Abstract
Purpose
Pregabalin is indicated for postherpetic neuralgia (PHN) in multiple countries, including China. This analysis compared pregabalin efficacy and safety in Chinese and international patients with PHN.
Patients and methods
Data from Chinese and international randomized, double-blind, placebo-controlled trials were compared. Pregabalin was administered at fixed (150, 300, or 600 mg/day) or flexible (150–600 mg/day) doses. The main efficacy measure was mean pain score change at endpoint on an 11-point numeric rating scale ranging from 0 = no pain to 10 = worst possible pain. Secondary efficacy measures included proportions of 30% and 50% pain responders, pain-related sleep interference (PRSI) scores, and proportions of Patient Global Impression of Change (PGIC) responders. The incidences of serious adverse events (SAEs) and adverse events (AEs) were used to assess safety. The effect of baseline pain severity on efficacy was tested. The proportions of patients with severe baseline pain who had moderate or mild pain at endpoint were also assessed.
Results
A total of 1166 patients were analyzed: 312 Chinese and 854 international. Overall, results were similar between Chinese and international patients. Pregabalin statistically significantly improved mean pain score versus placebo (least squares mean difference [95% CIs]: Chinese, −0.8 [–1.2, −0.5]; international, −1.3 [–1.6, −1.0]; both p<0.001). Pregabalin was statistically significantly better than placebo in Chinese and international patient groups in the proportions of 30% and 50% pain responders, PRSI scores, and proportions of PGIC responders. Baseline pain severity did not affect efficacy, except for some measures in Chinese patients with moderate baseline pain. Similar proportions of pregabalin-treated patients with severe baseline pain had moderate or mild pain at endpoint in both groups. SAE and AE profiles were comparable in Chinese and international patient groups, except incidences were commonly higher in international patients.
Conclusion
Chinese and international patients with PHN exhibit comparable pregabalin efficacy and safety, highlighting the utility of pregabalin for diverse PHN patient populations.
Keywords: China, pain, postherpetic neuralgia, pregabalin
Introduction
Postherpetic neuralgia (PHN) is a neuropathic pain condition that arises in response to acute herpes zoster infection.1,2 The reported incidence of PHN ranges from 5% to 30% of infected adults3–8 and increases with age.3,4,6–8 PHN-related pain negatively affects sleep as well as reducing quality of life, physical and social functioning, and psychological well-being.2,9–11
Pregabalin is an α2-δ calcium channel subunit ligand indicated globally for the treatment of PHN-related pain, including in the USA12 and across Europe.13 The efficacy and safety of pregabalin for PHN have been demonstrated in multiple international clinical trials.14–17 In these trials, pregabalin statistically significantly improved pain and disrupted sleep versus placebo, and patients were more likely to report a 30% or 50% improvement in pain, and global improvement, with pregabalin over placebo.14–17 However, few patients in these international trials were Asian or of Asian origin. More recently, clinical trials on pregabalin efficacy and safety for PHN have been conducted specifically in Chinese patients.18,19 In these Chinese trials, pregabalin also statistically significantly improved pain and disrupted sleep versus placebo, and patients were more likely to report a 30% improvement in pain, or global improvement, with pregabalin over placebo.18,19 Pregabalin is also indicated for PHN-related pain in China.20 Although pregabalin is minimally metabolized and therefore there is no reason to anticipate that its effectiveness would be different in different patient populations,12 whether the efficacy and safety profiles of pregabalin are similar in Chinese and international patient populations has not been tested. The objective of this post-hoc analysis was to compare the efficacy and safety of pregabalin in Chinese patients with PHN with an international patient population with PHN. The impact of baseline pain severity on efficacy responses in the two patient groups was also examined.
Materials and methods
Studies included in the post-hoc analysis
In total, 13 randomized, double-blind, placebo- controlled trials were considered for inclusion in the current study. Two trials were specifically conducted in Chinese patients (ClinicalTrials.gov identifiers: NCT00301223 and NCT01455428),18,19 and both were included in the analysis. Of the remaining 11 trials under consideration for inclusion in the international patient group, seven were excluded for the following reasons: did not complete (one trial); tested a controlled release formulation of pregabalin (one trial); duration shorter than 8 weeks (three trials); patient populations ethnically similar to Chinese patients (Japan and Korea, one trial each). As a result of exclusions, four trials were included in the international patient group. These trials were conducted in patients from Australia, Europe, and North America.14–17 The international trials were conducted before trial registration was required, so no ClinicalTrials. gov identifiers are available. All six of the trials included in this post-hoc analysis were originally approved by the institutional review board or ethics committee for each participating investigational center, and were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Each patient provided written informed consent to participate in the trial. No new patients were recruited for this post-hoc analysis.
The Chinese trials took place between February 2006 and January 2014, and the international trials between February 1999 and December 2002. One of the Chinese trials was in patients with PHN only, whereas the second was in patients with PHN or painful diabetic peripheral neuropathy (pDPN). Of the international trials, three were in PHN patients only, and one was in patients with PHN or pDPN. In those studies with a mixed PHN/pDPN patient sample, only those patients with PHN were included in the current analysis. Patient inclusion and exclusion criteria were similar across all the trials. Briefly, male or female patients aged ≥18 years were required to have neuropathic pain associated with PHN for >3 months after the healing of acute herpes zoster rash. Patients in the international trials could be of any race, but patients in the Chinese trials had to be ethnically Chinese. At screening and randomization, patients had to score ≥40 mm on the 100 mm visual analog scale of the Short Form-McGill Pain Questionnaire. Furthermore, patients at randomization were required to have completed ≥4 daily pain diaries over the previous 7 days and have a mean daily pain score of ≥4, based on an 11-point numeric rating scale (NRS) ranging from 0 = no pain to 10 = worst possible pain. Exclusion criteria included a mean pain score <4 on the NRS for the 7 days prior to randomization and the presence of other severe pain that, in the opinion of the investigator, may have impaired the assessment of pain due to PHN. Medications commonly used for treatment of PHN, and antiepileptics, including gabapentin, were excluded. Patients with clinically significant conditions such as respiratory, hematologic, hepatic, or cardiovascular disease, or those with clinically significant or unstable medical or psychological conditions, were also excluded. The double-blind treatment phase for each of the Chinese trials lasted for 8 weeks. In the international trials, double-blind treatment lasted for 8–13 weeks. In Chinese trials, patients were randomized to receive placebo or pregabalin at a fixed dose of 300 mg/day or a flexible dose of 150–600 mg/day. In the international trials, patients were randomized to receive placebo or pregabalin at fixed doses of 150, 300, or 600 mg/day or a flexible dose of 150–600 mg/day. The primary efficacy endpoint for all the trials was the endpoint mean pain score based on the NRS.
Efficacy measures
All efficacy measures were assessed at endpoint, which was set at 8 weeks for each patient group to enable simple comparison between groups. The main efficacy measure was the change in mean pain score from baseline, based on the NRS. The least squares (LS) mean difference and associated 95% CIs for pregabalin versus placebo were calculated. Secondary measures included the proportions of 30% and 50% pain responders, ie, those patients who reported a ≥30% or ≥50% improvement in mean pain score from baseline. These are clinically meaningful improvements in pain relief corresponding to moderate (≥30%) and substantial improvements (≥50%).21,22 The time to onset of pain relief was defined as the first day on which the patient’s daily pain score decreased by ≥1 point relative to baseline, and who had ≥30% pain response at endpoint.23 Median values were not available for all treatment arms in each patient group, therefore the time to onset for the first quartile of patients, ie, the first 25% of patients who experienced pain relief as defined previously, are presented. Sleep disruption was assessed using a daily pain-related sleep interference (PRSI) score, based on an 11-point NRS, ranging from 0 = pain does not interfere with sleep, to 10 = pain completely interferes with sleep. The LS mean difference and associated 95% CIs for pregabalin versus placebo were calculated. Overall improvement was assessed using the 7-point Patient Global Impression of Change (PGIC) (scores range from “very much worse” to “very much improved”). PGIC responders were identified as those patients reporting symptoms as “much improved” or “very much improved” at endpoint, corresponding to moderate and substantial overall improvements, respectively.21
Safety measures
The incidences of treatment-emergent serious adverse events (SAEs) and adverse events (AEs) reported throughout the duration of the trials were used to assess safety. SAEs and AEs were summed separately for the Chinese and international patient groups. Those AEs occurring in ≥5% of any treatment arm (placebo or pregabalin) of any patient group (Chinese or international) were included for comparison. Discontinuation rates, including discontinuations due to lack of efficacy and AEs related to study drug, were also calculated for each treatment group in the Chinese and international patient groups.
Statistical analysis
Mean pain score and PRSI score at endpoint were analyzed using a mixed model repeated measures analysis, with baseline pain or sleep, treatment, and study as variables. Missing data were imputed by the last observation carried forward method. Time to onset of pain relief was assessed using Kaplan-Meier analysis, with statistical significance determined using a log-rank test. Statistical comparisons were made for pregabalin versus placebo, with significance set at p<0.05. Statistical comparisons between the Chinese and international patient groups were not made.
To examine the effect of baseline pain severity on efficacy outcomes, patients were stratified by baseline mean pain score. Patients with a mean pain score ≥4 to <7 were categorized as having moderate baseline pain, and those with a score ≥7 to 10 were categorized as having severe baseline pain. Because patients were required to have a baseline mean pain score ≥4 to participate in the original clinical trials, patients with mild pain, ie, a score <4, were excluded from this analysis. The proportions of patients with severe baseline pain who had moderate or mild pain at endpoint were assessed. Patients were analyzed by treatment and by patient group. Missing data were imputed by the baseline observation carried forward method. No statistical comparisons were made for this analysis.
Results
Data from 1166 patients with PHN were included in the analysis; 312 were Chinese and 854 were international patients. Of the Chinese patients, 141 (45.2%) received placebo and 171 (54.8%) received pregabalin. Of the international patients, 273 (32.0%) received placebo and 581 (68.0%) received pregabalin. Table 1 shows the patient demographic and clinical characteristics at baseline. In general, Chinese patients were mostly younger than the international patients, whilst the vast majority of international patients were white. A larger proportion of Chinese patients were male than international patients. Baseline mean pain scores and PRSI scores were slightly lower in Chinese patients, indicating less disruptive pain and sleep. A larger proportion of patients in the Chinese group had moderate compared with severe baseline pain. In the international group, the proportions of patients with moderate and severe baseline pain were similar. Baseline mean pain scores in patients with moderate pain were similar in the Chinese and international groups, as were scores in patients with severe pain. Similarly, PRSI scores at baseline in patients with moderate pain were similar in the Chinese and international patient groups, as were scores in the patients with severe pain. In pregabalin-treated Chinese patients, 111 (64.9%) were taking a fixed dose and 60 (35.1%) a flexible dose. In pregabalin-treated international patients, 546 (94.0%) were taking a fixed dose and 35 (6.0%) a flexible dose. In Chinese patients, the mean (SD) daily pregabalin dose (flexible and fixed dose) was 364.5 (135.2) mg. In international patients, the mean daily dose was 332.4 (174.4) mg.
Table 1.
Demographic and clinical characteristics at baseline
| Characteristic | Chinese (N = 312)
|
International (N = 854)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 141)
|
Pregabalin (n = 171)
|
Placebo (n = 273)
|
Pregabalin (n = 581)
|
|||||||||
| All | Moderate pain | Severe pain | All | Moderate pain | Severe pain | All | Moderate pain | Severe pain | All | Moderate pain | Severe pain | |
| Number of patients, n (%) | 141 | 90 | 51 | 171 | 114 | 57 | 273 | 149 | 124 | 581 | 296 | 285 |
| (100) | (63.8) | (36.2) | (100) | (66.7) | (33.3) | (100) | (54.6) | (45.4) | (100) | (50.9) | (49.1) | |
| Male, n (%) | 80 | 49 | 31 | 87 | 56 | 31 | 133 | 71 | 62 | 264 | 128 | 136 |
| (56.7) | (54.4) | (60.8) | (50.9) | (49.1) | (54.4) | (48.7) | (47.7) | (50.0) | (45.4) | (43.2) | (47.7) | |
| Age, years, mean (SD) | 64.4 | 64.0 | 64.9 | 64.9 | 64.2 | 66.3 | 71.3 | 70.2 | 72.7 | 71.0 | 70.7 | 71.2 |
| (9.1) | (9.3) | (8.8) | (8.5) | (8.7) | (7.8) | (10.7) | (11.1) | (10.2) | (10.5) | (10.9) | (10.1) | |
| Race, n (%) | ||||||||||||
| White | 0 | 0 | 0 | 0 | 0 | 0 | 270 | 147 | 123 | 567 | 289 | 278 |
| (98.9) | (98.7) | (99.2) | (97.6) | (97.6) | (97.5) | |||||||
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 3 |
| (0.7) | (0.3) | (1.1) | ||||||||||
| Asian | 141 | 90 | 51 | 171 | 114 | 57 | 0 | 0 | 0 | 0 | 0 | 0 |
| (100) | (100) | (100) | (100) | (100) | (100) | |||||||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 10 | 6 | 4 |
| (1.1) | (1.3) | (0.8) | (1.7) | (2.0) | (1.4) | |||||||
| Baseline pain score, mean (SD) | 6.3 | 5.5 | 7.8 | 6.1 | 5.2 | 7.9 | 6.7 | 5.6 | 8.0 | 6.8 | 5.6 | 8.0 |
| (1.3) | (0.8) | (0.6) | (1.5) | (0.8) | (0.9) | (1.5) | (0.9) | (0.9) | (1.5) | (0.9) | (0.8) | |
| Baseline PRSI | 4.7 | 4.0 | 5.9 | 4.1 | 3.4 | 5.7 | 4.6 | 3.9 | 5.5 | 4.8 | 3.8 | 5.9 |
| score, mean (SD) | (2.0) | (1.9) | (1.8) | (2.5) | (2.0) | (2.6) | (2.6) | (2.1) | (3.0) | (2.6) | (2.1) | (2.5) |
Notes: Mean pain scores were based on an 11-point NRS, where 0 = no pain and 10 = worst possible pain. PRSI scores were based on an 11-point NRS, where 0 = pain does not interfere with sleep, and 10 = pain completely interferes with sleep. Patients categorized as having moderate pain had mean pain scores of ≥4 to <7 at baseline. Patients categorized as having severe pain had mean pain scores ≥7 to 10 at baseline. Patients with mild pain (mean pain score <4) were excluded from the study.
Abbreviations: NRS, numeric rating scale; PRSI, pain-related sleep interference.
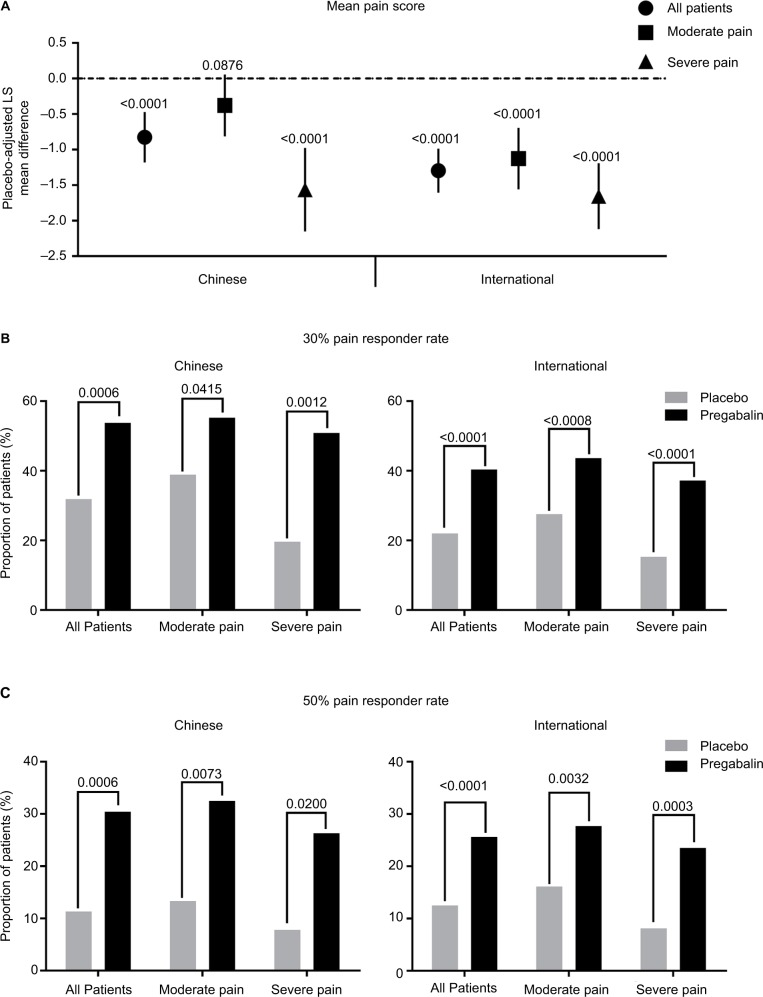
Overall, pregabalin efficacy was similar between the Chinese and international patient groups. At endpoint, pregabalin statistically significantly reduced (improved) mean pain score versus placebo in the total patient samples from the Chinese and international groups (both p<0.0001) (Figure 1A). The reduction in mean pain score was larger in the international patients compared with the Chinese patients. When patients were analyzed by baseline pain severity, pregabalin statistically significantly improved mean pain scores in Chinese patients with severe baseline pain and international patients with moderate or severe baseline pain (all p<0.0001). There was a trend toward improvement with pregabalin over placebo in Chinese patients with moderate baseline pain (p=0.0876).
Figure 1.
Placebo-adjusted mean pain scores, and 30% and 50% pain responder rates, at endpoint by patient group and baseline pain severity.
Notes: Comparison of endpoint placebo-adjusted mean pain scores (A), and 30% (B) and 50% (C) pain responder rates for pregabalin versus placebo treatment, between Chinese and international patients. Data in (A) are presented as the LS mean difference between pregabalin and placebo. Error bars are the 95% CIs. Lower scores in (A) indicate better pain relief. Comparison was made for the total patient sample in each group, and for those patients categorized as having moderate pain (mean pain score ≥4 to <7) or severe pain (mean pain score ≥7 to 10) at baseline. Patients with mild pain (mean pain score <4) were excluded from the study. p-values for pregabalin versus placebo are as indicated.
Abbreviation: LS, least squares.
The effect of pregabalin on pain relief was further assessed by examining the proportion of patients who achieved a 30% or 50% pain response with pregabalin or placebo treatment (Figure 1B and C); 30% and 50% reductions in pain are considered clinically meaningful.21 Results were comparable between Chinese and international patient groups. In both the Chinese and international total patient samples, the proportions of 30% and 50% pain responders were statistically significantly greater for pregabalin versus placebo (all p<0.001). Pain responder rates were also statistically significantly greater for pregabalin versus placebo in the Chinese and international patient groups irrespective of baseline pain severity (all p<0.05). A larger proportion of Chinese patients were 30% and 50% pain responders with pregabalin compared with international patients, in the total patient sample and irrespective of baseline pain severity.
Based on Kaplan-Meier analysis, the time to onset of pain relief for the first quartile of patients was statistically significantly faster for pregabalin versus placebo in the total patient samples of the Chinese and international groups (both p<0.0001) (Table 2). The time to onset of pain relief was also statistically significantly faster for pregabalin versus placebo in the Chinese and international patient groups irrespective of baseline pain severity (all p<0.01). The time to onset of pain relief with pregabalin was similar between the Chinese and international patient groups, in the total patient samples and irrespective of baseline pain severity.
Table 2.
Kaplan-Meier estimates of the time to onset of pain relief by patient group and pain severity at baseline
| Patient group | Time to onset (days)
|
||
|---|---|---|---|
| First quartilea | 95% CI | p-value | |
| Chinese | |||
| All patients | |||
| Placebo | 13 | 7–n/a | |
| Pregabalin | 3 | 3–5 | <0.0001 |
| Moderate pain | |||
| Placebo | 11 | 6–21 | |
| Pregabalin | 4 | 3–8 | <0.01 |
| Severe pain | |||
| Placebo | n/a | 12–n/a | |
| Pregabalin | 3 | 2–3 | 0.0001 |
| International | |||
| All patients | |||
| Placebo | n/a | 26–n/a | |
| Pregabalin | 3 | 2–3 | <0.0001 |
| Moderate pain | |||
| Placebo | 26 | 5–n/a | |
| Pregabalin | 2 | 2–3 | <0.0001 |
| Severe pain | |||
| Placebo | n/a | n/a | |
| Pregabalin | 3 | 2–3 | <0.0001 |
Notes:
The first 25% of patients who had onset of pain relief by the day listed. Time to onset of pain relief defined as the first day on which the patient’s daily pain score decreased by ≥1 point relative to baseline, and who had ≥30% pain response at endpoint. Patients categorized as having moderate pain had mean pain scores of ≥4 to <7 at baseline. Patients categorized as having severe pain had mean pain scores ≥7 to 10 at baseline. Patients with mild pain (mean pain score <4) were excluded from the study. p-values are for pregabalin versus placebo.
Abbreviation: n/a, not available.
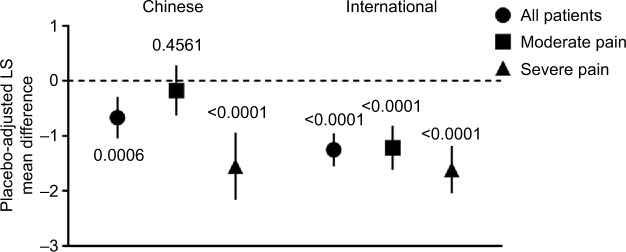
Pregabalin statistically significantly reduced (improved) PRSI score versus placebo in the total patient samples of the Chinese and international groups (both p<0.001) (Figure 2). The improvement in PRSI scores was greater in the international patients compared with the Chinese patients. Pregabalin also statistically significantly improved PRSI scores versus placebo in Chinese patients with severe baseline pain and in international patients with moderate or severe baseline pain (all p<0.0001). In Chinese patients with moderate baseline pain, there was no statistical difference between pregabalin and placebo (p=0.4561).
Figure 2.
Placebo-adjusted PRSI scores at endpoint by patient group and baseline pain severity.
Notes: Comparison of endpoint placebo-adjusted PRSI scores between Chinese and international patients. Data are presented as the LS mean difference between pregabalin and placebo. Error bars are the 95% CIs. Lower scores indicate less disrupted sleep. Comparison was made for the total patient sample in each group, and for those patients categorized as having moderate pain (mean pain score ≥4 to <7) or severe pain (mean pain score ≥7 to 10) at baseline. Patients with mild baseline pain (mean pain score <4) were excluded from the study. p-values for pregabalin versus placebo are as indicated.
Abbreviations: LS, least squares; PRSI, pain-related sleep interference.
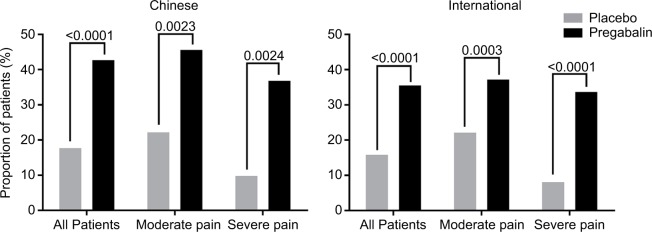
The proportion of PGIC responders was statistically significantly greater for pregabalin versus placebo in the total patient samples of the Chinese and international groups (both p<0.0001) (Figure 3). The proportion of PGIC responders was also statistically significantly greater for pregabalin versus placebo in Chinese and international patients irrespective of baseline pain severity (all p<0.01). A greater proportion of Chinese patients were PGIC responders with pregabalin than international patients, in the total patient samples and irrespective of baseline pain severity.
Figure 3.
Comparison of PGIC responder rates at endpoint by patient group and baseline pain severity.
Notes: Comparison of endpoint PGIC responder rates between Chinese and international patients. Comparison was made for the total patient sample in each group, and for those patients categorized as having moderate pain (mean pain score ≥4 to <7) or severe pain (mean pain score ≥7 to 10) at baseline. Patients with mild baseline pain (mean pain score <4) were excluded from the study. PGIC responders were classified as patients reporting symptoms as “much improved” or “very much improved” at endpoint. p-values for pregabalin versus placebo are as indicated.
Abbreviation: PGIC, Patient Global Impression of Change.
To further examine the clinical effectiveness of pregabalin, we determined the proportion of patients with severe baseline pain (mean pain score ≥7 to 10 on an 11-point NRS) who shifted pain severity category to moderate pain (mean pain score ≥4 to <7) or mild pain (mean pain score <4), at study endpoint. The proportion of Chinese or international patients with severe baseline pain who had moderate or mild pain at endpoint following treatment is shown in Figure 4. In both Chinese and international patients, a greater proportion of patients had shifted from severe pain at baseline to moderate or mild pain at endpoint with pregabalin compared with placebo. In the Chinese patient group, 68.4% of pregabalin-treated patients with severe baseline pain had moderate or mild pain at endpoint, compared with 46.7% of pregabalin-treated patients with severe baseline pain in the international group.
Figure 4.
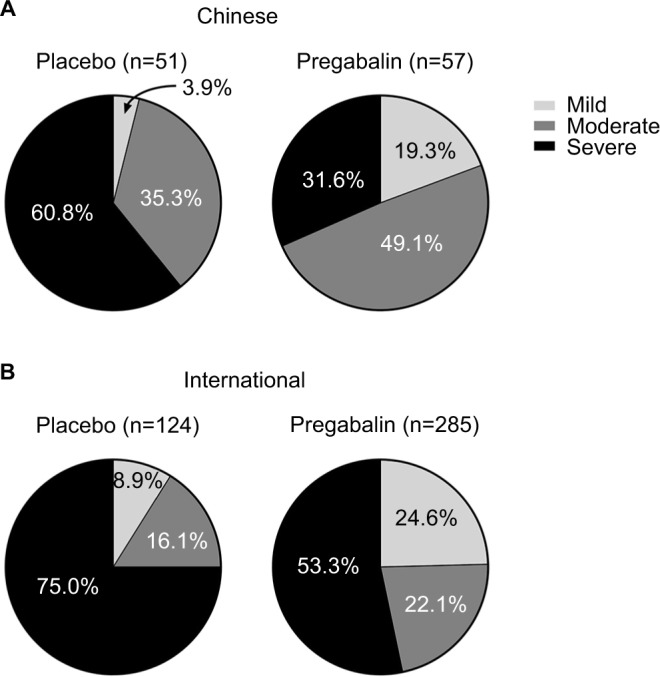
Pain severity category at endpoint of patients categorized as having severe pain at baseline, by patient group and by treatment.
Notes: Proportion of Chinese (A) and international (B) patients with baseline severe pain (mean pain score ≥7 to 10) who had moderate or mild pain at endpoint following placebo or pregabalin treatment. Moderate pain was defined as a mean pain score ≥4 to <7 and mild pain as a mean pain score <4. Key for pain severity category in (A) also applies to (B). No statistical comparisons were made.
In the Chinese patient group, no SAEs occurred in patients receiving placebo, whereas three (1.8%) pregabalin-treated patients experienced SAEs. In the international patient group, eight (2.9%) patients receiving placebo experienced SAEs compared with 25 (4.3%) pregabalin-treated patients. Table 3 shows the incidences of AEs occurring in ≥5% of patients with either pregabalin or placebo treatment for the total patient samples of the Chinese and international groups. Dizziness, peripheral edema, somnolence, constipation, and dry mouth were among the most common AEs with pregabalin treatment in the Chinese and international patient groups. Increased weight, headache, and blurred vision were also among the commonest AEs in international patients. No major differences were observed in the pregabalin AE profiles between the Chinese and international patient groups, except that incidences were generally higher in international patients. Discontinuation rates were generally lower in Chinese patients than in international patients. In Chinese patients, 26 (18.4%) patients who received placebo discontinued, and 20 (11.7%) pregabalin-treated patients discontinued. In international patients, 72 (26.4%) patients who received placebo discontinued, and 173 (29.8%) of pregabalin-treated patients discontinued. In pregabalin-treated Chinese patients, 0 patients and seven (4.1%) patients discontinued due to a lack of efficacy and pregabalin-related AEs, respectively. In pregabalin-treated international patients, 45 (7.7%) patients and 88 (15.1%) patients discontinued due to a lack of efficacy and pregabalin-related AEs, respectively.
Table 3.
Incidences of AEs by patient group
| Event, n (%) | Chinese (N = 312)
|
International (N = 854)
|
||
|---|---|---|---|---|
| Placebo (n = 141) | Pregabalin (n = 171) | Placebo (n = 273) | Pregabalin (n = 581) | |
| Dizziness | 6 (4.3) | 30 (17.5) | 33 (12.1) | 160 (27.5) |
| Peripheral edema | 2 (1.4) | 12 (7.0) | 9 (3.3) | 63 (10.8) |
| Somnolence | 5 (3.5) | 10 (5.8) | 15 (5.5) | 103 (17.7) |
| Constipation | 2 (1.4) | 7 (4.1) | 10 (3.7) | 31 (5.3) |
| Dry mouth | 3 (2.1) | 7 (4.1) | 9 (3.3) | 51 (8.8) |
| Nasopharyngitis | 9 (6.4) | 5 (2.9) | 6 (2.2) | 22 (3.8) |
| Fatigue | 1 (0.7) | 5 (2.9) | 15 (5.5) | 23 (4.0) |
| Increased weight | 2 (1.4) | 3 (1.8) | 3 (1.1) | 40 (6.9) |
| Headache | 2 (1.4) | 3 (1.8) | 14 (5.1) | 40 (6.9) |
| Blurred vision | 3 (2.1) | 3 (1.8) | 4 (1.5) | 31 (5.3) |
| Nausea | 2 (1.4) | 0 | 14 (5.1) | 9 (1.5) |
Notes: AEs occurring in ≥5% of patients in any treatment group. The total number of patients with events per treatment and patient group are reported. AEs are ordered by decreasing frequency in the Chinese patients who received pregabalin.
Abbreviation: AEs, adverse events.
Discussion
The results of this post-hoc analysis show that the efficacy and safety of pregabalin for PHN are similar in Chinese and international patient populations. In the Chinese and international total PHN patient samples, pregabalin improved pain relief and disrupted sleep versus placebo, and more patients reported to be 30% and 50% pain responders, and PGIC responders, for pregabalin over placebo. The time to onset of pain relief was faster for pregabalin versus placebo. Finally, the AE profiles of pregabalin were similar between the Chinese and international patient groups.
Some differences were observed between the total patient samples for the Chinese and international patient groups. Pregabalin improved mean pain scores and PRSI scores to a lesser extent in the Chinese patients compared with the international patients. This may be because mean pain scores and PRSI scores at baseline were slightly lower in the Chinese patients, so there was less room for improvement. It could also be because there were fewer Chinese patients than international patients. However, the proportions of pain responders in the total patient samples of the Chinese and international patient groups were similar and appeared to be larger in the Chinese patients. Thus, despite the fact that the absolute change in mean pain score was smaller in Chinese patients compared with the international patients, a considerable proportion of Chinese patients exhibited clinically meaningful improvements in pain relief. Also, a larger proportion of Chinese patients with severe baseline pain had moderate or mild pain at endpoint compared with international patients. In addition, no pregabalin-treated Chinese patients discontinued treatment because of a lack of efficacy compared with 45 (7.7%) of international pregabalin-treated patients, further highlighting the apparent effectiveness of pregabalin in the Chinese patient population. The pain data are supported by the larger proportion of Chinese patients exhibiting clinically meaningful overall improvement compared with international patients, as assessed by PGIC responder rates. The pregabalin AE profiles of the total patient samples were very similar, except that incidences were generally higher in the international patients, including for SAEs. Discontinuation rates were also higher in the international patients compared with the Chinese patients. This may be due to the generally shorter duration of the Chinese trials and smaller Chinese patient sample. The international patients were on average older than the Chinese patients, which may also have affected the incidences of AEs and SAEs.
In international patients, pregabalin was significantly better than placebo for all efficacy measures irrespective of baseline pain severity. The same was not true for the Chinese patient group. Although Chinese patients with severe baseline pain also exhibited statistically significantly better efficacy with pregabalin over placebo for all efficacy measures, there was no statistical difference between pregabalin and placebo in improving pain relief or sleep disruption in patients with moderate baseline pain. However, Chinese patients with moderate baseline pain had similar pain and PGIC responder rates to Chinese patients with severe baseline pain, which indicates that those with moderate baseline pain were experiencing clinically meaningful improvements in pain, and overall. The reason for lack of effect of pregabalin on mean pain scores and PRSI scores in Chinese patients with moderate baseline pain is not clear, but it may be due to smaller room for improvement in these patients versus their counterparts with severe pain. Chinese patients with moderate baseline pain had larger placebo responses for these efficacy endpoints compared with Chinese patients with severe baseline pain, which may also have contributed to pregabalin’s lack of effect in the former. Further analysis in a larger sample of Chinese patients with moderate baseline pain may help to answer these questions.
These findings extend results from previous studies of pregabalin for PHN. A systematic review and meta-analysis of four trials for doses of pregabalin ≥300 mg/day reported a reduction in pain NRS scores of −1.57 for pregabalin versus placebo.24 This is similar to the placebo-adjusted pain improvement reported here in international patients, but greater than that observed in Chinese patients. In a post-hoc analysis of five trials, the time to onset of pain relief in the first quartile of patients was 2 days for pregabalin doses of 150, 300, and 600 mg/day compared with 18 days for placebo.23 The values for pregabalin are remarkably similar to those reported here. Importantly, the definition of time to onset of pain relief in the article by Sharma et al23 is identical to that used in the current analysis. Two post-hoc analyses have examined the effect of sleep disturbance on pregabalin efficacy in more detail. Data from seven trials showed that pregabalin statistically significantly improved pain scores in the total PHN cohort versus placebo (placebo-adjusted LS mean difference, −1.08), and in patients categorized with mild, moderate, or severe baseline sleep disruption.25 The greatest improvement occurred in patients with severe sleep disruption. In a separate analysis of the same seven trials, the time to improvement in PRSI scores for the first quartile of patients with pregabalin was 2 days for all doses of pregabalin combined (75, 150, 300, 600, and flexible 150–600 mg/day).26 A previous analysis of nine studies also examined the shift in pain severity category from baseline to endpoint in a mixed PHN/DPN population.27 In this analysis, approximately two thirds of PHN/DPN patients with severe baseline pain who received a flexible dose of pregabalin (150–600 mg/day) had moderate or mild pain at baseline. These data are similar to the findings in the current study.
This study had several limitations. Patients were subject to the strict inclusion and exclusion criteria of the original clinical trials, therefore the findings may not be generalizable to the wider PHN population. Although the international patient group did not contain any Asian patients, some may have been of Asian origin. The length of the trials was different between the two patient groups, although the same 8-week endpoint was used for all efficacy analyses in both patient groups. The Chinese group had considerably fewer patients than the international group. Aside from race, there were minor differences in the baseline demographic and clinical characteristics between the patient groups, for instance gender and age. The proportions of patients with moderate or severe baseline pain differed between the patient groups. Finally, patients with mild pain were excluded from the study.
Conclusion
In summary, the efficacy and safety of pregabalin for PHN are similar between Chinese and international patients. Although the magnitude of absolute pain reduction and sleep disruption improvement was greater in international patients compared with Chinese patients, greater proportions of Chinese patients were pain responders and PGIC responders at endpoint, and a greater proportion of Chinese patients with severe baseline pain had moderate or mild pain with pregabalin at endpoint compared with international patients. Baseline pain severity did not appear to affect pregabalin efficacy, except for some measures in Chinese patients with moderate baseline pain. These data highlight the utility of pregabalin for diverse patient groups for the treatment of PHN.
Acknowledgments
Medical writing support was provided by David Cope, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Footnotes
Disclosure
This study was sponsored by Pfizer. The original trials of pregabalin efficacy and safety for PHN were also sponsored by Pfizer. Bruce Parsons, YuXuan Chen, Marie Ortiz, and Ed Whalen are employees of, and have stock options with, Pfizer. Li Xie was an employee of Pfizer during the development of part of this paper. The authors report no other conflicts of interest.
References
- 1.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 2.Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20(3):17. doi: 10.1007/s11916-016-0548-x. [DOI] [PubMed] [Google Scholar]
- 3.Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 4.Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137(1):38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 5.Ultsch B, Köster I, Reinhold T, et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ. 2013;14(6):1015–1026. doi: 10.1007/s10198-012-0452-1. [DOI] [PubMed] [Google Scholar]
- 6.Cebrián-Cuenca AM, Díez-Domingo J, San-Martín-Rodríguez M, et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia among patients treated in primary care centres in the Valencian community of Spain. BMC Infect Dis. 2011;11:302. doi: 10.1186/1471-2334-11-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gialloreti LE, Merito M, Pezzotti P, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: patient-reported outcomes in six European countries. Z Gesundh Wiss. 2012;20(4):441–451. doi: 10.1007/s10389-011-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–1736. doi: 10.1503/cmaj.091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(Suppl 1):S9–S27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- 12.Lyrica® (pregabalin) [prescribing information] New York: Pfizer Inc; 2016. [Accessed August 18, 2017]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=561. [Google Scholar]
- 13.Lyrica® (pregabalin) [summary of product characteristics] Sandwich, UK: Pfizer Ltd; 2017. [Accessed August 18, 2017]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. [Google Scholar]
- 14.Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60(8):1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 15.Sabatowski R, Gálvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1–2):26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 17.van Seventer R, Feister HA, Young JP, Jr, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22(2):375–384. doi: 10.1185/030079906x80404. [DOI] [PubMed] [Google Scholar]
- 18.Guan Y, Ding X, Cheng Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011;33(2):159–166. doi: 10.1016/j.clinthera.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Chen H, Xi L, et al. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of Chinese patients. Pain Pract. 2017;17(1):62–69. doi: 10.1111/papr.12413. [DOI] [PubMed] [Google Scholar]
- 20.Lyrica® (pregabalin) [prescribing information] Shanghai, China: Pfizer Ltd; 2016. [Accessed August 18, 2017]. Available from: http://apac.ecf.pfizer.com/sites/china/wrs/ProductsLibrary/Forms/AllItems.aspx?RootFolder=%2fsites%2fchina%2fwrs%2fProductsLibrary%2fAcute%20Disease%20Business%20Group&FolderCTID=0x012000601361A8EA01D74B8D23E133BD95537F. [Google Scholar]
- 21.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 23.Sharma U, Griesing T, Emir B, Young JP., Jr Time to onset of neuropathic pain reduction: a retrospective analysis of data from nine controlled trials of pregabalin for painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther. 2010;17(6):577–585. doi: 10.1097/MJT.0b013e3181d5e4f3. [DOI] [PubMed] [Google Scholar]
- 24.Snedecor SJ, Sudharshan L, Cappelleri JC, et al. Systematic review and meta-analysis of pharmacological therapies for pain associated with postherpetic neuralgia and less common neuropathic conditions. Int J Clin Pract. 2014;68(7):900–918. doi: 10.1111/ijcp.12411. [DOI] [PubMed] [Google Scholar]
- 25.Vinik A, Emir B, Parsons B, Cheung R. Prediction of pregabalin-mediated pain response by severity of sleep disturbance in patients with painful diabetic neuropathy and post-herpetic neuralgia. Pain Med. 2014;15(4):661–670. doi: 10.1111/pme.12310. [DOI] [PubMed] [Google Scholar]
- 26.Parsons B, Emir B, Knapp L. Examining the time to improvement of sleep interference with pregabalin in patients with painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther. 2015;22(4):257–268. doi: 10.1097/MJT.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 27.Parsons B, Argoff CE, Clair A, Emir B. Improvement in pain severity category in clinical trials of pregabalin. J Pain Res. 2016;9:779–785. doi: 10.2147/JPR.S102696. [DOI] [PMC free article] [PubMed] [Google Scholar]