In the 19th century through the early 20th century, an adequate diet was thought to be composed of proteins, minerals, fats, and carbohydrates. However, experiments in the early 20th century showed that animals fed these purified components alone did not survive. Hence the search was on for ‘‘vital amines,’’ elements present in foods that were required for health and survival and later termed vitamins,1 of which vitamin D was the fourth to be discovered. We now know that human subjects get vitamin D not only from the diet but also from sun exposure.
The investigation of the role of vitamin D as a treatment for asthma and allergies dates back to the 1930s, soon after the isolation of vitamin D, when viosterol (activated ergosterol) in large doses (at least 120,000 IU daily) was compared with corn oil in adults with asthma and hay fever.2 Because the mechanism of vitamin D in asthma and allergies could not be determined, this avenue of investigation in human subjects was largely abandoned. Investigations in animal models continued, showing that exposure to vitamin D by the developing lung in utero led to improved lung maturation and surfactant production and diminished airway inflammation after a viral infection and led to decreased airway smooth muscle mass (reviewed by Litonjua3). In addition, studies in immune cells showed that vitamin D is important in the immune response.4
Based on our epidemiologic studies and review of animal experiments of the developing lung, we hypothesized in 20075 that vitamin D deficiency could have a role in the increase in asthma and allergies, and the number of reports in the literature dramatically increased. The observational human studies investigating vitamin D in the development of asthma were understandably mixed, with studies using estimates of maternal intake of vitamin D generally showing a protective effect of higher vitamin D levels during pregnancy and studies using one measure of vitamin D status (25-hydroxyvitamin D [25OHD]) generally showing no effects,6 likely because the intake estimates were reflective of longer-term intakes, whereas the 25OHD level was reflective of shorter-term exposures. Clinical trials were supposed to clarify the role of vitamin D in the prevention of asthma and allergies in young children.
The Vitamin D Antenatal Asthma Reduction Trial (VDAART)7 and the Copenhagen Prospective Studies on Asthma in Childhood 20108 trial, both trials of prenatal supplementation of vitamin D to prevent asthma, wheezing, and allergies in young children, were recently published. Although strict statistical significance was not met in either trial, suggesting that power was an issue, there were strikingly identical effects of vitamin D supplementation on asthma and recurrent wheeze in the offspring: about a 20% reduction in the risk for these disorders by 3 years of age. However, it turns out that statistical power was likely not the main problem in the design of these clinical trials.
Heaney9 recently showed that nutrient trials, such as for vitamin D, are different than drug trials in many ways. Primary among these are that unlike drug trials, nutrient trials do not have a real placebo arm because of the fact that all subjects are exposed to some level of the nutrient and thus have some circulating level of the nutrient, leading to different starting points at the time of supplementation. We have shown this to be the case in VDAART and have shown potentially different effects of supplementation depending on the initial level of 25OHD on entry into the trial.10 Other factors influencing the results of VDAART have been summarized previously.11
In this issue, Hollams et al12 add another piece of information to our growing understanding about the effects of vitamin D on asthma occurrence. The investigators followed a birth cohort at high risk for asthma and allergies through the first 10 years of life, with assessment of vitamin D status at birth (cord blood), 6 months, and 1, 2, 3, 4, 5, and 10 years of age. They found that there were consistent inverse associations of 25OHD levels with allergic sensitization in early life, whereas there were no cross-sectional associations of 25OHD levels with asthma. However, they also found that over the 10-year period, the number of times a child was found to be deficient in vitamin D (defined as a level of <50 nmol/L, which is equivalent to <20 ng/mL) was positively associated with the risk for asthma and wheeze at age 10 years, suggesting that prevention of vitamin D deficiency throughout childhood was necessary to prevent asthma. These findings are consistent with the results of null cross-sectional reports of the association between vitamin D and asthma and reports in which only 1 measurement of vitamin D status was used. These findings also make sense because the many mechanisms of vitamin D that might prevent asthma (eg, effect on gene expression, effect on postviral inflammatory state, balance of TH1 and TH2 responses, and effect on smooth muscle inflammation and remodeling) are dynamic processes responsive to changes in the environment. Thus maintenance of adequate vitamin D status would logically lead to these beneficial effects.
Other important findings in this study need to be highlighted. First, the authors found that vitamin D status in early life was inversely associated with nasopharyngeal colonization with Streptococcus species and age of first febrile lower respiratory illness. The authors’ findings suggest that vitamin D might be important in helping to establish a beneficial airway microbiome and might have effects on early-life respiratory tract infections. This is consistent with the experimental findings that vitamin D is an important player in stabilization of the intestinal mucosa,13 and our group has also found that vitamin D status at birth is a determinant of the early-life intestinal microbiome.14 Although clinical trials of vitamin D to prevent respiratory tract infections have been disappointing, this might again be due to the initial levels of 25OHD and the low doses of vitamin D used.15 A longitudinal observation study suggested that maintenance of 25OHD levels of at least 95 nmol/L (38 ng/mL)16 was necessary for prevention of viral respiratory illnesses.
Second, the authors found that the majority of children could be defined as having vitamin D deficiency at birth, suggesting that pregnant mothers were also likely to be deficient. As suggested by results of recent trials, improving vitamin D status in pregnancy might have provided additional benefits.
Third, there was marked variability in 25OHD levels over time (intraclass correlation, 0.31), which is consistent with previous studies,17 emphasizing the fact that one measurement is not sufficient as a measure of long-term exposure and that longitudinal studies with multiple measures of exposure are more informative than cross-sectional studies.
The biology of vitamin D is complex, and we are learning more about it every day. Interpretation of data from this and other studies suggests a dynamic process of asthma and allergy inception that begins in the womb and continues throughout childhood and likely through adulthood (Fig 1). Because of flaws in the design of current human studies that have led to conflicting results or inadequate statistical significance, some have called for tempering the enthusiasm for vitamin D. On the contrary, we believe that there is much more to uncover, such as the dose and timing of supplementation and the level that maximizes protection. Although we have surmised that adequate vitamin D status from birth throughout life is necessary, the article by Hollams et al12 has finally given us a part of the answer: we at least need to eliminate vitamin D deficiency in childhood to prevent asthma. Rather than tempering the enthusiasm, we need to redouble our efforts to continue to define the role of vitamin D in the inception of asthma. Only then will we be able to make inroads in solving the asthma puzzle and ultimately achieve the holy grail of preventing the disorder.
FIG 1.
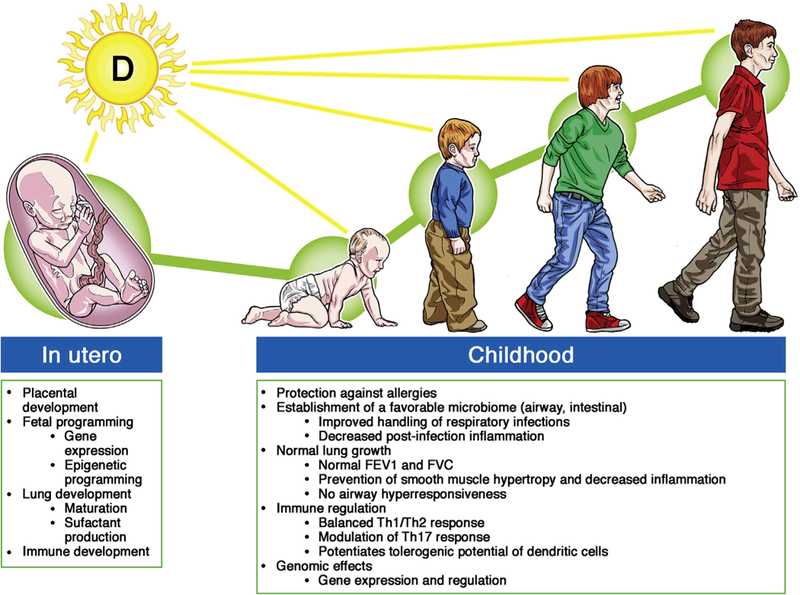
Adequate vitamin D status prenatally and throughout childhood in the prevention of asthma and allergies. FVC, Forced vital capacity.
Acknowledgments
Supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grant HL091528 and NIH grant 1UG3OD023268.
Footnotes
Disclosure of potential conflict of interest: A. A. Litonjua has received grants from the National Institutes of Health, has received royalties from UpToDate and Springer Humana Press, and has received consultant fees from AstraZeneca AIR. S. T Weiss declares that he has no relevant conflicts of interest.
Contributor Information
Augusto A. Litonjua, From the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital. Boston, Mass.
Scott T. Weiss, From the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital. Boston, Mass.
REFERENCES
- 1.Deluca HF. History of the discovery of vitamin D and its active metabolites. Bone-key Rep 2014;3:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rappaport BZ, Reed CI, Hathaway ML, Struck HC. The treatment of hay fever and asthma with viosterol of high potency. J Allergy 1934;5:541–53. [Google Scholar]
- 3.Litonjua AA. The role of vitamin D in the development, exacerbation, and severity of asthma and allergic diseases. In: Litonjua AA, editor. Vitamin D and the lung: mechanisms and disease associations New York: Humana Press; 2012. [Google Scholar]
- 4.Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015;7:8251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007;120:1031–5. [DOI] [PubMed] [Google Scholar]
- 6.Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr Opin Allergy Clin Immunol 2012;12:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawes BL, Bonnelykke K, Stokholm J, Vissing HH, Bjarnadottir E, Schoos AM, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016;315:353–61. [DOI] [PubMed] [Google Scholar]
- 9.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014;72:48–54. [DOI] [PubMed] [Google Scholar]
- 10.Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, O’Connor GT, Sandel M, et al. Vitamin D supplementation in pregnant women of different races and the risk of asthma/recurrent wheeze in the child: findings from the Vitamin D Antenatal Asthma Reduction Trial (VDAART) [abstract]. Paper presented at: American Thoracic Society International Conference 2016; 2016; San Francisco, Calif. [Google Scholar]
- 11.Weiss ST, Litonjua AA. Can we prevent childhood asthma before birth? Summary of the VDAART results so far. Expert Rev Respir Med 2016;10:1039–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollams EM, Teo SM, Kusel M, Holt BJ, Holt KE, Inouye M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. J Allergy Clin Immunol 2017;139:472–81. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, Immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med 2014;239: 1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3–6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 15.Weiss ST, Litonjua AA. Vitamin D dosing for infectious and immune disorders. Thorax 2015;70:919–20. [DOI] [PubMed] [Google Scholar]
- 16.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 2010;5:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasky-Su J, Lange N, Brehm JM, Damask A, Soto-Quiros M, Avila L, et al. Genome-wide association analysis of circulating vitamin D levels in children with asthma. Hum Genet 2012;131:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]


