Abstract
Background
lncRNA ANCR is proved to be a tumor suppressor gene only in colorectal cancer and breast cancer. Our study aimed to explore the possible involvement of ACNR in non-small cell lung cancer (NSCLC).
Material/Methods
In this study, we first detected the expression of ACNR in lung biopsies and plasma of both NSCLC patients and healthy controls. The diagnostic value of ANCR for NSCLC was analyzed by ROC curve analysis. Follow-up data of NSCLC patients was analyzed and the prognostic value of ANCR was analyzed by survival curve analysis. ANCR expression vector was transfected into cells of human NSCLC cell lines, and the effects on cell migration and invasion were explored by Transwell migration and invasion assays, respectively. TGF-β1 expression after ANCR overexpression was detected by Western blot analysis.
Results
ANCR was significantly downregulated in NSCLC patients compared with healthy controls in lung biopsies and plasma. Downregulated expression of ANCR distinguishes NSCLC patients from healthy controls and low NSCLC expression level indicates shorter postoperative survival time of NSCLC patients. ANCR overexpression inhibited NSCLC cell migration and invasion and downregulated TGF-β1 expression, while TGF-β1 treatment showed no significant effects on ANCR expression but promoted NSCLC cell migration and invasion. In addition, TGF-β1 treatment also attenuated the inhibitory effects of ANCR overexpression on NSCLC cell migration and invasion.
Conclusions
ANCR can inhibit NSCLC cell migration and invasion by downregulating TGF-β1 expression.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Cell Migration Inhibition; Receptors, Transforming Growth Factor beta; RNA, Long Noncoding
Background
Lung cancer is a frequently diagnosed human malignancy that is a leading cause of cancer-related deaths in China and world-wide [1,2]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and accounts for more than 85% of all cases [3]. In spite of progress made in the treatment and prevention of NSCLC, the prognosis of this disease is still poor. Surgical resection is the only radical treatment, but it is not suitable for most patients with NSCLC by the time of diagnosis due to distant tumor metastasis [4], which is reported to be responsible for more than 90% deaths among NSCLC patients [5]. Therefore, in-depth studies on the molecular mechanism of the metastasis of NSCLC may provide new insights into its treatment.
TGF-β signaling pathway interacts with multiple signaling pathways to participate in the development and progression of different types of human malignancies [6]. TGF-β functions as a tumor suppressor gene in the early stage of tumor development by inhibiting cell proliferation [7] and TGF-β also induces epithelial-mesenchymal transition (EMT) to promote tumor cell invasion [8]. TGF-β signaling in some cases achieves its biological functions through interactions with lncRNAs [9]. lncRNA ANCR is a tumor suppressor gene only in breast cancer and colorectal cancer [10,11]. In breast cancer, it has been reported that downregulation of ANCR is related to accelerated TGF-β-induced EMT and metastasis [10]. In our study, we observed that ANCR is likely an upstream inhibitor of TGF-β in NSCLC. Our study first investigated the functionality of ANCR in NSCLC and provides evidence for the possible mechanism of its action in this disease.
Material and Methods
Patients and specimen collection
Clinical data of all NSCLC (adenocarcinoma) patients who were diagnosed and treated in the Second Affiliated Hospital of Zhejiang University School of Medicine from January 2011 to March 2013 were reviewed and 72 of those patients were included in this study according to inclusion and exclusion criteria. Inclusion criteria: 1) patients pathologically diagnosed as having NSCLC; 2) patients diagnosed and treated for the first time; 3) patients with complete clinical data; 4) patients with high-quality tumor biospies and plasma samples in the specimen labrotary of the Second Affiliated Hospital of Zhejiang University School of Medicine; 5) patients who completed the whole treatment and follw-up procdedure in the Second Affiliated Hospital of Zhejiang University School of Medicine. Exclusion criteria: 1) patients without tumor biospies and plasma samples or the quality of samples was poor; 2) patients had from other maligancies or lung diseases; 3) patients recived any treatment before admission; 4) patients died of other casues during follow-up. Among thhe 72 patients who were included in the study, 44 were males and 28 were females, and the ages ranged from 22 to 68 years, with an average age of 44.1±6.1 years. There were 8 patients in AJCC stage IIB, 6 in AJCC stage IIIA, 2 in stage AJCC stage IIIB, 4 in AJCC stage IIIC, 32 in AJCC stage IVA, and 22 in AJCC stage IVB. Patients received chemotherapy or radiation therapy. Surgical resection was only performed in 19 cases. At the same time, biospies and plasma samples of 44 healthy people were also obtained from the specimen labrotary of the Second Affiliated Hospital of Zhejiang University School of Medicine to serve as a control group. Those healthy people received lung biopsy to detect potentoal lung lesions, which were finally excluded by pathological examanitions. The control group included 30 males and 14 females, age range 26–69 years, average age 45.4±5.7 years. No significant differences in age, sex, histories of diseases, living habits (including drinking and smoking) or other basic characteristics were found between the 2 groups. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine, and all participants and/or their families signed informed consent.
Real-time quantitative PCR (qRT-PCR)
Total RNA extractions were performed using Trizol reagent (Invitrogen, USA) in strict accordance with the instructions of the kit. A NanoDrop™ 2000 Spectrophotometer (Thermo Fisher Scientific, USA) was used to measure RNA concentration and detect RNA quality. cDNA was synthesized through reverse transcription. SYBR Green PCR Master Mix (Thermo Fisher Scientific) was used to prepare the PCR reaction system. Primers used in PCR reactions were: 5′-GCCACTATGTAGCGGGTTTC-3′ (upstream) and 5′-ACCTGCGCTAAGAACTGAGG-3′ (downstream) for human ANCR; 5′-GACCTCTATGCCAACACAGT3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA3′ (reverse) for β-actin. PCR reactions were performed on an ABI PRISM 7500 qRT-PCR device (Applied Biosystems, Rockford, IL, USA). Conditions of PCR reactions were: 40 s at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 60°C. 2−ΔΔCT method was utilized to analyze all Ct values, and ANCR expression was normalized to β-actin endogenous control.
Cell line, cell culture and transfection
Human NSCLC (adenocarcinoma) cell lines NCI-H23 [H23] (ATCC® CRL-5800™) and NCI-H522 [H522] (ATCC® CRL-5810™) were purchased from ATCC. Cells of both lines were cultured with ATCC-formulated RPMI-1640 medium (ATCC 30-2001) containing 10% fetal bovine serum (FBS, ATCC 30–2020) in an incubator (37°C, 5% CO2). The lncRNA ANCR overexpression vector was constructed by inserting ANCR cDNA into pIRSE2-EGFP vector (Clontech, Palo Alto, CA, USA). Lipofectamine 2000 reagent (11668-019, Invitrogen, Carlsbad, CA, USA) was used to transfect vector (negative control) into 5×105 cells. Empty pIRSE2-EGFP vector transfection was performed to serve as a negative control. Cells without transfection were used as controls.
Transwell migration and invasion assay
After transfection, cells of the 2 cell lines were collected and mixed with RPMI-1640 medium containing 1% FBS to make a single-cell suspension with a final density of 4×104 cells/ml. Transwell migration and invasion assays were then performed to evaluate cell migration and invasion abilities. In the migration assay, 4×103 cells in 0.1 ml cell suspension were transferred to the upper chamber, while RPMI-1640 medium (Thermo Fisher Scientific, USA) containing 20% FBS (Sigma-Aldrich, USA) was used to fill the lower chamber. Cell culture was performed for 24 h, followed by incubating membranes with 0.5% crystal violet (Sigma-Aldrich, USA) at room temperature for 15 min. The upper chamber was coated with Matrigel (356234, Millipore, USA) before adding cells in the invasion assay. Stained cells were counted under an optical microscope.
Western blot analysis
Protein extractions were performed using RIPA solution (Thermo Fisher Scientific) in strict accordance with the instructions of the kit. Protein concentrations were measured by BSA assay. After that, SDS-PAGE gel (10%) electrophoresis was performed with 20 μg protein per lane to separate proteins with different molecular weights. Gel transfer was performed to PVDF membranes (Bio-Rad, USA), followed by blocking with skimmed milk (5%) for 2 h at room temperature. After that, membranes were incubated with primary antibodies of TGF-β1 (rabbit anti-human, ab9758, 1: 1200; Abcam) and GAPDH (rabbit anti-human, ab9485, 1: 1400, Abcam) overnight at 4°C. IgG-HRP secondary antibody (1: 1000, MBS435036, MyBioSource) was then incubated with the membranes at room temperature for 3 h. ECL (Sigma-Aldrich, USA) was dropped on the membranes to develop signals, and TGF-β1 expression was normalized to GAPDH using Image J software.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 software. Expression and cell migration and invasion data were compared by one-way analysis of variance (among multiple groups) and the unpaired t test (between 2 groups). Correlations between ANCR expression and patients’ clinicopathological data were analyzed by chi-square test. p<0.05 was considered to be statistically significant.
Results
Comparison of ANCR expression in NSCLC patients and healthy controls
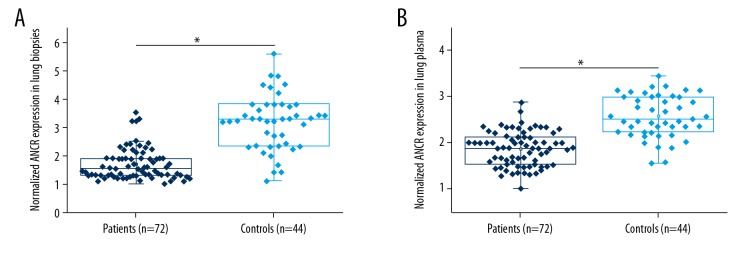
Differential expression of a gene in patients and healthy people indicates the potential involvement of this gene in certain diseases. As shown in Figure 1, expression of ANCR in NSCLC patients was significantly downregulated compared with healthy controls in lung biopsies (Figure 1A) and plasma (Figure 1B), which indicates that downregulation of ANCR is likely to be involved in the pathogenesis of NSCLC.
Figure 1.
Comparison of ANCR expression in NSCLC patients and healthy controls. This figure shows the comparison of ANCR expression in NSCLC patients and healthy controls in lung biopsies (A) and plasma (B). * p<0.05.
Potentials of the application of ANCR in the diagnosis of NSCLC
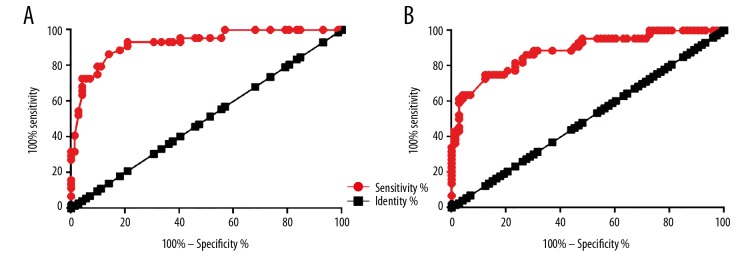
ROC curve analysis was performed to evaluate the diagnostic value of differential expression of ANCR in NSCLC patients and healthy controls for NSCLC. Regarding ANCR expression in lung biopsies, the area under the curve (AUC) was 0.9265, with standard error of 0.02464 and 95% confidence interval of 0.8782 to 0.9748 (p<0.0001) (Figure 2A). The AUC of plasma ANCR was 0.8831, with a standard error of 0.03253 and 95% confidence interval of 0.8193 to 0.9469 (p<0.0001) (Figure 2B).
Figure 2.
Potentials of the application of ANCR in the diagnosis of NSCLC. This figure shows ROC curve analysis of the potentials of the application of ANCR expression in lung biopsies (A) and plasma (B) for the diagnosis of NSCLC.
Correlations between ANCR expression and patients’ clinicopathological data
Correlations between ANCR expression and patients’ clinicopathological data were analyzed by chi-square test followed by Bonferroni correction. Results showed than ANCR expression in lung biopsies (Table 1) and plasma (Table 2) were not significantly correlated with patients’ age, sex, smoking and drinking habits, or tumor size (p>0.05). In contrast, ANCR expression in lung biopsies (Table 1) and plasma (Table 2) were found to be significantly correlated with distant tumor metastasis (p<0.05).
Table 1.
Correlations between ANCR expression in lung biopsies and patients’ clinicopathological data.
| Items | Groups | Cases | High-expression | Low-expression | χ2 | p Value |
|---|---|---|---|---|---|---|
| Age | >45 (years) | 38 | 17 | 21 | 0.89 | 0.37 |
| <45(years) | 34 | 19 | 15 | |||
| Sex | Males | 44 | 20 | 24 | 0.94 | 0.33 |
| Females | 28 | 16 | 12 | |||
| Smoking | Yes | 48 | 21 | 27 | 2.25 | 0.15 |
| No | 24 | 15 | 9 | |||
| Drinking | Yes | 43 | 20 | 23 | 0.52 | 0.48 |
| No | 29 | 16 | 13 | |||
| Primary tumor diameter | >4 cm | 46 | 20 | 26 | 2.17 | 0.15 |
| <4 cm | 26 | 16 | 10 | |||
| Distant tumor metastasis | Yes | 52 | 21 | 31 | 6.92 | 0.011 |
| No | 20 | 15 | 5 |
Table 2.
Correlations between ANCR expression in plasma and patients’ clinicopathological data.
| Items | Groups | Cases | High-expression | Low-expression | χ2 | p Value |
|---|---|---|---|---|---|---|
| Age | >45 (years) | 38 | 18 | 20 | 0.22 | 0.71 |
| <45(years) | 34 | 18 | 16 | |||
| Sex | Males | 44 | 21 | 23 | 0.02 | 0.82 |
| Females | 28 | 15 | 13 | |||
| Smoking | Yes | 48 | 21 | 27 | 2.25 | 0.14 |
| No | 24 | 15 | 9 | |||
| Drinking | Yes | 43 | 19 | 24 | 1.44 | 0.25 |
| No | 29 | 17 | 12 | |||
| Primary tumor diameter | >4 cm | 46 | 20 | 26 | 2.17 | 0.16 |
| <4 cm | 26 | 16 | 10 | |||
| Distant tumor metastasis | Yes | 52 | 20 | 32 | 9.97 | 0.002 |
| No | 20 | 16 | 4 |
Potential application of ANCR in the prognosis of NSCLC
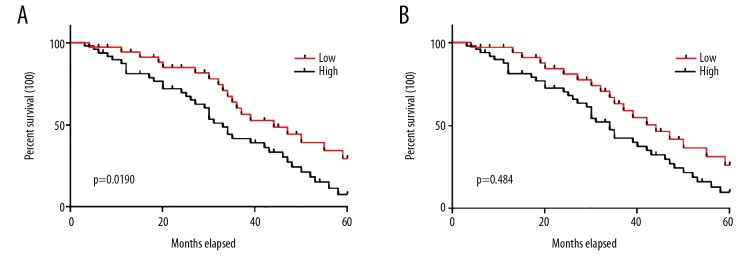
Expression levels of ANCR were significantly correlated with distant tumor metastasis, which is a major cause of death among patients with NSCLC. In this study, distant tumor metastasis was observed in 52 patients. Therefore, follow-up data of those 52 patients were analyzed. Those patients were divided into high- and low-expression groups (n=26) according to the median serum level of ANCR in lung biopsies (Figure 3A) and plasma (Figure 3B). No significant differences in disease stages and therapy options were found between high- and low-expression groups. Survival curves of these 2 groups of patients were plotted by Kaplan-Meier method. Survival curves were compared by log rank t test. As shown in Figure 3, the overall survival time of patients in the high-expression group was significantly longer than that of patients in the low-expression group (p<0.05).
Figure 3.
Potentials of the application of ANCR in the prognosis of NSCLC. This figure shows the comparison of survival curves of patients in groups with low and high expression of ANCR in biopsies (A) and plasma (B).
Effects of ANCR overexpression on TGF-β1 expression and migration and invasion of NSCLC cells
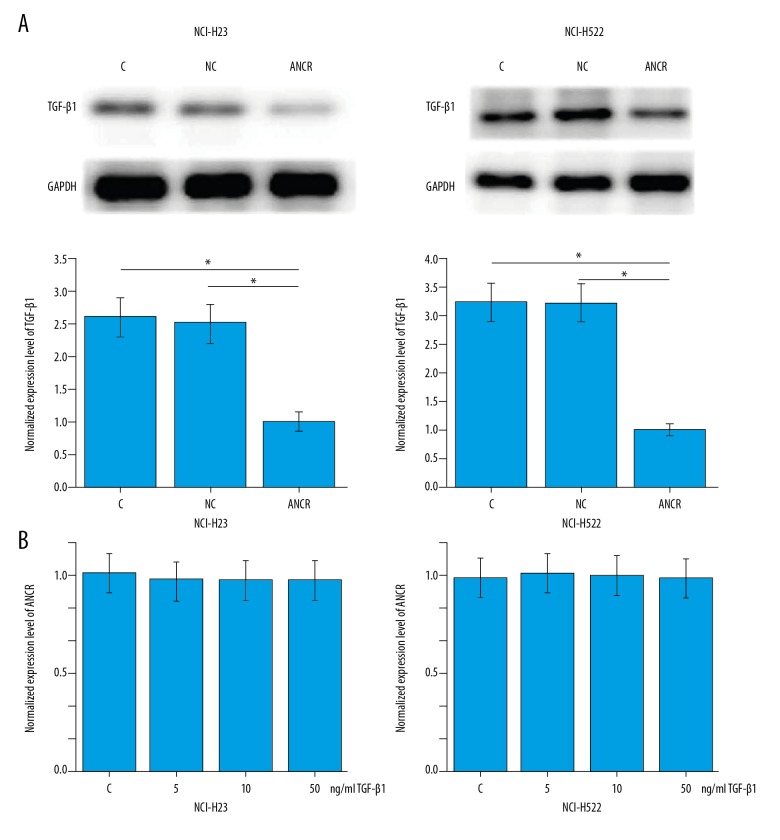
From the data in Tables 1 and 2, we can speculate that ANCR is likely to be involved in the regulation of tumor metastasis in NSCLC. Activation of TGF-β signaling plays pivotal roles in NSCLC metastasis [12]. Therefore, the potential interactions between ANCR and TGF-β1 were explored. As shown in Figure 4A, ANCR overexpression (confirmed by qRT-PCR) led to significantly downregulated expression of ANCR in cells of NSCLC cell lines NCI-H23 and NCI-H522 (p<0.05), while treatment with TGF-β1 (Sigma-Aldrich) for 12 h at the doses of 5, 10, and 50 ng per ml showed no significant effects on ANCR expression (Figure 4B). In addition, treatment with TGF-β1 at a dose of 10 ng/ml promoted the migration (Figure 5A) and invasion (Figure 5B) of cells of these 2 cell lines (p<0.05). In addition, compared with cells with ANCR overexpression only, cells with both ANCR overexpression and TGF-β1 treatment showed significantly increased migration (Figure 5A) and invasion (Figure 5B) ability (p<0.05).
Figure 4.
ANCR overexpression inhibited TGF-β1 expression. This figure shows the effects of ANCR overexpression on TGF-β1 expression (A) and the effects of TGF-β1 treatment on ANCR expression (B). * p<0.05.
Figure 5.
Effects of ANCR overexpression and TGF-β1 treatment on migration and invasion of NSCLC cells. Data here show the effects of ANCR overexpression and TGF-β1 treatment on migration (A) and invasion (B) of NSCLC cells. * p<0.05.
Discussion
The main finding of this study is that ANCR, which is a recognized tumor suppressor lncRNA in breast cancer and colorectal cancer [10,11], also inhibits the progression of NSCLC. ANCR is likely to be involved in the metastasis but not the growth of NSCLC. In addition, the action of ANCR in NSCLC is likely to be achieved through the inactivation of TGF-β signaling.
Differential expression of certain genes in lesion tissues and healthy tissues indicate the involvement of the genes in diseases. In addition, detecting the differential expression of genes also provides guidance for the diagnosis of human diseases. ANCR is a tumor suppressor gene that showed downregulated expression pattern in different types of malignancies [10,11,13,14]. In this study, expression of ANCR in lung tissues was found to be significantly downregulated in NSCLC patients compared with healthy controls, indicating the involvement of ANCR in this disease. Pathological examination through biopsies is the criterion standard for diagnosis of malignant diseases, but the application of this technique is challenged by its invasive nature. Therefore, circulating biomarkers, which are sensitive substances in blood that reflect the development of diseases, have been widely used to assist disease diagnosis [15]. In this study, ANCR was detected in plasma of all patients with NSCLC. ROC curve analysis showed that downregulated ANCR in plasma effectively distinguished patients with NSCLC from healthy controls. In addition, ANCR was not significantly correlated with age or smoking and drinking habits, which are known factors that affect the expression of certain genes [16–18], indicating the high stability of ANCR as a biomarker. In this manner, detecting plasma ANCR may be performed to assist the diagnosis of NSCLC.
ANCR in cancers has been proved to be involved in tumor metastasis of colorectal cancer [11] and breast cancer [13,14]. A recent study reported that ANCR may also be involved in tumor growth of osteosarcoma [14]. In our study, ANCR was found to be significantly correlated with tumor distant metastasis but not with tumor size. To our surprise, the difference between ANCR expression and metastasis is more significant in plasma than in biopsy. In theory, expression in biopsies should be more diagnostic of disease condition compared with expression in plasma. This outcome was possibly caused by the small sample size. In vitro cell invasion and migration assay further proved than ANCR is an inhibitor of NSCLC cell migration and invasion. Those data suggest the different functions of the same lncRNA in different types of cancers. However, all previous studies proved that ANCR is a tumor suppressor lncRNA in cancers.
TGF-β signaling promotes EMT in NSCLC [19]. TGF-β is a central signaling molecule in cancers that regulates tumor development and progression through a variety of intermediates, including lncRNAs [9]. However, studies on the role of lncRNAs as upstream regulators of TGF-β signaling are extremely rare. Another key finding of our study is that ANCR is likely to be an upstream inhibitor of TGF-β signaling. The role of ANCR in the regulation of NSCLC cell migration and invasion is at least partially achieved through the downregulation of TGF-β1. However, whether the regulatory role of ANCR on TGF-β1 is direct or indirect is still unknown. Our study only suggests the sequential ANCR – TGF-β1 signaling in a specific type of cancer.
Conclusions
Downregulation of ANCR is involved in the pathogenesis of NSCLC. Reduced ANCR expression level can be used to distinguish NSCLC patients from healthy people and it also predicts poor survival of NSCLC patients. ANCR inhibits NSCLC cell migration and invasion, at least partially by downregulating TGF-β1 expression.
Footnotes
Source of support: Key Science-Technology Innovation Team of Zhejiang Province (grant no. 2011R50016)
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669–92. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Park K, Bae C, Lee E. P1. 08–019 Risk Factors and Survival of Occult N2 Lymph Node Metastasis in NSCLC Patients with Clinical N0-1 Diagnosed by Preoperative PET-CT: Topic: Risk Assessment and Prognostic Factors. J Thorac Oncol. 2017;12(1):S741. [Google Scholar]
- 5.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 7.Lei Z, Xu G, Wang L, et al. MiR-142-3p represses TGF-β-induced growth inhibition through repression of TGFβR1 in non-small cell lung cancer. FASEB J. 2014;28(6):2696–704. doi: 10.1096/fj.13-247288. [DOI] [PubMed] [Google Scholar]
- 8.Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res. 2012;347(1):85–101. doi: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards EJ, Zhang G, Li ZP, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β: LncRNA-hit-mediated TGFβ-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290(11):6857–67. doi: 10.1074/jbc.M114.610915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Dong M, Fan D, et al. LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer. Oncotarget. 2017;8(40):67329–43. doi: 10.18632/oncotarget.18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZY, Yang F, Zhang YL, et al. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017;18(1):95–104. doi: 10.3233/CBM-161715. [DOI] [PubMed] [Google Scholar]
- 12.Daft PG, Meng X, Vander Ark A, et al. Abstract 1565: Cthrc1 mediated by myeloid-specific TGF-β signaling stimulates NSCLC bone metastasis. Cancer Res. 2016;76(14 Suppl):1565. [Google Scholar]
- 13.Li Z, Hou P, Fan D, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24(1):59–71. doi: 10.1038/cdd.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017;12(1):103. doi: 10.1186/s13018-017-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafari S, Backes C, Meese E, et al. Circulating biomarker panels in Alzheimer’s disease. Gerontology. 2015;61(6):497–503. doi: 10.1159/000375236. [DOI] [PubMed] [Google Scholar]
- 16.Bianchessi V, Badi I, Bertolotti M, et al. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J Mol Cell Cardiol. 2015;81:62–70. doi: 10.1016/j.yjmcc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Ijiri N, Panariti A, Mogas A, et al. Lncrna Neat1 promotes IL-8 expression in fibroblasts derived from COPD patients and in response to cigarette smoking. C74. Advances In Translational COPD. American Thoracic Society. 2017:A7676. [Google Scholar]
- 18.Mayfield RD. Emerging roles for ncRNAs in alcohol use disorders. Alcohol. 2017;60:31–39. doi: 10.1016/j.alcohol.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu RY, Zeng Y, Lei Z, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–51. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]