Abstract
Background
Endothelial dysfunction plays a central part in the pathogenesis of coronary atherosclerosis. The adipokine resistin is one of the key players in endothelial cell dysfunction. In addition, the role of epicardial fat in coronary artery endothelial dysfunction is also emphasized.
We investigated whether vasodilator-stimulated phosphoprotein (VASP) is involved in resistin-related endothelial dysfunction and the phenotype conversion of epicardial adipocytes.
Material/Methods
Cell proliferation and migration were evaluated by MTT and Transwell chamber assay, respectively. Next, we took epicardial fat samples from patients with valvular heart disease and non-coronary artery disease. Gene expression was determined by reverse transcription-quantitative polymerase chain reaction and relative abundance of the protein by Western blotting.
Results
Resistin induced endothelial proliferation and migration in a dose-dependent manner. Both resistin-induced cell proliferation and migration were effectively blocked by ablation of VASP. The brown adipose tissue-specific genes for uncoupling protein 1 (UCP-1) and PR-domain-missing16 (PRDM16) decreased, but the white adipose tissue-specific genes for resistin and RIP140 increased in VASP-deficient adipocytes compared with the LV-sicntr group. However, disruption of the Ras homolog gene family member A (RhoA) /Rho-associated kinase (ROCK) in VASP-deficient adipocytes with specific inhibitors inverted the adipocyte phenotype existing in VASP-deficient adipocytes. Furthermore, the expressions of proinflammatory cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and monocyte chemoattractantprotein-1 (MCP-1) in VASP-deficient adipocytes were markedly upregulated compared with the LV-sicntr group.
Conclusions
These results suggest a physiological role for VASP in coronary atherosclerosis through regulating adipokine resistin and phenotype conversion of epicardial adipose tissue.
MeSH Keywords: Adipocytes, Brown; Adipocytes, White; Cell Migration; Cell Proliferation; Coronary Artery Disease
Background
Coronary atherosclerosis remains the major cause of cardiovascular disease, despite major advances in diagnosis and treatment (mostly preventive) of known risk factors, which indicates the need to further explore the pathogenesis of atherosclerosis [1]. The pivotal role of angiogenesis in atherosclerotic lesions is firmly established [2]. There is no neovascularization in the intima and media of normal arteries, while neovascularization is seen in the intima of human atherosclerotic lesions. The pathological angiogenesis is characterized by persistent proliferation and faster migration of endothelial cells [3], leading to progression of plaque, plaque instability, intraplaque hemorrhage, and subsequently increased risk of athero-thrombotic events.
Epicardial adipose tissue (EAT), given its close anatomical affinity with the coronary, drew our attention. Many studies have demonstrated that EAT exhibits more characteristics of brown adipose tissue (BAT) than white adipose tissue (WAT) in non-coronary atherosclerotic heart disease [4]. BAT mainly produces heat and consumes energy, which is helpful for the maintenance of energy and metabolism balances. However, WAT can secret a variety of adipokines (such as resistin and leptin) and proinflammatory cytokines (such as IL-6 and IL-8) to facilitate endothelial dysfunction, resulting in progression of coronary atherosclerosis [5]. Resistin, a kind of adipokine or inflammatory cytokine, can accelerate atherosclerotic plaque progression through stimulating endothelial cells. In our previous studies, we demonstrated that during the progression of atherosclerosis, there is a phenotype conversion of EAT from BAT to WAT, which further promotes the focal occurrence and development of atherosclerosis [6]. However, the mechanism of adipokines resistin and phenotype conversion of epicardial adipocytes are unclear.
Vasodilatory-stimulated phosphoprotein (VASP) is an actin-binding protein that plays a crucial role in cell adhesion, proliferation, and migration by regulating F-actin cytoskeleton dynamics [7]. Recently, a body of evidence suggested that resistin can induce human endothelial cell proliferation and migration [8], thereby suggesting a potential for increased angiogenesis [9]. Therefore, we hypothesized that resistin potentiates angiogenesis via VASP signaling molecule in the human coronary endothelial cell line (HCAECs). VASP functions are regulated by phosphorylations, although the specific roles of phosphorylations on functional outcomes are unclear [10]. VASP phosphorylation at serine 239 is not only a marker of cyclic guanosine 3′,5′-monophosphate (cGMP)-dependent protein kinase (PKG) activation, but is also a mediator of relevant biological actions exerted by the nitric oxide (NO)/cGMP/PKG pathway [11]. Protein kinase G (PKG) is essential for brown fat cell differentiation and mitochondrial biogenesis [12]. VASP has recently emerged as an important regulator of differentiation of adipocytes and energy homeostasis [13]. Despite these early studies, the role of VASP in adipocytes is only emerging now, and the mechanism of phenotype conversion of human epicardial adipocytes has not been identified. The aim of our study was to determine the role of VASP I n function of resistin in HCAECs and phenotype conversion of epicardial adipose tissue. Our findings may provide evidence for the pathogenesis of coronary atherosclerosis and present new clues for the therapy of coronary atherosclerosis.
Material and Methods
Fat sample
Epicardial fat samples were taken from middle-aged patients (49.32±7.36 y) with valvular heart disease and non-coronary artery disease during heart valve replacement, and most were taken from near the origin of the right coronary artery. Informed consent was obtained from all patients and the study was approved by the Ethics Committee of Nanjing Jinling Hospital.
Cell isolation and adipogenic differentiation
Cell isolation of brown preadipocytes was performed as previously described. In brief, the adipose tissues were digested for 1 h in collagenase buffer [DMEM/F12 containing 0.1% collagenase type I]. After standing for 20 min on ice, cells were centrifuged and washed. The pellet was resuspended in DMEM/ F12 supplemented with 10% FBS and 1% P/S, then cells were seeded in 6 wells and grown at 37°C and 5% CO2. The morphology of primary preadipocytes from human EAT were round and had difference sizes. After 7–8 days, cells became oval monolayer-like. When they achieved 80–90% confluence, cells could be amplified by passage culture, but only within the cells of 4 generations.
These brown preadipocyte cells were differentiated by induced liquid of adipose tissue-derived mesenchymal stem cells following the manufacturer’s instructions. In brief, when brown preadipocyte cells reached confluence, the medium was refreshed with human adipose-derived stem cell adipogenic differentiation medium A, which mainly consists of glutamine, insulin, IBMX, rosiglitazone, and dexamethasone. After 3 days of induction, the medium was replaced with human adipose-derived stem cell adipogenic differentiation medium B for 1 days, which mainly includes glutamine and insulin. At 3–4 days, cells shrank and became round. After 6–8 days, lipid droplets were present around the nuclei and began to increase and enlarge. Then, the previous steps were repeated until 12–20 days. At 12–20 days, lipid droplets with different sizes were present in 80% of cells. After induction, cells were treated only with human adipose-derived stem cell adipogenic differentiation medium B for 4–7 days until lipid drops became big enough to be used in subsequent adipocyte-related experiments.
Oil red O staining
When the adipocytes became mature, the medium was removed, and cells were fixed in 10% formalin at room temperature for 1 h. Then, cells were stained with oil red solution for 30 min following manufacturer’s instructions. After washing, cells were observed under a light microscope. Oil red O staining showed red granules, which were found as lipid droplets.
Lentivectors construction and lentivirus production
The 2 pairs of shRNA targeting human VASP and a negative control shRNA listed in Table 1 were cloned into pCDH-U6-MCS-EF1-GreenPuro (SBI, Mountain View, CA, USA) to generate the pCDH-U6-VASP-sh1, pCDH-U6-VASP-sh2, and pCDH-U6-VASP-shN lentivectors, respectively. HEK-293T cells were transfected with the constructs along with 3 other plasmids (pGag/Pol, pRev, and pVSVG) into HEK293T cells using TurboFect (Thermo Scientific) according to the manufacturer’s protocol. The mature adipocytes or HCAECs were seeded in a 6-well plate. After 24 h, 50% of the medium in each well was removed and polybrene was added to a final concentration of 8 g/ml. The shRNA lentiviruses (MOI=1) were added to the cells. After an overnight incubation at 37°C, the medium was replaced with fresh medium and incubated for another 72 h. Then, cells were used to evaluate the expression of VASP or for further experiments.
Table 1.
Short hairpin RNA (shRNA) inserts.
| pCDH-U6-VASP-sh1S | GATCCGGGACTCATGGAAGAGATGAACAAGAGTTCATCTCTTCCATGAGTCCCTTTTTG |
| pCDH-U6-VASP-sh1A | AATTCAAAAAGGGACTCATGGAAGAGATGAACTCTTGTTCATCTCTTCCATGAGTCCCG |
| pCDH-U6-VASP-sh2S | GATCCGGACCTACAGAGGGTGAAACACAAGAGTGTTTCACCCTCTGTAGGTCCTTTTTG |
| pCDH-U6-VASP-sh2A | AATTCAAAAAGGACCTACAGAGGGTGAAACACTCTTGTGTTTCACCCTCTGTAGGTCCG |
| pCDH-U6-VASP-shNS | GATCCGATGAAATGGATAGAAGTACACAAGAGTGTACTTCTATCCATTTCATCTTTTTG |
| pCDH-U6-VASP-shNA | AATTCAAAAAGATGAAATGGATAGAAGTACACTCTTGTGTACTTCTATCCATTTCATCG |
Actin cytoskeletal staining
Actin cytoskeletal staining was conducted in the control group (LV-sicntr) and VASP-deficient group (LV-siVASP). Cells were cultured on coverslips in a 6-well plate. Cells were fixed with 4% paraformaldehyde for 30 min at room temperature, washed, and permeabilized with 0.5% Triton X-100 for 5 min. The cells were incubated with Actin Red at room temperature for 20 min. ProLong Gold Antifade reagent and DAPI (Invitrogen, Carlsbad, CA) were used to mount the coverslips to slides.
IL-6 or Y27632 2HCl treatment
The mature adipocytes with different conditions were treated with IL-6 at100 ng/ml in DMEM/F12 for 24 h at 37°C in an environment with 5% CO2. Total RNA was extracted, and RT-PCR was employed to detect the mRNA expressions of UCP-1, PRDM16, resistin, and RIP140. Y27632 2HCl, a selective ROCK1 inhibitor, was dissolved in DMSO to a concentration of 30 mM for conservation. Adipocytes were treated with 30 uM for 24 h.
Cell proliferation
The cell proliferation was measured by MTT assay (KeyGEN BioTECH). Serum-starved cells were seeded onto a 96-well plate at 10 000 cells/well. After 24 h, cells were serum-starved for 16 h and treated with various doses of resistin (0 ng/ml, 10 ng/ml, 50 ng/ml, and 100 ng/ml) for an additional 48 h. Then, MTT was added to each well, incubated for 4 h, and dissolved by DMSO at 37°C in a shaking bed. Last, the light density was measured at 490 wavelength using an enzyme meter.
Cell migration
Cell migration was measured using a Transwell chamber assay. Serum-free cell suspension (200 ul, 2×104 cells/well) containing various doses of resistin was added to the Transwell insert (8-um pore size, Corning, USA), and 10 ng PDGF-β (Sigma, USA) were applied to the bottom chamber as a chemoattractant (600 ul). After 12-h incubation, the chambers were fixed in 2% paraformaldehyde for 5 min and washed in PBS. The non-migrating cells in the upper chamber were wiped off using swabs and washed with PBS. Then, the numbers of cells with various morphologies were counted under a microscope.
RT-PCR
Total RNA was extracted from adipose cells using TRIZOL reagent (Invitrogen) according to the manufacturer’s protocol and reverse-transcribed into complementary DNA (cDNA). cDNA was synthesized using the PrimescriptTM RT reagent kit (Takara Bio, Dalian, China) according to the manufacturer’s instructions. cDNA served as a template for RT-PCR, and primers used in RT-PCR are shown in Table 2. RT-PCR was performed in 20 μl of the mixture and the amplification conditions were 95°C for 10 min, followed by 40 cycles at 95°C for 10 s, 58°C for 15 s, and 72°C for 15 s. β-actin served as a loading control.
Table 2.
Primers used in this study.
| Target gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | CCCGAGCCGTGTTTCCT | GTCCCAGTTGGTGACGATGC |
| UCP-1 | TTGGTGTCGGCTCTTATC | CCGTTGGTCCTTCGTT |
| PRDM16 | AGGTGCCTGTCTTCTATTCCC | GCTTCTTCTCCTGCATCCC |
| RIP140 | CTGCCACCTCACCTAA | TAACTGCCAACATCCTT |
| Resistin | GCAAGACCCTGTGCTCCA | CACTCCAGGCCAATGCTG |
| VASP | GACCACTTCCGAGACCCAACC | GAAGGCTTCAATGATTTCCTCT |
| IL-6 | AAGCAGCAAAGAGGCA | TCAAATCTGTTCTGGAGGT |
| MCP-1 | AGAATCACCAGCAGCAAG | GGAATCCTGAACCCACTT |
| IL-8 | AAACCTTTCCACCCC | ACTTCTCCACAACCCTC |
| TNF-a | CCACCACGCTCTTCTGC | GCTTGAGGGTTTGCTACAAC |
Western blot analysis
The treated cells were lysed in ice-cold cell lysis buffer containing a protease inhibitor cocktail (Sigma). The protein was separated on a 10% SDS-polyacrylamide gel. The separated proteins were then transferred onto a PVDF membrane (Millipore, MA, USA) and blocked with 5% non-fat dry milk prepared in 1×TBS. The membranes were incubated with the primary antibodies overnight at 4°C. The following antibodies were used: antibodies against UCP-1, PRDM16, and VASP (all from Abcam, Cambridge UK). After 3 washes with TBST, membranes were incubated with the appropriate HRP-conjugated secondary antibodies (Abcam, Cambridge, UK) for 2 h at room temperature, followed by detection with enhanced chemiluminescence (Millipore). The relative band intensity was measured using Image J software.
Statistical analysis
SPSS version 19.0 was used for statistical analysis. Data are expressed as mean ± standard deviation. One-way analysis of variance was used for comparisons among groups and the t test was used for comparisons between groups. A value of P<0.05 was considered statistically significant.
Results
Gene and protein expression after VASP silence
To test whether VASP is involved in the regulation of HCAECs proliferation and migration or phenotype conversion of epicardial adipocytes, knockdown of VASP by siRNA blocked its expression. Then, RT-PCR analysis and Western blot analysis with VASP-specific antibodies were used to assess expression of VASP. The data revealed that VASP content significantly decreased in LV-siVASP HCAECs compared with the LV-sicntr group (Figure 1).
Figure 1.
Knockdown of VASP by siRNA. Both gene and protein expressions were obviously lower after knockdown of VASP by siRNA compared with LV-sicntr group in HCAECs. * p<0.05.
Loss of VASP inhibited the effect of resistin on cell proliferation
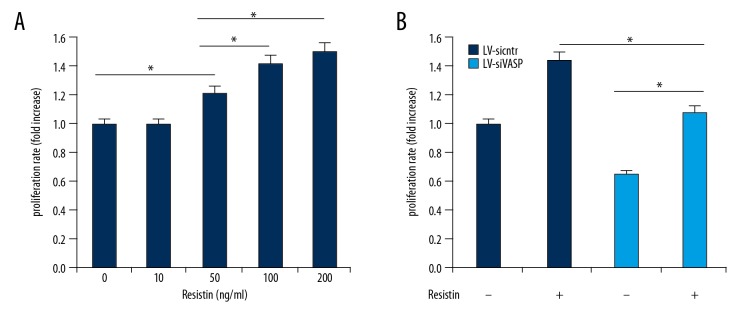
To evaluate the effects of VASP on resistin in proliferation, we first verified the effect of resistin on endothelial cell proliferation by MTT assay. The result showed that resistin significantly promoted HCAEC proliferation in a dose-dependent manner compared to the untreated control (Figure 2A). Next, we analyzed cell proliferation of the control group (LV-sicntr) and VASP-deficient group with or without resistin at 100 ng/ml. As shown in Figure 2B, ablation of VASP inhibited the effect of resistin on cell proliferation compared with the LV-sicntr group, showing that resistin induced HCAECs proliferation, at least in part, by interaction with the VASP molecule.
Figure 2.
Effect of VASP on proliferation of resistin. (A) After 24h incubation, resistin induced endothelial proliferation in a dose-dependent manner (0–200 ng/ml) with MTT assay. (B) Resistin-induced cell proliferation could be effectively blocked by ablation of VASP with or without 100 ng/ml of resistin. * p<0.05.
Ablation of VASP disrupts the effect of resistin on cell migration
Previous studies have confirmed that resistin and VASP play an important role in cell migration, but the involvement of VASP in the effect of resistin has not been elucidated. To investigate VASP-dependent regulation of resistin in endothelial cell migration, Transwell chamber assay was used to evaluate migration in various doses of resistin. In line with endothelial cell proliferation, resistin significantly increased HCAEC migration in a dose-dependent manner compared with the blank control group (Figure 3A). However, knockdown of VASP by siRNA abrogated the effect of resistin at 100ng/ml on endothelial cell migration compared with the LV-sicntr group (Figure 3B). Thus, loss of VASP can inhibit endothelial cell migration of resistin.
Figure 3.
Effect of VASP on migration of resistin. (A) HCAEC migration in different doses of resistin(0–200 ng/ml) was studied by transwell chamber assay. The results showed that resistin promoted migration of HCAECs after 12 h incubation. (B) Ablation of VASP disrupted the function of resistin on cell migration with or without 100 ng/ml of resistin. * p<0.05.
Knockdown of VASP promotes brown-to-white adipocyte trans-differentiation
Recent studies indicated that inflammation can induce brown-to-white adipocyte phenotype conversion [14]. To prove that there was phenotype conversion of adipocytes in IL-6, the mature adipocytes were treated with or without IL-6 at 100 ng/ml. We found that the abundance of BAT-specific genes such as UCP-1 and PRDM16 were significantly decreased, but the WAT-specific genes for resistin and RIP140 increased in IL-6 at 100 ng/ml (Figure 4A), which was consistent with previous studies.
Figure 4.
(A) IL-6 induced the differentiation of brown adipocytes into white adipocytes. (B) VASP contents were decreased during brown-to-white adipocyte trans-differentiation phenotype conversion. * p<0.05.
Therefore, we measured the contents of VASP during brown-to-white adipocyte trans-differentiation phenotype conversion by RT-PCR. Our results showed that VASP contents were decreased in IL-6 at 100 ng/ml compared with the control groups (Figure 4B). Next, to study the functional consequences of VASP on phenotype conversion of adipocytes, we knocked-down VASP by siRNA blocking its expression in mature brown adipocytes, and the inhibited effects were almost the same as in HCAEC (data no shown). Because proteins of the enabled (Ena)/vasodilator-stimulated phosphoprotein (VASP) family appear to promote actin filament formation [15], F-actin might exhibit dynamic instability (auto assembly-disassembly) in the absence of VASP. As shown in Figure 5A, fewer actin aggregates and bundled stress fibers were observed in VASP-deficient adipocytes, whereas stress fibers gathered by F-actin appeared long and regular in LV-sicntr adipocytes. Next, we investigated whether VASP was related with phenotype conversion of epicardial adipocytes. Surprisingly, we found obviously decreased expressions of UCP-1 and PRDM16 and increased expressions of resistin and RIP140 in VASP-deficient adipocytes compared with LV-sicntr (Figure 5B). These data show that ablation of VASP could not maintain brown phenotype of adipocytes, with a tendency to be easier to convert white adipocytes compared with the LV-sicntr group.
Figure 5.
Ablation of VASP promoted brown-to-white phenotype conversion of epicardial adipocytes. (A) Actin cytoskeletal staining with or without VASP. (B)The abundance of of UCP-1 and PRDM16 decreased, but the resistin and RIP140 expressions increased in VASP-deficient adipocytes compared with LV-sicntr group. * p<0.05.
Increased expressions of inflammatory adipocytokines in the absence of VASP
To identify the possible mechanism underlying the upregulation of resistin and RIP140 and downregulation of UCP-1 and PRDM16 in VASP-deficient adipocytes, we examined the expression of several proinflammatory cytokines associated with adipocyte dysfunction. IL-6 and TNF-α transcript expression were higher in VASP-deficient epicardial adipocytes as compared with the LV-sicntr group (Figure 6A, 6B). We also examined IL-8 expression and found high levels of IL-6 in VASP-deficient epicardial adipocytes (Figure 6C). MCP-1 is critically involved in the recruitment of macrophages to adipose tissues [16], and the result showed increased expressions in VASP-deficient epicardial adipocytes compared with the LV-sicntr group (Figure 6D). These results indicate that increased expressions of proinflammatory cytokines in VASP-deficient adipocytes might be responsible for brown-to-white adipocyte trans-differentiation in EAT.
Figure 6.
(A–D) Ablation of VASP increased expressions of inflammatory adipocytokines in epicardial adipose tissue. The mRNA expressions of IL-6, IL-8, TNF-α and MCP-1 increased in VASP-deficient adipocytes compared with LV-sicntr group. * p<0.05.
Disruption of RhoA/ROCK activity in VASP-deficient epicardial adipocytes can maintain brown phenotype of adipocytes
Previous studies have shown that RhoA/ROCK inhibits insulin signaling; however, insulin promoted brown adipogenesis and increases UCP-1 abundance [12]. To investigate the effect of RhoA/ROCK on phenotype conversion in VASP-deficient epicardial adipocytes, we inhibited RhoA/ROCK activity by Y-27632 at a concentration of 30 μM. As shown in Figure 7, the protein levels of UCP-1 and PRDM16 in Y-27632-treated adipocytes were significantly increased compared to untreated controls in VASP-deficient adipocytes, suggesting that VASP mediated phenotype conversion of EAT associated with RhoA/ROCK activity.
Figure 7.
RhoA/ROCK signal was involved in VASP-mediated phenotype conversion. After treatment with RhoA/ROCK specific blocker Y-27632 2HCL in VASP-deficient adipocytes, the expressions of UCP-1 and PRDM16 were significantly increased in protein levels compared with LV-sicntr group. * p<0.05.
Furthermore, there is increasing evidence that the RhoA signaling pathway plays a critical role in the inflammatory response [17]. In our study, blockade of ROCK activity by Y-27632 inhibited production of proinflammatory cytokines such as IL-6, IL-8, MCP-1, and TNF-α (Figure 6), which is consistent with previous studies. Thus, we demonstrated that disruption of RhoA/ROCK activity upregulates expressions of BAT-specific genes in VASP-deficient epicardial adipocytes involved in reduced inflammatory response.
Discussion
Treatment of coronary atherosclerosis has made little progress in recent years because of its complicated pathogenesis. In our study, we focused on VASP, an actin-binding protein, to provide evidence for the pathogenesis of coronary atherosclerosis and present new clues for the therapy of coronary atherosclerosis.
The VASP protein, a member of the Ena/VASP family, consists of 3 functional domains: an N-terminal Ena/VASP homology 1 (EVH1) domain, a central proline-rich domain (PRR), and a C-terminal Ena/VASP homology 2 (EVH2) domain [18]. These functional domains work together to enable VASP to modulate the actin ultrastructure and cellular functions [19] such as shape change, adhesion, migration, and proliferation, while resistin can facilitate angiogenesis by regulating cell migration and proliferation [9]. VASP has been shown to be overexpressed in the neointimal cell layer resulting from surgical endothelial denudation of rat carotid arteries [20]. Therefore, we hypothesized that VASP has crucial role in resistin-induced proliferation and migration in human coronary endothelial cells.
Resistin is an adipokine and was proposed originally to be a link between obesity and type 2 diabetes [21]. Recent experimental studies indicated that resistin aggravates atherosclerosis by inducing vascular inflammation and promoting angiogenesis [22]. It has been reported that lipopolysaccharide, interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α enhance resistin mRNA expression in human peripheral blood monocytes [23]. Sahmin et al. found that resistin directly binds to adenylyl cyclase-associated protein 1 (CAP1), a functional receptor for human resistin, and upregulate NF-κB-related transcription of inflammatory cytokines to modulate inflammatory action of monocytes [24]. However, Hong et al. found that resistin induced human endothelial cell proliferation and migration, promoted capillary-like tube formation, and upregulated the expression of vascular endothelial growth factor receptors (VEGFRs) and matrix metalloproteinases (MMPs) [9]. They also investigated potential signaling mechanisms and found that resistin treatment increased phosphorylated extracellular signal regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (p38MAPK, p38) in HCAECs; when specific inhibitors were used to block ERK1/2 and p38, the resistin-induced cell proliferation and migration previously observed were completely blocked [9]. Similarly, treatment of ovary carcinoma cells with resistin induced VEGF production to stimulate endothelial cell tube formation [25]. The effects of murine resistin and mouse resistin on angiogenesis have been investigated as well [26,27].
To investigate whether VASP is involved in the function of resistin, we observed cell proliferation and migration at various dose of resistin, although previous studies have revealed that resistin induced cell proliferation and migration in endothelial cells and some cancer cells [9,25,28]. In accordance with these results, our data showed that resistin promoted human coronary artery endothelial cells proliferation and migration in a dose-dependent manner. Based on these findings, knockdown of VASP by siRNA in HCAECs was carried out. Then, a fixed dose of resistin (100 ng/ml) was given to the LV-sicntr group and VASP-deficient group. We found that the effect of resistin on endothelial cell proliferation and migration was abolished after ablation of VASP compared with the LV-sicntr group. These data show that resistin induces human endothelial cell proliferation and migration, at least in part, by VASP protein.
Resistin is mainly produced by macrophages and white adipocytes in humans [23,29]. Human epicardial fat was previously referred to as “brown-like fat” and the brown adipose tissue can change into white adipose tissue with age and obesity [30]. Recently, VASP was proposed to participate in differentiation and VASP knockdown increased inflammation of adipocytes [13,31]. The relationship between VASP and risk factors for atherosclerosis such as hypertension and hyperglycemia have also been investigated [11,32,33]. These findings suggest VASP plays an important role in coronary atherosclerosis. In our study, we took epicardial fat samples from valvular heart disease patients during heart valve replacement to identify whether VASP was associated with phenotype conversion of epicardial adipose tissue. It is well known that the BAT-specific genes include UCP-1, PRDM16, and peroxisome proliferator activated receptor-γ coactivator 1-α (PGC-1α). The WAT-specific genes include RIP140, resistin, and leptin [6]. In our study, reverse transcription-quantitative polymerase chain reaction (RT-PCR) analysis revealed significantly decreased expression of BAT-specific genes and increased expression of WAT-specific genes in VASP-deficient adipose cell compared with the LV-sicntr group. Interestingly, NO and PKG signaling is required for BAT differentiation and deletion of VASP, which is a major substrate of PKG. Katja et al. found that VASP ablation enhanced BAT function and stimulated the development of brown-like adipocytes in WAT [13]. In contrast, Handa et al. reported that levels of mRNA encoding proinflammatory cytokines MCP-1, IL-6, and TNF-α were markedly elevated in VASP−/− mice compared with WT controls [31]. More remarkable, recent studies and the present study indicated that inflammation might induce the conversion of EAT from BAT into WAT [6, 14].
Next, we investigated the mechanisms responsible for brown-to-white phenotype conversion in VASP-deficient epicardial adipocytes. A potential reason could be the increased inflammation after ablation of VASP. Epicardial fat is known for its powerful proinflammatory properties associated with adipocyte dysfunction [34]. Secretion of antiinflammatory adiponectin is markedly reduced, whereas that of proinflammatory cytokines IL-6, IL-8, and MCP-1 is markedly increased in perivascular adipocytes surrounding human coronary arteries [35]. NO, produced by nitric oxide synthase (NOS), is involved in obesity-associated adipose tissue inflammation [36]. A growing body of evidence suggests VASP as a downstream mediator of NO signal transduction pathway exerts anti-obesity effects by improving inflammation in various tissues such as the liver, muscle, and adipose tissues) [37]. Consistent with these findings, we found that in VASP-deficient epicardial adipocytes, levels of mRNA encoding proinflammatory cytokines MCP-1, IL-6, TNF-α and IL-8 were significantly elevated compared with the LV-sicntr group. However, the result seemed to be contradictory to the fact that blocking VASP could ablate resistin-induced cell proliferation and migration, and resistin served as a pro-inflammation cytokine. Ena/VASP proteins negatively regulates fibroblast migration speed, while within living cells, Listeria speeds are reduced by an order of magnitude in the absence of Ena/VASP proteins [38]. Consequently, we suspected that the regulating mechanisms of VASP in different cell type might be different.
Ena/VASP and Rho GTPases are 2 classes of molecules that regulate the actin cytoskeleton, and a body of evidence suggests that Rho GTPases activity is regulated by Ena/VASP proteins [39,40]. Katja et al. confirmed that in VASP−/− cells, RhoA activity was decreased [13]. To investigate the role of RhoA/ROCK in VASP-deficient adipocytes in phenotype conversion, we disrupted the RhoA/ROCK activities by treating VASP-deficient cells with Y27632. Western blot analysis showed that RhoA/ROCK inhibition in VASP-deficient adipocytes restored reduced crucial markers (UCP-1, PRDM16) of brown fat cells in VASP-deficient adipocytes. After inhibiting RhoA/ROCK activity in VASP-deficient adipocytes for 24 h, increased proinflammatory cytokines such as IL-6, IL-8, TNF-α, and MCP-1 were observed. These findings prove that RhoA/ROCK plays important role in phenotype conversion of EAT in VASP-deficient adipocytes.
Conclusions
Here, we show for the first time that resistin induced HCAECs proliferation and migration, at least in part, by VASP protein. We also showed that VASP regulated phenotype conversion of human epicardial adipocyte associated with inflammation. Therefore, reduced VASP in epicardial adipose tissues might contribute to brown-to-white adipocyte phenotype conversion in the whole body through increased expressions of proinflammatory cytokines like resistin. Furthermore, resistin regulated endothelial cell dysfunction-associated VASP protein. Our study explains the involvement of VASP in the endothelium-driven development of arteriosclerotic diseases and offers new clues for the future therapy of metabolic diseases like obesity-related cardiovascular disease, but the specific mechanism of VASP in other kinds of cells needs further clarification. The modulation of VASP expression could be an opportunity for developing new arteriosclerosis therapies.
Footnotes
Source of support: The study was supported by the “Six Peaks of The Talented in Jiangsu Province” (WS-078) and “China’s Post-Doctoral Science Fund” (2016M603062)
References
- 1.Lloyd-Jones DM. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation. 2010;121:1768–77. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 2.Camare C, Pucelle M, Negre-Salvayre A, et al. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar B, Chile SA, Ray KB, et al. VEGF-C differentially regulates VEGF-A expression in ocular and cancer cells; Promotes angiogenesis via RhoA mediated pathway. Angiogenesis. 2011;14:371–80. doi: 10.1007/s10456-011-9221-5. [DOI] [PubMed] [Google Scholar]
- 4.Sacks HS, Fain JN, Bahouth SW, et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98:E1448–55. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee TK, Aronow BJ, Tong WS, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genom. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Chen D, Cheng XM, et al. Influence of phenotype conversion of epicardial adipocytes on the coronary atherosclerosis and its potential molecular mechanism. Am J Transl Res. 2015;7:1712–23. [PMC free article] [PubMed] [Google Scholar]
- 7.Galler AB, Garcia Arguinzonis MI, Baumgartner W, et al. VASP-dependent regulation of actin cytoskeleton rigidity, cell adhesion, and detachment. Histochem Cell Biol. 2006;125:457–74. doi: 10.1007/s00418-005-0091-z. [DOI] [PubMed] [Google Scholar]
- 8.Jamaluddin MS, Yan S, Lu J, et al. Resistin increases monolayer permeability of human coronary artery endothelial cells. PLoS One. 2013;8:e84576. doi: 10.1371/journal.pone.0084576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu H, Ohashi R, Yan S, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–57. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Benz PM, Blume C, Seifert S, et al. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–65. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo I, Viretto M, Doronzo G, et al. A short-term incubation with high glucose impairs VASP phosphorylation at serine 239 in response to the nitric oxide/cGMP pathway in vascular smooth muscle cells: role of oxidative stress. Biomed Res Int. 2014;2014 doi: 10.1155/2014/328959. 328959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas B, Mayer P, Jennissen K, et al. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal. 2009;2:ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- 13.Jennissen K, Siegel F, Liebig-Gonglach M, et al. A VASP-Rac-soluble guanylyl cyclase pathway controls cGMP production in adipocytes. Sci Signal. 2012;5:ra62. doi: 10.1126/scisignal.2002867. [DOI] [PubMed] [Google Scholar]
- 14.Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. 2014;66:95–103. doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Gunst SJ. Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism. J Biolog Chem. 2015;290:11403–16. doi: 10.1074/jbc.M115.645788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Zeng X, Halifu Y, et al. Blocking RhoA/ROCK inhibits the pathogenesis of pemphigus vulgaris by suppressing oxidative stress and apoptosis through TAK1/NOD2-mediated NF-kappaB pathway. Mol Cell Biochem. 2017;436:151–58. doi: 10.1007/s11010-017-3086-x. [DOI] [PubMed] [Google Scholar]
- 18.Pula G, Krause M. Role of Ena/VASP proteins in homeostasis and disease. Handb Exp Pharmacol. 2008;(186):39–65. doi: 10.1007/978-3-540-72843-6_3. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003;13:386–92. doi: 10.1016/s0962-8924(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 20.Monks D, Lange V, Silber RE, et al. Expression of cGMP-dependent protein kinase I and its substrate VASP in neointimal cells of the injured rat carotid artery. Eur J Clin Invest. 1998;28:416–23. doi: 10.1046/j.1365-2362.1998.00308.x. [DOI] [PubMed] [Google Scholar]
- 21.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 22.Jamaluddin MS, Weakley SM, Yao Q, et al. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–32. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaser S, Kaser A, Sandhofer A, et al. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–90. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Lee HC, Kwon YW, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014;19:484–97. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang L, Zhang Y, Yu Y, et al. Resistin promotes the expression of vascular endothelial growth factor in ovary carcinoma cells. Int J Mol Sci. 2013;14:9751–66. doi: 10.3390/ijms14059751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson SA, Rae CJ, Graham A. Induction of angiogenesis by murine resistin: Putative role of PI3-kinase and NO-dependent pathways. Regul Pept. 2009;152:41–47. doi: 10.1016/j.regpep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Robertson SA, Rae CJ, Graham A. Resistin: TWEAKing angiogenesis. Atherosclerosis. 2009;203:34–37. doi: 10.1016/j.atherosclerosis.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Di Simone N, Di Nicuolo F, Sanguinetti M, et al. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. Endocrinol. 2006;189:691–99. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz TU, Kerem M, Demirtas CY, et al. Increased resistin levels in intra-abdominal sepsis: correlation with proinflammatory cytokines and acute physiology and chronic health evaluation (APACHE) II scores. Sultan Qaboos Univ Med J. 2014;14:e506–12. [PMC free article] [PubMed] [Google Scholar]
- 30.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handa P, Tateya S, Rizzo NO, et al. Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding. Arterioscler Thromb Vasc Biol. 2011;31(12):2827–35. doi: 10.1161/ATVBAHA.111.236554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arlier Z, Basar M, Kocamaz E, et al. Hypertension alters phosphorylation of VASP in brain endothelial cells. Int J Neurosci. 2015;125:288–97. doi: 10.3109/00207454.2014.930740. [DOI] [PubMed] [Google Scholar]
- 33.Mori Y, Chiang S, Bendeck MP, et al. Insulin decreases atherosclerotic plaque burden and increases plaque stability via nitric oxide synthase in apolipoprotein E-null mice. Am J Physiol Endocrinol Metab. 2016;311:E335–45. doi: 10.1152/ajpendo.00320.2015. [DOI] [PubMed] [Google Scholar]
- 34.Fain JN, Sacks HS, Buehrer B, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–15. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–49. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang YM, Kim F, Lee WJ. Role of NO/VASP signaling pathway against obesity-related inflammation and insulin resistance. Diabetes Metab J. 2017;41:89–95. doi: 10.4093/dmj.2017.41.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause M, Dent EW, Bear JE, et al. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Ann Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 39.Doppler HR, Bastea LI, Lewis-Tuffin LJ, et al. Protein kinase D1-mediated phosphorylations regulate vasodilator-stimulated phosphoprotein (VASP) localization and cell migration. J Biol Chem. 2013;288:24382–93. doi: 10.1074/jbc.M113.474676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higashi M, Ishikawa C, Yu J, et al. Human Mena associates with Rac1 small GTPase in glioblastoma cell lines. PLoS One. 2009;4:e4765. doi: 10.1371/journal.pone.0004765. [DOI] [PMC free article] [PubMed] [Google Scholar]