Abstract
The murine cornea provides an excellent model to study wound healing. The cornea is the outermost layer of the eye, and thus is the first defense to injury. In fact, the most common type of eye injury found in clinic is a corneal abrasion. Here, we utilize an ocular burr to induce an abrasion resulting in removal of the corneal epithelium in vivo on anesthetized mice. This method allows for targeted and reproducible epithelial disruption, leaving other areas intact. In addition, we describe the visualization of the abraded epithelium with fluorescein staining and provide concrete advice on how to visualize the abraded cornea. Then, we follow the timeline of wound healing 0, 18, and 72 h after abrasion, until the wound is re-epithelialized. The epithelial abrasion model of corneal injury is ideal for studies on epithelial cell proliferation, migration and re-epithelialization of the corneal layers. However, this method is not optimal to study stromal activation during wound healing, because the ocular burr does not penetrate to the stromal cell layers. This method is also suitable for clinical applications, for example, pre-clinical test of drug effectiveness.
Keywords: Medicine, Issue 137, Cornea, epithelium, abrasion, ocular burr, wound healing, fluorescein, in vivo, mouse
Introduction
Epithelial layers of numerous organs are exposed to injuries. However, they also contain the ability to compensate for tissue loss through wound healing. The cornea offers an excellent model to study wound healing. It forms the external surface of the eye and provides a protective layer for the sensitive ocular machinery. Thus, cornea functions as a physical barrier to pathogens and water loss. It is composed of three layers; epithelium, stroma and endothelium. The epithelium of the cornea makes up the outermost layer of the cornea. Epithelial cells maintain the barrier function of the cornea by adhering strictly to each other through tight junctions1,2,3. An acellular corneal basement membrane, the Bowman's membrane, separates the epithelium from the extensive stroma, which contains refractory keratocytes. Under the stroma, endothelial cells channel nutrients, water, and oxygen to the upper layer.
Corneal abrasions are very common in the clinic4. Injuries to the cornea are diverse, but are largely caused by small particles such as dust or sand, scratches, or other foreign objects. The protocol described here aims at reproducing a clinically relevant type of corneal epithelial abrasion. In doing so, this protocol provides a controllable and seminal method for clinicians and corneal scientists to implement in their own studies. We have performed an in vivo injury repair assay on the murine cornea by abrading the tissue with a dulled ocular burr, the Algerbrush II. Here, we target the abrasion only to the central corneal epithelium and leave the other parts of the organ without damage. Thus, the protocol is ideal to study corneal epithelial cell dynamics or the basement membrane during re-epithelialization, cell migration, proliferation and differentiation in vivo5. Recently, this model was used to analyze progenitor cell dynamics in the murine cornea as well as to unveil the capacity of the differentiated corneal epithelial cells in re-establishing the corneal stem cell niche after injury6,7. Following abrasion, the cornea returns to its normal transparency and tensile strength. Interestingly, an in vitro study indicated that re-epithelialization occurs without increased cell proliferation8. This protocol describes the timeline of uninterrupted healing in the murine cornea. The method is thus applicable to test the effect of drugs on healing patterns and speed.
The cornea has been extensively used for wound healing studies. However, many studies have relied on other models of injury. A well-established model of corneal injury is the alkaline burn that is performed by applying sodium hydroxide (NaOH) with or without filter paper on the corneal surface9. Alkaline exposure results in a large and diffuse injury that affects not only the corneal epithelium, but also the conjunctiva and stroma9,10. Strong alkaline solutions have been shown to induce corneal ulcers, opacification, and neovascularization9. Inflammatory cells invade the stroma typically within 6 h and remain there until 24 h11. Thus, alkaline injury is an advisable method in studies related to stromal activation. Another type of chemical injury can be inflicted by applying dimethyl sulfoxide (DMSO) on the cornea9,10. Other commonly used injury models include incisional wounds that penetrate through the stroma and keratectomy wounds, which are limited to the upper portion of the stroma14,15. These methods are also useful to answer questions regarding stromal wound healing. Different injury models have their own advantages and disadvantages. Abrasion, or debridement, of the corneal epithelium was first developed using dulled scalpels or blades on ex vivo corneas16. This method has later been used in vivo on mouse, rat, and rabbit17,18,19,20,21,22. Using the ocular burr (Figure 1), we remove only a selected region of the epithelium, leaving the rest of the epithelium unaffected. This way, it is possible to precisely target the epithelial removal to different parts of the cornea. In addition, the abrasion size can be assessed with fluorescein staining. Furthermore, here we follow abrasion closure during the healing period.

This method poses several advantages, i) including precise location of abrasion site, which is not possible with chemical injury, ii) the abrasion is quick to perform, and iii) it is non-invasive. Herein, we describe the method using the outbred NMRI mouse as a model, however this could be applied to the vast array of mouse genetic models, as well as to the rat and rabbit, which are common models used to study human corneal disruption.
Protocol
All experiments are approved by the national animal experiment board.
1. Preparations
Prepare all solutions and keep in room temperature, unless otherwise indicated. Follow waste disposal regulations to dispose used materials and solutions.
Use NMRI and ICR outbred stocks, between ages 4-12 weeks and either gender. If using the C57BL/6 strain, follow the ketamine-medetomidine preparation method in step 1.3.2. For other steps, follow the instructions described in 1.3.3.-1.3.5.
- Prepare the veterinary medicine fresh and in time before starting the operation. Carprofen will be used later, thus it is not necessary to prepare it at this point.
- To prepare a solution of ketamine-medetomidine for anesthesia, mix 0.375 mL (stock concentration 50 mg/mL) of ketamine (Ketaminol Vet) with 0.25 mL (stock concentration 1 mg/mL) of medetomidine (Cepetor Vet) and add 1.25 mL of sterile 0.9% NaCl. Administer 0.15 mL/20 g of mouse weight (7.5 mg/kg, ketamine and 1 mg/kg, medetomidine).
- To prepare a more dilute solution of ketamine-medetomidine for anesthesia on C57BL/6 mice, mix 0.375 mL (stock concentration 50 mg/mL) of ketamine with 0.25 mL (stock concentration 1 mg/mL) of medetomidine and add 2.5 mL of sterile 0.9% NaCl. Administer 0.15 mL/20 g of mouse weight (3.75 mg/kg, ketamine and 0.5 mg/kg, medetomidine). NOTE: This is an alternative step, only for adult C57BL/6 mice.
- To prepare buprenorfin for analgesia, add 0.1 mL (stock concentration 0.3 mg/mL) of buprenorfin to 1.9 mL of sterile 0.9% NaCl. Administer 0.2 mL/20 g mouse weight (0.15 mg/kg).
- To prepare atipamezole for discontinuing anesthesia, add 0.1 mL (stock concentration 5 mg/mL) of atipamezole to 4.9 mL of 0.9% NaCl. Administer 0.15 mL/20 g mouse weight (0.75 mg/kg).
- For analgesia during post-anesthesia use carprofen. Add 0.1 mL (stock concentration 50 mg/mL) of carprofen to 4.9 mL of 0.9% NaCl. Administer 0.15 mL/20 g of mouse weight (7.5 mg/kg).
- Prepare fluorescent staining solution
- For visualizing the abrasion under the Cobalt blue light (Figure 1), use a fluorescent solution. Prepare a 0.1% fluorescein solution by first measuring 10 mg of fluorescein salt with a fine scale and then add it to 10 mL in phosphate-buffered saline solution (PBS).
- Protect the fluorescein solution from light and shake for 5 minutes on a shaker. NOTE: The fluorescein solution is light-sensitive; keep the solution covered from light. This solution can be stored in +4 °C for 2-3 days. For use, it is helpful to place the fluorescein solution in a dropper bottle that is used for eye drops. Use a sterile-filter when moving the solution to the dropper bottle.
Clean the ocular burr tip before and after use; first with PBS and then with 70% EtOH. If making an abrasion to several mice during the same operation, do the cleaning steps between each mouse.
Put the heated plate on (+37 °C) and place a paper towel on it.
2. Corneal Abrasion
CAUTION: Use protective wear (gloves, lab coat) when handling mice. Weigh the mouse to estimate the volume of medication to administer.
Use the one-handed scruffing method to handle the mouse23. Administer ketamine-medetomidine mixture intraperitoneally (i.p.) to the left lower abdomen. Place the mouse to an individual cage and wait for it to fall asleep. This typically takes less than 5 minutes. Then, administer first buprenorfin and then atipamezole via i.p. injections. Use the same handling technique. NOTE: Once anesthesia is given, the protocol should not be paused at any moment.
Before continuing, check that the heated plate is warm. Place the anesthetized mouse on the heated plate. The level of anesthesia is good if the tail reflex is absent, but the toe reflex is present. Check these reflexes by pinching the tail and any toe with fingers or forceps. Do not use excessive force. Anesthesia will last 20-25 minutes.
- To make an abrasion, use the cleaned ocular burr. Rotate the base of the burr to turn on the vibration that is essential to make the abrasion. Feel the vibration in hand, when the burr is functioning properly.
- Open one eye at a time by holding the eyelids separately with fingers. Then firmly touch the burr to the cornea and move the instrument back and forth as well as sideways on the ocular surface. The burr vibration will perform the scratch; do not press, shake or tear the cornea. In the central cornea, 20 abrading movements on the surface are sufficient to induce the wound. Do not lift the burr and try to stay in the region of the cornea to be abraded. Acquiring a standard-sized abrasion will require several rounds of practice.
3. Imaging the Abrasion
- Illuminate the abraded region using Cobalt blue pen light (Figure 1) and fluorescein solution. The ocular surface appears colorless in ambient light. After fluorescein application, the abraded region fluoresces in green when illuminating the eye with the pen light.
- If needed, open the abraded eye similarly as in 2.3 and administer one drop of fluorescein solution from the dropper bottle. Wash the eye once with PBS or 0.9% NaCl to reduce background of the fluorescein. Use a dropper bottle or a pipette for washing. Aspirate the liquid away with a soft wipe and clean the eyelashes, if needed. The excess liquid usually collects to the nasal border of the eye, close to the opening of the tear canal. CAUTION: Avoid touching the cornea while cleaining as minor abrasions might be induced.
- Take pictures of the corneal abrasion.
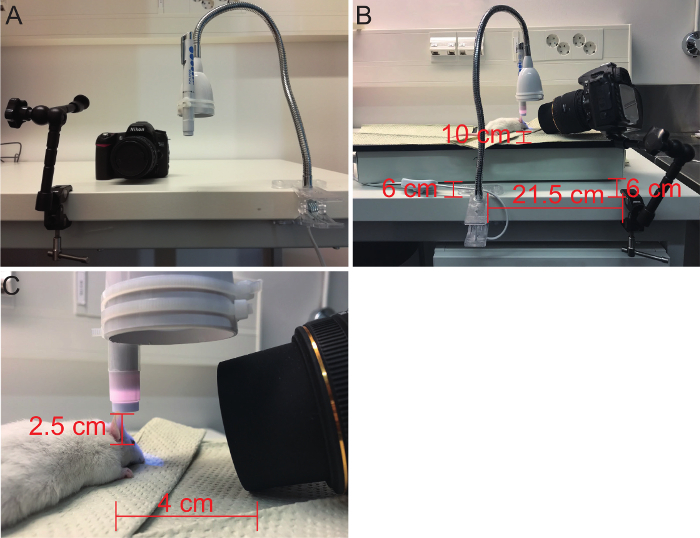
- To obtain similar images during each imaging session, use steady attachment tools for the Cobalt blue pen light and the SLR camera (Figure 2). This protocol provides a setup that is easy to repeat (Figure 2).
- To attach the pen light and the camera, use a table lamp with a flexible arm and clamp and an adjustable camera arm with a clamp, respectively. Cable-tie the pen light to the table lamp and position just above the mouse eye. Positioning the light differently might alter the visualization of the abrasion. A suggestion for positioning these tools is described in Figure 2.
- Use the SLR camera to take pictures of the eye. Place the animal in a desired distance and position and focus the camera (1:12) in room light. After that, close all other lights except the Cobalt blue pen light. NOTE: Tape the pen light handle down or use a rubber band to keep the light on all the time during imaging. This might help if the image becomes blurry. It is easiest to perform the imaging step with a colleague.
4. Waking up after Corneal Abrasion
Administer first buprenorfin and then atipamezole via i.p. injections. Use the same handling technique as in 2.1.
Place a drop of antibacterial, fucidin acid eye ointment (Isathal) to both abraded and non-abraded eyes to moisturize and keep the ocular surface clean from bacteria after anesthesia.
As the mouse will wake up in 5-20 minutes on the heated plate, observe the mouse during the post-anesthetic wake-up period. When it becomes mobile, place it in an individual cage. NOTE: If waking up takes longer, place the mouse in the cage with a heated pad or place the entire cage on the heated plate and monitor the mouse regularly. While the anesthesia wears off, the mouse typically shakes and reaches upwards with the anterior body. This behavior should return to normal in 3-4 hours and then you can place the mouse back to a shared cage.
Administer carprofen i.p. for analgesia and eye ointment on two consecutive days after the abrasion, similarly as in steps 2.2. and 4.2.
5. Imaging during Wound Healing
- In this protocol, use time points 18 h and 72 h after abrasion to observe the wound closure.
- For consecutive imaging, anesthetize the mice as explained in 2.2.
- Take pictures as explained in 3.
- Wake up the animals with atipamezole i.p. and monitor the wake-up period as explained in 4.3. NOTE: If not continuing the follow-up, euthanize the mouse right after 5.1.2. Euthanasia is described in 6.1.
6. Cornea Collection and Paraffin Embedding
After the last time point, sacrifice the animal. Place the mouse in a chamber that can be filled with CO2. Fill the chamber air with CO2. When the animal is immobile, dislocate the cervical vertebrae by pulling firmly from the base of the tail and pressing the neck region down at the same time. CAUTION: Always follow the guidelines of the local animal welfare body.
- Collect the whole eyeball by cutting an opening in the skin to the side of the eye with dissection scissors. Place the scissors under the eyeball and cut the ocular nerve to pop the eyeball out of the orbit. Use forceps if the eye does not come out easily.
- Make a hole on the back of the eye, on the retinal side, with a 26G needle to allow free penetration of solutions inside the eye. NOTE: Do not let the eye become dry, instead, place it in PBS until continuing with the protocol.
Collect the eyeball in a 2 mL tube with PBS. Do not pause the experiment at this point.
Remove PBS and fix eyeballs in 4% paraformaldehyde (PFA) in +4 °C for 4-5 hours.
- Move the fixed eyeball to a tissue cassette for tissue processing. Use a tissue processing machine and the following program:
- Rinse with PBS.
- Incubate 2 x 45 min in 70% ethanol at room temperature.
- Incubate 2 x 1 h in 94% ethanol at room temperature.
- Incubate 3 x 45 min in absolute ethanol at room temperature.
- Incubate 3 x 1 h in xylene, at room temperature and last xylene at 37 °C.
- Incubate 3 x 1 h in paraffin wax at 60 °C.
After tissue processing, embed the eyeball into liquid paraffin (+ 60 °C) using a tissue embedding block. Place the eye inside the block so that it is looking sideways and the peripheral border is facing upwards. Let the block solidify on a cool plate for 5-10 minutes.
7. Paraffin Sections of the Cornea
Prepare the microtome for sectioning according to the institution's or manufacturer's instructions. CAUTION: Be careful when handling the sharp microtome blade.
Place one water bath with room temperature ultrapure water beside the microtome and another with ultrapure water heated to +50 °C.
Make 5 µm thick sections of the eyeball. NOTE: The lens breaks easily during sectioning. To avoid this, wipe the cutting surface with a moist tissue each time a new row of sections is started. Use tap water to moisten the tissue.
Place the sections beside each other in the room temperature water bath and then move them to the hot water bath with the help of a glass slide. Stretch the sections in the hot water bath until all wrinkles relax and the sections become matte.
Dry the glass slides with sections overnight in +37 °C.
Attach the sections to the slides by keeping the slide for 1 minute on a heated plate in +60 °C. After this, the sections can be directly processed for staining, immunohistological methods or stored in +4 °C.
Representative Results
This protocol describes a model to inflict an abrasion injury to the mouse cornea and suggests how to follow and visualize the healing process after abrasion. Recently, we employed this method to study the role of corneal epithelial progenitor cells during wound healing6. The use of established tools is the key to a successful abrasion experiment. We, and others, have used the Algerbrush II ocular burr (Figure 1) to perform the abrasions6,7,24. This tool has a dulled burr tip of 0.5 mm in size that brushes rather than drills or shears the corneal epithelium away. Thus, this tool is the recommended choice for an abrasion injury on the cornea.
A central part of this model is that the affected region can be effortlessly visualized at desired intervals. In Figure 3A, we present the use of fluorescein staining in combination with a Cobalt blue light source (Figure 1) to display the abrasion site. In this experiment, we performed a large wound in the central cornea and left the peripheral cornea untouched (Figure 3A). Furthermore, we abraded only the left eye of each mouse and kept the right, bilateral eye as a non-abraded control. The bilateral eye samples remained negative for fluorescein signal, which suggests that they do not undergo any cell loss during the experiment and thus function well as control samples. However, green signal in the borders of the eye displayed the fluorescein solution that accumulated in the junction between the eye and the eyelid. The abrasion was largest at 0 h, immediately following the injury (Figure 3A). It was markedly smaller after 18 h and, in this case, located close to the upper border of the eye. However, the epithelium closes the exposed region at an equal speed from all sides of the abrasion. Notably, at 72 h post-wounding, no green signal was visible. This indicates that the corneal epithelium was fully re-epithelialized by 72 h after the abrasion.
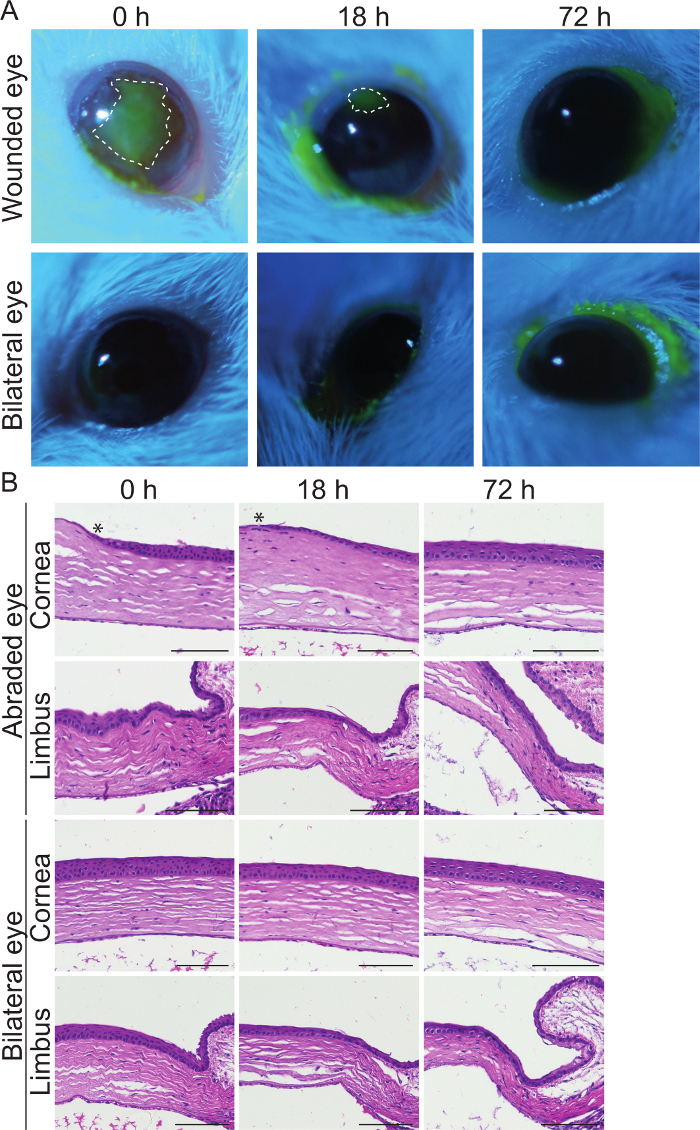
With this method, we aimed at focusing the abrasion only on the corneal epithelium, so that deeper layers of the cornea remained intact. The removal of the corneal epithelium was evident from histological sections in Figure 3B. At 0 h, the edge of the abrasion is shown as a narrow, acellular ledge that is continuous from a regular, 4-5 cell layers thick corneal epithelium. The limbus, peripheral border of the corneal epithelium, presents an example region that was not affected by the abrasion during the entire experimental timeline (72 h). In addition, histological sections indicated that the deeper layers of the cornea, the stroma and the endothelium, were not harmed by the ocular burr. These two tissues appeared similar in abraded and bilateral eye samples. At 18 h post-injury, the healing process is active and re-epithelialization is ongoing. This was suggested by the appearance of a leading edge. The leading edge contains only 1-2 epithelial cell layers and it covers the exposed region before re-stratification can occur25. In line with the results on Figure 3A, the surface was fully re-epithelialized by 72 h, when the migrating fronts had covered the wound and all the epithelial layers were again present. Histological sections from the bilateral eye confirmed (Figure 3A) that the control eyes remained intact during the observation period.
Lastly, we provide evidence that the abrasion model is restricted to the corneal epithelium and does not invoke a stromal response to injury, such as neovascularization. Figure 4 shows the murine cornea at 18 h after abrasion. Based on macroscopic view, this time point did not display a stromal response, thus indicating that our protocol does not disrupt the deeper corneal layers. In comparison, an alkaline injury with 0.75 mol/L NaOH immediately induced neovascularization in the stroma. Given the rapid neovascularization in alkaline injury, the time points in our method provide evidence to rule out the possibility of response in the stroma.
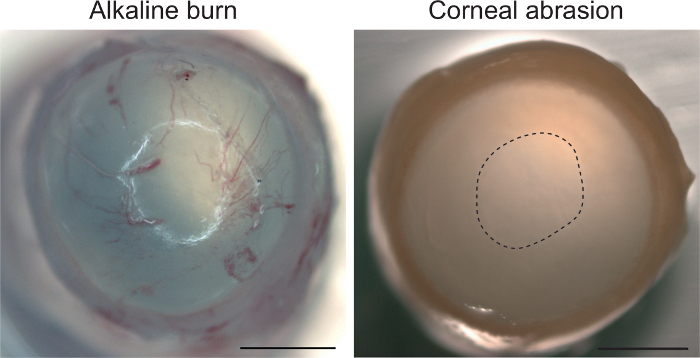
Figure 1: Essential tools for corneal epithelial abrasion. On the left is a vibrating ocular burr. The Cobalt blue pen light in the right is used to bring the fluorescein stain visible. Please click here to view a larger version of this figure.
Figure 2: Imaging the corneal abrasion. (A) Tools for imaging are an adjustable camera arm with clamp (left), the SLR camera (center) and a table lamp with flexible arm and clamp (right). The Cobalt blue pen light is tied to the dome of the lamp with two cable ties. (B) A general view of the imaging setup; the distance of each attachment tool is marked in the image in cm. Both clamps are 6 cm away from the heat plate and the mouse is placed 10 cm away from the heat plate edge. (C) A closer look at the abrasion imaging with proposed distance and position to the mouse eye in cm. Please click here to view a larger version of this figure.
Figure 3: Detection and localization of the corneal abrasion.(A) Mouse eyes at 0, 18, and 72 h after abrasion. Fluorescein signal marks the abraded region in bright green, whereas all other regions in the epithelium remain dark. Bilateral eyes serve as controls. Dashed line marks the borders of the wound and the white spot in each eye is the reflection from the camera. (B) Histologically sectioned and hematoxylin and eosin stained samples of the mouse eyes at 0, 18, and 72 h after abrasion. Limbus is the border that surrounds the corneal epithelium from all sides. Asterix marks the edge of the abrasion. Scale bar is 100 µm. Please click here to view a larger version of this figure.
Figure 4: Corneal abrasion does not activate a stromal response. Alkaline exposure with 0.75 mol/L NaOH followed by irrigation with 0.9% NaCl produces corneal neovascularization (eye collected immediately after treatment). Abrasion with the ocular burr shows no neovascularization even after 18 h. Abrasion site is marked with a dashed line. Scale bar 1 mm. Please click here to view a larger version of this figure.
Discussion
Wounding methods are popular tools to study different aspects of corneal homeostasis and pathologies. The abrasion model offers a well-controlled method to address relevant problems in ophthalmology. However, certain critical points in the protocol are worth emphasizing. Notably, the details outlined regarding the veterinary medicine, wound healing timeline, and outcome are optimized for use with outbred NMRI and ICR stocks, but may vary among strains of mice26. With this protocol, the experimental animals will remain anesthetized for approximately 20 minutes. This gives a short but sufficient window of time to perform the abrasion. However, when using the ocular burr, making the abrasion itself is a very quick operation and should be possible to perform in this time frame.
Easy and accessible visualization is one of the main advantages of the abrasion model. Combined with molecular biology methods, this opens possibilities to study the epithelial cells in great detail. Abraded corneas can be processed for histological or immunological staining's and proteins or nucleic acids can be collected and examined further. Pajoohesh-Ganji et al. showed that gene expression patterns in the corneal epithelial cells change upon corneal insult with a dulled blade27. To ensure controlled sampling, we recommend that images of the abrasions are taken immediately after the operation and sample collections follow exactly the planned timelines.
This protocol has uses beyond examination of corneal epithelialization. For example, abrasion of the corneal limbus, the stem cell niche of the cornea, may be used to study stem cell dynamics7. We showed that the non-abraded region of the epithelium remained normal during the healing process, however some authors claim that the basement membrane and the underlying stromal keratocytes are affected by the ocular burr24,28. The removal of the basement membrane can be estimated with specific basement membrane staining. Furthermore, repeated abrasions over a longer timeline could reveal interesting healing patterns. In this protocol, we did not observe the architecture of the epithelium over a long-term chase. Both dulled scalpel and ocular burr injuries have shown an increased incidence to corneal erosions after one to several weeks after injury24,29,30. This should be kept in mind when using the abrasion model for long-term studies. However, studies focusing on corneal erosions might find this protocol useful.
Other wounding models are described at length by Stepp et al. in a review5. Together, this protocol and the existing approaches provide versatile options to examine both fundamental biological questions as well as practical clinical problems.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We would like to thank Kaisa Ikkala for her invaluable technical assistance and insightful help when actualizing this method as well as later on during implementation to our central research questions. We would also like to thank the Laboratory Animal Center and Anna Meller for her help with planning the guidelines of the veterinary work.
References
- Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investigative Ophthalmology & Visual Science. 2000;41(13):4093–4100. [PubMed] [Google Scholar]
- Wang Y, Chen M, Wolosin JM. ZO-1 In Corneal Epithelium; Stratal Distribution and Synthesis Induction by Outer Cell Removal. Experimental Eye Research. 1993;57(3):283–292. doi: 10.1006/exer.1993.1126. [DOI] [PubMed] [Google Scholar]
- Sugrue SP, Zieske JD. ZO1 in Corneal Epithelium: Association to the Zonula Occludens and Adherens Junctions. Experimental Eye Research. 1997;64(1):11–20. doi: 10.1006/exer.1996.0175. [DOI] [PubMed] [Google Scholar]
- Jackson H. Effect of eye-pads on healing of simple corneal abrasions. British Medical Journal. 1960;2(5200):713. doi: 10.1136/bmj.2.5200.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, et al. Wounding the cornea to learn how it heals. Experimental Eye Research. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalha S, Shrestha B, Sanz Navarro M, Jones KB, Klein OD, Michon F. Bmi1+ Progenitor Cell Dynamics in Murine Cornea During Homeostasis and Wound Healing. Stem Cells. 2018. [DOI] [PMC free article] [PubMed]
- Nasser W, et al. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Reports. 2018;22(2):323–331. doi: 10.1016/j.celrep.2017.12.040. [DOI] [PubMed] [Google Scholar]
- Kaplan N, Fatima A, Peng H, Bryar PJ, Lavker RM, Getsios S. EphA2/Ephrin-A1 Signaling Complexes Restrict Corneal Epithelial Cell Migration. Investigative Ophthalmology & Visual Science. 2012;53(2):936. doi: 10.1167/iovs.11-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J-Q, Qin H-F, Zhao S-H. Research on mouse model of grade II corneal alkali burn. International Journal of Ophthalmology. 2016;9(4):487–490. doi: 10.18240/ijo.2016.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MF, et al. Protective effects of matrix metalloproteinase-12 following corneal injury. Journal of Cell Science. 2013;126:3948–3960. doi: 10.1242/jcs.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. American Journal of Pathology. 2009;175(2):571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai-Lange A, et al. A Method for Lineage Tracing of Corneal Cells Using Multi-color Fluorescent Reporter Mice. Journal of Visualized Experiments. 2015. p. e53370. [DOI] [PMC free article] [PubMed]
- Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage Tracing of Stem and Progenitor Cells of the Murine Corneal Epithelium. Stem Cells. 2015;33(1):230–239. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AEK, Stepp MA, Zieske JD. αVβ6 Integrin Promotes Corneal Wound Healing. Investigative Ophthalmology & Visual Science. 2011;52(11):8505. doi: 10.1167/iovs.11-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AEK, Zieske JD. Role of Thrombospondin-1 in Repair of Penetrating Corneal Wounds. Investigative Ophthalmology & Visual Science. 2013;54(9):6262. doi: 10.1167/iovs.13-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Kiorpes TC. Epithelial sheet movement: Protein and glycoprotein synthesis. Developmental Biology. 1982;92(1):259–262. doi: 10.1016/0012-1606(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Gipson IK. Actin "purse string" filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. Journal of Cell Science. 1998;111:3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo C-K. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. Journal of Biological Chemistry. 2005;280(22):21653–21660. doi: 10.1074/jbc.M500374200. [DOI] [PubMed] [Google Scholar]
- Nagata M, Nakamura T, Hata Y, Yamaguchi S, Kaku T, Kinoshita S. JBP485 promotes corneal epithelial wound healing. Science Reports. 2015;5:14776. doi: 10.1038/srep14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Zhu L, Cranfill R. Changes in beta 4 integrin expression and localization in vivo in response to corneal epithelial injury. Investigative Ophthalmology & Visual Science. 1996;37(8):1593–1601. [PubMed] [Google Scholar]
- Stepp MA, Zhu L. Upregulation of alpha 9 integrin and tenascin during epithelial regeneration after debridement in the cornea. Journal of Histochemistry & Cytochemistry. 1997;45(2):189–201. doi: 10.1177/002215549704500205. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Tadvalkar G, Jurjus RA, Zieske JD, Stepp MA. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Experimental Eye Research. 2008;87(5):478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JoVE Science Education Database. Lab Animal Research. Rodent Handling and Restraint Techniques. 2018. Available from: https://www.jove.com/science-education/10221/rodent-handling-and-restraint-techniques.
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA. Removal of the basement membrane enhances corneal wound healing. Experimental Eye Research. 2011;93(6):927–936. doi: 10.1016/j.exer.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Progress in Retinal and Eye Research. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Sato Y, Seo N, Kobayashi E. Genetic background differences between FVB and C57BL/6 mice affect hypnotic susceptibility to pentobarbital, ketamine and nitrous oxide, but not isoflurane. Acta Anaesthesiologica Scandinavica. 2006;50(5):553–556. doi: 10.1111/j.1399-6576.2006.001002.x. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. K14 + Compound niches are present on the mouse cornea early after birth and expand after debridement wounds. Developmental Dynamics. 2016;245(2):132–143. doi: 10.1002/dvdy.24365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boote C, et al. Quantitative Assessment of Ultrastructure and Light Scatter in Mouse Corneal Debridement Wounds. Investigative Ophthalmology & Visual Science. 2012;53(6):2786. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, et al. MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice. Journal of Cell Science. 2011;124(Pt 15):2666–2675. doi: 10.1242/jcs.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: alpha9beta1 integrin implicated in progression of the disease. Investigative Ophthalmology & Visual Science. 2004;45(6):1775–1788. doi: 10.1167/iovs.03-1194. [DOI] [PubMed] [Google Scholar]


