Abstract
Pregnant women are uniquely susceptible to adverse effects of air pollution exposure due to vulnerabilities and health consequences during pregnancy (e.g., hypertensive disorders of pregnancy [HDP]) compared to the general population. Because the Clean Air Act (CAA) creates a duty to protect at-risk groups, the regulatory assessment of at-risk populations has both policy and scientific foundations. Previously, pregnant women have not been specially protected in establishing the margin of safety for the ozone and particulate matter (PM) standards. Due to physiological changes, pregnant women can be at greater risk of adverse effects of air pollution and should be considered an at-risk population. Women with preexisting conditions, women experiencing poverty, and groups that suffer systematic discrimination may be particularly susceptible to cardiac effects of air pollutants during pregnancy. We rigorously reviewed 11 studies of over 1.3 million pregnant women in the United States to characterize the relationship between ozone or PM exposure and HDP. Findings were generally mixed, with a few studies reporting a joint association between ozone or PM and social determinants or pre-existing chronic health conditions related to HDP. Adequate evidence associates exposure to PM with an adverse effect of HDP among pregnant women not evident among non-gravid populations.
Keywords: air pollution, cardiovascular disease, pregnancy
Introduction
Populations that experience multiple social, physical, and chemical environmental stressors are at increased risk of disease (Brulle & Pellow, 2006; Morello-Frosch, Zuk, Jerrett, Shamasunder, & Kyle, 2011; Payne-Sturges et al., 2006; Vesterinen, Morello-Frosch, Sen, Zeise, & Woodruff, 2017). Disparities in health outcomes may be produced by both environmental (e.g., physical, chemical, and biological agents) and social factors (e.g., individual- and community-level traits such as socioeconomic position, education, psychosocial stress, coping resources and support systems, residential factors, cultural traditions, and institutional, structural, and political processes such as racism and classism) (Institute of Medicine, 1999). Residents of low to moderate income urban communities, who are likely to be non-Hispanic Black and Hispanic, are more likely to be exposed to multiple air pollutants in some areas of the United States (Gray, Edwards, & Miranda, 2013; Morello-Frosch, Jesdale, Sadd, & Pastor, 2010; Morello-Frosch et al., 2011; Schulz et al., 2016; Su, Jerrett, Morello-Frosch, Jesdale, & Kyle, 2012; U.S. EPA, 2015; Woodruff, Darrow, & Parker, 2008; Woodruff, Parker, Kyle, & Schoendorf, 2003). Residents of such communities experience higher rates of adverse health effects of air pollutants due to higher rates of pre-existing chronic conditions such as asthma, obesity, diabetes, and cardiovascular disease (Kannan, Misra, Dvonch, & Krishnakumar, 2006; MDOCH, 2007; Wasilevich, 2008). These vulnerabilities coincide with social stressors such as poverty, poor housing, reduced access to nutritious foods and health care, and psychosocial stress, which further exacerbate adverse health effects from air pollution (Bower, Thorpe, Rohde, & Gaskin, 2014; Kannan et al., 2010). Thus, to fully characterize the relationship between air pollution exposure and health and which populations are at risk, the role of multiple chemical and social stressors must be considered when creating policies that protect women susceptible to environmental exposures (National Research Council, 2009).
Despite the cumulative effects of multiple chemical and social exposures and underlying population burden of disease, many environmental policy decisions rely on risk assessments that consider single chemical exposures rather than combined exposures of chemicals and social stressors. This is in part a result of the nature of the available controls (e.g., engineering devices or strategies to ban or replace an individual chemical), legislative authority, or the methodological goal to isolate and establish causal connections between individual chemical exposures and health outcomes. Nevertheless, several science policy reports highlighted the need for more systematic consideration of combined risks from multiple chemical and social environmental stressors (Institute of Medicine, 1999; National Research Council, 1994, 2009; Omenn, 1997; Thurston et al., 2017). A joint European Respiratory Society/American Thoracic Society (ATS) policy statement notes that health equity and environmental justice considerations are relevant to population risk assessments (Thurston et al., 2017). One of these factors is higher burdens of chronic health conditions, such as diabetes and hypertension, that are socially patterned with higher risk among low-income people (Brummett et al., 2011; Menke, Casagrande, Geiss, & Cowie, 2015). Air pollution adds another burden to populations already at higher risk of disease from exposure to adverse social and economic contexts or with higher prevalence of chronic disease, and pollution exposures may have disparate impacts on people in higher risk groups (Institute of Medicine, 1999; Thurston et al., 2017). Researchers have proposed frameworks for studying the shared etiologic path ways of air pollution and negative social factors (Erickson, Ostry, Chan, & Arbour, 2016; Institute of Medicine, 1999; Payne-Sturges et al., 2006; Solomon, Morello-Frosch, Zeise, & Faust, 2016). To be useable in policy decisions, these research frameworks and resulting evidence must be connected to regulatory assessment methods (Koman & Mancuso, 2017).
In this study, to improve the identification of “at-risk” populations for regulatory risk assessment, we first critically examine regulatory methods used to set U.S. air pollution standards as they apply to pregnant women. We then evaluate the disease category of hypertensive disorders of pregnancy (HDP), as exemplars of maternal health outcomes that are currently undervalued in regulatory decision making. Further, HDP are important causes of morbidity and mortality for pregnant women (Dolea & AbouZahr, 2003), and as such, are major causes of disease and death in young women. Preeclampsia is a leading cause of maternal mortality and morbidity affecting 2–10 percent of pregnancies in the United States (ACOG, 2013a; Sibai, Dekker, & Kupferminc, 2005; Steegers, von Dadelszen, Duvekot, & Pijnenborg, 2010). HDP are associated with air pollution exposure (Hu et al., 2014; Pedersen et al., 2014) and are also associated with social determinants of health including residential poverty and race (Erickson & Arbour, 2014; Tanaka et al., 2007).
HDP can be a harbinger of poor long-term cardiovascular health for the mother (Amaral, Cunningham, Cornelius, & LaMarca, 2015; Lykke, Langhoff-Roos, Lockwood, Triche, & Paidas, 2010). HDP has been linked to development of cardiovascular diseases decades after pregnancy and therefore represents a risk factor that undermines long-term health in women who have experienced HDP in pregnancy (Amaral et al., 2015). Furthermore, in the decades following pregnancy, HDP is associated with smaller maternal brain volume and decreased performance on tests of processing speed (Mielke et al., 2016; Nelander et al., 2016). In addition to a predisposition to cardiovascular and neurological changes associated with HDP in later life, women experiencing HDP are at greater risk for end-stage renal disease (Wang et al., 2013) and diabetes (Theilen et al., 2016). The mechanisms of long-term consequences of HDP on health are not well elucidated but may be related to changes in anti-angiogenic and immune factors occurring during HDP (Amaral et al., 2015), which manifest as a disease threshold later in life (Arabin & Baschat, 2017). Pregnancy has long been described as a “stress test for life” because pregnancy complications such as HDP often manifest later in life as essential hypertension (Williams, 2003). In addition to the impacts on the mother’s health, preeclampsia is a major risk factor for adverse birth outcomes such as fetal growth restriction and preterm birth, which have health implications for both mother and fetus (Clausson, Cnattingius, & Axelsson, 1998; Ota et al., 2014). Racial disparities for birth outcomes have been documented, but not fully explained. Therefore, protection of pregnant women who are particularly vulnerable to hypertensive disorders or women who have higher odds of entering pregnancy with chronic hypertension due to susceptibilities that are patterned by race or ethnicity (Ghosh et al., 2014) should be a policy priority, particularly in instances where exposure to air pollutants is more likely.
In this paper, we first describe the policy framework and general evidence for designating pregnant women as at-risk populations under the Clean Air Act (CAA) regulatory framework. We use the regulatory criteria described below, with the goal of illustrating how the CAA regulatory framework can be used more effectively to identify and prevent adverse impacts within at-risk populations. Specifically, we focus on the evidence for joint effects of pollutant exposures and social determinants of health (e.g., socioeconomic status) on HDP among pregnant women. We also examine socially patterned pre-existing conditions that may predispose subpopulations of pregnant women to adverse health effects from pollution exposures. Accordingly, using the EPA’s four-part criteria for at-risk populations, our aim was to evaluate evidence about whether pregnant women with additional stressor(s) or pre-existing disease are more vulnerable to the effects of air pollution exposure than nongravid populations for HDP.
Policy and Scientific Foundations of At-Risk Populations in the Clean Air Act
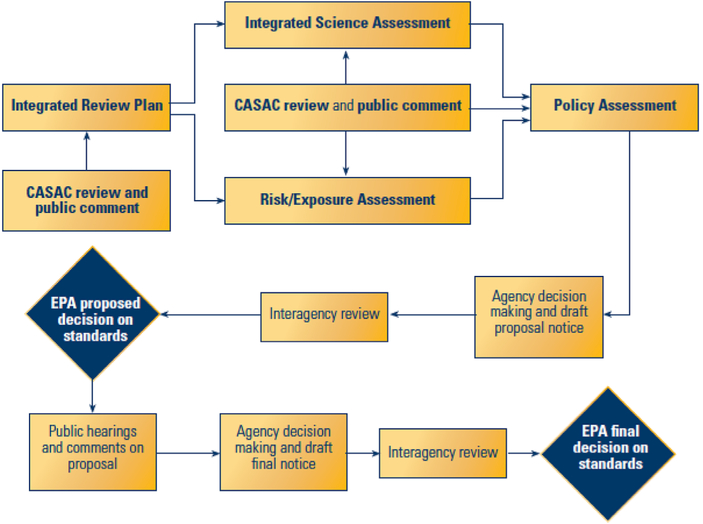
One prominent example of regulatory risk assessment used in policy decisions is the CAA national ambient air quality standards (NAAQS) (42 U.S.C. 7408). As shown in Figure 1, under CAA Section 109, the U.S. Environmental Protection Agency (EPA) sets standards for widespread “criteria” pollutants such as particulate matter (PM) and ozone to protect public health “with an adequate margin of safety.” In the most recent NAAQS review for ozone, the EPA enumerates how it interprets the law to create regulatory procedures to review scientific data to assess the adequacy of existing standards and to protect sensitive populations. Specifically, in developing an adequate margin of safety, the EPA Administrator’s judgment relies on “such factors as the nature and severity of health effects, the size of sensitive population(s) at risk, and the kind and degree of the uncertainties that must be addressed” (Federal Register 80 No. 206 October 26, 2015, Part I, Section A, p 65295, emphasis added). Grounded in legislative history, EPA designates at-risk populations as part of the standard-setting process (S. Rep. No. 91–1196, 91st Cong., 2d Sess. 10 [1970]) based on the idea that protecting sensitive groups would also lead to an adequate margin of safety for the entire population. A population is considered at-risk if the group meets any one of four criteria (Table 1) (Federal Register, 2013). Consequently, the NAAQS could be a powerful tool to address health disparities related to social determinants of health that help to define at-risk populations in cases where the scientific evidence supports it.
Figure 1.
U.S. Environmental Protection Agency’s Process for Reviewing Criteria for National Ambient Air Quality Standards.
Table 1.
U.S. Environmental Protection Agency’s Criteria for Designating At-Risk Populationsa
| Under the definition of at-risk population, based on the EPA Administrator’s judgment, a population need meet one of four criteria with adequate evidence (Federal Register, 2013): |
|---|
| (1) Higher levels of exposure; |
| (2) Higher dose at a given level of ambient exposure; |
| (3) Increased adverse effects at a given level of ambient exposure; and |
| (4) Increased health effects due to continuum of effect among sensitive members of a populationb |
| Different criteria for identifying and evaluating evidence for designation of vulnerable at-risk |
| populations were used in the two most recent NAAQS review processes. Specifically, in the Ozone NAAQS review (2014), EPA used a three-part definition for inclusion and did not account for more adverse effects due to already compromised health, a criterion articulated in the American Thoracic Society statement. The evidence was arrayed with a more informative four-level scale (adequate, suggestive, inadequate evidence, and evidence of no effect). By contrast, in the PM NAAQS review (2012), EPA used a broader set of criteria, but a binary scale to evaluate the evidence; essentially, a population was either considered an at-risk population or a healthy comparison group. |
In EPA’s Integrated Science Assessment, the staff practice is to require a demonstration of harm in the peer-reviewed literature as a screening step—that air pollution is harming a population—before assessing that group against the criteria.
In the ozone NAAQS review, EPA only included the first three criteria for the development of the revised ozone NAAQS. Although contained in the American Thoracic Society (ATS) statement (American Thoracic Society, 2000), EPA’s Ensuring Risk Reduction in Communities with Multiple Stressors: Environmental Justice and Cumulative Risks/Impact (NEJAC, 2004) and applied in previous NAAQS reviews, the fourth criterion was omitted from correspondence with EPA Administrator Lisa Jackson in the February 2013 memo to CASAC which documented Agency policy (U.S. EPA, 2013). The rationale for not including the fourth criterion of population consequence was not discussed.
We critically assess EPA’s regulatory methods by examining their adequacy for considering pregnant women as a candidate population for one category of health outcome. The first step is to understand how EPA’s methods are evolving. EPA updated how it designates at-risk populations in consultation with its peer reviewers on the Clean Air Science Advisory Committee in 2012 and 2013. Based on documents in EPA’s regulatory record, EPA used two approaches in the most recent PM and ozone NAAQS reviews. Table 2 presents the populations evaluated and the EPA’s two assessments of evidence based on exposure to a single pollutant. EPA staff practice in the Integrated Science Assessment (ISA) is that peer-reviewed studies must first demonstrate adequate evidence of harm for a health outcome (i.e., “likely causal” relationship) before EPA assesses a candidate group against its criteria. In applying these criteria, combinations of factors have been included in previous evaluations, such as of asthmatic children (developmental and disease status) (U.S. EPA, 2009, 2012). Social factors (e.g., low socioeconomic status) have also been assessed singly in EPA’s designations (U.S. EPA, 2009, 2012).
Table 2.
U.S. Environmental Protection Agency’s Designated At-Risk Populations for Particulate Matter and Ozone Exposure
| A. At-Risk Populations for Particulate Matter Exposure (2012 | |
| Category of Evidence | Population |
| Adequate evidence | People with heart and lung disease, asthmatics |
| Children under 18 years | |
| Older adults at and above 65 years | |
| Inadequate evidence | Pregnant women for effect on fetus |
| Obese populations for cardiac susceptibility | |
| Women (gender) | |
| Populations of low socioeconomic status | |
| Not assessed | Obese populations for pulmonary susceptibility |
| Overweight populations | |
| Outdoor athletes | |
| Populations with poor diets with nutritional (anti-oxidant or vitamin deficiencies) | |
| Racial/ethnic groups | |
| Smokers | |
| B. At-Risk Populations for Ozone Air Pollution (2015) | |
| Category of Evidence | Population |
| Adequate evidence | Asthmatics |
| Children under 18 years | |
| Older adults at and above 65 years | |
| Populations with poor diets with nutritional (anti-oxidant or vitamin deficiencies) | |
| Outdoor workers | |
| Suggestive evidence | Obese populations |
| Populations with genetic markers | |
| Women | |
| Populations of low socioeconomic status | |
| Inadequate evidence | Patients with the following: |
| Chronic obstructive pulmonary disease | |
| Cardiovascular disease | |
| Diabetes | |
| Hyperthyroidism | |
| Influenza and other respiratory infections | |
| Racial groups | |
| Smokers | |
| Evidence of no effect | — |
| Not assessed | Overweight populations |
| Pregnant women | |
| Outdoor athletes | |
In considering pregnant women in the 2012 PM NAAQS review, the EPA evaluated the effect of PM exposure on fetuses in studies of low birth weight and fetal growth restrictions. The EPA concluded there was suggestive evidence for an association of PM exposure during pregnancy with low birth weight and fetal growth restriction. Without stronger evidence of a population experiencing an adverse effect, a population would not be considered as “at-risk.” Importantly, the impact of air pollution on the mother’s health was not evaluated in the ISA (where staff make recommendations) or final rulemaking (where the Administrator’s judgment including public comment are documented) (U.S. EPA, 2009, 2011). Additionally, the effects of social determinants of health were not considered jointly with candidate populations in selecting at-risk populations in the PM NAAQS review. With regard to evidence of risk among pregnant women, all but one of the studies in this review were published after the cutoff date for inclusion in the 2012 PM NAAQS review. Pregnant women were not considered in the final Agency judgment of the margin of safety for the most recent PM NAAQS (Federal Register, 2013; U.S. EPA, 2009) or in any of the NAAQS since 1970.
Subsequently, in the ozone NAAQS review, the EPA considered all nonelderly adult women as a group, but did not consider adult pregnant women separately either in the ISA or in the proposal (U.S. EPA, 2012). Following public comment identifying evidence of associations of ozone exposure with HDP among pregnant women, the EPA conducted a provisional review of those studies. In the final ozone NAAQS decision by the Administrator, EPA noted that pregnant women were not designated as an at-risk population (Federal Register 80 No. 206 October 26, 2015, p. 65337). Therefore, with direct implications for policy, the EPA has not fully evaluated or designated pregnant women as at-risk in establishing the margin of safety for the PM NAAQS and has provisionally considered pregnant women for the ozone NAAQS.
Pregnant Women as a Candidate At-Risk Population
Adult pregnant women represent a large potentially at-risk population. The U.S. Department of Health and Human Services estimated nearly four million births in 2015 (Hamilton, Martin, & Osterman, 2015). This number underestimates the number of pregnancies but otherwise translates to pregnant women accounting for 1.25 percent of the population that year. We argue that this population has not been adequately considered in air pollution risk assessments or policy decisions to date.
Risk assessment of pregnant women exposed to air pollutants such as ozone and PM requires an understanding of normal adaptive changes to the pulmonary and cardiovascular system which occur during pregnancy (Tan & Tan, 2013). Whereas a number of respiratory parameters do not appear to be significantly altered during pregnancy, including respiratory rate, which increases only incrementally by 1–2 breaths/minute (Rees, Broughton Pipkin, Symonds, & Patrick, 1990), maternal minute ventilation (the volume of air inhaled/exhaled over one minute) increases by 40 percent via an increase in tidal volume (size of each breath), and oxygen consumption increases by 20 percent to accommodate the metabolic demands of pregnancy and oxygen transfer across the placenta (Hegewald & Crapo, 2011). Increased oxygen consumption is maintained by a 50 percent increase in cardiac output, which is accompanied by increased maternal blood volume, heart rate, and pulmonary circulation (Bobrowski, 2010; Tan & Tan, 2013). During pregnancy, increased cardiac output, defined as the amount of blood the heart pumps in one minute, is necessary to maintain sufficient blood flow to the uterus, lungs, kidneys, and skin. Factors inhibiting cardiac output during pregnancy may potentiate pulmonary edema, leading to respiratory distress and poor oxygenation of blood (Clark et al., 1989), as well as decreased uterine blood flow and placental perfusion, leading to fetal hypoxia (Soma-Pillay, Catherine, Tolppanen, & Mebazaa, 2016). Taken together, the compensatory changes that occur to the cardiopulmonary system during pregnancy are essential to maintaining pregnancy (May, 2015).
These same adaptations may also render pregnant women susceptible to additional health challenges, such as air pollution, and vulnerable to untoward cardiopulmonary effects, which contribute to poor health outcomes during pregnancy and beyond. It has been consistently documented, for example, that compared to other adults, pregnant women have greater inhalation uptake on a body weight basis and corresponding blood concentration of inhaled volatile organic compounds (Brochu, Bouchard, & Haddad, 2014; Valcke, Nong, & Krishnan, 2012). In addition, critical windows of exposure for the mother occur during the first trimester, involving implantation and placentation, as well as during labor. Other susceptibilities among pregnant women should also be considered that may be relevant to effects of air pollution, such as changes to the immune system, increased insulin resistance, or complications of maternal asthma. Relevant to the EPA’s first criterion for at-risk populations, disparities in exposure to air pollution among pregnant women by race/ethnicity, education, and income have been documented in the United States in non-Hispanic Black, Hispanic, and Asian/Pacific Islander mothers who have experienced higher mean levels of criteria air pollution and were more than twice as likely to live in the most polluted counties as non-Hispanic White mothers, after controlling for maternal risk factors, region, and educational status (Ponce, Hoggatt, Wilhelm, & Ritz, 2005; Woodruff et al., 2003).
Effects of PM and Ozone on Pregnant Women and Non-Gravid Populations
We argue that pregnant populations exposed to air pollution should be reclassified as at-risk populations under the current NAAQS. In non-gravid human populations, exposure to pollutants such as PM has been associated with increased cardiovascular mortality (Dabass et al., 2016; Krewski et al., 2009; Miller et al., 2007; Pope, Burnett, & Thurston, 2004) and morbidity, including onset of atrial fibrillation (Lin, Liu, Le, & Hwang, 2013) and decrease in high density lipoprotein functionality (Ramanathan et al., 2015). Similarly, ozone exposure has been associated with increased cardiovascular mortality among humans (Cakmak, Hebbern, Vanos, Crouse, & Burnett, 2016). Studies in rats genetically predisposed to lower antioxidant reserves show that they have greater susceptibility to the effects of ozone, suggesting that genetic and/or dietary factors may play a role in response to air pollutants (Dye, Costa, & Kodavanti, 2015). A large body of evidence suggests that ozone and PM exposures can each independently induce systemic inflammation and oxidative stress, and vascular endothelial injury (Brook et al., 2004) which are implicated in the etiologies of pregnancy complications, including preeclampsia and gestational hypertension (Erickson et al., 2016; Jauniaux, Poston, & Burton, 2006). In recent meta-analyses, Pedersen et al. (2014) reported a combined odds ratio (OR) of 1.57 (95% CI 1.26, 1.96) per 5 μg/m3 PM2.5 for the entire pregnancy for all hypertensive diseases of pregnancy; Hu et al. (2015) reported a combined OR of 1.18 (95% CI 0.98, 1.41) for a 5 μg/m3 increase in PM2.5 during the first trimester for HDP and 1.02 (95% CI 0.95, 1.08) for a 1 mg/m3 increase in PM10 during the first trimester for HDP. Similarly, the OR for a combined effect for a 10 ppb increase in ozone for the entire pregnancy was 1.06 (95% CI 0.99, 1.14) (p = 0.08) (Pedersen et al., 2014) and 1.09 (95% CI 0.95, 1.08) for a 10 ppb increase in ozone in the first trimester (Hu et al., 2015). This air pollution-associated increase in risk, while small, is important to public health due to widespread exposures. Taken together, these data support an increased risk with air pollution exposures among pregnant women.
Social Determinants of Health and Pre-Existing Disease
Social determinants of health are generally defined as social, political, and economic factors that are associated with health. Social factors, such as socioeconomic status (SES) and exposure to stressful life conditions, play a significant independent role in maternal health (Silva et al., 2008) and may be means of identifying policy-relevant sensitive subpopulations for protection from air pollution via CAA regulations. Measured at either the community or individual level, chronic stressors associated with low SES may contribute to psychosocial stress from lack of access to community resources such as education, nutritious food, health care, and adequate housing. Discrimination based on race, ethnicity, gender identity, or pregnancy status can also be a source of stress (Payne-Sturges et al., 2006; Solar & Irwin, 2010). These social stressors are hypothesized to increase allostatic load and cumulative biological damage through inflammatory and oxidative stress pathways, which are also central to the pathology of HDP. These pathways are also central mechanisms through which air pollution exerts adverse effects (Jauniaux et al., 2006). Thus, health outcomes associated with social stressors and air pollution may be mechanistically similar and target the same systems or processes (e.g., cardiovascular system, placentation) in pregnant women (Erickson & Arbour, 2014). Accordingly, it is well recognized that social and economic conditions can amplify the adverse effects of environmental agents on health (ACOG, 2013a; National Research Council, 2009; NEJAC, 2004; Payne-Sturgis & Martin, 2014; Thurston et al., 2017), which we hypothesize contributes to excess risk to pregnant women exposed to air pollutants.
The combined effects of multiple social, physical, and chemical exposures, along with reduced access to resources necessary to maintain health (e.g., nutritious foods, health care, adequate housing), have important implications for federal policy on ubiquitous air pollution exposures in sensitive populations (Schwartz, Bellinger, & Glass, 2011; Solar & Irwin, 2010). The health effects of exposures to ambient air pollution among the general non-gravid population also depend on underlying health status, living in poor neighborhoods, and the presence of co-pollutants (Bell & Dominici, 2008; Burra, Moineddin, Agha, & Glazier, 2009; Cakmak et al., 2009; Morello-Frosch et al., 2010). Women of childbearing age who are also members of vulnerable populations are more likely to have one or more chronic health conditions. That is, pre-existing conditions manifest at younger ages among women with low income and education, and among racial groups that are disproportionately exposed to poverty, reduced educational opportunities, and higher levels of pollutants from occupations and community ambient exposures. However, these combined exposures—which have implications for risk—are rarely considered a priori in defining at-risk populations under the CAA.
Materials and Methods
We examined whether relationships between air pollution exposure and HDP are affected by (i) social determinants of health such as poverty, race/ethnicity, psychosocial stress, access to nutrition and access to health care; and (ii) pre-existing chronic conditions such as diabetes, hypertension, or asthma. We searched the literature for studies conducted with women in the United States in order to maximize the relevance to the U.S. social, economic, and regulatory context, and to avoid unanticipated heterogeneity that might be introduced due to global variations in air pollutant levels or variations in social determinants of health (e.g., economic development, forms and patterns of racial discrimination, social or legal patterns of women’s access to resources) (Hajat, Hsia, & O’Neill, 2015), as well as global variations in the incidence of HDP (Dolea & AbouZahr, 2003).
We developed a “Participants,” “Exposure,” “Comparator,” and “Outcomes” (PECO) statement (Table 3). To be included, the study must have explicitly examined cardiovascular health in pregnant women in the United States in relation to either (i) the joint contribution of at least one social factor and at least one pollutant exposure or (ii) at least one pre-existing chronic condition and at least one pollutant exposure. We included studies with tests of statistical interaction between air pollution exposure and at least one factor; stratified analyses that examined the influence of air pollution exposure by levels of a social factor or chronic disease; and studies of mediation (i.e., intermediate steps in the causal pathway between exposure and outcome). Studies examining the independent main effects but not the joint or combined effects of environmental and social factors were excluded.
Table 3.
Participants, Exposure, Comparator, and Outcomes (PECO) Statement
| Category | Description |
|---|---|
| Participants | Pregnant women |
| Exposure | Any pregnancy exposure to air pollution and social determinant of health (SDOH) or preexisting condition that occurred prior to the preeclampsia (PE) or gestational hypertension (GH) assessment. |
| 1) “Any pregnancy exposure” is defined as maternal exposure incurred any time in proximity to conception (as defined by authors of the included study) through birth. | |
| 2) “Air pollution” is defined as any indoor or outdoor source of any inhaled airborne particulate matter or ozone, excluding active and passive smoking. | |
| 3) “SDOH” in our study is limited to a set of exposures determined a priori: poverty, race/ethnicity, psychosocial stress, access to nutrition, access to health care; SDOH exposures can be at the community or individual level. | |
| 4) “Pre-existing condition” is a pregnant woman with a health condition such as diabetes, chronic hypertension, obesity/overweight, or asthma. | |
| 5) Exposures “prior to the PE or GH assessment” include direct and proxy measures for this time period, such as trimesters or entire pregnancy. Note that there is uncertainty about the relevant exposure window for the development of PE or GH, but one hypothesis focuses on conception through first trimester as the most relevant period. | |
| Comparator | Pregnant women exposed to lower levels of air pollution than the more highly exposed women. Pregnant women exposed to lower levels of social stressors than the more highly exposed women. Pregnant women compared to nonpregnant women. |
| 1) This definition is intended to include groups defined by PE or GH case-control studies; for instance, comparing the air pollution exposure levels for people with PE or GH versus those without. | |
| 2) We distinguish the comparator for evaluation within a study used here from the comparison group for the policy synthesis (e.g., the non-at-risk population) | |
| Outcome | Any clinical diagnosis or other continuous or dichotomous scale assessment of preeclampsia or gestational hypertension.a |
Preeclampsia is generally defined as new hypertension (blood pressure >140/90mm Hg) and proteinuria (≥300mg in 24 hours) at or after 20 weeks’ gestation and affects 2–10 percent of pregnancies and only resolves with delivery. Gestational hypertension is pregnancy-induced hypertension without proteinuria.
Because several recent articles have reviewed the primary relationship between air pollution exposure and pregnancy outcomes, we started with these reviews (Hu et al., 2014; Menke et al., 2015; Pedersen et al., 2014; Sacks et al., 2011; U.S. EPA, 2009, 2012). We performed keyword searches and examined citations for additional studies. We selected studies examining a maternal outcome (preeclampsia [PE], gestational hypertension [GH], or the HELLP syndrome of hemolysis, elevated liver enzymes, and a low platelet count [a severe variant of preeclampsia]) assessing PM or ozone exposure in the United States in the past 15 years published in English that examined the effects of or interactions between selected social determinants of health (e.g., race/segregation, SES/poverty/income, education, nutrition/obesity/access to food, housing quality, access to medical care, psychosocial stress) and the relationship between air pollution exposure and maternal health. We considered studies published since the beginning of the previous ozone NAAQS review (15 years ago) to harmonize our search with the regulatory review decisions we assessed. We used the Medical Subject Headings (MeSH) database to compile synonyms for air pollution and PE/GH-related outcomes (https://www.ncbi.nlm.nih.gov/mesh/68000397).
We used key words to identify additional epidemiologic studies of pregnant women, with a particular focus on studies examining joint effects of air pollution and social determinants on cardiovascular effects of the mother. Specifically, relevant Medical Subject Headings (MeSH) keyword and reference lists searches using PubMed were conducted using the key words, medical headings, and medical subject headings from three groups connected with “AND”: (i) air pollution or air pollutants, adverse effects; (ii) pregnancy; (iii) social determinants (race/segregation, poverty, SES/income, education, nutrition/obesity/access to food, housing quality, access to medical care, psychosocial stress); and (iv) preeclampsia, gestational hypertension, blood pressure, hypertension, HELLP syndrome, cardiovascular, adverse effects. Searches were conducted on December 1, 2015 and updated on August 29, 2016, and July 20, 2017. Physiological and biomedical studies of ozone and PM exposure studies in humans were reviewed for factors that may modify the relationship between ozone and PM exposure and health effects. We examined EPA documents from the record of decision on at-risk population designations (U.S. EPA, 2009, 2012).
For the selected studies, we extracted information related to study design; sample size; outcomes assessed; definition, measurement, and timing of air pollution and social factor exposure; descriptions of analyses of how joint contributions of air pollution and social factors were tested; and primary findings. We evaluated case ascertainment and exposure metrics. We did not conduct a meta-analysis due to the heterogeneity of exposures assessed across studies (exposure averaging times, social determinants). Instead, this review provides a descriptive summary and synthesis. We first reviewed the evidence for a main association between air pollution and preeclampsia or gestational hypertension in our geographically limited pool of studies. Second, we examined evidence for independent effects and interactions with each social factor. We critically assessed metrics employed, averaging time for the factor, and statistical approach.
To examine these complex phenomena in a policy-relevant framework, we employed EPA’s criteria and four-part definition used to designate at-risk populations in the NAAQS review. As noted above, several recent reviews have addressed criteria 1 and 2 (e.g., Hu et al., 2014; Pedersen et al., 2014). Our review focused on papers that might shed light on pregnant women as an at-risk population using criteria 3 or 4 (Table 1). The first step in EPA’s process is to identify an adverse health effect and direct comparisons of groups (Sacks et al., 2011; Vinikoor-Imler et al., 2014). We identified three HDP: preeclampsia, gestational hypertension and elevated blood pressure during labor and delivery as outcomes. We compared pregnant women to the non-gravid not-at-risk population. Next, we applied the four-part criteria to describe to what extent the geographically limited epidemiologic evidence supported a designation as an at-risk population, noting that a full assessment would include other relevant health effects; not limit the geographic scope; and would synthesize evidence from exposure science, dosimetry, and toxicology.
Results
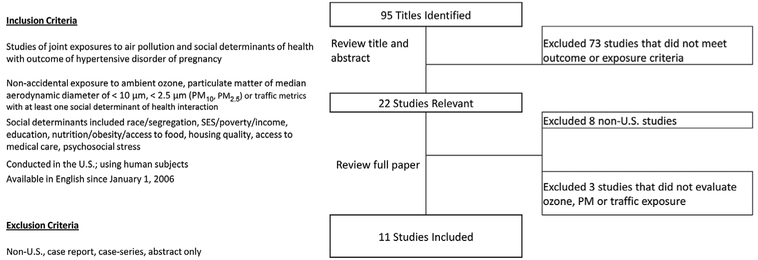
We identified 95 studies in our initial literature search (Figure 2). Following screening of titles and abstracts for ozone or PM exposure among pregnant adults and one of our HDP outcomes, we excluded eight hypertension and air pollution studies that did not meet our geographic criteria (Malmqvist, Jakobsson, Tinnerberg, Rignell-Hydbom, & Lars Rylander, 2013; Olsson, Mogren, & Forsberg, 2013; Parker et al., 2011; Pereira et al., 2013; van den Hooven et al., 2012; Vigeh, Yunesian, Shariat, Niroomanesh, & Ramezanzadeh, 2011; Yorifuji et al., 2013) and three studies which controlled for social determinants as an independent predictor of the outcome but did not evaluate PM or ozone exposure (Friberg et al., 2016; Woodruff et al., 2003; Zhai et al., 2012).
Figure 2.
Literature Search Strategy.
In total, we identified 11 relevant studies reporting on the main effects of air pollution and social stressor exposures among over 1.3 million pregnant women in the United States (Table 4). Some studies examined more than one pollutant and single studies assessed multiple endpoints or geographic scopes (Supporting Information Table S1). Seven studies analyzed odds of preeclampsia, three examined odds of gestational hypertension, four examined odds of HDP, one examined hypertension at labor and delivery, and one reported blood pressure change during pregnancy using a variety of definitions for case ascertainment (Tables 5 and S2 in Supporting Information). Exposure period varied from the entire pregnancy to different trimesters, or using monthly and hourly exposures. Using these studies, we examined the primary air pollution–HDP relationship and then the combined relationships of air pollution with social factors and preexisting chronic conditions.
Table 4.
Summary of Studies of Joint Consideration of Air Pollution Exposure and Social Determinant and/or Chronic Condition with HDP Among U.S. Pregnant Women (Primary Association of Air Pollution Exposure)
| Study | Location | Population | Covariates | Social Stressors or Chronic Condition | Outcome(s) | Primary Association | |
|---|---|---|---|---|---|---|---|
| PM/Traffic | Ozone | ||||||
| Wu et al. (2009) | Los Angeles and Orange Counties, USA (1997–2006) | N=81,186 pregnancies (n=2,442 PE cases) Included singleton births. Excluded subjects with missing residential data | Age, education, race/ethnicity, diabetes, parity, prenatal care insurance type, poverty, and season of conception | Stratification by study region, race, poverty, insurance type, and diabetes status | Preeclampsia | ⇈ | N/A |
| Wu et al. (2011) | Los Angeles County, USA (1997–2006) | N=38,709 pregnancies (n=1,303 PE cases) Included singleton births. Excluded subjects with missing residential data | Age, education, race/ethnicity, diabetes, parity, prenatal care insurance type, poverty, and season of conception | Stratification by study region, race, poverty (% living below poverty level based on U.S. Census block group for 2000) | Preeclampsia | ⇈ | ↔ |
| Wu et al. (2011) | Orange County, USA (1997–2006) | N=42,477 pregnancies (n=1,139 PE cases) Included singleton births. Excluded subjects with missing residential data | Age, education, race/ethnicity, diabetes, parity, prenatal care insurance type, poverty, and season of conception | Stratification by study region, race, poverty (% living below poverty level based on U.S. Census block group for 2000) | Preeclampsia | ⇈ | ⇈ |
| Rudra et al. (2011) | Washington, USA (1996–2006) | N=3,509 (n=117) Eligible subjects attending prenatal care before week 20. Excluded subjects with maternal aged <18 years; non-English language and planned delivery outside study area | Age, education, race/ethnicity, parity, prepregnancy body mass index (BMI), physical activity, employment, household income, marital status, history of asthma; diabetes or chronic hypertension; smoking; season and year of conception | Stratification by age, BMI (<25 kg/m2), ever smoking and ETS, employment | Preeclampsia | ↑↔ | N/A |
| Vinikoor-Imler et al. (2012) | North Carolina, USA (2000–2003) | N=222,775 (n=12,085) Excluded multiple births, infants with Congenital abnormalities, birth weight <42 g, missing covariates data and chronic hypertension | Age, education, ethnicity, marital status, neighborhood deprivation index (NDI), parity and smoking | Interaction of PM and NDI category using loglikelihood test. NDI by census tract constructed from % households in poverty, % femaleheaded households, % household income <$30,000, % households on public assistance, % males in management, % crowded households, % unemployed, % <high school education | Gestational hypertension | ⇈ | N/A |
| Lee et al. (2012) | Pittsburgh (Allegheny County), PA USA (1997–2001) | N=1,684 Excluded multiple births, women with chronic hypertension and/or diabetes, gestational age 45 weeks and residential location outside study area | Age, race/ethnicity, parity, smoking (number of cigarettes), vitamin use, BMI, temperature, season of birth and year of conception from hospital-based records | Stratification by race/ethnicity (Caucasian and African American) | Increase in systolic blood pressure (SBP) Increase in diastolic blood pressure (DBP) |
↑↔ ↑↔ |
⇈ ↑↔ |
| Lee et al. (2013) | Pittsburgh (Allegheny County), PA USA (1997–2002) | N=34,705: PE (n=1,141) and GH (n=2,078). Excluded multiple births, women with chronic hypertension and/or diabetes, gestational age 45 weeks and residential location outside study area | Age, race/ethnicity, nulliparity, smoking (number of cigarettes), season of birth and year of conception from hospital-based records | Stratification by race/ethnicity (Caucasian and African American) | Preeclampsia Gestational |
↑↔ ↑↔ |
↑↔ ↑↔ |
| Miranda et al. (2013) | North Carolina, USA (2004–2008) | N=468,517 (n=25,768) entire state. Included singleton births, subjects with birth number 1–4, non- Hispanic White, non- Hispanic Black, Hispanic, aged 15–40. Excluded infants with congenital anomalies, birth weight <400 g and/or missing covariates; excluded women with chronic hypertension | Age, education, race/ethnicity, marital status, parity, smoking, maternal nativity, season of birth, tract-level median income and urbanization | Controlled for as confounder, but did not directly test education, race/ethnicity, poverty (community census tract- level) income, population density; excluded women with chronic hypertension from study | Hypertensive disorders of pregnancy (HDP) | ↑↔ | N/A |
| Mobasher et al. (2013) | Los Angeles, CA, USA (1996–2008) | N=298 (n=136) predominantly Hispanic women. Excluded multiple pregnancies, women with lupus, chronic renal disease, sickle cell disease or trait | Age, race/ethnicity, parity, exposure to second hand smoke, parity, smoking and year of conception BMI (note 17 missing BMI measures), chronic hypertension;asthma measured but not included in models | BMI—Stratified by obesity category (BMI ≥30 kg/m2) and likelihood ratio test for interaction | Hypertensive disorders of pregnancy (HDP) | ⇈ | ⇈ |
| Xu et al. (2014) | Jacksonville, FL, USA (2004–2005) | N=22,041 (n=1,037) Included live born singleton births. Excluded infants with congenital abnormalities, low birth weight, gestational age <24 weeks or >42 weeks, previous preterm birth, chronic hypertension, missing residential data and living far from monitor | Age, ethnicity, education, marital status, prenatal care, season of conception, smoking and track median household income | Multiple pollutants at low concentrations, and stratified by race/ethnicity, diabetes status, and education level. Stratified by race/ethnicity (non- Hispanic White, non-Hispanic Black, Others). Stratified by education status (<high school, high school graduate, >high school). Preexisting condition, stratified by gestational diabetes status N=568, n(GD+HDP)=58 | Hypertensive disorders of pregnancy (HDP) | ⇈ | ↔ |
| Savitz et al. (2015) | New York City, NY, USA (2008–2010) | N=268,601; mild PE n=6,940, severe PE n=4,226, GH n=5,834, and total HDP n=17,000 from 41 hospitals; excluded smokers and those with chronic hypertension and multiple births | Age, parity (0, 1, or ≥2), conception year. BMI. BMI2, and Medicaid status as proxy for SES. hospital, social deprivation index | Access to nutrition (BMI), education, SES (based on government insurance eligibilty), social deprivation index (SDI). SDI was comprised of % with college degree, % unemployment, % management/professional occupation, % residential crowding, % below 200% of the federal poverty line, % of households receiving public assistance, and % nonwhite race | Gestational hypertension Total HDP |
↑↔ ↑↔ |
N/A N/A |
| Männistö et al. (2015) Consortium on Safe Labor | 12 centersa (19 hospitals; 15 hospital referral regions) across USA (2002–2008) | N=151,276 births at ≥23 weeks gestation assembled using hospital delivery admission electronic medical records (both mother and neonate charts) excluded multi-fetal pregnancies, deliveries <37 weeks, women with eclampsia and missing variables | Age, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Hispanic, Asian/Pacific Islander, Other, Unknown), prepregnancy BMI category (underweight <18.5, normal weight 18.5 to <25 kg/m2, overweight 25 to <30 kg/m2, obese 30 to 34.9 kg/m2, severty obese >35.0 kg/m2, unknown), parity insurance status (public/self-pay, private, other, unknown), smoking during pregnancy, antihypertensive medication use, admission time, site, spontaneous labor, and number of pregnancies in the cohort were all derived from the electronic medical records | Modeled interaction between hypertensive disorder diagnosis and air pollution | Hypertensive blood pressure upon admission to labor and delivery | ↔ | ↔ |
| Medical condition of gestational hypertension, preeclampsia, chronic hypertension or superimposed preeclampsia (both hypertension from different cause and preeclampsia) | |||||||
|
Mendola et al. (2016) Consortium on Safe Labor/Air Quality and Reproductive Health Study |
12 centersa (19 hospitals; 15 hospital referral regions) across USA (2002–2008) | N=228,438 births at ≥23 weeks gestation assembled using hospital delivery admission electronic medical records (both mother and neonate charts) | Age, race/ethnicity (White, Black, Hispanic, Asian/Pacific Islander, Other/Unknown), prepregnancy BMI category (underweight <18.5, normal weight 18.5 to <25, overweight 25 to <30, obese ≥30 kg/m2. unknown), parity (nulliparous, primiparous, multiparous), marital status (married, divorced/widowed, single, unknown), insurance status (public, private, other, unknown), smoking and alcohol use during pregnancy (both, yes/no) were all derived from the electronic medical record | Access to nutrition preexisting condition (asthma) | Preeclampsia | ↑↔ | ↓↔ |
⇈ At least one model with a positive association where lower confidence bound does not include 1.0 for OR or RR (or 0.0 for continuous variables).
↑↔ Positive association where lower confidence bound includes 1.0 for OR or RR (or 0.0 for continuous variables).
⇊ Negative association where lower confidence bound does not include 1.0 for OR or RR (or 0.0 for continuous variables).
↓↔ Negative association where lower confidence bound includes 1.0 for OR or RR (or 0.0 for continuous variables).
↔ Null association.
Consortium on Safe Labor include Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville, MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, TX.
Table 5.
Metrics and Approaches to Evaluate the Combined Association of Air Pollution with HDP by Social Determinant or Pre-Existing Chronic Condition among U.S. Pregnant Women
| Gestational Hypertension | |||||||
| Study | Main Air Pollution Exposure | Pollutant | PM Metric | ||||
| Ozone | PM | PM2.5 | PM10 | Traffic | |||
| Vinikoor-Imler et al. (2012) | ⇈ | ⇈ | ⇈ | ||||
| Lee et al. (2013) | ↑↔ | ↑↔ | ↑↔ | ↑↔ | |||
| Savitz et al. (2015) | ↑↔ | ↑↔ | |||||
| Social Factors Effects Modification of Air Pollution—Gestational Hypertension Relationship | |||||||
| Social Factor | Study | Metric (Approach) | |||||
| Poverty |
Vinikoor-Imler et al. (2012) Savitz et al. (2015) |
Neighborhood Deprivation Index (NDI) NDI |
↔ |
↔ |
|||
| Education | Vinikoor-Imler et al. (2012) | Completed HS v. Completed College | |||||
| Racial Discrimination | Vinikoor-Imler et al. (2012) | Race/ethnicity: non-Hispanic Black v. non-Hispanic White | |||||
| Vinikoor-Imler et al. (2012) | Race/ethnicity: Hispanic v. non-Hispanic White | ||||||
| Lee et al. (2013) | Race category: Caucasian and African American (Stratified) | ↑↔ | ↑↔ | ↑↔ | |||
| Pyschosocial Stress | No U.S. studies | ||||||
| Access to Nutrition | No U.S. studies | ||||||
| Access to Healthcare | No U.S. studies | ||||||
| Medical Condition Effect Modification of Air Pollution-Gestational Hypertension Relationship | |||||||
| No U.S. studies examined joint chronic conditions and air pollution with GH | |||||||
| Preeclampsia | |||||||
| Pollutant | PM Metric | ||||||
| Study | Main Air Pollution Exposure | Ozone | PM | PM2.5 | PM10 | Traffic | |
| Wu et al. (2009) | ⇈ | ⇈ | |||||
| Wu et al. (2011) (Los Angeles) | ↔ | ⇈ | ↔ | ↔ | ⇈ | ||
| Wu et al. (2011) (Orange County) | ⇈ | ⇈ | ↔ | ↔ | ⇈ | ||
| Rudra et al. (2011) | ↑↔ | ↑↔ | |||||
| Lee et al. (2013) | ↑↔ | ↑↔ | ↑↔ | ↔ | |||
| Savitz et al. (2015) (Mild PE and PE) | ↓↔ | ↓↔ | |||||
| Savitz et al. (2015) (Severe PE and eclampsia) | ↑↔ | ↑↔ | |||||
| Mendola et al. (2016) | ↓↔ | ↑↔ | ↑↔ | ↔ | ⇈ | ||
| Social Factor Effect Modification of Air Pollution–Preeclampsia Relationship | |||||||
| Social Factor | Study | Metric (Approach) | |||||
| Poverty | Wu et al. (2009) | Insurance type (Stratified) | ↔ | ↔ | |||
| Wu et al. (2011) | Insurance type (Stratified) | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Wu et al. (2011) | % Poverty by block group (Stratified, data not shown) | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Savitz et al. (2015) | NDI (Interaction not reported) | ↔ | ↔ | ||||
| Education | No U.S. studies | ||||||
| Racial Discrimination | Wu et al. (2009) | Race/ethnicity (Stratified, data not shown) | ↔ | ↔ | |||
| Wu et al. (2011) | Race/ethnicity (Stratified, data not shown) | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Lee et al. (2013) | Race category: Caucasian and African American (Stratified) | ↔ | ↔ | ↔ | ↔ | ||
| Psychosocial Stress | No U.S. studies | ||||||
| Access to Nutrition | No U.S. studies | ||||||
| Access to Healthcare | Wu et al. (2009) | Insurance type (Stratified) | ↓↔ | ↓↔ | |||
| Wu et al. (2011) | Insurance type (Stratified) | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Preeclampsia | |||||||
| Pollutant | PM Metric | ||||||
| Ozone | PM | PM2.5 | PM10 | Traffic | |||
| Study | Medical Condition Effect Modification of Air Pollution–Preeclampsia Relationship | ||||||
| Obesity | Rudra et al. (2011) | BMI ≥25 kg/m2 (Stratified) | ↔ | ↔ | |||
| Mendola et al. (2016) | BMI ≥30 kg/m2 (Stratified, results not shown) | ↔ | ↔ | ↔ | ↔ | ||
| Wu et al. (2009, 2011) | Diabetes status (Stratified, data not shown) | ↔ | |||||
| Mendola et al. (2016) | Asthma status (Test for interaction) | ||||||
| All Hypertensive Disorders of Pregnancy | |||||||
| Study | Main Air Pollution Exposure | Pollutant | PM Metric | ||||
| Ozone | PM | PM2.5 | PM10 | Traffic | |||
| Miranda et al. (2013) | ↑↔ | ↑↔ | |||||
| Mobasher et al. (2013) | ⇈ | ⇈ | ⇈ | ↑↔ | |||
| Xu et al. (2014) | ↔ | ⇈ | ⇈ | ||||
| Savitz et al. (2015) | ↑↔ | ↑↔ | |||||
| Social Factor Effect Modification of Air Pollution–Hypertensive Disorders of Pregnancy Relationship | |||||||
| Social Factor | Study | Metric (Approach) | |||||
| Poverty | Savitz et al. (2015) | NDI (Interaction not reported) | ↔ | ↔ | |||
| Education | Xu et al. (2014) | Education category (<HS, HS graduate, >HS) (Stratified) | ↔ | ↓↔ | ↓↔ | ||
| Racial Discrimination | Xu et al. (2014) | Race/ethnicity Non-Hispanic Black and non-Hispanic White (Stratified) | ↓↔ | ⇊ | ⇊ | ||
| Race/ethnicity Other and non-Hispanic White (Stratified) | ↔ | ⇈ | |||||
| All Hypertensive Disorders of Pregnancy | |||||||
| Social Factor | Study | Metric (Approach) | Pollutant | PM Metric | |||
| Ozone | PM2.5 | PM2.5 | PM10 | Traffic | |||
| Mobasher et al. (2013) | Primarily Hispanic women (>96%), lacks comparison group | N/A | N/A | N/A | |||
| Pyscho-social Stress | No U.S. studies | Primarily Hispanic women (>96%), lacks comparison group | |||||
| Access to Nutrition | No U.S. studies | ||||||
| Access to Healthcare | No U.S. studies | ||||||
| Medical Condition Effect Modification of Air Pollution–Hypertension Disorders of Pregnancy Relationship | |||||||
| Obesity | Mobasher et al. (2013) | BMI >30 kg/m2 (Likelihood ratio test for interaction) | |||||
⇈ At least one model with a positive association where lower confidence bound does not include 1.0.
↑↔ Positive association where lower confidence bound includes 1.0.
⇊ Negative association where lower confidence bound does not include 1.0.
↓↔ Negative association where lower confidence bound includes 1.0.
↔ Null association.
![]() Indicates formal test for interaction (multiplicative effect).
Indicates formal test for interaction (multiplicative effect).
![]() Indicates additive effect.
Indicates additive effect.
![]() No shading indicates stratification, often used to examine confounding factor
No shading indicates stratification, often used to examine confounding factor
Primary Association of Air Pollution Exposures and Measures of Hypertensive Disorders of Pregnancy
We examined U.S. studies that also examined social factors or pre-existing conditions for primary associations of HDP with pollutant exposure with ozone and various indicators of PM. With respect to ozone exposure, we observed some limited evidence of a positive association with ozone exposure, but fewer studies analyzed ozone exposure than PM. Two analyses of ozone exposure with any HDP yielded positive significant associations (Mobasher et al., 2013; Wu, Wilhelm, Chung, & Ritz, 2011), four analyses reported positive associations of ozone exposure with any HDP but the 95 percent confidence interval includes 1.0 (Lee et al., 2012, 2013; Männistö et al., 2015; Mobasher et al., 2013), and six studies reported null results (Table 4).
Results for the main adjusted effect of PM2.5 or PM10 exposure on HDP among pregnant women in the United States yielded generally positive but not always significant associations (Table 5). Six analyses of PM exposure with various measures of HDP yielded positive significant associations and eight analyses reported positive associations but the lower 95 percent confidence interval includes 1.0. Because the pathophysiology of HDP is thought to begin early in pregnancy during placentation, we would expect first trimester exposures to be relevant.
We identified three large, high-quality studies published subsequent to the two meta-analyses (Männistö et al., 2015; Mendola et al., 2016; Pedersen et al., 2014; Savitz et al., 2015; Xu et al., 2014). A large multi-center birth record study in New York City (Savitz et al., 2015) reported PM2.5 exposure in the first and second trimesters were positively related to risk of gestational hypertension (first trimester OR 1.4 [95% CI 1.2, 1.5] and second trimester OR 1.4 [95% CI 1.3, 1.5]) and inversely related to risk of mild PE in models not adjusted for delivery hospital. Additional adjustment for delivery hospital as a potential confounder resulted in a null association. This adjustment needs additional investigation: It may represent procedures related to administrative coding, as the authors note, or despite adjustments for neighborhood deprivation index and other confounders, may reflect unmeasured characteristics of the hospital catchment areas. The two additional studies associated with the 12-center Consortium on Safe Labor used modeled air quality to explore additional pollutants and much shorter averaging times (0–4 hours).
Proximity to vehicle traffic is another metric for exposure to primary PM and other emissions from motor vehicles in which ozone levels would be expected to be low. Two studies reported positive associations between traffic and preeclampsia in southern California (Wu et al., 2009, 2011) and with gestational hypertension in North Carolina (Vinikoor-Imler et al., 2012), whereas a study in North Carolina of combined preeclampsia and gestational hypertension did not observe significant associations with distance to roadways (Miranda et al., 2013). The Wu et al. (2011) studies focused on exposure assessment; they reported no association of hypertensive disorders with total PM2.5 but did find an association with traffic-specific PM, indicating that effects may be masked by exposure misclassification or that high levels may be an important factor.
To examine publication bias, Pedersen et al. (2014) performed funnel plots and Egger tests; the authors did not report any publication bias for either PM or ozone exposures with studies from all geographic locations. Hu et al. (2015) reported similar results. Among the U.S. studies identified in our review, investigators employed standard approaches for long-term air pollution exposure assessment including nearest regulatory air quality monitor, monitoring data supplemented with modeling (e.g., kriging and CMAQ fused with modeling data), modeling alone (e.g., CALine4 and CMAQ) and distance to roadway as a proxy for traffic-related PM (see Table S2).
Combined Association of Air Pollution and Social Stressors on Hypertensive Disorders of Pregnancy
The studies in this review examined three of the five measures of social factors using a wide array of metrics with varying degrees of quality (Supporting Information Table S3). The studies examined interactions of or stratifications by the air pollution–HDP relationships with social factors or pre-existing condition. One study discussed mediation of blood pressure increases (Lee et al., 2013). Three studies directly assessed interactions (Mendola et al., 2016; Mobasher et al., 2013; Vinikoor-Imler et al., 2012), and the other studies largely performed stratifications to assess confounders, reporting few differences by category (Table 5). All studies assumed that the exposure period for social factors was the same as for pollutants. Among five studies of pre-existing conditions, air pollution exposure effects were considered jointly with obesity, asthma, gestational diabetes, and gestational hypertension (Table S3). Studies used various measurements to quantify social determinants of health including poverty, education, race/ethnicity, and access to health care (Table 5, see also Table S3). As metrics of poverty, for example, authors tested income-dependent insurance of the mother (Wu et al., 2011), percent poverty by block group (Wu et al., 2009), maternal education (Vinikoor-Imler et al., 2012; Xu et al., 2014), and two types of neighborhood deprivation indices (Savitz et al., 2015; Vinikoor-Imler et al., 2012).
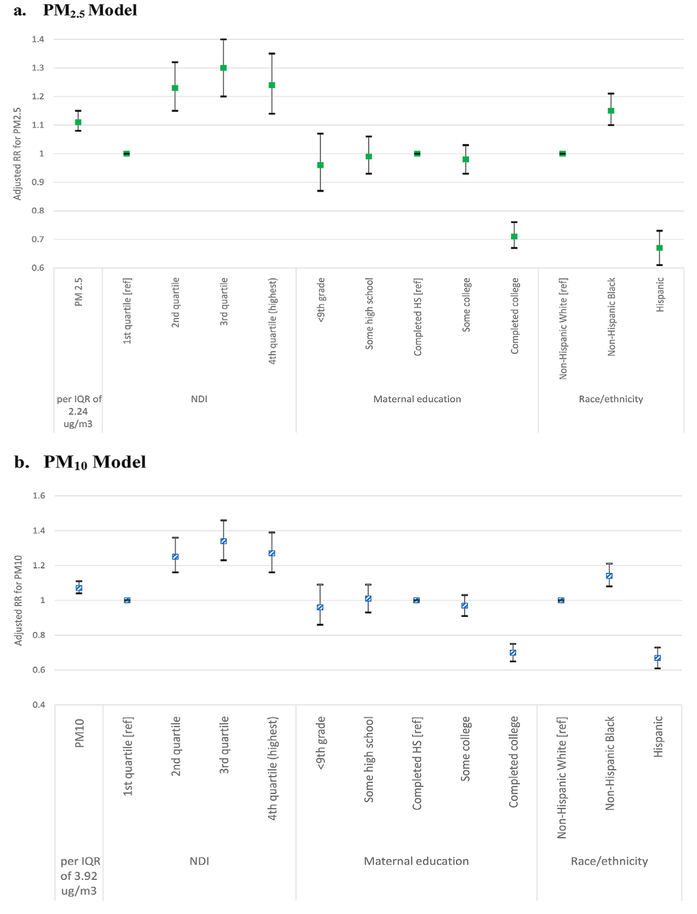
A majority of epidemiologic studies presented air pollution findings adjusted for social determinants of health. For example, Wu et al. (2011) adjusted for poverty and insurance type, but did not report results for these variables (i.e., they only adjusted results for associations of air pollutants with outcomes). Similarly, Savitz et al. (2015) adjusted for both neighborhood deprivation index and delivery hospital, but they did not report results for effect sizes. As a result, these studies are not reported in Figure 3, which highlights an additive effect of social factors including neighborhood deprivation, poverty, maternal education and race and ethnicity, adjusting for air pollution and other factors (Vinikoor-Imler et al., 2012) (see also Tables 5 and S3). In the only study we identified that directly tested for differences across racial and ethnic groups, Vinikoor-Imler et al. (2012) reported a main effect of race and ethnicity, adjusting for PM2.5 and other covariates (Figure 3). Specifically, they report relative risk of gestational hypertension was 15 percent higher among non-Hispanic black women compared to non-Hispanic white women (OR 1.15 [95% CI 1.10, 1.21]) and 33 percent lower among Hispanic women compared to non-Hispanic White women (OR 0.67 [95% CI 0.61, 0.73]) (Vinikoor-Imler et al., 2012). Effects were similar for models with PM10. A case-control study in Los Angeles of predominantly Hispanic women reported increased odds of preeclampsia with second trimester ozone exposures and with first trimester PM2.5 exposures (Mobasher et al., 2013); however, no comparison racial group was included in the study.
Figure 3.
Adjusteda Risk Ratios (RR) for the Associations between Particulate Pollution, Neighborhood Deprivation Index (NDI), Maternal Education, and Race/Ethnicity and Maternal Gestational Hypertension (Vinikoor-Imler et al., 2012).
aAdjusting for IQR PM, NDI, maternal age category, maternal education category, smoking during pregnancy, nulliparity, race/ethnicity, marital status. NDI variable constructed from census tract-level percent households in poverty, percent female-headed households, percent household income <$30,000, percent households on public assistance, percent males in management, percent crowded households, percent unemployed, percent < high school education. The IQR for PM2.5 was 2.24 μg/m3 and PM10 was 3.92 μg/m3 during pregnancy.
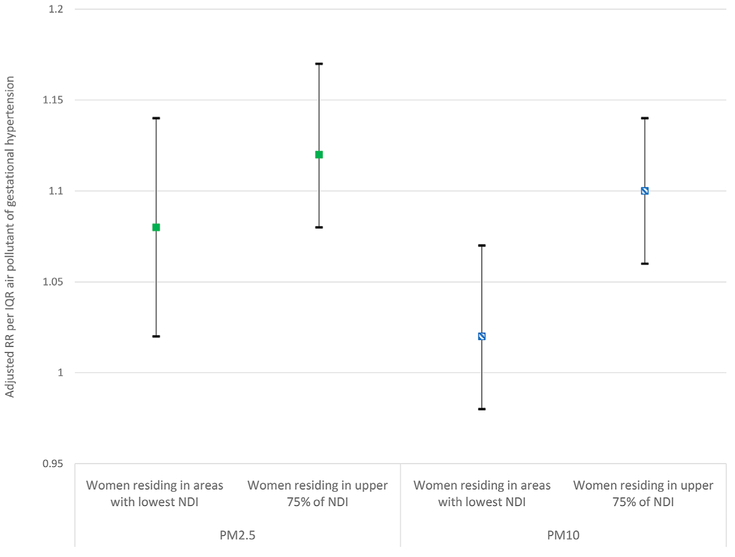
Only one study used interaction terms to test for effect modification. Vinikoor-Imler et al. (2012) tested whether residing in neighborhoods with high levels of neighborhood deprivation, assessed using a dichotomized neighborhood deprivation index (NDI), modified associations between PM2.5 and PM10 exposure and gestational hypertension (Figure 4). Women residing in areas with the most deprivation (upper 75 percent deprivation indices) had a higher risk of gestational hypertension per interquartile range (IQR) PM10 (OR 1.10 (95% CI 1.06, 1.14)) compared to women in the lowest quartile NDI (OR 1.02 (95% CI 0.98, 1.07)). There was a positive interaction for NDI and PM10 exposure (p < 0.05), but the interaction was not significant between NDI and PM2.5 (p 0.24).
Figure 4.
Comparison of Adjusteda RR for Gestational Hypertension per IQR Increase in Particulate Pollution among Women Residing in Neighborhoods with Lower Neighborhood Deprivation Index (NDI) Compared to Upper 75th Percentile NDI (Interaction) (Vinikoor-Imler et al., 2012).
aThe model contained an interaction between a binary term for NDI and air pollution concentration and loglikelihood ratio test was employed. Adjusting for IQR PM, NDI category, maternal age category, maternal education category, smoking during pregnancy, nulliparity, race/ethnicity, marital status. NDI variable constructed from census tract-level percent households in poverty, percent female-headed households, percent household income <$30,000, percent households on public assistance, percent males in management, percent crowded households, percent unemployed, percent < high school education. The IQR for PM2.5 was 2.24 μg/m3 and PM10 was 3.92 μg/m3 during pregnancy.
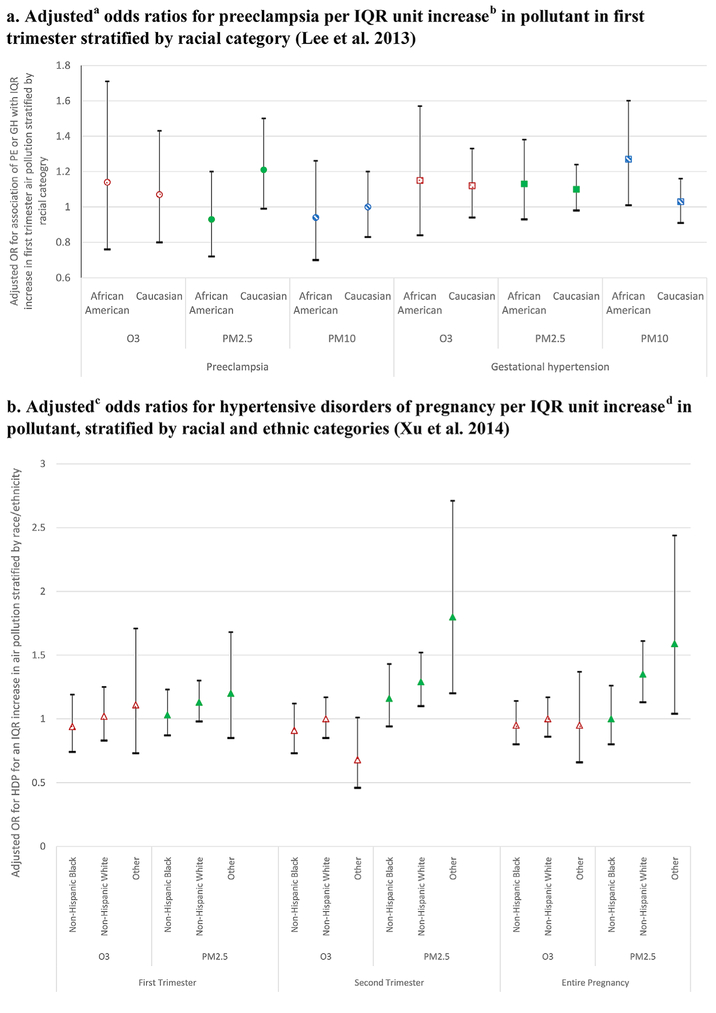
Two studies stratified results by maternal race/ethnicity (Lee et al., 2013; Xu et al., 2014), and one evaluated maternal race/ethnicity as an independent risk factor (Vinikoor-Imler et al., 2012). As shown in Figure 5 (see also Table 5), Lee et al. (2013) reported no significant associations between ozone or PM10 with PE among Caucasian and African American women in Pennsylvania. They reported positive but not significant associations with PM2.5 exposure and PE among Caucasian but not African American women. In addition, they reported positive effects of PM10 exposure on gestational hypertension among African American but not Caucasian women. In a relatively small sample of women in Florida, Xu et al. (2014) reported no significant associations between long-term ozone exposure and HDP for any racial or ethnic group, or between PM2.5 and HDP among non-Hispanic Black women. They reported significant associations between PM2.5 and HDP among both non-Hispanic White and “Other” women associated with exposures in the second trimester and the entire pregnancy, but not in the first trimester (see Figure 5b).
Figure 5.
Relationships Between Air Pollution and Hypertensive Disorders of Pregnancy (HDP), Stratified by Race/Ethnicity and Education Categories.
aAdjusting for interquartile range (IQR) zipcode level pollutant, maternal age, number of cigarettes smoked during pregnancy, nulliparity, race/ethnicity, season of birth, year of conception from hospital-based records. bThe IQR for O3 was 16.9 ppb, for PM2.5 was 4.0 μg/m3, and for PM10 was 7.7 μg/m3 during first trimester. cAdjusting for IQR pollution, maternal age, maternal education category, smoking, race/ethnicity, marital status, prenatal care, season of conception, census tract median household income. dThe IQR for O3 was 30 ppb, and for PM2.5 was 0.67 μg/m3 during entire pregnancy.
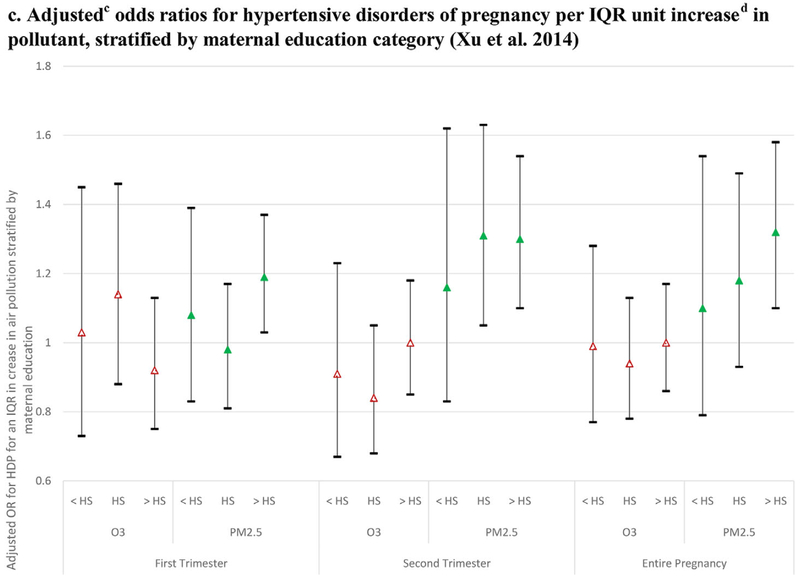
Maternal education has been used as a metric to approximate SES (Figures 3 and 5c). A study conducted in Jacksonville, Florida, which had relatively low levels of pollution, examined effects of ozone and PM2.5 exposure on HDP using stratified models to assess association by level of maternal education (Xu et al., 2014). No significant associations were reported for ozone for women in any educational level. Increased PM2.5 exposure was significantly associated with increased risk of HDP in the second trimester among high school graduates, and with increased risk of HDP in the first and second trimesters and entire pregnancy among those with more than a high school education. Among those with less than a high school education, trends toward increased risk of HDP were not statistically significant (Xu et al., 2014).
Combined Association of Air Pollution and Pre-Existing Co-Morbidities on Pregnancy Hypertension Disorders
With respect to pre-existing co-morbidities, studies examined obesity, diabetes, and asthma with a variety of pollutants, averaging times, outcomes, and covariates (see Table S3). As with analyses in the previous section, investigators generally reported only adjusted models or used stratification for sensitivity analysis but did not report specific coefficients for individual variables.
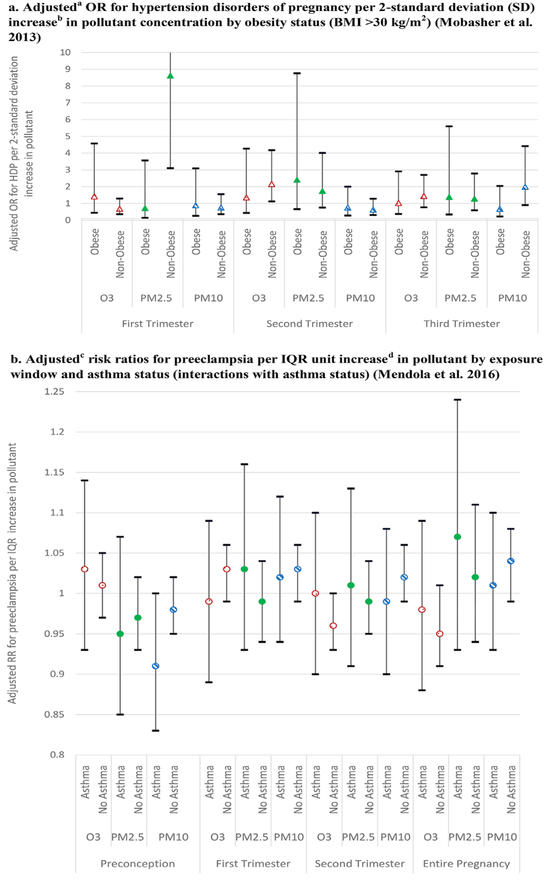
With respect to excess weight, one study directly tested for an interaction; specifically, obesity (pre-pregnancy BMI ≥30 kg/m2) had a protective effect for odds of preeclampsia with ozone during the third trimester and suggestive evidence for a protective effect for PM10 and PM2.5 exposures in some trimesters (Mobasher et al., 2013) (Figure 6a). Other studies stratified by various BMI categories. Analyses restricted by BMI (stratified on pre-pregnancy BMI ≥25 kg/m2 only and BMI ≥30 kg/m2) generally yielded similar results as the main analyses, although with a loss of precision due to the smaller sample (Mendola et al., 2016). Rudra et al. (2011) reported no differences in models stratified by BMI status in their finding of no association of PM2.5 with either preterm delivery or preeclampsia.
Figure 6.
Chronic Conditions and Effect Modification of Air Pollution–HDP Relationships.
aAdjusting for 2-SD pollutant, maternal age, smoking and second hand smoke, parity, and race/ethnicity. b2-SD for O3 was 15 ppb, for PM2.5 was 7 μg/m3, and for PM10 was 13 μg/m3. cAdjusting for interquartile range (IQR) modeled pollutant, maternal age, smoking and alcohol use, nulliparity, race/ethnicity, pre-pregnancy BMI category, insurance status, marital status and study site. dIQR for O3 was 7.9 ppb, PM2.5 was 4.7 μg/m3, and PM10 was 4.5 μg/m3 during entire pregnancy.
Wu et al. (2009) reported no difference in PM2.5 exposure association with preeclampsia when stratified by diabetes status. With respect to asthma status, one study reported no associations or interaction by asthma status with preeclampsia for ozone exposure (Mendola et al., 2016) (Figure 6b). However, for elemental carbon exposure (a subset of PM and often associated with diesel emissions) during full pregnancy, the odds of preeclampsia was significantly higher among those women with asthma (OR 1.11 [95% CI 1.03, 1.21] compared to those without asthma; OR 1.03 [95% CI 0.99, 1.06]) (Mendola et al., 2016).
Discussion
We critically evaluated the regulatory framework that established criteria for identifying and designating at-risk populations for inclusion in the margin of safety decisions using the CAA NAAQS. We considered pregnant women as a candidate at-risk population and one disease category (i.e., HDP). Our goal was to characterize how the relationships between air pollution exposure and HDP are affected (i) by social determinants of health such as poverty, race/ethnicity, psychosocial stress, access to nutrition, and access to health care; and (ii) by the pre-existence of chronic conditions (e.g., diabetes, obesity/overweight, or asthma). Recent reviews have examined the combined effect of chemical and social stressors on birth outcomes among fetuses or infants (e.g., low birth-weight and fetal growth restriction [Dadvand et al., 2013; Parker et al., 2011; Vesterinen et al., 2017; Woodruff, Carlson, Schwartz, & Giudice, 2008; Woodruff et al., 2003]), whereas this paper focuses on pregnant women themselves from a population perspective via the policy lens of the CAA designation of at-risk populations.
Evaluation of Pregnant Women as an At-Risk Population
As part of the EPA’s future NAAQS review process, the EPA would synthesize all evidence from basic biological studies, dosimetry, toxicology, exposure science, and epidemiology for all relevant health outcomes. This full assessment is beyond the limited scope of this paper. Even with a limited review of the epidemiologic literature for a single endpoint, we argue for the routine inclusion of pregnant women as a candidate at-risk population in the NAAQS review process from two perspectives: policy and scientific risk assessment.
From a policy perspective, the legislative history and regulatory practice envisions an analysis of populations in which sensitive groups of people are protected, thereby leading to fuller risk reduction across the population, while acknowledging that the standards are not intended to be risk-free. The conceptualization of the scope or definition of the candidate populations are arguably a policy choice informed by scientific data. The first step in the EPA’s process is to identify an adverse health effect and direct comparisons of groups. Relying solely on scientific studies to identify vulnerable populations is flawed because essentially these groups must first experience and demonstrate harm before being afforded protection under the law. The EPA’s procedures are inconsistent with the statutory language in the CAA that articulates the goal of preventing likely harms among sensitive groups from air pollution exposures. Furthermore, these groups may be especially difficult to study. For example, Institutional Review Boards may require extra steps to study vulnerable groups (including pregnant women), or administrative data sets may exclude people without an address or may not contain detailed information about severe poverty or racial discrimination. Policy and scientific framings may not align with each other to define a population. For instance, a study may not have the resources to include another group or may choose to focus on only one population (e.g., Mobasher et al., 2013). The goal of a scientific study may not consider its subsequent use in policy so the hypotheses, methods, or reporting may not align with the regulatory needs (e.g., not reporting the regression coefficients for poverty variables separately).
From a physiological perspective, we argue that there are sufficient scientific data to consider pregnant women as a candidate at-risk population that should be fully evaluated in formal risk assessment. Pregnant women are more susceptible to the adverse effects of criteria air pollution on their own health than nonpregnant populations due to sensitive maternal exposure periods, increased cardiac output, increased minute ventilation, and other adaptations of pregnancy, and additional adverse health endpoints (e.g., preeclampsia, gestational hypertension, gestational diabetes) not experienced in other reference populations (Di Renzo et al., 2015; Erickson & Arbour, 2014). Reviewed elsewhere, additional evidence should be considered regarding birth outcomes that can have both negative health repercussions for the fetus and physical and mental health consequences for the mother (Shah & Balkhair, 2011; Srám, Binkov a, Dejmek, & Bobak, 2005; Stieb, Chen, Eshoul, & Judek, 2012).
Moreover, we recommend taking a population perspective to evaluate the need to consider further subsets of at-risk populations due to social factors (e.g., low SES, racism) that might contribute to adversity of effect or lack of ability to recover from exposure consistent with ATS’s statement and environmental justice perspectives (Assibey-Mensah et al., 2016; Institute of Medicine, 1999; Thurston et al., 2017).1
Associations of Air Pollution Exposure and Hypertensive Disorders of Pregnancy
Regarding primary air pollution–HDP relationships, we report mixed findings for associations with ozone exposures. Results reported here are suggestive of positive associations of PM with preeclampsia, gestational hypertension, and blood pressure changes during pregnancy, and positive but not consistently significant associations between proximity to traffic and maternal health outcomes. Few studies reported either main (additive) effects of air pollution and social factors to assess joint effects, and few reported explicit tests for effect modification (interactions). The former is necessary to establish cumulative risk, while the latter is necessary to establish susceptibility. For example, additive effects for social factors in models with both PM2.5 and PM10 were associated with living in deprived neighborhoods, but confounding could not be ruled out (Savitz et al., 2015). Findings for level of education were inconclusive, however, a study that directly assessed the effect of education reported women with lower education had increased odds of gestational hypertension with PM exposure compared to women with higher education (Vinikoor-Imler et al., 2012). One study (Xu et al., 2014) stratified by education level and reported variations in associations between PM and HDP for different education strata. Future studies which directly test for effect modification are needed to further assess these associations. There is limited evidence suggesting racial differences in associations between PM and preeclampsia. Taken together, these studies offer limited evidence that there is increased risk among women living in deprived neighborhoods and among those with less than high school education. Relatively few studies have examined whether women with pre-existing conditions are at heightened risk of HDP associated with PM or ozone.
Critical analysis across studies is needed to inform future research and policy. Findings are inconclusive with limitations in techniques and metrics used to assess air pollution, social factors, and case ascertainment; general lack of reporting of coefficients; and with small sample size of subgroupings upon stratification. In these studies, air pollution exposure assignment generally followed accepted techniques. Studies that used exposure metrics that specifically linked maternal residences (e.g., Miranda et al., 2013; Vinikoor-Imler et al., 2012) yield more precise estimates than those using zip codes (e.g., Lee et al., 2012) or regional hospital catchment zones (Männistö et al., 2015). Studies using geocoded maternal residence at birth omitted residential addresses that could not be geocoded. There may be unmeasured sources of bias resulting from this process. Similarly, a majority of studies using maternal residential address used the address reported at birth as a single exposure point, but women spend time away from home. In addition, this process may contribute to exposure misclassification bias if women moved during pregnancy. These air pollution exposure assessment techniques have been shown to be relatively comparable and any exposure misclassification biases findings toward the null. For example, Wu et al. (2011) evaluated the validation of PM measured by different models and found comparable results between air pollution and PE when using dispersion models, land use regression, or a more simplistic method such as nearest monitor in a well characterized air basin of Los Angeles.
Methods of case ascertainment and pre-existing diseases varied; studies used birth certificate records, hospital records, and specific clinical measurements, with inclusion criteria varying across studies. Previous reviews have discussed limitations of case ascertainment including that different administrative agencies define HDP differently and generally lack detailed date of HDP diagnosis (Hu et al., 2014). The clinical definitions of HDP have undergone significant revision over the past 5 years, and given the variety of data sources in the relevant literature, the definitions have not been used consistently (ACOG, 2013b). Techniques varied to determine the exposure period of interest with highest reliability coming from the combined date of last menstrual period from birth or hospital records combined with ultrasound measurements. Most studies averaged air quality over a trimester (Lee et al., 2012; Mobasher et al., 2013; Wu et al., 2011; Xu et al., 2014) or an entire pregnancy (Vinikoor-Imler et al., 2012; Wu et al., 2011; Xu et al., 2014), which might miss critical peaks or shorter exposure periods. One study examined modeled air quality exposure 0–4 hours preceding hospital admission blood pressure reading (Männistö et al., 2015). Most studies stratified by the social factors to examine differences in associations, rather than directly addressing effect modification. Stratification reduces sample sizes and thus may reduce statistical power to detect associations. Furthermore, stratification may introduce unintended bias into the samples, limiting the ability to draw strong conclusions. Studies generally did not discuss the exposure period for social factors and assumed the same timing as the pollutant exposures. Together, these methodological differences contribute to challenges in identifying clear patterns of findings. However, despite these challenges, there is evidence in the papers reviewed here of significant associations between air pollution (especially PM/traffic exposures) and adverse maternal health effects, and suggestive evidence from a limited number of studies that have directly assessed joint additive effects of air pollutants and social determinants of health (e.g., Vinikoor-Imler et al., 2012). In addition, while findings to date are sparse and inconclusive, these results suggest the need to further consider the combined effect of air pollution and other chronic conditions that are also systematically related to social conditions.
There has been increasing attention to the extent to which these joint effects have a cumulative effect on health outcomes and explain, in part, observed health disparities. To examine the evidence against the EPA’s third or fourth criteria for at-risk populations, our review explicitly examined the joint effects of these exposures during pregnancies in the United States. We concluded that there is insufficient literature to examine (by meta-analysis) a combined effect of one air pollutant and social stressor exposure on preeclampsia or gestational hypertension (Table 5). The studies we evaluated have generally been limited in one or more of the following features: Quality of air pollution exposure assessment (relying on sparse regulatory air pollution monitoring data or 3-month averages which may not be adequately temporally resolved), quality of the measurement (e.g., relying on pre-pregnancy BMI for obesity rather than waist circumference; or available metrics for individual SES such as insurance provider or maternal education that may be measured with error, biasing toward the null), quality of outcome assessment (often relying on birth certificate data), or limited control for confounders.
Similarly, the literature provides evidence for a combined effect of a single air pollutant and a pre-existing chronic condition on maternal health, but the data are limited in scope. No evidence of an increase in risk of preeclampsia with increases in PM exposure was observed in a test of interaction by maternal asthmatic status (Mendola et al., 2016).
For obesity, few studies reported either primary effects of air pollution and excess weight to assess joint effects (e.g., not reporting the beta coefficient for obesity), and few reported explicit tests for effect modification (interactions). Among those studies that did examine interactions, findings regarding differences in risk by maternal BMI are mixed, with two studies reporting no associations between air pollution and preeclampsia by BMI category using stratification, and one study that directly tested for differences reporting lower risk of preeclampsia among obese women (with pre-pregnancy BMI ≥30 kg/m2) (Mobasher et al.,2013). A protective effect of obesity was unexpected, as obese women are at higher risk for chronic hypertension and HDP generally and obese populations receive a greater dose of ozone and PM for the same ambient concentrations compared to other populations (Gidding et al., 2004; Koman & Mancuso, 2017; Lin & Lin, 2012; Salome, King, & Berend, 2010). Thus, we would have expected a higher risk of air pollution-related preeclampsia with increasing weight, which was not observed in these studies (Mendola et al., 2016; Mobasher et al., 2013; Rudra et al., 2011). These mixed results for interactions suggest further investigation to clarify etiology and mechanism of disease.
Limitations and Bias
Our review limited the study locations to the United States for ease of comparison of social factors. A further limitation is that our study does not conduct a meta-analysis, primarily due to the heterogeneity of measures and exposure periods of both social determinants and pre-existing conditions in the available studies. We did not assess for publication bias due to our limited geographic coverage, although two previous reviews reported no evidence of publication bias (Hu et al., 2015; Pedersen et al., 2014). We did not conduct a formal evaluation of study quality.
We observed little methodological consistency across studies in their measurement of social determinants, which also prevented a meta-analysis. Two studies examined neighborhood deprivation (Savitz et al., 2015; Vinikoor-Imler et al., 2012), but used different indices and methodologies. None of the studies measured stress, racial discrimination, or access to nutrition or health care directly. A limitation of the interpretation of the studies is the reliance on broad social factors such as race or SES, which may operate on maternal health via multiple pathways. For example, these pathways may include stress, housing security, quality of housing or health care, ability to afford healthy food, and race-based residential segregation and implications for material living conditions. Another limitation is that in considering poverty or SES, the studies generally did not consider programs for pregnant women to ameliorate their effects on health (e.g., income-tested programs to increase access to nutrition, knowledge about mitigation, or health care for pregnant women or women with children), which may lead to exposure misclassification and difficulties in interpreting the results. Our ability to assess combined effects is further complicated by the lack of consistent air pollution exposures and exposure periods in studies that evaluate combined effects with social stressors. Some of the social factors are short term and others are long term in exposure period, a discrepancy that is seldom addressed in air pollution studies.
Despite these limitations, failure to assess the population effects of joint exposures, particularly in populations that already experience compromised health due to multiple exposures, may underestimate these effects and may under-represent the size of at-risk populations. For example, a multicity time series study followed four cohorts of Medicare enrollees with chronic conditions that might predispose them to ozone related effects from 1985 to 2006 (Zanobetti & Schwartz, 2005). This study reported an association between long-term (warm season) exposure to ozone and elevated risk of mortality in the cohort that had previously experienced an emergency hospital admission due to chronic obstructive pulmonary disease (COPD). Given socioeconomic, racial, and ethnic disparities in risk factors for COPD, these findings suggest that failure to apply consistent population risk criteria for vulnerability may underestimate adverse health effects in communities where residents experience the combined effects of multiple exposures and vulnerabilities (Brulle & Pellow, 2006; Farley et al., 2006; O’Neill, Breton, Devlin, & Utell, 2012; U.S. EPA, 2015; Woodruff et al., 2003).
Research Needs
We identified a number of gaps in the current literature that limit the ability to draw conclusions regarding population vulnerability, and thus to create scientifically informed policies that assure adequate protection of vulnerable populations. First, a majority of studies to date have been able to identify main effects of air pollutants on HDP, and they have treated individual maternal characteristics (e.g., race, BMI) or neighborhood characteristics (e.g., neighborhood deprivation) as confounders. Most commonly, these studies report air pollution effects adjusted for maternal or neighborhood characteristics but do not report the main effects of these factors. As a result, the ability to observe independent or additive risks associated with joint exposures and vulnerabilities is limited.
More direct measures of exposure to racial discrimination or experiences of psychosocial stress are needed. Many studies used administrative hospital record data or data from the Census or American Community Survey (ACS), which are limited in specificity, often serving as proxies or crude indicators of social determinants of health. Furthermore, additional studies that explicitly model neighborhood characteristics as social determinants of health are needed to disentangle the effects of the geographic distribution of social determinants of health from individual characteristics. For example, the pathways linking individual race to HDP (e.g., exposure to interpersonal racism, housing discrimination) are likely to differ from those linking neighborhood racial composition to HDP (e.g., neighborhood economic disinvestment) and with differential implications for associations between air pollutant exposures and HDP. Additional research that carefully examines individual and neighborhood level indicators of social determinants of health is warranted.
Relatively few studies have directly addressed the question of effect modification. Several studies stratified by race to assess the extent to which effects of air pollutant exposure on HDP were consistent across strata. Stratification has important limitations, including introduction of bias in the subsamples and limitations on sample sizes that may influence results. Only one study included in this review included a direct test of interactions between social determinants of health (assessed as neighborhood deprivation index) and air pollution. Additional studies that directly test the hypothesis that social determinants of health modify associations between air pollution and HDP are needed in order to strengthen the evidence base.
Among the relatively few studies that have more directly assessed questions related to the joint effects of social determinants of health and air pollutants, or of individual vulnerability (e.g., chronic conditions) and air pollution, there are wide variations in the air pollutant exposure period (e.g., four hours, trimesters, full length of pregnancy), and limited attention to the exposure periods for social determinants of health. This contributes to challenges in comparisons across studies and use in policy decisions. Moreover, a systematic review could identify methodological weaknesses.
In sum, there is a strong need for additional studies that directly assess the independent and joint effects associated with exposure to air pollutants in conjunction with social determinants of health. Such studies should be designed to include explicit estimates of social determinants of health, reporting coefficients for these variables in addition to air pollutant variables included in the models. Because these joint effects may be additive or interactive, researchers should identify potential pathways and test additive or interactive effects (effect modifiers) as appropriate. Finally, more attention to the issue of exposure windows for both air pollutant exposures, and social determinants of health, is needed to develop plausible pathways and developmental windows of exposure to advance research and inform policy.
Policy Implications
Because the CAA creates a duty to protect at-risk groups, the framing of the assessment of at-risk populations has both policy and scientific foundations. We contend that a plain reading of the CAA does not envision that sensitive groups must first experience and demonstrate harm before they are afforded protection under the law, but instead implies a preventative approach that could anticipate the need to assess all relevant groups, including pregnant women.
A key finding of this review is that pregnant women ought to be assessed directly as a candidate at-risk population for consideration in the margin of safety of the NAAQS due to critical windows of vulnerability to exposure for the woman during conception, placenta implantation, artery remodeling, and labor; increased demands on the cardiovascular system during pregnancy; and association of air pollution exposure and adverse endpoints that are unique to pregnancy such as preeclampsia and gestational hypertension. Based on this review, there is adequate evidence that exposure to PM is associated with an adverse effect among pregnant women (e.g., HDP) not evident among non-gravid populations. There is suggestive evidence that exposure to ozone is associated with adverse effects among pregnant women (e.g., HDP) not evident among non-gravid populations. This evidence should be weighed in conjunction with data on social factors to identify subsets among pregnant women that may be especially at risk.
The policy implication is that the EPA’s risk assessment techniques could be improved to consider how broader socioeconomic factors impact the relationships between air pollution and adverse health effects. The standards set without these considerations are inadequate to protect health in communities with multiple risk factors and likely underestimates the adverse health effects of criteria pollutants, particularly for populations with multiple exposures and vulnerabilities. Among the factors we assessed, there is some evidence for increase air pollution-associated risk with chronic conditions with social patterns such as obesity, and social factors such as neighborhood deprivation. However, the literature regarding the joint exposure of air pollution and social stressors among pregnant women in the United States is sparse and underdeveloped for quantitative risk assessment.
There are few consistently designed HDP studies in the United States from which to sufficiently evaluate the possible interaction between air pollution and social stressors and their effects on pregnancy. We concluded that the findings were generally mixed, with a small portion of the studies in this emerging literature reporting evidence in support of a joint contribution between ozone or PM and social determinants or pre-existing chronic health conditions related to HDP in the United States. Additionally, the interplay between social and environmental exposures is likely to be varied and nuanced. A challenge for interpreting both the primary and joint effect is a mismatch between the time period over which air pollution is hypothesized to play a role in the development of pregnancy-related hypertension versus the time horizon for the effects of social determinants. Similarly, few studies examining the joint effect of air pollution exposure and pre-existing chronic conditions reported evidence in support of a joint contribution.
Conclusions
The Clean Air Act NAAQS could be a powerful tool to address health disparities related to social determinants of health in cases where the scientific evidence supports it. In keeping with recent scientific guidance (Thurston et al., 2017), the NAAQS review process could be enhanced by routinely and explicitly including pregnant women as a candidate at-risk population and by evaluating social determinants of health, especially for vulnerable subpopulations, during risk assessment (e.g., pregnant women living in poor neighborhood conditions). Under the history of the CAA, the EPA has a duty to protect at-risk populations with an adequate margin of safety. To do so, the EPA must fully assess the risk to populations experiencing the cumulative effects of multiple social and chemical exposures, and, particularly, among pregnant women.
Supplementary Material
Footnotes
Conflicts of interest: None declared.
The population risk criterion in the ATS statement may be especially relevant for sensitive health outcomes (e.g., biomarkers, blood pressure changes, lung function decrements) that may or may not constitute an adverse effect in and of themselves. The criterion is also helpful for considering population risk from combined air pollution exposure and social stressors since its foundation is a population health perspective. The guidance from ATS states, “Exposure to air pollution that increases the risk of an adverse effect to the entire population is adverse, even though it may not increase the risk of any individual to an unacceptable level. For example, a population of asthmatics could have a distribution of lung function such that no individual has a level associated with clinically important impairment. Exposure to air pollution could shift the distribution to levels that still do not bring any individuals to a level that is associated with clinically relevant effects. However, this would be considered to be adverse because individuals within the population would have diminished reserve function, and therefore would be at increased risk for further environmental insult” (Part II, Section B, Part 3) (American Thoracic Society, 2000).
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
Contributor Information
Patricia D. Koman, University of Michigan School of Public Health, Environmental Health Sciences Department in Ann Arbor, Michigan..
Kelly A. Hogan, University of Michigan School of Public Health, Environmental Health Sciences Department in Ann Arbor, Michigan, and presently a research fellow in the Department of Biochemistry and Molecular Biology and the Robert and Arlene Kogod Center on Aging at Mayo Clinic, Rochester, Minnesota..
Natalie Sampson, University of Michigan-Dearborn, Department of Health & Human Services in Dearborn, Michigan..
Rebecca Mandell, Arbor Research Collaborative for Health in Ann Arbor, Michigan..
Chris M. Coombe, University of Michigan School of Public Health, Department of Health Behavior & Health Education in Ann Arbor, Michigan..
Myra M. Tetteh, University of Michigan School of Public Health, Department of Health Behavior & Health Education in Ann Arbor, Michigan..
Yolanda R. Hill-Ashford, Detroit Health Department in Detroit, Michigan..
Donele Wilkins, Green Door Initiative in Detroit, Michigan.
Marya G. Zlatnik, University of California San Francisco, Department of Obstetrics, Gynecology and Reproductive Sciences in San Francisco, California..
Rita Loch-Caruso, University of Michigan School of Public Health, Environmental Health Sciences Department and director of the Michigan Center on Lifestage Environmental Exposures and Disease and director of the Environmental Toxicology and Epidemiology Program in Ann Arbor, Michigan..
Amy J. Schulz, Department of Health Behavior and Health Education, associate director for the Center for Research on Ethnicity, Culture and Health, and co-lead for the Community Engagement Core for the Michigan Center on Lifestage Environmental Exposures and Disease at the University of Michigan School of Public Health..
Tracey J. Woodruff, University of California, San Francisco in the Department of Obstetrics, Gynecology, and Reproductive Sciences and Philip R. Lee Institute for Health Policy Studies and the director of the Program on Reproductive Health and the Environment in San Francisco, California..
References
- American College of Obstetrics and Gynecology (ACOG). 2013a. “Hypertension in Pregnancy.” Obstetrics and Gynecology 122 (5): 1122–31. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology (ACOG). 2013b. Hypertension in Pregnancy. Washington, DC: [Online]. https://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. [Google Scholar]
- Amaral Lorena M., Cunningham Mark W., Cornelius Denise C., and Babbette LaMarca. 2015. “Preeclampsia: Long-Term Consequences for Vascular Health.” Vascular Health and Risk Management 11: 403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society (ATS). 2000. “What Constitutes an Adverse Health Effect of Air Pollution? This Official Statement of the American Thoracic Society Was Adopted by the ATS Board of Directors, July 1999.” [Online]. http://hero.epa.gov/index.cfm?action=reference.details&reference_id=11738. Accessed November 20, 2012. [Google Scholar]
- Arabin Birgit, and Baschat Ahmet A.. 2017. “Pregnancy: An Underutilized Window of Opportunity to Improve Long-Term Maternal and Infant Health-An Appeal for Continuous Family Care and Interdisciplinary Communication.” Frontiers in Pediatrics 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assibey-Mensah Vanessa, Liu Kaibo, Thurston Sally W., Stevens Timothy P., Zhang Junfeng, Zhang Jinliang, Kane Cathleen, Pan Ying, Weinberger Barry, Ohman-Strickland Pamela, Woodruff Tracey, et al. 2016. “Impact of the 2008 Beijing Olympics on the Risk of Pregnancy Complications.” Archives of Environmental & Occupational Health 71 (4): 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell Michelle L., and Dominici Francesca. 2008. “Effect Modification by Community Characteristics on the Short-Term Effects of Ozone Exposure and Mortality in 98 US Communities.” American Journal of Epidemiology 167 (8): 986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski Renee A. 2010. “Pulmonary Physiology in Pregnancy.” Clinical Obstetrics and Gynecology 53 (2): 285–300. [DOI] [PubMed] [Google Scholar]
- Bower Kelly M., Thorpe Roland J., Rohde Charles, and Gaskin Darrell J.. 2014. “The Intersection of Neighborhood Racial Segregation, Poverty, and Urbanicity and Its Impact on Food Store Availability in the United States.” Preventive Medicine 58: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochu Pierre, Bouchard Michèle, and Haddad Sami. 2014. “Physiological Daily Inhalation Rates for Health Risk Assessment in Overweight/Obese Children, Adults, and Elderly.” Risk Analysis 34 (3): 567–82. [DOI] [PubMed] [Google Scholar]
- Brook Robert D., Franklin Barry, Cascio Wayne, Hong Yuling, Howard George, Lipsett Michael, Luepker Russell, Mittleman Murray, Samet Jonathan, Smith Sidney C., and Tager Ira. 2004. “Air Pollution and Cardiovascular Disease: A Statement for Healthcare Professionals from the Expert Panel on Population and Prevention Science of the American Heart Association.” Circulation 109 (21): 2655–71. [DOI] [PubMed] [Google Scholar]
- Brulle Robert J., and Pellow David N. 2006. “Environmental Justice: Human Health and Environmental Inequalities.” Annual Review of Public Health 27 (1): 103–24. [DOI] [PubMed] [Google Scholar]
- Brummett Beverly H., Babyak MA, Siegler IC, Shanahan M, Harris KM, Elder GH, and Williams RB. 2011. “Systolic Blood Pressure, Socioeconomic Status, and Biobehavioral Risk Factors in a Nationally Representative US Young Adult Sample.” Hypertension 58 (2): 161–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra Tara A., Moineddin Rahim, Agha Mohammad M., and Glazier Richard H.. 2009. “Social Disadvantage, Air Pollution, and Asthma Physician Visits in Toronto, Canada.” Environmental Research 109 (5): 567–74. [DOI] [PubMed] [Google Scholar]
- Cakmak Sabit, Dales Robert E., Gultekin Timur, Vidal Claudio Blanco, Farnendaz Marcelo, Rubio Maria Angelico, and Oyola Pedro. 2009. “Components of Particulate Air Pollution and Emergency Department Visits in Chile.” Archives of Environmental & Occupational Health 64 (3): 148–55. [DOI] [PubMed] [Google Scholar]
- Cakmak Sabit, Hebbern Chris, Vanos Jennifer, Crouse Dan L., and Burnett Rick. 2016. “Ozone Exposure and Cardiovascular-Related Mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by Spatial Synoptic Classification Zone.” Environmental Pollution 214: 589–99. [DOI] [PubMed] [Google Scholar]
- Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J, Hankins GDV, et al. 1989. “Central Hemodynamic Assessment of Normal Term Pregnancy.” American Journal of Obstetrics and Gynecology 161 (6 Pt 1): 1439–42. [DOI] [PubMed] [Google Scholar]
- Clausson Britt, Cnattingius Sven, and Axelsson Ove. 1998. “Preterm and Term Births of Small for Gestational Age Infants: A Population-Based Study of Risk Factors Among Nulliparous Women.” BJOG: An International Journal of Obstetrics and Gynaecology 105 (9): 1011–17. [DOI] [PubMed] [Google Scholar]
- Dabass Arvind, Talbott Evelyn O., Bilonick Richard A., Rager Judith R., Venkat Arvind, Marsh Gary M., Duan Chunzhe, and Xue Tao. 2016. “Using Spatio-Temporal Modeling for Exposure Assessment in an Investigation of Fine Particulate Air Pollution and Cardiovascular Mortality.” Environmental Research 151: 564–72. [DOI] [PubMed] [Google Scholar]
- Dadvand Payam, Parker Jennifer, Bell Michelle L., Bonzini Matteo, Brauer Michael, Darrow Lyndsey A., Gehring Ulrike, Glinianaia Svetlana V., Gouveia Nelson, Ha Eun-hee, Leem Jong Han, et al. 2013. “Maternal Exposure to Particulate Air Pollution and Term Birth Weight: A Multi-Country Evaluation of Effect and Heterogeneity.” Environmental Health Perspectives 121 (3): 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzo Di, Carlo Gian, Conroy Jeanne A., Blake Jennifer, Di Francesco Mark S., DiNicola Nathaniel, Martin James M. Jr., McCue Kelly A., Richmond David, Shaw Abid, Sutton Patrice, Woodruff Tracey J., et al. 2015. “International Federation of Gynecology and Obstetrics Opinion on Reproductive Health Impacts of Exposure to Toxic Environmental Chemicals.” International Journal of Gynecology & Obstetrics 131 (3): 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolea Carmen, and Carla AbouZahr. 2003. Global Burden of Hypertensive Disorders of Pregnancy in the Year 2000. Geneva, Switzerland: WHO; www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpregnancy.pdf [Google Scholar]
- Dye Janice A., Costa Daniel L., and Kodavanti Urmila P.. 2015. “Executive Summary: Variation in Susceptibility to Ozone-Induced Health Effects in Rodent Models of Cardiometabolic Disease.” Inhalation Toxicology 27 (Suppl 1): 105–15. [DOI] [PubMed] [Google Scholar]
- Erickson Anders C., and Arbour Laura. 2014. “The Shared Pathoetiological Effects of Particulate Air Pollution and the Social Environment on Fetal-Placental Development.” Journal of Environmental and Public Health 2014: 901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson Anders C., Ostry Aleck, Chan Laurie H. M., and Arbour Laura. 2016. “The Reduction of Birth Weight by Fine Particulate Matter and Its Modification by Maternal and Neighbourhood-Level Factors: A Multilevel Analysis in British Columbia, Canada.” Environmental Health: A Global Access Science Source 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley Thomas A., Mason Karen, Rice Janet, Habel Joanna D., Scribner Richard, and Cohen Deborah A.. 2006. “The Relationship Between the Neighbourhood Environment and Adverse Birth Outcomes.” Paediatric and Perinatal Epidemiology 20 (3): 188–200. [DOI] [PubMed] [Google Scholar]
- Federal Register. 2013. “January 15, 2013 Vol. 78, No. 10”: 3086–3287. http://www.gpo.gov/fdsys/pkg/FR-2013-01-15/pdf/2012-30946.pdf. [Google Scholar]
- Friberg Mariel D., Zhai Xinxin, Holmes Heather A., Chang Howard H., Strickland Matthew J., Stefanie Ebelt Sarnat Paige E. Tolbert, Russell Armistead G., and Mulholland James A.. 2016. “Method for Fusing Observational Data and Chemical Transport Model Simulations To Estimate Spatiotempo-rally Resolved Ambient Air Pollution.” Environmental Science & Technology 50 (7): 3695–705. [DOI] [PubMed] [Google Scholar]
- Ghosh Gaurav, Grewal Jagteshwar, Mannisto Tuija, Mendola Pauline, Chen Zhen, Xie Yunlong, and Laughon S. Katherine. 2014. “Racial/ethnic Differences in Pregnancy-Related Hypertensive Disease in Nulliparous Women.” Ethnicity & Disease 24 (3): 283–89. [PMC free article] [PubMed] [Google Scholar]
- Gidding SS, Samuel S, Nehgme Rodrigo, Heise Charles, Muscar Carol, Linton Annie, and Hassink Sandra. 2004. “Severe Obesity Associated with Cardiovascular Deconditioning, High Prevalence of Cardiovascular Risk Factors, Diabetes Mellitus/hyperinsulinemia, and Respiratory Compromise.” Journal of Pediatrics 144 (6): 766–69. [DOI] [PubMed] [Google Scholar]
- Gray Simone C., Edwards Sharon E., and Miranda Marie Lynn. 2013. “Race, Socioeconomic Status, and Air Pollution Exposure in North Carolina.” Environmental Research 126: 152–58. [DOI] [PubMed] [Google Scholar]
- Hajat Anjum, Hsia Charlene, and O’Neill Marie S. 2015. “Socioeconomic Disparities and Air Pollution Exposure: A Global Review.” Current Environmental Health Reports 2 (4): 440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton Brady E., Martin Joyce A., and Osterman Michelle J. K. S.. 2015. “Births: Preliminary Data for 2015.” National Vital Statistics Reports 65 (3): 1–15. [PubMed] [Google Scholar]
- Hegewald Matthew J., and Crapo Robert O.. 2011. “Respiratory Physiology in Pregnancy.” Clinics in Chest Medicine 32 (1): 1–13. [DOI] [PubMed] [Google Scholar]
- Hu Hui, Ha Sandie, Roth Jeffrey, Kearney Greg, Talbott Evelyn O., and Xu Xiaohui. 2014. Ambient Air Pollution and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis.” Atmospheric Environment 97: 336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Hui, Ha Sandie, Henderson Barron H., Warner Tamara D., Roth Jeffrey, Kan Haidong, and Xu Xiaohui. 2015. “Association of Atmospheric Particulate Matter and Ozone with Gestational Diabetes Mellitus.” Environmental Health Perspectives 123 (9): 853–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. 1999. Toward Environmental Justice. Washington, D.C.: National Academies Press; [Online]. http://www.nap.edu/catalog/6034. Accessed November 3, 2016. [Google Scholar]
- Jauniaux Eric, Poston Lucilla, and Burton Graham J.. 2006. “Placental-Related Diseases of Pregnancy: Involvement of Oxidative Stress and Implications in Human Evolution.” Human Reproduction Update 12 (6): 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan Srimathi, Dvonch Timothy J., Schulz Amy J., Israel Barbara A., Mentz Graciela, House James, Max Paul, and Reyes Angela G.. 2010. “Exposure to Fine Particulate Matter and Acute Effects on Blood Pressure: Effect Modification by Measures of Obesity and Location.” Journal of Epidemiology and Community Health 64 (1): 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan Srimathi, Misra Dawn P., Dvonch J. Timothy, and Krishnakumar Ambika. 2006. “Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition.” Environmental Health Perspectives 114 (11): 1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koman Patricia Diane, and Mancuso Peter. 2017. “Ozone Exposure, Cardiopulmonary Health, and Obesity: A Substantive Review.” Chemical Research in Toxicology 30 (7): 1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski Daniel, Jerrett Michael, Burnett Richard T., Ma Renjun, Hughes Edward, Shi Yuanli, Turner Michelle C., Pope C. Arden III, Thurston George, Calle Eugenia E., Thun Michael J., et al. 2009. “Extended Follow-up and Spatial Analysis of the American Cancer Society Study Linking Particulate Air Pollution and Mortality.” Research Report (Health Effects Institute) (140): 5–114. [PubMed] [Google Scholar]
- Lee Pei-Chen, Talbott Evelyn O., Roberts James M., Catov Janet M., Bilonick Richard A., Stone Roslyn A., Sharma Ravi K., and Ritz Beate. 2012. “Ambient Air Pollution Exposure and Blood Pressure Changes During Pregnancy.” Environmental Research 117: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Pei-Chen, Roberts James M., Catov Janet M., Talbott Evelyn O., and Ritz Beate. 2013. “First Trimester Exposure to Ambient Air Pollution, Pregnancy Complications and Adverse Birth Outcomes in Allegheny County, PA.” Maternal and Child Health Journal 17 (3): 545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Ching-Kai, and Lin Ching-Chi. 2012. “Work of Breathing and Respiratory Drive in Obesity.” Respirology 17 (3): 402–11. [DOI] [PubMed] [Google Scholar]
- Lin Shao, Liu Xiu, Le Linh H., and Hwang Syni-an. 2013. “Children‘S Health Chronic Exposure Among Children to Ambient Ozone and Asthma Hospital Admissions.” Environmental Health Perspectives 116 (12): 1725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke Jacob A., Jens Langhoff-Roos Charles J. Lockwood, Triche Elizabeth W., and Paidas Michael J.. 2010. “Mortality of Mothers from Cardiovascular and Non-Cardiovascular Causes Following Pregnancy Complications in First Delivery.” Paediatric and Perinatal Epidemiology 24 (4): 323–30. [DOI] [PubMed] [Google Scholar]
- Malmqvist Ebba, Jakobsson Kristina, Tinnerberg Håkan, Rignell-Hydbom Anna, and Rylander Lars. 2013. “Gestational Diabetes and Preeclampsia in Association with Air Pollution at Levels Below Current Air Quality Guidelines.” Environmental Health Perspectives 121 (4): 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männistö Tuija, Mendola Pauline, Liu Danping, Leishear Kira, Sherman Seth, and Laughon S. Katherine. 2015. “Acute Air Pollution Exposure and Blood Pressure at Delivery Among Women with and Without Hypertension.” American Journal of Hypertension 28 (1): 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May Linda. 2015. “Cardiac Physiology of Pregnancy” In Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, Inc., 1325–44. [DOI] [PubMed] [Google Scholar]
- MDOCH. 2007. Heart Disease Deaths and Death Rates Three Year Moving Average 2005–2007. Lansing, MI: http://www.mdch.state.mi.us/pha/osr/CHI/CRI/frame.asp. [Google Scholar]
- Mendola Pauline, Wallace Maeve, Liu Danping, Robledo Candace, Männistö Tuija, and Grantz Katherine L.. 2016. “Air Pollution Exposure and Preeclampsia Among US Women with and Without Asthma.” Environmental Research 148: 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke Andy, Casagrande Sarah, Geiss Linda, and Cowie Catherine C.. 2015. “Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012.” JAMA 314 (10): 1021–29. [DOI] [PubMed] [Google Scholar]
- Mielke Michelle M., Milic Natasa M., Weissgerber Tracey L., White Wendy M., Kantarci Kejal, Mosley Thomas H., Windham B. Gwen, Simpson Brittany N., Turner Stephen T., and Garovic Vesna D.. 2016. “Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders.” Circulation. Cardiovascular Quality and Outcomes 9 (2 Suppl 1): S70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Kristin A., Siscovick David S., Sheppard Lianne, Shepherd Kristen, Sullivan Jeffrey H., Anderson Garnet L., and Kaufman Joel. 2007. “Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women.” New England Journal of Medicine 356 (5): 447–58. [DOI] [PubMed] [Google Scholar]
- Miranda Marie Lynn, Edwards Sharon E., Chang Howard H., and Auten Richard L.. 2013. “Proximity to Roadways and Pregnancy Outcomes.” Journal of Exposure Science and Environmental Epidemiology 23 (1): 32–38. [DOI] [PubMed] [Google Scholar]
- Mobasher Zahra, Salam Muhammad T., Goodwin T. Murphy, Lurmann Frederick, Ingles Sue A., and Wilson Melissa L.. 2013. “Associations Between Ambient Air Pollution and Hypertensive Disorders of Pregnancy.” Environmental Research 123: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch Rachel, Miriam Zuk, Michael Jerrett, Bhavna Shamasunder, and Amy D. Kyle. 2011. “Understanding the Cumulative Impacts of Inequalities in Environmental Health: Implications for Policy.” Health Affairs (Project Hope) 30 (5): 879–87. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch Rachel, Jesdale Bill M., Sadd James L., and Pastor Manuel. 2010. “Ambient Air Pollution Exposure and Full-Term Birth Weight in California.” Environmental Health 9 (1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academies Press; [Online]. http://www.nap.edu/catalog/2125. Accessed November 17, 2016. [PubMed] [Google Scholar]
- National Research Council. 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- NEJAC. 2004. Ensuring Risk Reduction in Communities with Multiple Stressors: Environmental Justice and Cumulative Risks. Washington, DC: https://www.epa.gov/sites/production/files/2015-04/documents/ensuringriskreducationnejac.pdf [Google Scholar]
- Nelander M, Cnattingius S, Åkerud H, Wikstrom J, Pedersen NL, and Wikstrom A-K. 2016. “Pregnancy Hypertensive Disease and Risk of Dementia and Cardiovascular Disease in Women Aged 65 Years or Older: A Cohort Study.” BMJ Open 6 (1): e009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill Marie S., Breton Carrie V., Devlin Robert B., and Utell Mark J.. 2012. “Air Pollution and Health: Emerging Information on Susceptible Populations.” Air Quality, Atmosphere & Health 5 (2): 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson David, Mogren Ingrid, and Forsberg Bertil. 2013. “Air Pollution Exposure in Early Pregnancy and Adverse Pregnancy Outcomes: A Register-Based Cohort Study.” BMJ Open 3 (2): e001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn Gilbert S. 1997. Framework for Environmental Health Risk Management: Final Report by the Presidential/Congressional Commission on Risk Assessment and Risk Management. Washington, DC: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=55006. [Google Scholar]
- Ota Erika, Ganchimeg Togoobaatar, Morisaki Naho, Vogel Joshua P., Pileggi Cynthia, Eduardo Ortiz-Panozo João P. Souza, Mori Rintaro, on behalf of the WHO Multi-Country Survey on Maternal and Newborn Health Research Network. 2014. “Risk Factors and Adverse Perinatal Outcomes Among Term and Preterm Infants Born Small-for-Gestational-Age: Secondary Analyses of the WHO Multi-Country Survey on Maternal and Newborn Health.” PLoS ONE 9 (8): e105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker Jennifer D., Rich David Q., Glinianaia Svetlana V., Jong Han Leem Daniel Wartenberg, Bell Michelle L., Bonzini Matteo, Brauer Michael, Darrow Lyndsey, Gehring Ulrike, Gouveia Nelson, et al. 2011. “The International Collaboration on Air Pollution and Pregnancy Outcomes: Initial Results.” Environmental Health Perspectives 119 (7): 1023–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne-Sturges Devon, Gee Gilbert C., Crowder Kirstin, Hurley Bradford J., Lee Charles, Rachel Morello-Frosch Arlene Rosenbaum, Schulz Amy, Wells Charles, Woodruff Tracey, and Zenick Hal. 2006. “Workshop Summary: Connecting Social and Environmental Factors to Measure and Track Environmental Health Disparities.” Environmental Research 102 (2): 146–53. [DOI] [PubMed] [Google Scholar]
- Payne-Sturgis Devon, and Martin Lawrence. 2014. The Cumulative Risk Assessment Webinar Series: What We Learned from Cumulative Risk Technical Panel. Washington, DC: U.S. EPA. [Google Scholar]
- Pedersen M, Leslie Stayner R Slama emy, Sørensen Mette, Figueras Francesc, Nieuwenhuijsen Mark J., Raaschou-Nielsen Ole, and Dadvand Payam. 2014. “Ambient Air Pollution and Pregnancy-Induced Hypertensive Disorders: A Systematic Review and Meta-Analysis.” Hypertension 64 (3): 494–500. [DOI] [PubMed] [Google Scholar]
- Pereira Gavin, Haggar Fatima, Shand Antonia W., Bower Carol, Cook Angus, and Nassar Natasha. 2013. “Association Between Pre-Eclampsia and Locally Derived Traffic-Related Air Pollution: A Retrospective Cohort Study.” Journal of Epidemiology and Community Health 67 (2): 147–52. [DOI] [PubMed] [Google Scholar]
- Ponce NA, Hoggatt Katherine J., Wilhelm Michelle, and Ritz Beate. 2005. “Preterm Birth: The Interaction of Traffic-Related Air Pollution with Economic Hardship in Los Angeles Neighborhoods.” American Journal of Epidemiology 162 (2): 140–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. Arden III, Burnett Richard T., and Thurston George D.. 2004. “Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution.” Circulation 109 (1): 71–77. [DOI] [PubMed] [Google Scholar]
- Ramanathan Gajalakshmi, Yin Fen, Speck Mary, Tseng Chi-hong, Brook Jeffrey R., Silverman Frances, Urch Bruce, Brook Robert D., and Araujo Jesus A.. 2015. “Effects of Urban Fine Particulate Matter and Ozone on HDL Functionality.” Particle and Fibre Toxicology 13 (1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees Gareth B., Fiona Broughton Pipkin Edwin M. Symonds, and Patrick John M.. 1990. “A Longitudinal Study of Respiratory Changes in Normal Human Pregnancy with Cross-Sectional Data on Subjects with Pregnancy-Induced Hypertension.” American Journal of Obstetrics and Gynecology 162 (3): 826–30. [DOI] [PubMed] [Google Scholar]
- Rudra Carole B., Williams Michelle A., Sheppard Lianne, Koenig Jane Q., and Schiff Melissa A.. 2011. “Ambient Carbon Monoxide and Fine Particulate Matter in Relation to Preeclampsia and Preterm Delivery in Western Washington State.” Environmental Health Perspectives 119 (6): 886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks Jason D., Lindsay Wichers Stanek Thomas J. Luben, Johns Douglas O., Buckley Barbara J., Brown James S., and Ross Mary. 2011. “Particulate Matter-Induced Health Effects: Who Is Susceptible?” Environmental Health Perspectives 119 (4): 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome Cheryl M., King Gregory G., and Berend Norbert. 2010. “Physiology of Obesity and Effects on Lung Function.” Journal of Applied Physiology 108 (1): 206–11. [DOI] [PubMed] [Google Scholar]
- Savitz David A., Elston Beth, Bobb Jennifer F., Clougherty Jane E., Dominici Francesca, Ito Kazuhiko, Johnson Sarah, McAlexander Tara, Ross Zev, Shmool Jessie L. C., Matte Thomas D., et al. 2015. “Ambient Fine Particulate Matter, Nitrogen Dioxide, and Hypertensive Disorders of Pregnancy in New York City.” Epidemiology 26 (5): 748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz Amy J., Mentz Graciela B., Sampson Natalie, Ward Melanie, Anderson Rhonda, Ricardo deMajo Barbara A. Israel, Lewis Toby C., and Wilkins Donele. 2016. “Race and the Distribution of Social and Physical Environmental Risk: A Case Example from the Detroit Metropolitan Area.” Du Bois Review 13 (2): 285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Joel, Bellinger David, and Glass Thomas. 2011. ”Expanding the Scope of Environmental Risk Assessment to Better Include Differential Vulnerability and Susceptibility.” American Journal of Public Health 101 (Suppl): S88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Prakesh S., and Balkhair Taiba. 2011. “Air Pollution and Birth Outcomes: A Systematic Review.” Environment International 37 (2): 498–516. [DOI] [PubMed] [Google Scholar]
- Sibai Baha, Dekker Gus, and Kupferminc Michael. 2005. “Pre-Eclampsia.” The Lancet 365 (9461): 785–99. [DOI] [PubMed] [Google Scholar]
- Silva Lindsay M., Coolman Marianne, Steegers Eric A. P., Jaddoe Vincent W. V., Moll Henriëtte A., Hofman Albert, Mackenbach Johan P., and Raat Hein. 2008. “Low Socioeconomic Status Is a Risk Factor for Preeclampsia: The Generation R Study.” Journal of Hypertension 26 (6): 1200–08. [DOI] [PubMed] [Google Scholar]
- Solar Orielle, and Irwin Alec. 2010. A Conceptual Framework for Action on the Social Determinants of Health. World Health Organization. [Online]. http://www.who.int/social_determinants/publications/9789241500852/en/. Accessed November 6, 2016. [Google Scholar]
- Solomon Gina M., Rachel Morello-Frosch Lauren Zeise, and Faust John B.. 2016. “Cumulative Environmental Impacts: Science and Policy to Protect Communities.” Annual Review of Public Health 37: 83–96. [DOI] [PubMed] [Google Scholar]
- Soma-Pillay Priya, Catherine N, Tolppanen Heli, and Mebazaa Alexandre. 2016. “Physiological Changes in Pregnancy.” Cardiovascular Journal of Africa 27 (2): 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srám Radim J., Blanka Binková Jan Dejmek, and Bobak Martin. 2005. “Ambient Air Pollution and Pregnancy Outcomes: A Review of the Literature.” Environmental Health Perspectives 113 (4): 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers Eric A. P., Peter von Dadelszen Johannes J. Duvekot, and Pijnenborg Robert. 2010. “Pre-Eclampsia.” The Lancet 376 (9741): 631–44. [DOI] [PubMed] [Google Scholar]
- Stieb David M., Chen Li, Eshoul Maysoon, and Judek Stan. 2012. “Ambient Air Pollution, Birth Weight and Preterm Birth: A Systematic Review and Meta-Analysis.” Environmental Research 117: 100–11. [DOI] [PubMed] [Google Scholar]
- Su Jason G., Jerrett Michael, Rachel Morello-Frosch Bill M. Jesdale, and Kyle Amy D.. 2012. “Inequalities in Cumulative Environmental Burdens Among Three Urbanized Counties in California.” Environment International 40: 79–87. [DOI] [PubMed] [Google Scholar]
- Tan Eng Kien, and Tan Eng Loy. 2013. “Alterations in Physiology and Anatomy During Pregnancy.” Best Practice & Research. Clinical Obstetrics & Gynaecology 27 (6): 791–802. [DOI] [PubMed] [Google Scholar]
- Tanaka Masako, Jaamaa Gundegmaa, Kaiser Michelle, Hills Elaine, Soim Aida, Zhu Motao, Shcherbatykh Ivan Y., Samelson Renee, Bell Erin, Zdeb Michael, and McNutt Louise-Anne. 2007. “Racial Disparity in Hypertensive Disorders of Pregnancy in New York State: A 10-Year Longitudinal Population-Based Study.” American Journal of Public Health 97 (1): 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen Lauren H., Fraser Alison, Hollingshaus Michael S., Schliep Karen C., Varner Michael W., Smith Ken R., and Esplin M. Sean. 2016. “All-Cause and Cause-Specific Mortality After Hypertensive Disease of Pregnancy.” Obstetrics and Gynecology 128 (2): 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston George D., Kipen Howard, Annesi-Maesano Isabella, Balmes John, Brook Robert D., Cromar Kevin, De Matteis Sara, et al. 2017. “A Joint ERS/ATS Policy Statement: What Constitutes an Adverse Health Effect of Air Pollution? An Analytical Framework.” European Respiratory Journal 49 10.1183/13993003.00419-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 2009. Integrated Science Assessment for Particulate Matter. Research Triangle Park, NC: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 2011. Policy Assessment for the Review of the Particulate Matter National Ambient Air Quality Standards. Research Triangle Park, NC: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 2012. Integrated Science Assessment of Ozone and Related Photochemical Oxidants (EPA 600/R-10/076F). Research Triangle Park, NC: [Online]. http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=242490. Accessed November 6, 2012. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 2013. Letter from Administrator Lisa Jackson to Dr. Chris Frey, CASAC Chair. Washington, DC: Author. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 2015. Regulatory Impact Assessment of Final Revisions to the National Ambient Air Quality Standards for Ground Level Ozone EPA-452/R-15-007. Research Triangle Park, NC: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. [Google Scholar]
- Valcke Mathieu, Nong Andy, and Krishnan Kannan. 2012. “Modeling the Human Kinetic Adjustment Factor for Inhaled Volatile Organic Chemicals: Whole Population Approach Versus Distinct Subpopulation Approach.” Journal of Toxicology 2012: 404329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven Edith H., Pierik Frank H., de Kluizenaar Yvonne, Hofman Albert, van Ratingen Sjoerd W., Zandveld Peter Y. J., Russcher Henk, Lindemans Jan, Miedema Henk M. E, Steegers Eric A. P., and Jaddoe Vincent W. V.. 2012. “Air Pollution Exposure and Markers of Placental Growth and Function: The Generation R Study.” Environmental Health Perspectives 120 (12): 1753–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen Hanna M., Rachel Morello-Frosch Saunak Sen, Zeise Lauren, and Woodruff Tracey J.. 2017. “Cumulative Effects of Prenatal-Exposure to Exogenous Chemicals and Psychosocial Stress on Fetal Growth: Systematic-Review of the Human and Animal Evidence” ed. Meliker Jaymie. PLOS ONE 12 (7): e0176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeh Mohsen, Yunesian Masoud, Shariat Mamak, Niroomanesh Shireen, and Ramezanzadeh Fateme. 2011. “Environmental Carbon Monoxide Related to Pregnancy Hypertension.” Women & Health 51 (8): 724–38. [DOI] [PubMed] [Google Scholar]
- Vinikoor-Imler Lisa C., Owens Elizabeth O., Nichols Jennifer L., Ross Mary, Brown James S., and Sacks Jason D.. 2014. “Evaluating Potential Response-Modifying Factors for Associations Between Ozone and Health Outcomes: A Weight-of-Evidence Approach.” Environmental Health Perspectives 122 (11): 1166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor-Imler Lisa C., Gray Simone C., Edwards Sharon E., and Miranda Marie Lynn. 2012. “The Effects of Exposure to Particulate Matter and Neighbourhood Deprivation on Gestational Hypertension.” Paediatric and Perinatal Epidemiology 26 (2): 91–100. [DOI] [PubMed] [Google Scholar]
- Wang I-Kuan, Muo Chih-Hsin, Chang Yi-Chih, Liang Chih-Chia, Chang Chiz-Tzung, Lin Shih-Yi, Yen Tzung-Hai, Chuang Feng-Rong, Chen Pei-Chun, Huang Chiu-Ching, Wen Chi-Pang, et al. 2013. “Association Between Hypertensive Disorders During Pregnancy and End-Stage Renal Disease: A Population-Based Study.” CMAJ: Canadian Medical Association journal journal de l’Association medicale canadienne 185(3): 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilevich E 2008. Detroit—The Epicenter of Asthma Burden, in Epidemiology of Asthma in Michigan,. Lansing, MI: Michigan Department of Community Health. [Google Scholar]
- Williams David. 2003. “Pregnancy: A Stress Test for Life.” Current Opinion in Obstetrics & Gynecology 15 (6): 465–71. [DOI] [PubMed] [Google Scholar]
- Woodruff Tracey J., Carlson Alison, Schwartz Jackie M., and Giudice Linda C.. 2008. “Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: Executive Summary.” Fertility and Sterility 89 (2): e1–20. [DOI] [PubMed] [Google Scholar]
- Woodruff Tracey J., Darrow Lyndsey A., and Parker Jennifer. 2008. “Air Pollution and Postneonatal Infant Mortality in the United States, 1999–2002.” Environmental Health 116 (1): 110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff Tracey J., Parker Jennifer D., Kyle Amy D., and Schoendorf Kenneth C.. 2003. “Disparities in Exposure to Air Pollution During Pregnancy.” Environmental Health Perspectives 111 (7): 942–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Jun, Ren Cizao, Delfino Ralph J., Chung Judith, Wilhelm Michelle, and Ritz Beate. 2009. “Association Between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California.” Environmental Health Perspectives 117 (11): 1773–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Jun, Wilhelm Michelle, Chung Judith, and Ritz Beate. 2011. “Comparing Exposure Assessment Methods for Traffic-Related Air Pollution in an Adverse Pregnancy Outcome Study.” Environmental Research 111 (5): 685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Xiaohui, Hu Hui, Ha Sandie, and Roth Jeffrey. 2014. “Ambient Air Pollution and Hypertensive Disorder of Pregnancy.” Journal of Epidemiology and Community Health 68 (1): 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji Takashi, Naruse Hiroo, Kashima Saori, Takao Soshi, Murakoshi Takeshi, Doi Hiroyuki, and Kawachi Ichiro. 2013. “Residential Proximity to Major Roads and Adverse Birth Outcomes: A Hospital-Based Study.” Environmental Health 12 (1): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti Antonella, and Schwartz Joel. 2005. “The Effect of Particulate Air Pollution on Emergency Admissions for Myocardial Infarction: A Multicity Case-Crossover Analysis.” Environmental Health Perspectives 113 (8): 978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Desheng, Guo Yanfang, Smith Graeme, Krewski Daniel, Walker Mark, and Wu Wen Shi. 2012. “Maternal Exposure to Moderate Ambient Carbon Monoxide Is Associated with Decreased Risk of Preeclampsia.” American Journal of Obstetrics and Gynecology 207 (1): 57.e1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.