association between spontaneous abortion rates and high TCS urine concentrations. An increase in TCS urinary concentrations to levels above 248 nM was related to an increased time to pregnancy (decrease in fecundity) (Velez et al 2015). Several investigators showed an association between high TCS urine levels and diminished head circumference in boys (Lassen et al. 2016; Philippat et al. 2014). Etzel et al (2017) found a rise in urinary TCS concentration was inversely correlated with newborn head circumference, weight, and length, regardless of gender. Li et al (2015) also noted a reduction in BMI with elevated urinary TCS concentrations, which may also be related to birth defects. Enhanced TCS sera levels obtained from human mothers was associated with birth defects (Wei et al. 2017). As previously indicated, several studies showed that TCS exhibited endocrine disrupting effects, which may account for a portion of the adverse reproductive outcomes observed in the epidemiological studies. Mitochondrial disruption by antimicrobial (Weatherly et al. 2016; Ajao et al. 2015; Newton et al. 2005) may also contribute to adverse reproductive effects as proper mitochondrial function is required for embryonic development (Chen et al. 2003).
Similar reproductive adverse effects were also reported in animal studies. An association between high TCS concentrations and spontaneous abortion was shown in mice (Wang et al. 2015). Treatment with TCS on certain gestational days decreased implantation rates in mice (Crawford and Decatanzaro 2012). An increase in spontaneous abortion rates occurred in pregnant rats exposed to TCS and the placenta was found to accumulate compound after treatment (Feng et al. 2016). In the human and the animal studies, there is no apparent molecular explanation provided for the observed effects of TCS at the organismal level.
Several human epidemiological studies noted associations between TCS exposure and allergies and asthma (Clayton et al. 2011; Spanier et al. 2014; Hong et al. 2014). Urinary TCS concentrations were correlated with increased allergies diagnoses in the under 18 age group (Clayton et al. 2011). An elevated level of TCS may lead to an enhanced risk of asthma, allergies, and food sensitization (Spanier et al. 2014). In addition Hong et al (2014) suggested an association between a high use of antimicrobial products with wheezing and allergic rhinitis
Several animal and in vitro studies with respect to the immune system show similar results. Rat in vivo studies noted a fall in IL-25 (pro-inflammatory), IL-33 (both pro and anti-inflammatory), and IL-1α (pro-inflammatory) but an rise in pro-inflammatory cytokine IL-1β and TNF-α expression following 3% TCS dermal exposure (Marshall et al. 2015). In primary human epidermis tissue, 0.1% TCS elevated TSLP, IL-1β, and IL-1α gene expression (Marshall et al. 2015). Further, antimicrobial also increased the number of immune cells in the skin draining lymph node after dermal application to mice (Anderson et al. 2016).
However, several other studies reported that TCS alleviated skin reactions, including allergic skin reactions upon irritation (Barkvoll and Rolla 1995; Kjaerheim et al. 1995; Tan et al. 2010; Waaler et al. 1993). In addition, TCS was found to produce immunosuppression of natural killer cells via inhibition of their lytic function (Udoji et al. 2010). The mechanism underlying the observed decrease in skin reactions was investigated by examining the effects of TCS on mast cells, which are involved in allergic diseases. Palmer et al. (2012) found that TCS diminished mast cell degranulation stimulated by multivalent antigen (Ag). Further, it was determined that TCS produced an enhanced effect on mast cell degranulation upon calcium ionophore stimulation, leading to the postulation that the antimicrobial was inhibiting mast cell degranulation via a target after the influx of calcium across the Ca2+ release-activated Ca2+ (CRAC) channel (Palmer et al. 2012). These data provide a mechanism underlying TCS-mediated inhibition of allergic skin reactions and immunosuppression seen in clams.
Conclusions
TCS was recently been banned by the FDA but only from certain soap products. This antimicrobial still remains in many consumer products, including popular toothpaste. Consumers are exposed via these products as evident by numerous studies showing detectable levels of TCS in skin, urine, and plasma ranging from nM to μM concentrations. There remains some controversy regarding whether TCS concentrations absorbed into the human body might induce adverse effects noted in lab studies. Here, comparing TCS levels absorbed into human skin to compound concentrations reported to produce adverse effects, it was found that antimicrobial levels absorbed via the skin are comparable to those needed to induce effects seen in lab studies such as mitochondrial dysfunction. It is also important to note that TCS is not rapidly metabolized dermally and the majority remains as the parent compound for at least 24 hr. Several studies demonstrated TCS-mediated effects following just a few hr following chemical exposure. TCS might undergo both biotic and abiotic transformation, leading to production of a multitude of by-products. Many of these by-products have not been thoroughly investigated while others were found to be more toxic than parent TCS compound. TCS was reported to be an endocrine disrupting chemical in multiple species. TCS might also affect immune responses, ROS production, and cardiovascular functions. There has been a recent increase in the number of epidemiology studies examining TCS with effects shown in Figure 1. Many of these studies observed an association between a rise in TCS exposure and reproductive and developmental defects in infants. Taking into consideration these significant findings, incorporation of this antimicrobial into readily available consumer products, not just in soap, needs to be re-evaluated, and biological effects of its breakdown products and metabolites need to be investigated.
Acknowledgments
Funding
This work was supported by the National Institute of Environmental Health Sciences: [Grant Number R15ES24593].
References
- Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DP, Gee SJ, and Hammock BD. 2008. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens.Environ Health Persp 116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajao C, Andersson MA, Teplova VV, Nagy S, Gahmberg CG, Andersson LC, Hautaniemi M, Kakasi B, Roivainen M, and Salkinoja-Salonen M. 2015. Mitochondrial toxicity of triclosan on mammalian cells.Toxicol Rep 2: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rajab AJ, Sabourin L, Scott A, Lapen DR, and Topp E. 2009. Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarban in an agricultural soil.Sci Total Environ 407: 5978–5985. doi: 10.1016/j.scitotenv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Al Habashneh R, Farasin R, and Khader Y. 2017. The effect of a triclosan/copolymer/fluoride toothpaste on plaque formation, gingivitis, and dentin hypersensitivity: A single-blinded randomized clinical study. Quintessence Int 48: 123–130. doi: 10.3290/j.qi.a37384. [DOI] [PubMed] [Google Scholar]
- Allard AS, Remberger M, and Neilson AH. 1987. Bacterial O-methylation of halogen-substituted phenols. Appl Environ Microbiol 53: 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, and Sandborgh-Englund G. 2006. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ 372: 87–93. [DOI] [PubMed] [Google Scholar]
- Alvarez-Rivera G, Llompart M, Garcia-Jares C, and Lores M. 2016. Pressurized liquid extraction-gas chromatography-mass spectrometry for confirming the photo-induced generation of dioxin-like derivatives and other cosmetic preservative photoproducts on artificial skin. J Chromatogr A 1440:37–44. doi: 10.1016/j.chroma.2016.02.066. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Franko J, Kashon ML, Anderson KL, Hubbs AF, Lukomska E, and Meade BJ. 2013. Exposure to triclosan augments the allergic response to ovalbumin in a mouse model of asthma. Toxicol Sci 132: 96–106. doi: 10.1093/toxsci/kfs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ, Long CM, Lukomska E, and Marshall NB. 2016. Investigations of immunotoxicity and allergic potential induced by topical application of triclosan in mice. J Immunotoxicol 13:165–172. doi: 10.3109/1547691x.2015.1029146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranami K, and Readman JW. 2007. Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 66: 1052–1056. doi: 10.1016/j.chemosphere.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Boberg J, Vinggaard AM, Christiansen S, and Hass U. 2013. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem Toxicol 59: 534–40. doi: 10.1016/j.fct.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Bagley DM, and Lin YJ. 2000. Clinical evidence for the lack of triclosan accumulation from daily use in dentifrices. Am J Dent 13: 148–152. [PubMed] [Google Scholar]
- Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, Muller MD, and Buser HR. 2004. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol 38: 390–395. [DOI] [PubMed] [Google Scholar]
- Barkvoll P, and Rolla G. 1995. Triclosan reduces the clinical symptoms of the allergic patch test reaction (APR) elicited with 1% nickel sulphate in sensitised patients. J Clin Periodontol 22: 485–487. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, and Green H. 1985. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci U S A 82: 5390–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile ER, Avery HW, Bien WF, and Keller JM. 2011. Diamondback terrapins as indicator species of persistent organic pollutants: Using Barnegat Bay, New Jersey as a case study. Chemosphere 82: 137–144. doi: 10.1016/j.chemosphere.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Bedoux G, Roig B, Thomas O, Dupont V, and Le Bot B. 2012. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res Int 19: 1044–10465. doi: 10.1007/s11356-011-0632-z. [DOI] [PubMed] [Google Scholar]
- Bernot MJ, Smith L, and Frey J. 2013. Human and veterinary pharmaceutical abundance and transport in a rural central Indiana stream influenced by confined animal feeding operations (CAFOs). Sci Total Environ 445-446: 219–230. doi: 10.1016/j.scitotenv.2012.12.039. [DOI] [PubMed] [Google Scholar]
- Bester K 2003. Triclosan in a sewage treatment process--balances and monitoring data. Water Res 37: 3891–3896. doi: 10.1016/s0043-1354(03)00335-x. [DOI] [PubMed] [Google Scholar]
- Bester K 2005. Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol 49: 9–17. doi: 10.1007/s00244-004-0155-4. [DOI] [PubMed] [Google Scholar]
- Black JG, Howes D, and Rutherford T. 1975. Percutaneous absorption and metabolism of Irgasan DP300. Toxicology 3: 33–47. [DOI] [PubMed] [Google Scholar]
- Braoudaki M, and Hilton AC. 2004. Low level of cross-resistance between triclosan and antibiotics in Escherichia coli K-12 and E. coli O55 compared to E. coli O157. FEMS Microbiol Lett 235: 305–309. doi: 10.1016/j.femsle.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Brenwald NP, and Fraise AP. 2003. Triclosan resistance in methicillin-resistant Staphylococcus aureus (MRSA). J Hosp Infect 55: 141–144. [DOI] [PubMed] [Google Scholar]
- Bukowska B 2003. Effects of 2,4-D and its metabolite 2,4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes.Comp Biochem Physiol C Toxicol Pharmacol 135: 435–441. [DOI] [PubMed] [Google Scholar]
- Buth JM, Grandbois M, Vikesland PJ, McNeill K, and Arnold WA. 2009. Aquatic photochemistry of chlorinated triclosan derivatives: Potential source of polychlorodibenzo-p-dioxins. Environ Toxicol Chem 28: 2555–2563. doi: 10.1897/08-490.1. [DOI] [PubMed] [Google Scholar]
- Butler E, Whelan MJ, Sakrabani R, and van Egmond R. 2012. Fate of triclosan in field soils receiving sewage sludge. Environ Pollut 167: 101–109. doi: 10.1016/j.envpol.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, and Needham LL. 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Persp 116: 303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosa P, Morales S, Rodriguez I, Rubi E, Cela R, and Gomez M. 2005. Aquatic degradation of triclosan and formation of toxic chlorophenols in presence of low concentrations of free chlorine. Anal Bioanal Chem 383: 1119–1126. doi: 10.1007/s00216-005-0116-4. [DOI] [PubMed] [Google Scholar]
- Chai L, Chen A, Luo P, Zhao H, and Wang H. 2017. Histopathological changes and lipid metabolism in the liver of Bufo gargarizans tadpoles exposed to triclosan. Chemosphere 182: 255–266. doi: 10.1016/j.chemosphere.2017.05.040. [DOI] [PubMed] [Google Scholar]
- Chedgzoy P, Winckle G, and Heard CM. 2002. Triclosan: release from transdermal adhesive formulations and in vitro permeation across human epidermal membranes. Int J Pharm 235: 229–236. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, and Chan DC. 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jiang J, Zhang F, Yu H, and Zhang J. 2004. Cytotoxic effects of environmentally relevant chlorophenols on L929 cells and their mechanisms. Cell Biol Toxicol 20: 183–196. [DOI] [PubMed] [Google Scholar]
- Chen X, Nielsen JL, Furgal K, Liu Y, Lolas IB, and Bester K. 2011. Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 84: 452–456. doi: 10.1016/j.chemosphere.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Chen X, Richard J, Liu Y, Dopp E, Tuerk J, and Bester K. 2012. Ozonation products of triclosan in advanced wastewater treatment. Water Res 46: 2247–2256. doi: 10.1016/j.watres.2012.01.039. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pi B, Zhou H, Yu Y, and Li L. 2009. Triclosan resistance in clinical isolates of Acinetobacter baumannii . J Med Microbiol 58:1086–1091. doi: 10.1099/jmm.0.008524-0. [DOI] [PubMed] [Google Scholar]
- Cherednichenko G, Zhang R, Bannister RA, Timofeyev V, Li N, Fritsch EB, Feng W, Barrientos GC, Schebb NH, Hammock BD, Beam KG, Chiamvimonvat N, and Pessah IN. 2012. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc Natl Acad Sci U S A 109: 14158–14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, and Schweizer HP. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: Exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother 45: 428–432. doi: 10.1128/aac.45.2.428-432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciusa ML, Furi L, Knight D, Decorosi F, Fondi M, Raggi C, Coelho JR, Aragones L, Moce L, Visa P, Freitas AT, Baldassarri L, Fani R, Viti C, Orefici G, Martinez JL, Morrissey I, and Oggioni MR. 2012. A novel resistance mechanism to triclosan that suggests horizontal gene transfer and demonstrates a potential selective pressure for reduced biocide susceptibility in clinical strains of Staphylococcus aureus. Int J Antimicrob Agents 40: 210–220. doi: 10.1016/j.ijantimicag.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Clayborn AB, Toofan SN, and Champlin FR. 2011. Influence of methylation on the antibacterial properties of triclosan in Pasteurella multocida and Pseudomonas aeruginosa variant strains. J Hosp Infect 77: 129–133. doi: 10.1016/j.jhin.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Clayton EM, Todd M, Dowd JB, and Aiello AE. 2011. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Persp 119: 390–396. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan MA, Edziyie RE, La Point TW, and Venables BJ. 2007. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67: 1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Coogan MA, and La Point TW. 2008. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem 27: 1788–1793. doi: 10.1897/07-374.1. [DOI] [PubMed] [Google Scholar]
- Crawford BR, and Decatanzaro D. 2012. Disruption of blastocyst implantation by triclosan in mice: Impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol 34: 607–613. doi: 10.1016/j.reprotox.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Davis EF, Klosterhaus SL, and Stapleton HM. 2012. Measurement of flame retardants and triclosan in municipal sewage sludge and biosolids. Environ Int 40:1–7. doi: 10.1016/j.envint.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Deepa PR, Vandhana S, Jayanthi U, and Krishnakumar S. 2012. Therapeutic and toxicologic evaluation of anti-lipogenic agents in cancer cells compared with non-neoplastic cells. Basic Clin Pharmacol Toxicol 110: 494–503. doi: 10.1111/j.1742-7843.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- DeLeo P, Pawlowski S, Barton C, and Fort DJ. 2011. Comment on “Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems”. Environ Sci Technol 45: 10283–10284; author reply 10285–7. doi: 10.1021/es202937q. [DOI] [PubMed] [Google Scholar]
- DeSalva SJ, Kong BM, and Lin YJ. 1989. Triclosan: A safety profile. Am J Dent 2 Spec No:185–96. [PubMed] [Google Scholar]
- Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledon M, Verma M, and Surampalli RY. 2015. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health 12: 5657–5684. doi: 10.3390/ijerph120505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury B, Scott J, Rosi-Marshall EJ, and Kelly JJ. 2013. Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ Sci Technol 47: 8923–8930. doi: 10.1021/es401919k. [DOI] [PubMed] [Google Scholar]
- Enan E, Lasley B, Stewart D, Overstreet J, and Vandevoort CA. 1996. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) modulates function of human luteinizing granulosa cells via cAMP signaling and early reduction of glucose transporting activity. Reprod Toxicol 10: 191–198. [DOI] [PubMed] [Google Scholar]
- Escarrone AL, Caldas SS, Primel EG, Martins SE, and Nery LE. 2016. Uptake, tissue distribution and depuration of triclosan in the guppy Poecilia vivipara acclimated to freshwater. Sci Total Environ 560–561: 218–224. doi: 10.1016/j.scitotenv.2016.04.039. [DOI] [PubMed] [Google Scholar]
- Etzel TM, Calafat AM, Ye X, Chen A, Lanphear BP, Savitz DA, Yolton K, and Braun JM. 2017. Urinary triclosan concentrations during pregnancy and birth outcomes. Environ Res 156: 505–511. doi: 10.1016/j.envres.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair PA, Lee HB, Adams J, Darling C, Pacepavicius G, Alaee M, Bossart GD, Henry N, and Muir D. 2009. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ Pollut 157: 2248–2254. doi: 10.1016/j.envpol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Fang JL, Stingley RL, Beland FA, Harrouk W, Lumpkins DL, and Howard P. 2010. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C Environ Carcinogen Ecotoxicol Rev 28: 147–171. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- Fang JL, Vanlandingham M, da Costa GG, and Beland FA. 2016. Absorption and metabolism of triclosan after application to the skin of B6C3F1 mice. Environ Toxicol 3: 609–623. doi: 10.1002/tox.22074. [DOI] [PubMed] [Google Scholar]
- Fang Y, Gao X, Zhao F, Zhang H, Zhang W, Yang H, Lin B, and Xi Z. 2014. Comparative proteomic analysis of ovary for Chinese rare minnow (Gobiocypris rarus) exposed to chlorophenol chemicals. J Proteom 110:172–182. doi: 10.1016/j.jprot.2014.07.026. [DOI] [PubMed] [Google Scholar]
- FDA. “Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Proposed Amendment of the Tentative Final Monograph.”. 2013 https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/UCM379555.pdf.
- Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z, and Shao B. 2016. Endocrine disrupting effects of triclosan on the placenta in pregnant rats. PLoS One 11: e0154758. doi: 10.1371/journal.pone.0154758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Zhang X, Chen P, and Zhang Y. 2016. Endoplasmic reticulum stress is involved in 2,4-dichlorophenol-induced hepatotoxicity. J Toxicol Sci 41:745–756. doi: 10.2131/jts.41.745. [DOI] [PubMed] [Google Scholar]
- Gangadharan PVP, Nadaraja AV, Bhasi A, Khan S, and Bhaskaran K. 2012. Degradation of triclosan under aerobic, anoxic, and anaerobic conditions. Appl Biochem Biotechnol 167:1603–1612. doi: 10.1007/s12010-012-9573-3. [DOI] [PubMed] [Google Scholar]
- Gaume B, Bourgougnon N, Auzoux-Bordenave S, Roig B, Le Bot B, and Bedoux G. 2012. In vitro effects of triclosan and methyl-triclosan on the marine gastropod Haliotis tuberculata. Comp Biochem Physiol C Toxicol Pharmacol 156: 87–94. doi: 10.1016/j.cbpc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Geens T, Neels H, and Covaci A. 2012. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 87: 796–802. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, de Muinck Keizer-Schrama SM, Hooijkaas H, Steegers EA, Hofman A, Verhulst FC, van der Ende J, de Rijke YB, and Tiemeier H. 2011. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res 69: 454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ 1987. The oral clearance of zinc and triclosan after delivery from a dentifrice. J Pharm Pharmacol 39: 480–483. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, Fraser SB, and van der Ouderaa FJ. 1987. Oral disposition of triclosan (2,4,4’-trichloro-2’-hydroxydiphenyl ether) delivered from a dentifrice. Caries Res 21: 29–36. [DOI] [PubMed] [Google Scholar]
- Goodchild CG, Frederich M, and Zeeman SI. 2016. Is altered behavior linked to cellular energy regulation in a freshwater mussel (Elliptio complanata) exposed to triclosan? Comp Biochem Physiol C Toxicol Pharmacol 179:150–157. doi: 10.1016/j.cbpc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Haggblom MM, Nohynek LJ, and Salkinoja-Salonen MS. 1988. Degradation and O-methylation of chlorinated phenolic compounds by Rhodococcus and Mycobacterium strains. Appl Environ Microbiol 54: 3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Nishimura T, and Ando M. 1997. Effect of 2,4,4’-trichloro-2’-hydroxydiphenyl ether on cytochrome P450 enzymes in the rat liver. Chemosphere 34 : 719–730. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Baduel C, Toms LM, Calafat AM, Ye X, Hobson P, Broomhall S, and Mueller JF. 2015. Use of pooled samples to assess human exposure to parabens, benzophenone-3 and triclosan in Queensland, Australia. Environ Int 85: 77–83. doi: 10.1016/j.envint.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler J, and Halden RU. 2007. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 66: 362–369. doi: 10.1016/j.chemosphere.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Hinther A, Bromba CM, Wulff JE, and Helbing CC. 2011. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol 45: 5395–5402. doi: 10.1021/es1041942. [DOI] [PubMed] [Google Scholar]
- Hollowell JG Jr., Garbe PL, and Miller DT. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341: 2016–2017. [PubMed] [Google Scholar]
- Hong S, Kwon HJ, Choi WJ, Lim WR, Kim J, and Kim K. 2014. Association between exposure to antimicrobial household products and allergic symptoms. Environ Health Toxicol 29: e2014017. doi: 10.5620/eht.e2014017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, and Arizono K. 2004. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol 67: 167–179. doi: 10.1016/j.aquatox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- James MO, Marth CJ, and Rowland-Faux L. 2012. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat Toxicol 124–125: 72–82. doi: 10.1016/j.aquatox.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Jones RD, Jampani HB, Newman JL, and Lee AS. 2000. Triclosan: A review of effectiveness and safety in health care settings. Am J Infect Control 28: 184–196. [PubMed] [Google Scholar]
- Juncker J-C. 2016. Commission impementing decision not approving triclosan as an existing active substance for use in biocidal products for product-type 1. In 528/2012, edited by Union European. Brussels: Jean-Claude Juncker. [Google Scholar]
- Kanetoshi A. 1992. Acute toxicity, percutaneous absorption and effects on hepatic mixed function oxidase activities of 2,4,4’-trichloro-2’-hydroxydiphenyl ether (Irgasan DP300) and its chlorinated derivatives. Environ Contam Toxicol 23: 91–98. [DOI] [PubMed] [Google Scholar]
- Kanetoshi A, Katsura E, Ogawa H, Ohyama T, Kaneshima H, and Miura T. 1992. Acute toxicity, percutaneous absorption and effects on hepatic mixed function oxidase activities of 2,4,4’-trichloro-2’-hydroxydiphenyl ether (Irgasan DP300) and its chlorinated derivatives. Arch Environ Contam Toxicol 23: 91–98. [DOI] [PubMed] [Google Scholar]
- Kanetoshi A, Ogawa H, Katsura E, Kaneshima H, and Miura T. 1988. Formation of polychlorinated dibenzo-p-dioxins upon combustion of commercial textile products containing 2,4,4’-trichloro-2’-hydroxydiphenyl ether (Irgasan DP300). J Chromatogr 442: 289–299. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yi BR, Go RE, Hwang KA, Nam KH, and Choi KC. 2014. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ Toxicol Pharmacol 37: 1264–1274. doi: 10.1016/j.etap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Kim SA, Moon H, Lee K, and Rhee MS. 2015. Bactericidal effects of triclosan in soap both in vitro and in vivo. J Antimicrob Chemother 70: 3345–3352. doi: 10.1093/jac/dkv275. [DOI] [PubMed] [Google Scholar]
- Kim YM, Murugesan K, Schmidt S, Bokare V, Jeon JR, Kim EJ, and Chang YS. 2011. Triclosan susceptibility and co-metabolism--a comparison for three aerobic pollutant-degrading bacteria. Bioresour Technol 102: 2206–2212. doi: 10.1016/j.biortech.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Werner SL, and Cahill JD. 2006. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ Toxicol Chem 25: 317–326. [DOI] [PubMed] [Google Scholar]
- Kjaerheim V, Barkvoll P, Waaler SM, and Rolla G. 1995. Triclosan inhibits histamine-induced inflammation in human skin. J Clin Periodontol 22: 423–426. [DOI] [PubMed] [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, and Meeker JD. 2013. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ 445–446: 299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, and Buxton HT. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36:1202–1211. [DOI] [PubMed] [Google Scholar]
- Kux L 2016. “Federal Register Vol. 81 No. 126 https://www.gpo.gov/fdsys/pkg/FR-2016-06-30/pdf/2016-15410.pdf. [Google Scholar]
- Lan Z, Hyung Kim T, Shun Bi K, Hui Chen X, and Sik Kim H. 2015. Triclosan exhibits a tendency to accumulate in the epididymis and shows sperm toxicity in male Sprague-Dawley rats. Environ Toxicol 30: 83–91. doi: 10.1002/tox.21897. [DOI] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Kyhl HB, Swan SH, Main KM, Andersson AM, Lind DV, Husby S, Wohlfahrt-Veje C, Skakkebaek NE, and Jensen TK. 2016. Prenatal triclosan exposure and anthropometric measures including anogenital distance in Danish infants. Environ Health Persp 124:1261–1268. doi: 10.1289/ehp.1409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latch DE, Packer JL, Arnold WA, and McNeill K. 2003. Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solution. J Photochem Photobiol A-Chem 158:63–66. doi: 10.1016/s1010-6030(03)00103-5. [DOI] [Google Scholar]
- Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, and McNeill K. 2005. Aqueous photochemistry of triclosan: Formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem 24: 517–25. [DOI] [PubMed] [Google Scholar]
- Lawrence JR, Topp E, Waiser MJ, Tumber V, Roy J, Swerhone GD, Leavitt P, Paule A, and Korber DR. 2015. Resilience and recovery: the effect of triclosan exposure timing during development, on the structure and function of river biofilm communities. Aquat Toxicol 161:253–266. doi: 10.1016/j.aquatox.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Lee DG, and Chu KH. 2013. Effects of growth substrate on triclosan biodegradation potential of oxygenase-expressing bacteria. Chemosphere 93: 1904–1911. doi: 10.1016/j.chemosphere.2013.06.069. [DOI] [PubMed] [Google Scholar]
- Lee HR, Hwang KA, Nam KH, Kim HC, and Choi KC. 2014. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol 27: 834–842. doi: 10.1021/tx5000156. [DOI] [PubMed] [Google Scholar]
- Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, and Rafferty JB. 1999. Molecular basis of triclosan activity. Nature 398: 383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang X, Qiu Q, An Z, Qi Y, Huang D, and Zhang Y. 2013. 2,4-dichlorophenol induces apoptosis in primary hepatocytes of grass carp (Ctenopharyngodon idella) through mitochondrial pathway. Aquat Toxicol 140–141:117–122. doi: 10.1016/j.aquatox.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao J, Wang G, Zhu Y, Rabito F, Krousel-Wood M, Chen W, and Whelton PK. 2015. Urinary triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: Experience in NHANES 2003–2010. Int J Hyg Environ Health 218: 401–406. doi: 10.1016/j.ijheh.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, and Hua T. 2010. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 72: 825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- Lin D, Li Y, Zhou Q, Xu Y, and Wang D. 2014. Effect of triclosan on reproduction, DNA damage and heat shock protein gene expression of the earthworm Eisenia fetida. Ecotoxicology 23: 1826–1832. doi: 10.1007/s10646-014-1320-9. [DOI] [PubMed] [Google Scholar]
- Lin YJ 2000. Buccal absorption of triclosan following topical mouthrinse application. Am J Dent 13: 215–217. [PubMed] [Google Scholar]
- Lindstrom A, Buerge IJ, Poiger T, Bergqvist PA, Muller MD, and Buser HR. 2002. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36: 2322–2329. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang Y, Fillgrove KL, and Anderson VE. 2002. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother Pharmacol 49:187–193. doi: 10.1007/s00280-001-0399-x. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL. 2000. Molecular Cell Biology. 4th ed New York: W. H. Freeman. [Google Scholar]
- Lores M, Llompart M, Sanchez-Prado L, Garcia-Jares C, and Cela R. 2005. Confirmation of the formation of dichlorodibenzo-p-dioxin in the photodegradation of triclosan by photo-SPME. Anal Bioanal Chem 381: 1294–1298. doi: 10.1007/s00216-004-3047-6. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, and Stoker TE. 2017. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J Toxicol Environ Health A:1–14. doi: 10.1080/15287394.2017.1287029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano N, Rice CP, Ramirez M, and Torrents A. 2013. Fate of triclocarban, triclosan and methyltriclosan during wastewater and biosolids treatment processes. Water Res 47 : 4519–4527. doi: 10.1016/j.watres.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Lv W, Chen Y, Li D, Chen X, and Leszczynski J. 2013. Methyl-triclosan binding to human serum albumin: Multi-spectroscopic study and visualized molecular simulation. Chemosphere 93:1125–1130. doi: 10.1016/j.chemosphere.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Lv Y, Rui C, Dai Y, Pang Q, Li Y, Fan R, and Lu S. 2016. Exposure of children to BPA through dust and the association of urinary BPA and triclosan with oxidative stress in Guangzhou, China. Environ Sci Process Impacts 18: 1492–1499. doi: 10.1039/c6em00472e. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu C, Lam PK, Wu RS, Giesy, Hecker M, Zhang X, and Zhou B. 2011. Modulation of steroidogenic gene expression and hormone synthesis in H295R cells exposed to PCP and TCP. Toxicology 282: 146–153. doi: 10.1016/j.tox.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Macedo S, Torres T, and Santos MM. 2017. Methyl-triclosan and triclosan impact embryonic development of Danio rerio and Paracentrotus lividus. Ecotoxicology. doi: 10.1007/s10646-017-1778-3. [DOI] [PubMed] [Google Scholar]
- Macherius A, Eggen T, Lorenz W, Moeder M, Ondruschka J, and Reemtsma T. 2012. Metabolization of the bacteriostatic agent triclosan in edible plants and its consequences for plant uptake assessment. Environ Sci Technol 46: 10797–10804. doi: 10.1021/es3028378. [DOI] [PubMed] [Google Scholar]
- Macherius A, Lapen DR, Reemtsma T, Rombke J, Topp E, and Coors A. 2014. Triclocarban, triclosan and its transformation product methyl triclosan in native earthworm species four years after a commercial-scale biosolids application. Sci Total Environ 472:235–238. doi: 10.1016/j.scitotenv.2013.10.113. [DOI] [PubMed] [Google Scholar]
- Manevski N, Swart P, Balavenkatraman KK, Bertschi B, Camenisch G, Kretz O, Schiller H, Walles M, Ling B, Wettstein R, Schaefer DJ, Itin P, Ashton-Chess J, Pognan F, Wolf A, and Litherland K. 2015. Phase II metabolism in human skin: Skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation. Drug Metab Dispos 43: 126–139. doi: 10.1124/dmd.114.060350. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Long CM, Kashon ML, Sharpnack DD, Nayak AP, Anderson KL, Jean Meade B, and Anderson SE. 2015. Triclosan induces thymic stromal lymphopoietin in skin promoting Th2 allergic responses. Toxicol Sci 147:127–139. doi: 10.1093/toxsci/kfv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Zapata M, Aristizabal C, and Penuela G. 2013. Photodegradation of the endocrine-disrupting chemicals 4n-nonylphenol and triclosan by simulated solar UV irradiation in aqueous solutions with Fe(III) and in the absence/presence of humic acids. J Photochem Photobiol A-Chem 251: 41–49. doi: 10.1016/j.jphotochem.2012.10.009. [DOI] [Google Scholar]
- Mathews S, Henderson S, and Reinhold D. 2014. Uptake and accumulation of antimicrobials, triclocarban and triclosan, by food crops in a hydroponic system. Environ Sci Pollut Res Int 21: 6025–6033. doi: 10.1007/s11356-013-2474-3. [DOI] [PubMed] [Google Scholar]
- Matozzo V, Costa Devoti A, and Marin MG. 2012. Immunotoxic effects of triclosan in the clam Ruditapes philippinarum. Ecotoxicology 2: 66–74. doi: 10.1007/s10646-011-0766-2. [DOI] [PubMed] [Google Scholar]
- McAvoy DC, Schatowitz B, Jacob M, Hauk A, and Eckhoff WS. 2002. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem 21:1323–1329. [PubMed] [Google Scholar]
- Meador JP, Yeh A, Young G, and Gallagher EP. 2016. Contaminants of emerging concern in a large temperate estuary. Environ Pollut 213: 254–267. doi: 10.1016/j.envpol.2016.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezcua M, Gomez MJ, Ferrer I, Aguera A, Hernando MD, and Fernandez-Alba AR. 2004. Evidence of 2,7/2,8-dibenzodichloro-p-dioxin as a photodegradation product of triclosan in water and wastewater samples. Anal Chim Acta 524: 241–247. [Google Scholar]
- Moss T, Howes D, and Williams FM. 2000. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4’-trichloro-2’-hydroxydiphenyl ether). Food Chem Toxicol 38: 361–370. [DOI] [PubMed] [Google Scholar]
- Mulla SI, Wang H, Sun Q, Hu A, and Yu CP. 2016. Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci Rep 6: 21965. doi: 10.1038/srep21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HP, Barrieshi-Nusair KM, Kononen E, and Yang M. 2006. Effect of triclosan/copolymer-containing toothpaste on the association between plaque and gingival bleeding: a randomized controlled clinical trial. J Clin Periodontol 33: 811–818. doi: 10.1111/j.1600-051X.2006.00993.x. [DOI] [PubMed] [Google Scholar]
- Narrowe AB, Albuthi-Lantz M, Smith EP, Bower KJ, Roane TM, Vajda AM, and Miller CS. 2015. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome 3:6. doi: 10.1186/s40168-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon R, Hernandez-Ruiz S, Chorover J, and Chefetz B. 2011. Interactions of carbamazepine in soil: Effects of dissolved organic matter. J Environ Qual 40:942–948. doi: 10.2134/jeq2010.0446. [DOI] [PubMed] [Google Scholar]
- Newton AP, Cadena SM, Rocha ME, Carnieri EG, and Martinelli de Oliveira MB. 2005. Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett 160: 49–59. doi: 10.1016/j.toxlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Nietch CT, Quinlan EL, Lazorchak JM, Impellitteri CA, Raikow D, and Walters D. 2013. Effects of a chronic lower range of triclosan exposure on a stream mesocosm community. Environ Toxicol Chem 32: 2874–2887. doi: 10.1002/etc.2385. [DOI] [PubMed] [Google Scholar]
- Olaniyan LW, Mkwetshana N, and Okoh AI. 2016. Triclosan in water, implications for human and environmental health. Springerplus 5:1639. doi: 10.1186/s40064-016-3287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK, Hutchinson LM, Burpee BT, Tupper EJ, Pelletier JH, Kormendy Z, Hopke AR, Malay ET, Evans BL, Velez A, and Gosse JA. 2012. Antibacterial agent triclosan suppresses RBL-2H3 mast cell function. Toxicol Appl Pharmacol 258: 99–108. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, and Crofton KM. 2012. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: A dynamic and kinetic evaluation of a putative mode-of-action. Toxicology 300: 31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, and Crofton KM. 2010. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young Long-Evans rats. Toxicol Sci 113: 367–79. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perencevich EN, Wong MT, and Harris AD. 2001. National and regional assessment of the antibacterial soap market: A step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control 29: 281–283. [DOI] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles MA, and Slama R. 2014. Prenatal exposure to phenols and growth in boys. Epidemiology 25: 625–635. doi: 10.1097/ede.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher G, Berube R, Dumas P, Bienvenu JF, Gaudreau E, Belanger P, and Ayotte P. 2014. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography-tandem mass spectrometry. J Chromatogr A 1348: 97–104. doi: 10.1016/j.chroma.2014.04.072. [DOI] [PubMed] [Google Scholar]
- Pycke BF, Geer LA, Dalloul M, Abulafia O, Jenck AM, and Halden RU. 2014. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol 48: 8831–8838. doi: 10.1021/es501100w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao XL, Zheng XD, Xie Q, Yang XH, Xiao J, Xue WF, and Chen JW. 2014. Faster photodegradation rate and higher dioxin yield of triclosan induced by cationic surfactant CTAB. J Hazard Mater 275: 210–214. doi: 10.1016/j.jhazmat.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, Bastian B, Abdel-Tawab M, and Fuhr U. 2010. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother 54: 570–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut SA, and Angus RA. 2010. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem 29: 1287–1291. doi: 10.1002/etc.150. [DOI] [PubMed] [Google Scholar]
- Riley P, and Lamont T. 2013. Triclosan/copolymer containing toothpastes for oral health. Cochrane Database Syst Rev 12: Cd010514. doi: 10.1002/14651858.CD010514.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel H, Bohmer W, Muller M, Fliedner A, Ricking M, Teubner D, and Schroter-Kermani C. 2013. Retrospective study of triclosan and methyl-triclosan residues in fish and suspended particulate matter: Results from the German Environmental Specimen Bank. Chemosphere 91: 1517–1524. doi: 10.1016/j.chemosphere.2012.12.030. [DOI] [PubMed] [Google Scholar]
- Rule KL, Ebbett VR, and Vikesland PJ. 2005. Formation of chloroform and chlorinated organics by free-chlorine-mediated oxidation of triclosan. Environ Sci Technol 39: 3176–3185. [DOI] [PubMed] [Google Scholar]
- Ruszkiewicz JA, Li S, Rodriguez MB, and Aschner M. 2017. Is triclosan a neurotoxic agent? J Toxicol Environ Health B 20: 104–117. doi: 10.1080/10937404.2017.1281181. [DOI] [PubMed] [Google Scholar]
- Sadowski MC, Pouwer RH, Gunter JH, Lubik AA, Quinn RJ, and Nelson CC. 2014. The fatty acid synthase inhibitor triclosan: Repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 5: 9362–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Prado L, Llompart M, Lores M, Garcia-Jares C, Bayona JM, and Cela R. 2006. Monitoring the photochemical degradation of triclosan in wastewater by UV light and sunlight using solid-phase microextraction. Chemosphere 65:1338–1347. doi: 10.1016/j.chemosphere.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, and Ekstrand J. 2006. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A 69: 1861–1873. [DOI] [PubMed] [Google Scholar]
- Schmid B, Rippmann JF, Tadayyon M, and Hamilton BS. 2005. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun 328 : 1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- Schnitzler JG, Frederich B, Dussenne M, Klaren PH, Silvestre F, and Das K. 2016. Triclosan exposure results in alterations of thyroid hormone status and retarded early development and metamorphosis in Cyprinodon variegatus. Aquat Toxicol 181:1–10. doi: 10.1016/j.aquatox.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Shala L, and Foster GD. 2010. Surface water concentrations and loading budgets of pharmaceuticals and other domestic-use chemicals in an urban watershed (Washington, DC, USA). Arch Environ Contam Toxicol 58: 551–561. doi: 10.1007/s00244-009-9463-z. [DOI] [PubMed] [Google Scholar]
- Shim J, Weatherly LM, Luc RH, Dorman MT, Neilson A, Ng R, Kim CH, Millard PJ, and Gosse JA. 2016. Triclosan is a mitochondrial uncoupler in live zebrafish. J Appl Toxicol 36:1662–1667. doi: 10.1002/jat.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui WH. 1979. Pharmacokinetics of triclosan in rat after intravenous and intravaginal administration. J Environ Pathol Toxicol 2: 861–871. [PubMed] [Google Scholar]
- Singer H, Muller S, Tixier C, and Pillonel L. 2002. Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36: 4998–5004. [DOI] [PubMed] [Google Scholar]
- Sorg O 2014. AhR signalling and dioxin toxicity. Toxicol Lett 230: 225–233. doi: 10.1016/j.toxlet.2013.10.039. [DOI] [PubMed] [Google Scholar]
- Spanier AJ, Fausnight T, Camacho TF, and Braun JM. 2014. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc 35: 475–481. doi: 10.2500/aap.2014.35.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statista. “Sales growth of the leading toothpaste brands in the United States in 2016 (change to prior sales year).”. 2016a https://www.statista.com/statistics/195651/us-sales-growth-of-toothpaste-brands-in-2007-and-2008/.
- Statista. “Sales of the leading toothpaste brands in the United States in 2016 (in million U.S. dollars).”. 2016b https://www.statista.com/statistics/195650/leading-us-toothpaste-brands-in-2007-and-2008-based-on-sales/.
- Suller MT, and Russell AD. 2000. Triclosan and antibiotic resistance in Staphylococcus aureus. J Antimicrob Chemother 46: 11–18. [DOI] [PubMed] [Google Scholar]
- Tal T 2017. Developmental exposure to triclosan alters microbiota commnity structure and locomotor activity in larval zebrafish Society of Toxicology, Baltimore, MA. [Google Scholar]
- Tamura I, Kanbara Y, Saito M, Horimoto K, Satoh M, Yamamoto H, and Oyama Y. 2012. Triclosan, an antibacterial agent, increases intracellular Zn(2+) concentration in rat thymocytes: Its relation to oxidative stress. Chemosphere 86: 70–75. doi: 10.1016/j.chemosphere.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Tan WP, Suresh S, Tey HL, Chiam LY, and Goon AT. 2010. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol 35: e109–e112. [DOI] [PubMed] [Google Scholar]
- Tavakoly Sany SB, Hashim R, Salleh A, Rezayi M, Karlen DJ, Razavizadeh BB, and Abouzari-Lotf E. 2015. Dioxin risk assessment: Mechanisms of action and possible toxicity in human health. Environ Sci Pollut Res Int 22: 19434–50. doi: 10.1007/s11356-015-5597-x. [DOI] [PubMed] [Google Scholar]
- Tessier L, Boisvert JL, Vought LB, and Lacoursie JO. 2000. Effects of 2,4-dichlorophenol on the net-spinning behavior of hydropsyche slossonae larvae (Trichoptera; Hydropsychidae), an early warning signal of chronic toxicity. Ecotoxicol Environ Saf 46 : 207–217. doi: 10.1006/eesa.1999.1897. [DOI] [PubMed] [Google Scholar]
- Tixier C, Singer HP, Canonica S, and Muller SR. 2002. Phototransfomation of ticlosan in surface waters: a relevant elimination process for this widely used biocide--laboratory studies, field measurements, and modeling. Environ Sci Technol 36: 3482–3489. [DOI] [PubMed] [Google Scholar]
- Tulp MTM , Sundstrom G, Martron LB and O. Huntzinger1979. Metabolism of chlorodiphenyl ethers and Irgasan DP300. Xenobiotica 9: 65–77. [DOI] [PubMed] [Google Scholar]
- Udoji F, Martin T, Etherton R, and Whalen MM. 2010. Immunosuppressive effects of triclosan, nonylphenol, and DDT on human natural killer cells in vitro. J Immunotoxicol 7: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez MP, Arbuckle TE, and Fraser WD. 2015. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 103:1011–1020.e2. doi: 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Waaler SM, Rolla G, Skjorland KK, and Ogaard B. 1993. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: a pilot study. Scand J Dent Res 101: 192–195. [DOI] [PubMed] [Google Scholar]
- Wang CF, and Tian Y. 2015. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environ Pollut 206:195–201. doi: 10.1016/j.envpol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Falany CN, and James MO. 2004. Triclosan as a substrate and inhibitor of 3’-phosphoadenosine 5’-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos 32: 1162–1169. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, and Chen L. 2015. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci Rep 5:18252. doi: 10.1038/srep18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Kennedy RH, Shim J, and Gosse JA. 2013. A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: Effects of triclosan without use of an organic solvent. J Vis Exp: e50671. doi: 10.3791/50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shim J, Hashmi HN, Kennedy RH, Hess ST, and Gosse JA. 2016. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol 36: 777–789. doi: 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Qiao P, Shi Y, Ruan Y, Yin J, Wu Q, and Shao B. 2017. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin Chim Acta 466:133–137. doi: 10.1016/j.cca.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Weiss L, Arbuckle TE, Fisher M, Ramsay T, Mallick R, Hauser R, LeBlanc A, Walker M, Dumas P, and Lang C. 2015. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health 218: 507–513. doi: 10.1016/j.ijheh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Willey JC, Saladino AJ, Ozanne C, Lechner JF, and Harris CC. 1984. Acute effects of 12-O-tetradecanoylphorbol-13-acetate, teleocidin B, or 2,3,7,8-tetrachlorodibenzo-p-dioxin on cultured normal human bronchial epithelial cells. Carcinogenesis 5:209–215. [DOI] [PubMed] [Google Scholar]
- Winitthana T, Lawanprasert S, and Chanvorachote P. 2014. Triclosan potentiates epithelial-to-mesenchymal transition in anoikis-resistant human lung cancer cells. PLoS One 9: e110851. doi: 10.1371/journal.pone.0110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witorsch RJ 2014. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit Rev Toxicol 44: 535–555. doi: 10.3109/10408444.2014.910754. [DOI] [PubMed] [Google Scholar]
- Wu C, Spongberg AL, and Witter JD. 2009. Adsorption and degradation of triclosan and triclocarban in soils and biosolids-amended soils. J Agric Food Chem 57: 4900–4905. doi: 10.1021/jf900376c. [DOI] [PubMed] [Google Scholar]
- Wu JL, Liu J, and Cai Z. 2010. Determination of triclosan metabolites by using in-source fragmentation from high-performance liquid chromatography/negative atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Commun Mass Spectrom 24: 1828–1834. doi: 10.1002/rcm.4558. [DOI] [PubMed] [Google Scholar]
- Wu Q, Shi H, Adams CD, Timmons T, and Ma Y. 2012. Oxidative removal of selected endocrine-disruptors and pharmaceuticals in drinking water treatment systems, and identification of degradation products of triclosan. Sci Total Environ 439: 18–25. doi: 10.1016/j.scitotenv.2012.08.090. [DOI] [PubMed] [Google Scholar]
- Wu X, Ernst F, Conkle JL, and Gan J. 2013. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ Int 60: 15–22. doi: 10.1016/j.envint.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Wu Y, Beland FA, Chen S, and Fang JL. 2015. Extracellular signal-regulated kinases 1/2 and Akt contribute to triclosan-stimulated proliferation of JB6 Cl 41–5a cells. Arch Toxicol 89: 1297–1311. doi: 10.1007/s00204-014-1308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chitranshi P, Loukotkova L, Gamboa da Costa G, Beland FA, Zhang J, and Fang JL. 2017. Cytochrome P450-mediated metabolism of triclosan attenuates its cytotoxicity in hepatic cells. Arch Toxicol.91: 2405–2423. doi: 10.1007/s00204-016-1893-6. [DOI] [PubMed] [Google Scholar]
- Yang B, Ying GG, Zhao JL, Zhang LJ, Fang YX, and Nghiem LD. 2011. Oxidation of triclosan by ferrate: Reaction kinetics, products identification and toxicity evaluation. J Hazard Mater 186: 227–235. doi: 10.1016/j.jhazmat.2010.10.106. [DOI] [PubMed] [Google Scholar]
- Yeh A, Marcinek DJ, Meador JP, and Gallagher EP. 2017. Effect of contaminants of emerging concern on liver mitochondrial function in Chinook salmon. Aquat Toxicol 190: 21–31. doi: 10.1016/j.aquatox.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Wei L, Shi Y, Zhang J, Wu Q, and Shao B. 2016. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails.Environ Geochem Health 38: 1125–1135. doi: 10.1007/s10653-015-9777-x. [DOI] [PubMed] [Google Scholar]
- Ying GG, and Kookana RS. 2007. Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int 3: 199–205. doi: 10.1016/j.envint.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ying GG, Yu XY, and Kookana RS. 2007. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut 150:300–305. doi: 10.1016/j.envpol.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Yu BJ, Kim JA, and Pan JG. 2010. Signature gene expression profile of triclosan-resistant Escherichia coli. J Antimicrob Chemother 65:1171–1177. doi: 10.1093/jac/dkq114. [DOI] [PubMed] [Google Scholar]
- Yu JC, Kwong TY, Luo Q, and Cai Z. 2006. Photocatalytic oxidation of triclosan. Chemosphere 65: 390–2399. doi: 10.1016/j.chemosphere.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Yueh MF, Taniguchi K, Chen S, Evans RM, Hammock BD, Karin M, and Tukey RH. 2014. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc Natl Acad Sci U S A 111:17200–17205. doi: 10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Ma H, Pan S, You J, Zhang G, Yu Z, Sheng G, and Fu J. 2016. LINE-1 gene hypomethylation and p16 gene hypermethylation in HepG2 cells induced by low-dose and long-term triclosan exposure: The role of hydroxyl group. Toxicol In Vitro 34:35–44. doi: 10.1016/j.tiv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang H, and Huang CH. 2003. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ Sci Technol 37: 2421–2430. [DOI] [PubMed] [Google Scholar]
- Zhang L, Niu J, and Wang Y. 2016. Full life-cycle toxicity assessment on triclosan using rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 127:30–35. doi: 10.1016/j.ecoenv.2015.12.043. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, and Wang L. 2010. Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: From source to the receiving environment. J Hazard Mater 179: 215–222. doi: 10.1016/j.jhazmat.2010.02.082. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, and Stoker TE. 2009. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci 107: 56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]


