Abstract
Timely and efficient reperfusion of the occluded coronary artery is the best strategy for decreasing myocardial infarct size in patients with a ST-segment elevated myocardial infarction. However, reperfusion per se can result in further cardiomyocyte death, a phenomenon known as reperfusion injury. The opening of the mitochondrial permeability transition pore (mPTP), with the decrease of the mitochondrial membrane potential (MMP), or mitochondrial depolarization, is universally recognized as the final step of reperfusion injury and is responsible for mitochondrial and cardiomyocyte death. JC-1 is a lipophilic cationic dye that accumulates in mitochondria depending on the value of MMP. The higher the MMP is, the more JC-1 accumulates in the mitochondria. The increasing amounts of JC-1 in mitochondria can be reflected by a fluorescence emission shift from green (~530 nm) to red (~590 nm). Therefore, the reduction of the red/green fluorescence intensity ratio can indicate the depolarization of mitochondria. Here, we take advantage of JC-1 to measure MMP, or the opening of mPTP in human cardiac myocytes after hypoxia/reoxygenation, detected by flow cytometry.
Keywords: Behavior, Issue 137, Mitochondrial permeability transition pore, mitochondrial membrane potential, JC-1 assay, reperfusion injury, cardiac myocytes, flow cytometry
Introduction
Coronary heart disease is the leading cause of death worldwide. The treatment of choice for reducing ischemic injury and limiting infarct size in patients with ST-segment elevated myocardial infarction is timely and effective myocardial reperfusion via primary percutaneous coronary intervention (PCI)1,2. However, reperfusion causes additional damage, which can account for up to 30 percent of the final infarct size3. It is universally acknowledged that the mitochondrial permeability transition pore (mPTP) is not only central in mitochondrial damage and cell death during ischemia/reperfusion (I/R), but is also a converging target of cardioprotective signaling4,5. As the mPTP opening would bring about depolarization of the inner mitochondrial membrane potential (MMP)4, we detected mPTP opening using the 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanineiodide (JC-1) assay.
The JC-1 assay is a cytofluorimetric method that is both qualitative and quantitative, and it has been further validated by analyzing the MMP at the level of a single mitochondria6. JC-1 exists as an aggregated form, yielding a red to orange colored emission (590 ± 17.5 nm) in the matrix of mitochondria with normal MMP; with the loss of MMP, JC-1 is converted to the monomeric form that yields green fluorescence with an emission of 530 ± 15 nm. Therefore, a decrease in the red/green fluorescence intensity ratio could indicate the reduction of MMP in conditions such as ischemia/reperfusion (I/R).
In addition to JC-1, MMP has also been studied with membrane-permeable lipophilic cations such as Rhodamine 123 and 3,3′-dihexyloxadicarbocyanine iodide [DiOC6(3)]. However, compared to these two probes, JC-1 is more reliable for analyzing MMP. Rhodamine 123 has relatively poor sensitivity (especially in quenching mode7,8) and poor specificity. The shift in rhodamine 123 sometimes is so small that it is hard for researchers or equipment to observe/detect. Besides, in a single cell, there are different mitochondrion binding sites for rhodamine 123 and so that it may have different fluorescence emissions9. DiOC6(3) is not recommended for detecting MMP either as it reacts sensitively to the depolarization of plasma membrane10.
Therefore, here we use the JC-1 assay to assess the MMP of HCMs after being exposed to hypoxia/reoxygenation with or without a protective agent.
Protocol
1. Preparation of Reagents and Solutions
Prepare human cardiac myocytes (HCMs) complete medium by adding 25 mL of Myocyte Growth Medium supplement mix to 500 mL of Myocyte Growth Medium as per the manufacturer’s instructions. Store at 4 °C and warm to 37 °C before using.
Prepare Tongxinluo (TXL) Solution by dissolving TXL ultrafine powder in serum/glucose-free Dulbecco’s modified Eagle’s medium (DMEM) and adjust the concentration of TXL to 400 µg/mL by adding DMEM (for more details, see11). Store at 4 °C and warm to 37 °C before using.
Prepare 5 mL of JC-1 working solution by adding 25 µL of JC-1 stock solution (200x), 4 mL of ultrapure water, and 1 mL of stock staining buffer (5x) in a 15 mL centrifuge tube in the dark. Pipette the mixture up and down several times and warm to 37 °C before using.
Prepare 30 mL of working staining buffer by adding 24 mL of ultrapure water to 6 mL of stock staining buffer (5x) in a 50 mL centrifuge tube. Use this solution ice-cold.
2. Establishment of Hypoxia/Reoxygenation Model
Plate 1.0 x 105 cells per well with HCM complete medium in a 6 well plate. Grow to reach a confluency of about 70% in a cell incubator containing 5% CO2 and 95% air at 37 °C.
Wash HCMs with phosphate buffer saline (PBS). Expose them to different treatments (400 µg/mL TXL or not) in 2 mL of serum/glucose-free DMEM for 30 min prior to hypoxia in a cell incubator containing 5% CO2 and 95% air at 37 °C.
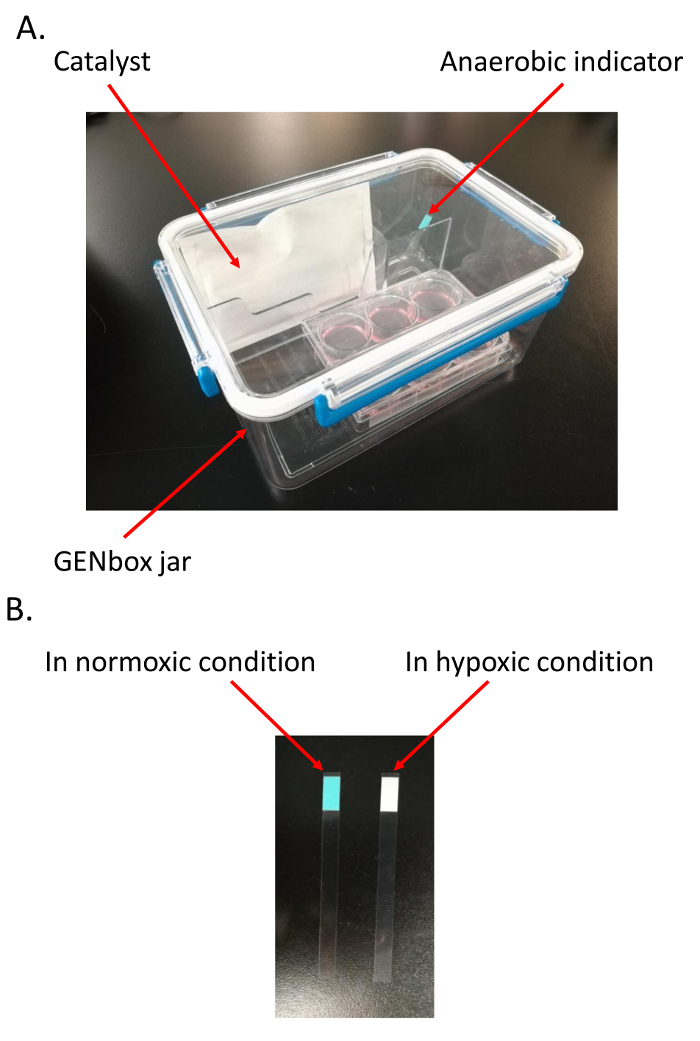
Incubate HCMs in an airtight and hypoxic jar (2.5 L) fitted with a catalyst to scavenge free oxygen and induce hypoxia for 18 h (Figure 1A), and an anaerobic indicator to measure the oxygen tension in the medium11,12,13, in a cell incubator containing 5% CO2 and 95% air at 37 °C. Note: It takes about 1.5 h for the catalyst to scavenge free oxygen in the jar. Therefore, the HCMs are kept in the jar for 19.5 h before the jar is opened. The color of anaerobic indicator changes from light blue in normoxic environment to pale white in hypoxic condition (Figure 1B).
Reoxygenate HCMs by moving the 6 well plate into an incubator set at 37 °C containing 5% CO2 and 95% air for 2 h.
3. Preparation of HCMs for Flow Cytometry
Warm the 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) solution and PBS to 37 °C.
Aspirate medium from each well, rinse the cells with 1 mL of PBS and add 0.5 mL of 0.25% trypsin along the wall of the well.
Incubate at 37 °C until all HCMs are almost detached (about 1 min).
Add 1 mL of DMEM complete medium (DMEM medium with 10% fetal bovine serum) to the wells to neutralize the trypsin. Transfer the cell suspension to a 15 mL centrifuge tube and pellet HCMs by centrifuging at 500 x g for 3 min at room temperature.
Carefully aspirate the supernatant without disturbing the pellet.
Add 0.5 mL of HCM complete medium and 0.5 mL of JC-1 working solution to each tube. Resuspend the HCMs by pipetting up and down.
Cover the whole tubes with aluminum foil to prevent bleaching of the fluorescent indicator and incubate HCMs in normal conditions (at 37 °C containing 5% CO2 and 95% air) for 20 min.
Pellet HCMs by centrifuging at 500 x g for 3 min at 4 °C.
Rinse HCMs twice with 2 mL of ice-cold JC-1 staining buffer.
Resuspend HCMs in a final volume of 300 µL of ice-cold staining buffer in the sample tube (12 mm x 75 mm) and use the cells for analysis within 30 min. Note: Use carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a potent inhibitor of respiratory electron transport chain, to treat HCMs for 1 h at a concentration of 20 µM, for the positive control.
4. Flow Cytometer Setup
Start the flow cytometer and ensure that the software is connected to it.
Perform fluidics startup, start the stream and set up the breakoff.
- Open two dot plot windows in the flow cytometry software.
- Select forward scattered light (FSC) on the X axis and side scattered light (SSC) on the Y axis in the first dot plot to represent the characteristics of the cells in terms of their size and their granularity, respectively.
- Select the PE (575 ± 26 nm) detection channel for the Y axis and FITC (530 ± 30 nm) for the X axis using a logarithmic scale in the second dot plot, which is used to measure the fluorescence intensity of JC-1 dye in the cells.
5. Flow Cytometry Measurement and Data Analysis
Click Unload to let out the tube support arm and position the Blank (HCMs that have not been dyed with JC-1) sample tube on it. Click Load to return the tube support arm to its working position.
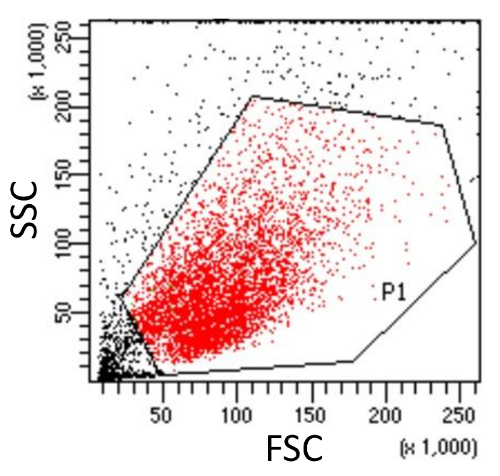
Click Record to collect particles from suspension in the Blank sample tube and then gate the cell population (P1) for further analysis in the first dot plot (Figure 3).
Collect HCMs in other sample tubes and optimize the measurement by adjusting the voltages of fluorescence channels, to make sure that the cell dot is located in the central area of the second dot plot.
Display the statistics of each sample tube and calculate the MMPs of each sample by dividing the ratio of red fluorescence intensity by that of green fluorescence intensity.
Representative Results
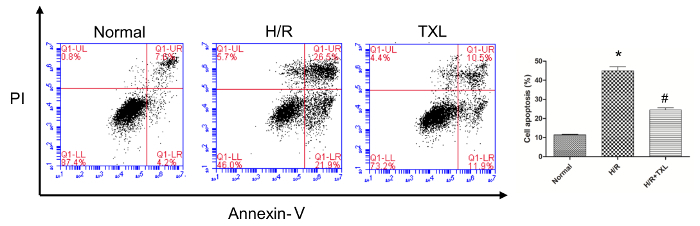
Before performing the JC-1 assay to evaluate the changes of MMP, it is highly recommended that experiments be carried out to confirm the conditions successfully set by the researchers. As shown by the flow cytometry results (Figure 2), compared with the normal group, hypoxia/reoxygenation (H/R) significantly induced the apoptosis of HCMs (Annexin V + /PI±), indicating that we had established a cell-based model of I/R (45.00 ± 2.13% vs. 11.50 ± 0.18% in the normal group, p <0.05). Tongxinluo, a Chinese traditional medicine with cardioprotective effects, prevented the apoptosis of HCMs in condition of H/R (24.50 ± 1.13% vs. 45.00 ± 2.13% in the H/R group, p <0.05).
CCCP is a protonophore that can inhibit oxidative phosphorylation in mitochondria by uncoupling the proton gradient (loss of MMP) established during the normal activity of electron carriers in the electron transport chain. Consequently, HCMs treated with CCCP was set as positive control. As shown in Figure 4, the red/green ratio in the CCCP-treated group was almost zero. Consistent with the results from the apoptosis assay, the H/R-treated group displayed a significantly lower red/green ratio compared with the normal group (0.69 ± 0.07 vs. 5.64 ± 0.21 in the normal group, p <0.05) and Tongxinluo reversed such a ratio change (2.92 ± 0.22 vs. 0.69 ± 0.07 in the H/R group, p<0.05).
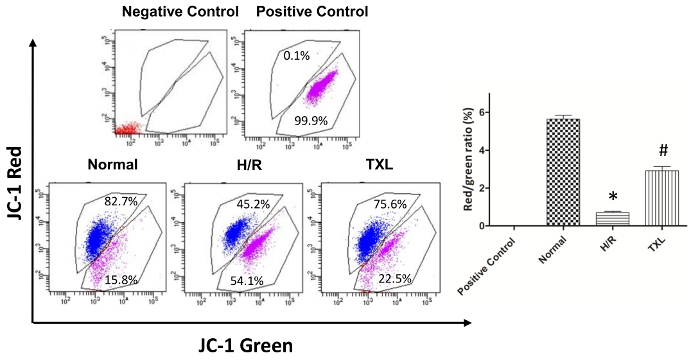
Figure 1. Establishment of hypoxia/reoxygenation (H/R) model. A. Materials needed to establish the H/R model; B. Confirmation of the hypoxic environment in the jar. The color of anaerobic indicator changes from light blue in normoxic environment to pale white in hypoxic condition. Please click here to view a larger version of this figure.
Figure 2. Validation of the establishment of hypoxia/reoxygenation (H/R) model by flow cytometry. Compared with the normal group, the apoptotic rate of human cardiac myocytes was significantly higher in the H/R group, while Tongxinluo reversed this change (*p <0.05 vs. Normal; # p <0.05 vs. H/R; n = 3)11. Please click here to view a larger version of this figure.
Figure 3. Gating cell population (P1, Red) in the SSC versus FSC dot plot for further analysis. The integrity of the cell population is assessed using side scatter (SSC) and forward scatter (FSC). Cell debris (Outside the gate P1, Black), which have reduced light scattering, are excluded. Please click here to view a larger version of this figure.
Figure 4. Evaluation of mitochondrial membrane potential in human cardiac myocytes. Compared with the normal group, the H/R group displayed a significantly lower red/green ratio, while Tongxinluo reversed this trend (Red: cells with no fluorescence; Blue, cells with red fluorescence; Purple, cells with green fluorescence. Negative Control, blank and no staining; Positive Control, cells treated with CCCP; *p <0.05 vs. Normal; #p <0.05 vs. H/R; n = 3)11. Please click here to view a larger version of this figure.
Discussion
Here, we present a protocol that uses JC-1 dye to assess the MMP of cells after being exposed to H/R. Detected by JC-1 assay, the MMP of cells is independent of factors such as mitochondrial size, shape, and density that may influence single-component fluorescence signals14. Consequently, the results of JC-1 assay are relatively reliable. Besides, it is convenient and time-saving to perform the JC-1 assay. This assay has a low requirement for materials and reagents, and generally, it takes less than 1.5 h to do the staining, from cell detachment to MMP measurement.
A few critical steps cannot be overstated when performing the JC-1 assay if researchers want to obtain reliable and satisfactory results. First of all, the cells loaded with JC-1 should be stored in ice-cold staining buffer until they are collected by the flow cytometer. Temperature has a considerable effect on the stability of MMP and the value of one sample varies a lot after a brief time in room temperature. In addition, preliminary experiments are recommended to determine the pretreating time and concentration of CCCP, ensuring that the red/green ratio of the positive control is ~0. It is noteworthy that carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), which can also eliminate MMP and inhibit oxidative phosphorylation, is an alternative to CCCP and can also be used to set up the positive control10.
The JC-1 assay has a major limitation in that other fluorochromes cannot be combined with JC-1, for its fluorescence emission spans the green, yellow, and part of the red wavelengths of the spectrum. Additionally, JC-1 assay must be combined with one or more methods, such as using calcein-acetoxymethylester15,16 or even genetic manipulation17,18, to determine if the opening of mPTP accounts for the decrease of MMP. This is because the opening of other mitochondrial pores like the ATP-sensitive K+ channels19,20 can also lead to the depolarization of mitochondria.
The JC-1 assay is best suited to more coarse "yes/no" assessments with the aim of evaluating whether mitochondria are largely polarized (e.g., apoptosis-related experiments)8. It has also been applied to measure MMP in a variety of cell types other than cardiomyocytes, including neurons21, hepatocytes22, renal cells23 and even isolated mitochondria24. In summary, we describe a quick, simple and sensitive method for the detection of MMP in HCMs after H/R.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by grants from The National Key Research and Development Program of China (No. 2017YFC1700503), the National Basic Research Program (973 Program) of China (No.2012CB518602), the National Natural Science Foundation of China (No. 81370223 and No. 81573957), and the Postgraduates' Innovative Research Foundation of Peking Union Medical College (2016-1002-01-02).
References
- Anderson JL, Morrow DA. Acute Myocardial Infarction. New England Journal of Medicine. 2017;376(21):2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- Ibanez B, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) European Heart Journal. 2017;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. New England Journal of Medicine. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. Journal of Molecular and Cellular Cardiology. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circulation Research. 2015;116(4):674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Ceccarelli D, Masini A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Experimental Cell Research. 1996;222(1):84–94. doi: 10.1006/excr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Ward MW, Rego AC, Frenguelli BG, Nicholls DG. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. Journal of Neuroscience. 2000;20(19):7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Salvioli S. Flow cytometric analysis of mitochondrial membrane potential using JC-1. Current Protocols in Cytometry. 2001. p. 14. Chapter 9 Unit 9. [DOI] [PubMed]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Letters. 1997;411(1):77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Chen GH, et al. Inhibition of miR-128-3p by Tongxinluo Protects Human Cardiomyocytes from Ischemia/reperfusion Injury via Upregulation of p70s6k1/p-p70s6k1. Frontiers in Pharmacology. 2017;8:775. doi: 10.3389/fphar.2017.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, et al. Induction of autophagy by Tongxinluo through the MEK/ERK pathway protects human cardiac microvascular endothelial cells from hypoxia/reoxygenation injury. Journal of Cardiovascular Pharmacology. 2014;64(2):180–190. doi: 10.1097/FJC.0000000000000104. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 2008;26(1):135–145. doi: 10.1634/stemcells.2007-0098. [DOI] [PubMed] [Google Scholar]
- Chazotte B. Labeling mitochondria with JC-1. Cold Spring Harbor Protocols. 2011. [DOI] [PubMed]
- Pravdic D, et al. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111(2):267–274. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. Suppression of Excessive Histone Deacetylases Activity in Diabetic Hearts Attenuates Myocardial Ischemia/Reperfusion Injury via Mitochondria Apoptosis Pathway. Journal of Diabetes Research. 2017;2017:8208065. doi: 10.1155/2017/8208065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, et al. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochemical and Biophysical Research Communications. 2014;448(1):15–21. doi: 10.1016/j.bbrc.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Zhen YF, et al. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. Journal of Cell Physiology. 2014;229(10):1475–1483. doi: 10.1002/jcp.24589. [DOI] [PubMed] [Google Scholar]
- Nazarewicz RR, Dikalova AE, Bikineyeva A, Dikalov SI. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305(8):H1131–H1140. doi: 10.1152/ajpheart.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang ZX, Xu YJ. Effect of mitochondrial KATP channel on voltage-gated K+ channel in 24 hour-hypoxic human pulmonary artery smooth muscle cells. Chinese Medical Journal (Engl) 2005;118(1):12–19. [PubMed] [Google Scholar]
- Kuter N, Aysit-Altuncu N, Ozturk G, Ozek E. The Neuroprotective Effects of Hypothermia on Bilirubin-Induced Neurotoxicity in vitro. Neonatology. 2018;113(4):360–365. doi: 10.1159/000487221. [DOI] [PubMed] [Google Scholar]
- Zheng YY, Wang M, Shu XB, Zheng PY, Ji G. Autophagy activation by Jiang Zhi Granule protects against metabolic stress-induced hepatocyte injury. World Journal of Gastroenterology. 2018;24(9):992–1003. doi: 10.3748/wjg.v24.i9.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gamal H, Eid AH, Munusamy S. Renoprotective Effects of Aldose Reductase Inhibitor Epalrestat against High Glucose-Induced Cellular Injury. Biomed Research International. 2017;2017:5903105. doi: 10.1155/2017/5903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Luna-Vargas MP, Chipuk JE. Mouse Liver Mitochondria Isolation, Size Fractionation, and Real-time MOMP Measurement. Bio-Protocols. 2016;6(15) doi: 10.21769/BioProtoc.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]


