Abstract
IMPORTANCE
There is growing evidence that statins may protect against the development or worsening of open-angle glaucoma (OAG). As researchers plan clinical trials to more definitively study whether statins indeed protect against OAG, it would be helpful to know whether specific daily dosages or types of statin confer a greater protective effect than others.
OBJECTIVE
To assess whether the protective effect of statins on the risk of glaucoma varies depending on the daily dosage or type of statin taken.
DESIGN, SETTING, AND PARTICIPANTS
Using claims data from January 2001 to December 2009, we observed 25 420 patients with no preexisting glaucoma and quantified exposure to statins and other cholesterol-lowering medications. Using multivariable regression modeling, we assessed the hazard of developing OAG and how it varied by the daily dosage or type of statin and whether any protective effect persists after accounting for baseline low-density lipoprotein level.
EXPOSURES
Different daily dosages and types of statins.
MAIN OUTCOMES AND MEASURES
Hazard ratios (HRs) for developing OAG with 95% CIs.
RESULTS
Of the 25 420 patients who met the eligibility criteria for study inclusion, the mean (SD) age was 66.1 (5.8) years, and 14 112 (55.5%) were female. Additionally, 19 232 patients (84.1%) were white, 1252 (5.5%) were black, and 1558 (6.8%) were Latino. After accounting for baseline low-density lipoprotein levels, persons who filled prescriptions for statins continuously for 2 years had a 21% reduced risk of glaucoma compared with nonusers (adjusted HR, 0.79; 95% CI, 0.66–0.96; P = .02). There was no additional protective effect associated with taking the highest dosage of statins (80 mg) compared with a lower dosage (40 mg) (HR, 1.03; 95% CI, 0.59–1.80; P = .91). The protective effect of the following statins on OAG risk did not differ compared with atorvastatin, an inexpensive generic statin: lovastatin (HR, 1.09; 95% CI, 0.71–1.68; P = .69), cerivastatin (HR, 0.61; 95% CI, 0.09–4.41; P = .63), rosuvastatin (HR, 0.83; 95% CI, 0.48–1.44; P = .51), fluvastatin (HR, 0.89; 95% CI, 0.39–2.02; P = .78), pravastatin (HR, 1.29; 95% CI, 0.93–1.79; P = .13), and simvastatin (HR, 1.03; 95% CI, 0.83–1.29; P = .78).
CONCLUSIONS AND RELEVANCE
Even after accounting for baseline low-density lipoprotein level, statin exposure continued to be associated with a reduction in OAG risk. Our study helps inform researchers of a reasonable daily dosage and type of statin to use when designing randomized clinical trials to assess the association between statin use and glaucoma.
Evidence suggests that statins may protect against open-angle glaucoma (OAG).1–11 In 2012, we assessed the association between statins and OAG among more than 500000 managed-care enrollees with hyperlipidemia.11 We found that persons with hyperlipidemia who took statins continuously for 2 years had an 8% decreased OAG risk relative to nonusers (P = .006) and a 9% decreased risk of progressing from glaucoma suspect to OAG compared with nonusers (P = .006).11While these findings support other studies demonstrating that statins may help protect against OAG,1–10 the definitive means of assessing the role of statins in glaucoma care is to perform a randomized clinical trial (RCT). Before embarking on an RCT,there are additional questions that can be explored using claims data. Here, we use the same data source as our previous study11 to assess whether (1) the apparent beneficial effect of statins on OAG risk is limited to persons with elevated low-density lipoprotein (LDL) cholesterol levels, (2) the use of a particular daily dosage of statin is required to achieve a beneficial effect, and (3) the use of a particular statin type protects best against OAG.
Methods
We used the Clinformatics Data Mart (OptumInsight),aclaims database of enrollees in a nationwide managed-care network.Tokeepanalysesconsistentwithourpastwork,11we used data from January 2001 to December 2009.Thisdatabasecaptures ocular and nonocular diagnoses by using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)12 codes and contains information on enrollees’ sociodemographic characteristics, outpatient prescriptions filled, and laboratory test results. All enrollees in the medical plan were also in the pharmacy plan. The University of Michigan Institutional Review Board approved this study, and because data were taken from a deidentified database,informed consent was waived.
Weidentifiedenrollees60yearsandolderwithhyperlipidemia (ICD-9-CM code 272.x) and no preexisting OAG diagnosis in the previous 2 years. Those with less than 2 years of plan enrollment, any noncontinuous enrollment, or no ophthalmologist or optometrist visits were excluded. Each enrollee had to have at least 1 LDL measurement within 6 months of the index date (2 years after plan enrollment). Multivariable Cox proportional hazards regression modeling assessed the hazard for developing OAG.We created 3 models.For each model,enrollees were observed from the index date until OAG onset or their last eye-care visit. The outcome of interest was diagnosis of OAG(ICD-9-CM codes 365.1,365.10–12,and 365.15). Covariates included sociodemographic characteristics (age, sex, race/ethnicity, household net worth, education level, and region of residence), medical comorbidities (systemic hypotension, obesity, sleep apnea, migraine, diabetes, and hypertension), ocular comorbidities (cataract, pseudophakia/ aphakia, macular degeneration, and diabetic retinopathy), other cholesterol-lowering medication use, and Charlson Comorbidity Index score,13 which measures disease burden. Additional methodological details appear elsewhere.11
All 3 models assessed the possible associations among the use of statins, use of other cholesterol-lowering drugs, and baseline LDL measurements and the hazard for OAG.Model1 included statin use as a time-dependen tvariable.Fo revery day after the index date, we calculated the number of days (later converted to months) each beneficiary received a statin over the previous 24months.Model2 assessed whether daily statin dosage was associated with the hazard for OAG.Here,we compared users who filled prescriptions for exclusively 10 mg, 20 mg, and 80 mg of statins daily with users of 40 mg daily. Persons who filled prescriptions for multiple different strengths of statins(4942[19.4%])and nonusers(9522[37.5%])were excluded from Model 2.Model 3 assessed whether statin type was associated with the hazard for OAG.Statinusers were categorized in to those who exclusively filled prescriptions for lovastatin, cerivastatin, atorvastatin, rosuvastatin, fluvastatin, pravastatin,and simvastatin;for each group,the OAGhazard was calculated relative to atorvastatin users. Users of multiple statin types(7704[30.3%])and nonusers(9522[37.5%]) were excluded.In all models,baseline LDL level was grouped inquartiles.For those with multiplere corded LDL values,we used the reading closest to the index date. Checks for correlation of model covariates identified no concerns. Statistical significance was set at P = .05.
Results
Among the 25420 eligible enrollees, 15898 persons (62.5%) had at least 1 record of statin use. The mean (SD) patient age was 66.1 (5.8) years; 14112 patients (55.5%) were female, 19232 (84.1%) were white, 1252 (5.5%) were black, and 1558 (6.8%) were Latino. A total of 1218 persons (4.8%) developed OAG over a median follow-up of 440 days (Table 1). Among statin users, 3099 (19.5%) filled prescriptions exclusively for 10 mg daily, 2882 (18.1%) for 20 mg daily, 1857 (11.7%) for 40 mg daily, and 356 (2.2%) for 80 mg daily; the remaining 7704 (48.5%) received more than 1 strength. The most widely used statin agent was atorvastatin (5492 [34.6%]), followed by simvastatin (3271 [20.6%]), pravastatin (968 [6.1%]), lovastatin (616 [3.9%]), rosuvastatin (387 [2.4%]), fluvastatin (191 [1.2%]), and cerivastatin (31 [0.2%]). The remaining patients (4942 [31.1%]) used multiple agents. The mean (SD) LDL value among patients was 112 (33) mg/dL (to convert to millimoles per liter, multiply by 0.0259).
Table 1.
Characteristics of the Study Participants
| No. (%) |
||||
|---|---|---|---|---|
| Characteristic | Overall | No OAG | OAG | P Value |
| Total | 25420 (100) | 24202 (95.2) | 1218 (4.8) | |
| Age, mean (SD), y | 66.1 (5.8) | 66.1 (5.8) | 66.6 (5.9) | .003 |
| Race/ethnicitya | ||||
| White | 19 232 (84.1) | 18 398 (84.6) | 834 (75.5) | |
| Black | 1252 (5.5) | 1127 (5.2) | 125(11.3) | <.001 |
| Latino | 1558 (6.8) | 1458 (6.7) | 100 (9.1) | |
| Asian | 600 (2.6) | 565 (2.6) | 35 (3.2) | |
| Other | 218 (1.0) | 208 (1.0) | 10 (0.9) | |
| Sex | ||||
| Male | 11308 (44.5) | 10767 (44.5) | 541 (44.4) | .96 |
| Female | 14112 (55.5) | 13435 (55.5) | 677 (55.6) | |
| Any statin usedb | 15 898 (62.5) | 15152 (62.6) | 746 (61.3) | .34 |
| Type of statin used exclusivelyb | ||||
| Lovastatin | 616 (3.9) | 586 (3.9) | 30 (4.0) | |
| Cerivastatin | 31 (0.2) | 29 (0.2) | 2 (0.3) | |
| Atorvastatin | 5492 (34.6) | 5253 (34.7) | 239 (32.0) | |
| Rosuvastatin | 387 (2.4) | 371 (2.5) | 16(2.1) | .59 |
| Fluvastatin | 191 (1.2) | 182 (1.2) | 9(1.2) | |
| Pravastatin | 968 (6.1) | 921 (6.1) | 47 (6.3) | |
| Simvastatin | 3271 (20.6) | 3126 (20.6) | 145 (19.4) | |
| Using multiple statin types | 4942 (31.1) | 4684 (30.9) | 258 (34.6) | |
| Other cholesterol-lowering medication use | 6356 (25.0) | 6062 (25.1) | 294 (24.1) | .47 |
| Statin strength used exclusively, mgb | ||||
| 10 | 3099 (19.5) | 2972 (19.6) | 127 (17.0) | |
| 20 | 2882 (18.1) | 2751 (18.2) | 131 (17.6) | .38 |
| 40 | 1857 (11.7) | 1768 (11.7) | 89(11.9) | |
| 80 | 356 (2.2) | 336 (2.2) | 20 (2.7) | |
| Using multiple statin strengths | 7704 (48.5) | 7325 (48.3) | 379 (50.8) | |
| Baseline LDL cholesterol level, mg/dL | ||||
| <82 | 4541 (17.9) | 4326 (17.9) | 215(17.7) | |
| 82–130 | 13 623 (53.6) | 12 988 (53.7) | 635 (52.1) | .62 |
| 130–177 | 6339 (24.9) | 6017 (24.9) | 322 (26.4) | |
| 177–225 | 917 (3.6) | 871 (3.6) | 46 (3.8) | |
Abbreviations: LDL, low-density lipoprotein; OAG, open-angle glaucoma.
Excluding 2560 with missing data on race/ethnicity.
Excluding 9522 who were not taking statins.
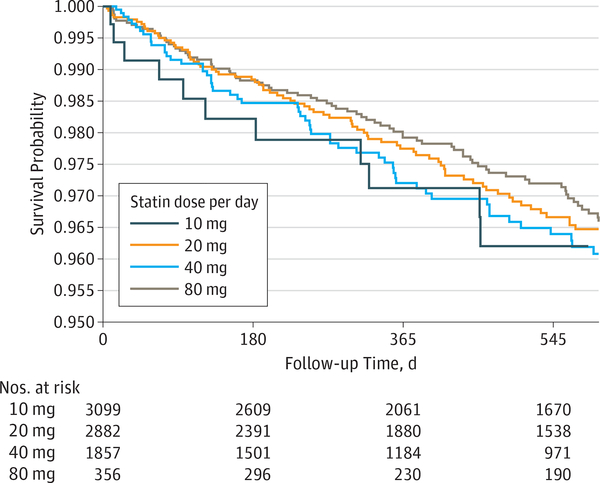
After adjusting for possible confounders and baseline LDL level, every additional month of statin use was associated with a 1% decreased hazard for OAG (hazard ratio [HR], 0.990; 95% CI, 0.983–0.998; P = .02). Thus, those who took statins continuously for 2 years had a 21% decreased hazard for OAG compared with nonusers. The difference between users and nonusers of other cholesterol-lowering medications was not significant for OAG risk (HR, 1.00; 95% CI, 0.99–1.01; P = .69). The OAG hazard among persons in the lowest baseline LDL quartile did not differ with persons in any other LDL quartile (P > .05 for all comparisons) (Table 2). The hazard did not differ between users of 40 mg daily and those taking 10 mg (HR, 0.82; 95% CI, 0.61–1.11; P = .20), 20 mg (HR, 0.92; 95% CI, 0.69–1.23; P = .58), or 80 mg (HR, 1.03; 95% CI, 0.59–1.80; P = .91) daily (Figure; Table 2). Compared with atorvastatin users, the risk of OAG was similar for persons taking lovastatin (HR, 1.09; 95% CI, 0.71–1.68; P = .69), cerivastatin (HR, 0.61, 95% CI, 0.09–4.41; P = .63), rosuvastatin (HR, 0.83; 95% CI, 0.48–1.44; P = .51), fluvastatin (HR, 0.89; 95% CI, 0.39–2.02; P = .78), pravastatin (HR, 1.29; 95% CI, 0.93–1.79; P = .13), or simvastatin (HR, 1.03; 95% CI, 0.83– 1.29; P = .78) (Table 2).
Table 2.
Factors Affecting the Hazard of Developing Open-Angle Glaucomaa
| Variable | Adjusted HR (95% CI) | P Value |
|---|---|---|
| Model 1b | ||
| TD statin use | 0.99 (0.98–1.00) | .02 |
| TD use of other cholesterol-lowering medications | 1.00 (0.99–1.01) | .69 |
| LDL cholesterol level, mg/dL | ||
| <82 | 1 [Reference] | NA |
| 82–130 | 0.88 (0.74–1.04) | .13 |
| 130–177 | 0.93 (0.76–1.13) | .43 |
| 177–225 | 0.94 (0.66–1.34) | .74 |
| Model 2 | ||
| 40 mg of statins daily | 1 [Reference] | NA |
| 10 mg of statins daily | 0.82 (0.61–1.11) | .20 |
| 20 mg of statins daily | 0.92 (0.69–1.23) | .58 |
| 80 mg of statins daily | 1.03 (0.59–1.80) | .91 |
| Model 3 | ||
| Atorvastatin | 1 [Reference] | NA |
| Lovastatin | 1.09 (0.71–1.68) | .69 |
| Cerivastatin | 0.61 (0.09–4.41) | .63 |
| Rosuvastatin | 0.83 (0.48–1.44) | .51 |
| Fluvastatin | 0.89 (0.39–2.02) | .78 |
| Pravastatin | 1.29 (0.93–1.79) | .13 |
| Simvastatin | 1.03 (0.83–1.29) | .78 |
Abbreviations: HR, hazard ratio; LDL, low-density lipoprotein; NA, not applicable; TD, time-dependent.
All 3 models adjusted for age (implicitly), sex, race/ethnicity, household net worth, education level, region of residence, systemic hypotension, obesity, sleep apnea, migraine, diabetes, hypertension, cataract, pseudophakia/ aphakia, macular degeneration, diabetic retinopathy, and Charlson Comorbidity Index score.
The risk reduction of the use of statins and other cholesterol-lowering medications in the model is for each additional month of continuous use of these medications.
Figure. Probability of Developing Open-Angle Glaucoma Among Users of Different Daily Dosages of Statins.
Survival probability is the unadjusted probability of not developing open-angle glaucoma by that time. Although not shown, the confidence bands for the 4 daily dosages overlap for nearly the entire length of follow-up, indicating no statistically significant differences.
Discussion
This study offers use ful in formation a bout key parameters to consider in planning an RCT to test whether statins protect against OAG development or progression. The risk of developing OAG does not appear to be associated with the daily dosage consumed (10, 20, or 80 mg vs 40 mg). Because the risk of adverse effects rises with increasing daily statin dosage,14 exposing RCT participants to a relatively high dosage of 80mg would seem unnecessary.A daily dosage of 40 mg or less may be more appropriate. Additionally, compared with atorvastatin, no other statin type was more protective against OAG. Because some statins are costlier than others, it seems reasonable to select an inexpensive generic agent like atorvastatin for an RCT. Finally, we found that the apparent protective effect of statins against OAG observed in our earlier study appears to persist in the present analyses after adjusting for baseline LDL levels.There was no significant difference in OAG risk between those in the highe stand lowest baseline LDL quartile safter we accounted foruse of statin and other cholesterollowering medications. This suggests that the potential beneficial effect of statins on OAG risk is associated less with lowering lipid levels and more with other properties of statins, such as outflow enhancement by affecting rho kinase activity or neuroprotection.13–15Infact,the risk reduction of 2years’ continuous statin use was 8% before baseline LDL level adjustment and 21% afterward
Limitations
This study had limitations.Unfortunately,claims data lackin formation on clinical variables,such a sintraocular pressures, to consider in our models. Also, we assume that filling medication prescriptions is a reasonable surrogate for medication consumption.However,if persons are filling prescriptions but not actually taking the medications, then this would bias the results to the null.
Conclusions
These study findings provide researchers with useful information about an appropriate daily dosage and type of statin to select for an RCT to explore whether statins can prevent the development of OAG or halt OAG progression.
Key Points.
Question
Does the protective effect of statins affect the risk of glaucoma depending on the daily dosage or type of statin taken?
Findings
This study found that after accounting for baseline low-density lipoprotein levels, persons who filled prescriptions for statins continuously for 2 years had a 21% reduced risk of glaucoma vs nonusers. There was no additional protective effect associated with taking the highest dosage of statins (80 mg) compared with a lower dosage (40 mg) or with other types compared with atorvastatin.
Meaning
When planning clinical trials to study the effect of statins on glaucoma, it is reasonable to use a generic statin like atorvastatin and a 40mg or lower daily dosage.
Acknowledgments
Funding/Support: This study was supported by grant R34 EY022399, a clinical trial planning grant from the National Eye Institute (Drs Musch and Stein).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Stein has received the Physician-Scientist award from Research to Prevent Blindness as well as support from the W. K. Kellogg Foundation. No other disclosures were reported
Concept and design: Talwar, Stein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Talwar, Stein. Critical revision of the manuscript for important intellectual content: Talwar, Musch.
Statistical analysis: Talwar.
Obtained funding: Musch, Stein.
Administrative, technical, or material support: Musch, Stein.
Contributor Information
Nidhi Talwar, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Center for Eye Policy and Innovation, University of Michigan, Ann Arbor.
David C. Musch, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Center for Eye Policy and Innovation, University of Michigan, Ann Arbor; Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor.
Joshua D. Stein, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Center for Eye Policy and Innovation, University of Michigan, Ann Arbor; Department of Health Management and Policy, University of Michigan School of Public Health, Ann Arbor..
REFERENCES
- 1.Nagaoka T, Takahashi A, Sato E, et al. Effect ofsystemic administration of simvastatin on retinal circulation. Arch Ophthalmol. 2006;124(5): 665–670. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal G Jr, Chatterjee A, Oh SS, Oh DJ,Rhee DJ. Pharmacological regulation of SPARC by lovastatin in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014;55(3):1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao VP, Epstein DL. Rho GTPase/rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21(3):167–177. [DOI] [PubMed] [Google Scholar]
- 4.Zacco A, Togo J, Spence K, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23(35):11104–11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bösel J, Gandor F, Harms C, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92(6):1386–1398. [DOI] [PubMed] [Google Scholar]
- 6.Honjo M, Tanihara H, Nishijima K, et al. Statininhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol. 2002;120(12):1707–1713. [DOI] [PubMed] [Google Scholar]
- 7.McGwin G Jr, McNeal S, Owsley C, Girkin C,Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122(6): 822–826. [DOI] [PubMed] [Google Scholar]
- 8.Leung DYL, Li FCH, Kwong YYY, Tham CC,Chi SC, Lam DS. Simvastatin and disease stabilization in normal tension glaucoma: a cohort study. Ophthalmology. 2010;117(3):471–476. [DOI] [PubMed] [Google Scholar]
- 9.Marcus MW, Müskens RP, Ramdas WD, et al. Cholesterol-lowering drugs and incident open-angle glaucoma: a population-based cohort study. PLoS One. 2012;7(1):e29724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Castro DK, Punjabi OS, Bostrom AG, et al. Effect of statin drugs and aspirin on progression in open-angle glaucoma suspects using confocal scanning laser ophthalmoscopy. Clin Exp Ophthalmol. 2007;35(6):506–513. [DOI] [PubMed] [Google Scholar]
- 11.Stein JD, Newman-Casey PA, Talwar N, Nan B,Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119(10):2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 14.de Lemos JA, Blazing MA, Wiviott SD, et al. ;Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316 [DOI] [PubMed] [Google Scholar]