Abstract
Purpose
Zinc finger protein 259 (ZNF259), also known as ZPR1, is a zinc finger-containing protein that can bind the intracellular tyrosine kinase domain of EGFR. At present, our knowledge on ZNF259 in cancers is limited. Here, we aimed to explore the biological functions of ZNF259 in breast cancer and reveal their mechanisms.
Patients and methods
The expression of ZNF259 was measured in 133 cases of breast cancer by immunohistochemistry. The online database Kaplan–Meier (KM) Plotter Online Tool was used to analyze the relationship between ZNF259 expression and breast cancer patient survival prognosis. Plasmid transfection and small interfering RNA and inhibitor treatments were carried out to explore the functions of ZNF259 in breast cancer cell lines and its potential mechanism. Matrigel invasion and wound healing assays were performed to detect the invasion and migration ability of cancer cells. In addition, protein expressions in tissues and cells were determined by Western blotting.
Results
ZNF259 expression was much higher in breast cancer cells than in the adjacent normal breast duct glandular epithelial cells (75.94% vs 7.52%, P<0.001) and was closely related to the breast cancer patients’ TNM stages (P=0.013) and lymph node metastasis (P=0.021). Knockdown of ZNF259 could downregulate p-ERK, p-GSK3β, and Snail expression, and upregulate the expression of E-cadherin and ZO-1, and then it also inhibited invasion and migration by the breast cancer cell lines MCF-7 and MDA-MB-231. Correspondingly, ZNF259 transfection could upregulate p-ERK, p-GSK3β, and Snail expression, and downregulate E-cadherin and ZO-1 expression, which led to stronger invasion and migration abilities of cancer cells. Furthermore, the ERK inhibitor U0126 could reverse all these effects induced by ZNF259 transfection.
Conclusion
ZNF259 could promote breast cancer cell invasion and migration by activating the ERK/GSK3β/Snail signaling pathway.
Keywords: ZPR1, E-cadherin, ZO-1, U0126, p-ERK, p-GSK3β
Introduction
Despite great advances in diagnostic and therapeutic strategies in recent decades, breast cancer is still listed as the leading cause of cancer-related death in women worldwide,1–3 largely due to breast cancer cell invasion and metastasis.4,5 It has great significance to elucidate the invasion and metastasis mechanism of breast cancer cells, and to find new biological molecules for the development of new targeted drugs.
Zinc finger protein 259 (ZNF259), also known as ZPR1, was first identified in mammalian cells as a cytoplasmic protein that could bind the intracellular domain of the epidermal growth factor receptor (EGFR).6 ZNF259 is an evolutionarily conserved protein with 2 C4 zinc fingers and 2 specific conserved homology domains.6,7 ZNF259 has essential roles in embryonic development and cell apoptosis, and can translocate to the nucleus during the S phase to regulate the cell cycle progression and cell proliferation.8–12 However, so far, our knowledge on ZNF259 expression and biological functions in malignant tumors is limited.
In this study, we explored ZNF259 expression in breast cancer tissue and breast cancer cell lines for the first time, and investigated the roles of ZNF259 in the invasion and migration by breast cancer cells. We found that ZNF259 could activate extracellular signal-regulated kinase (ERK)/glycogen synthase kinase 3β (GSK3β)/Snail signaling, which plays a vital role in breast cancer cell invasion and migration.13,14 In ERK/GSK3β/Snail signaling, activated ERK inhibits the function of GSK3β in promoting Snail degradation and leads to upregulation of Snail.15–17 The inhibition of adhesion factors such as E-cadherin by Snail can directly lead to the invasion and metastasis by cancer cells.18–21 Our results reveal the functions and mechanism of ZNF259 in breast cancer cell invasion and migration, and provide a new potential molecular target for the therapy of breast cancer patients.
Materials and methods
Patients and specimens
This study was approved by the local Institutional Review Board of the Cancer Hospital of China Medical University and Liaoning Cancer Hospital and Institute. The patients whose tissue samples were used in this study provided written informed consent. Primary tumor specimens were obtained from 133 patients who were diagnosed with invasive ductal carcinoma and underwent complete resection at the Cancer Hospital of China Medical University and Liaoning Cancer Hospital and Institute between 2011 and 2013. The patients who underwent neoadjuvant radiotherapy and/or chemotherapy were excluded from the cohort of the present study. All patients received standard chemotherapy after surgery. The mean age of the patients was 51 years (range, 29–83 years). Lymph node metastases were identified in 61 of the 133 patients. The pathological Tumor-Node-Metastasis (p-TNM) staging system of the International Union Against Cancer (seventh edition) was used to classify specimens as stages I (n=29), II (n=50), and III (n=54). In addition, 12 pairs of fresh specimens, including both breast cancer and paired adjacent nontumor tissues, were stored at −80°C immediately after resection, for protein extraction.
Immunohistochemistry
All tissue specimens were fixed in neutral formaldehyde, embedded in paraffin, and sectioned (thickness, 4 µm). The streptavidin-peroxidase immunohistochemical method was used to improve staining. Tissue sections were incubated at 4°C overnight with ZNF259 rabbit monoclonal antibody (1:200, ab134970, Abcam, Cambridge, UK); PBS was used as a blank control. Sections were then incubated with biotin-labeled secondary antibodies (Ultrasensitive; MaiXin, Fuzhou, People’s Republic of China) at 37°C for 30 minutes, followed by diaminobenzidine for coloration. Finally, samples were lightly counterstained with hematoxylin, dehydrated in alcohol, and mounted. Two investigators blinded to the clinical data semi-quantitatively scored the slides by evaluating the staining intensity and percentage of stained cells in representative areas. The staining intensity was scored as 0 (no signal), 1 (weak), 2 (moderate), or 3 (high). The percentage of cells stained was scored as 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), or 4 (76%–100%). A final score of 0–12 was obtained by multiplying the intensity and percentage scores. ZNF259 stain with a score ≥4 was considered positive. Tumor samples with scores between 0 and 3 were defined as negative expression.
Cell culture
MCF-10A, T47D, MCF-7, BT-549, MDA-MB-231, and MDA-MB-468 cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum (Invitrogen), 100 international units (IU)/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA), and 100 µg/mL streptomycin (Sigma-Aldrich). Cells were grown on sterilized culture dishes and were passaged every 2 days following 0.25% trypsin (Invitrogen) digestion.
Plasmid transfection and small interfering RNA (siRNA) treatment
Plasmids pCMV6-ddk-myc and pCMV6-ddk-myc-ZNF259 were purchased from Origene (RC205721, Rockville, MD, USA). ZNF259-siRNA (sc-35282) and NC-siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Transfection was carried out using the Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s instructions.
Western blotting
Total protein was extracted using a lysis buffer (Pierce, Rockford, IL, USA) and quantified with the Bradford method. Total protein samples (30 µg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were incubated overnight at 4°C with the following primary antibodies: ZNF259 (rabbit monoclonal antibody, 1:200, ab134970; Abcam; mouse monoclonal antibody, 1:200, sc-398491; Santa Cruz Biotechnology), phosphorylated mitogen-activated protein kinase (p-MEK), MEK, p-ERK, ERK, p-GSK3β, GSK3β, and Snail (1:500; Cell Signaling Technology, Danvers, MA, USA), E-cadherin (1:500; BD Transduction Laboratories, Lexington, KY, USA), zonula occludens protein 1 (ZO-1) (1:500; Proteintech, Chicago, IL, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000; Sigma-Aldrich). Membranes were washed and subsequently incubated with peroxidase-conjugated anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology) at 37°C for 2 hours. Bound proteins were visualized using electrochemiluminescence (Pierce) and detected with a bioimaging system (DNR Bio-Imaging Systems, Jerusalem, Israel).
Matrigel invasion
Cell invasion assay was performed using a 24-well Transwell chamber with 8 µm pores (Costar, Cambridge, MA, USA). The inserts were coated with 20 µL Matrigel (1:3 dilution; BD Biosciences, San Jose, CA, USA). Twenty-four hours after transfection, cells were trypsinized, and 3×105 cells in 100 µL of serum-free medium were transferred to the upper Matrigel chamber for 18 hours. Media supplemented with 10% fetal bovine serum were added to the lower chamber as a chemoattractant. After incubation, cells that passed through the filter were fixed with 4% paraformaldehyde and stained with hematoxylin. The invasive cells were microscopically counted in 10 randomly selected high-power fields.
Wound healing assay
In cultures with cell density below 90%, 48 hours after transfection, wounds were created in confluent areas using a 1,000 µL pipette tip. Wound healing within the scrape line was observed at both 0 and 24 hours, and representative scrape lines for each cell line were photographed. Duplicate wells were examined for each condition, and each experiment was repeated 3 times. The optical wound distances were measured using Image J software (National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Statistical Package for Social Science version 23.0 for Mac (IBM Corporation, Armonk, NY, USA) was used for all analyses. The Pearson’s χ2 test was performed to assess possible correlations between ZNF259 and clinicopathological factors. Mann–Whitney U test was performed for the image analysis of Western blotting results and invasive assay results. P<0.05 was considered to indicate statistically significant differences.
Results
ZNF259 is related to the malignant progress of breast cancer
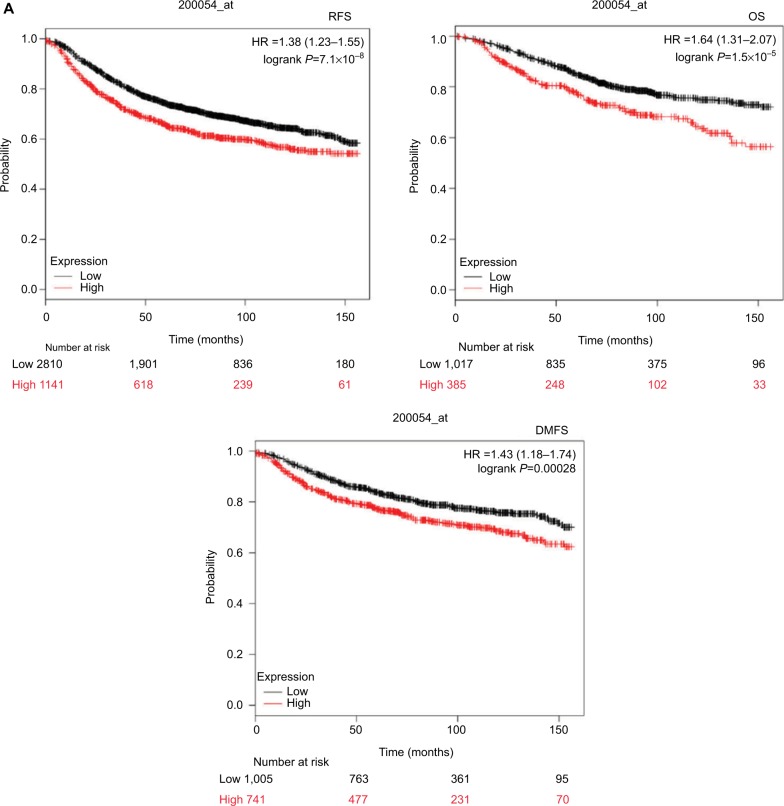
To investigate the functions of ZNF259 in malignant tumors, we first analyzed the relationship between its expression and different cancer patients’ survival prognosis, using an online database Kaplan–Meier (KM) Plotter Online Tool (http://www.kmplot.com). The data showed that ZNF259 gene expression significantly correlated with the breast cancer patients’ relapse-free survival (n=3,951, P=7.1×10−8), overall survival (n=1,402, P=1.5×10−5), and distant metastasis-free survival (n=1,746, P=0.00028) (Figure 1A).
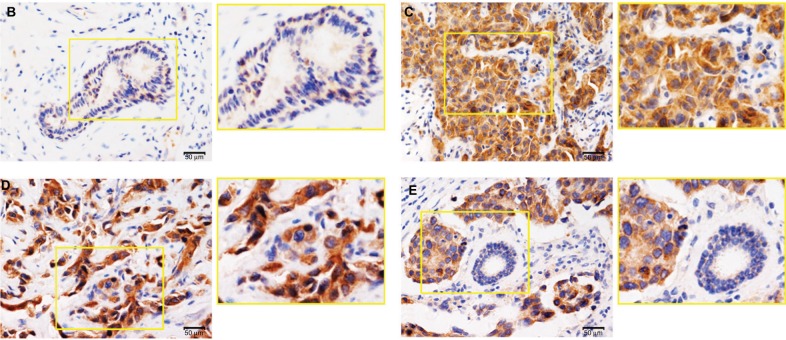
Figure 1.
Zinc finger protein 259 (ZNF259) was upregulated in breast cancer tissues and related to poor prognosis.
Notes: (A) Data from KM Plotter Online Tool showed that ZNF259 expression significantly correlated with breast cancer patients’ poor survival (RFS, n=3,951, P=7.1×10−8 OS, n=1,402, P=1.5×10−5; DMFS, n=1,746, P=0.00028). (B) ZNF259 was very weakly stained in normal breast duct glandular epithelium cells and myoepithelial cells. (C) ZNF259 was moderately positive in the cytoplasm of breast cancer cells. (D) ZNF259 showed strong positive staining in the cytoplasm of cancer cells, and scattered nucleus-stained cells could also be observed although not very obviously. (E) ZNF259 expression in breast cancer cells was much higher than that in adjacent normal breast duct glandular epithelium cells in the same field of view (400× and partial enlargement).
Abbreviations: DMFS, distant metastasis-free survival; OS, overall survival; RFS, relapse-free survival; ZNF259, zinc finger protein 259.
We performed immunohistochemistry staining to analyze the expression of ZNF259 in 133 cases of breast cancer and adjacent noncancerous tissue specimens. The results showed that ZNF259 was very weakly positively stained in normal breast duct glandular epithelium cells (10/133, 7.52%, Figure 1B, Table 1). In breast cancer tissues, ZNF259 stain was much stronger than that in normal breast duct glandular epithelium cells, showing moderate to strong positive stain in the cytoplasm of cancer cells (101/133, 75.94%, P<0.001, Figure 1C and D, Table 1). Scattered nuclear stained cells were rarely visible, but not very obviously (Figure 1D). As Figure 1E shows, ZNF259 expression in breast cancer cells was much higher than that in adjacent normal breast duct glandular epithelium cells in the same field of view. Statistical analysis results indicated that ZNF259 expression was significantly correlated to the breast cancer patient’s TNM stages (P=0.013) and lymph node metastasis (P=0.021) but had no significant association with age (P=0.206, Table 2).
Table 1.
ZNF259 expression in adjacent normal breast tissue and breast cancer tissue
| Positive | Negative | |
|---|---|---|
| Breast duct glandular epithelial cells | 10 (7.52%) | 123 (92.48%) |
| Breast cancer cells | 101 (75.94%) | 32 (24.06%)a |
Note:
P<0.001, indicating statistical significance.
Abbreviation: ZNF259, zinc finger protein 259.
Table 2.
The relevance of ZNF259 expression to clinicopathological characteristics of breast cancer patients
| Clinicopathological feature | N | ZNF259
|
χ2 | P-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| All cases | |||||
| Age (years) | 133 | 101 | 32 | ||
| ≤51 | 66 | 47 | 19 | 1.603 | 0.206 |
| >51 | 67 | 54 | 13 | ||
| TNM stage | |||||
| I + II | 79 | 54 | 25 | 6.127 | 0.013 |
| III | 54 | 47 | 7 | ||
| Lymph node metastasis | |||||
| Negative | 72 | 49 | 23 | 5.341 | 0.021 |
| Positive | 61 | 52 | 9 | ||
Abbreviation: ZNF259, zinc finger protein 259.
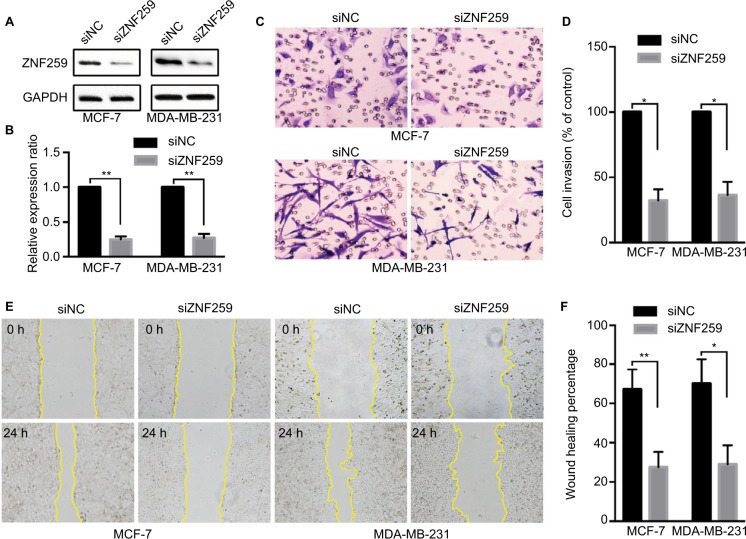
ZNF259 knockdown could inhibit invasion and migration by breast cancer cells
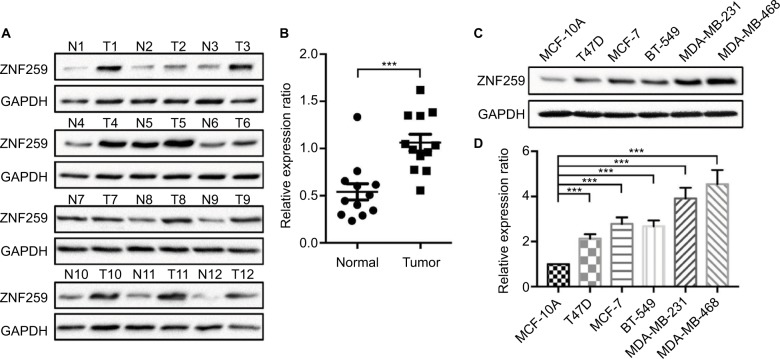
We measured the expression of ZNF259 in breast cancer tissues and cell lines by Western blotting. The results showed that in 12 pairs of fresh tissues, the relative expression rate of ZNF259 in breast cancer tissues was significantly higher than that in adjacent nontumor tissues (P<0.001, Figure 2A and B). In addition, in 5 breast cancer cell lines, T47D, MCF-7, BT-549, MDA-MB-231, and MDA-MB-468, ZNF259 expression was significantly higher than that in normal human mammary epithelial cell line MCF-10A (all P<0.001, Figure 2C and D). Furthermore, we knocked down ZNF259 with siRNAs in MCF-7 and MDA-MB-231 cell lines (MCF7, P=0.0011; MDA-MB-231, P=0.0021, Figure 3A and B). Transwell analysis showed that ZNF259 knockdown could inhibit invasion by the MCF-7 and MDA-MB-231 cells (MCF-7, P=0.0242; MDA-MB-231, P=0.0273, Figure 3C and D). Wound healing assay showed that the migration abilities were also suppressed by ZNF259 knockdown in MCF-7 and MDA-MB-231 cells (MCF-7, P=0.006; MDA-MB-231, P=0.0107, Figure 3E and F).
Figure 2.
ZNF259 expression increased in breast cancer tissues and cell lines.
Notes: (A) Western blotting was performed on 12 pairs of fresh breast cancer tissues and adjacent nontumor tissues. ZNF259 expression in most breast cancer tissues was higher than that in the corresponding adjacent nontumor tissues (11/12, 91.67%). (B) The relative expression ratio of ZNF259 in breast cancer tissues was significantly higher than that in the adjacent nontumor tissues (bars indicate the mean ± standard error of the mean, ***P<0.001). (C, D) ZNF259 expression in the breast cancer cell lines T47D, MCF-7, BT-549, MDA-MB-231, and MDA-MB-468 was significantly higher than that in the normal human mammary epithelial cell MCF-10A (bars represent the mean ± standard deviation, ***P<0.001).
Abbreviation: ZNF259, zinc finger protein 259.
Figure 3.
ZNF259 knockdown inhibited the invasion and migration abilities of breast cancer cells.
Notes: (A, B) ZNF259 was knocked down by siRNAs in MCF-7 and MDA-MB-231 cell lines (mean ± standard deviation, **P<0.01). (C, D) Transwell analysis showed that ZNF259 knockdown inhibited invasion by MCF-7 and MDA-MB-231 cells (mean ± standard error of the mean, *P<0.05). (E, F) Wound healing assay revealed that silencing ZNF259 expression also inhibited migration by MCF-7 and MDA-MB-231 cells (mean ± standard error of the mean, *P<0.05; **P<0.01).
Abbreviation: ZNF259, zinc finger protein 259.
ZNF259 knockdown could upregulate E-cadherin and ZO-1 expression and downregulate p-MEK, p-ERK, p-GSK3β, and Snail expression
Western blot results showed that ZNF259 knockdown in both MCF-7 and MDA-MB-231 cell lines could downregulate p-MEK, p-ERK, p-GSK3β (Ser 9), and Snail expression, and then upregulate E-cadherin and ZO-1 expression, which could greatly affect the invasion and migration abilities of tumor cells (Figures 4 and S1). The total MEK, ERK, and GSK3β expression showed no obvious change.
Figure 4.
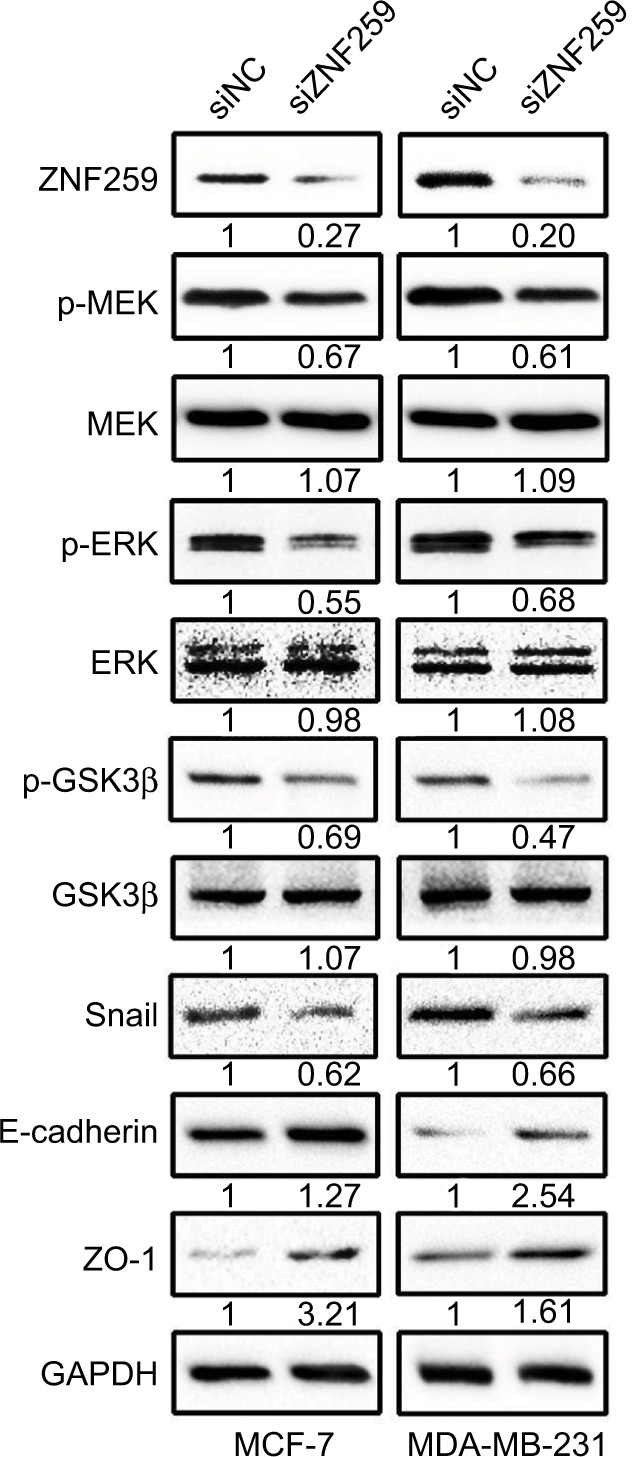
ZNF259 knockdown could downregulate phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-glycogen synthase kinase 3β (p-GSK3β), and Snail expression, and upregulate E-cadherin and zonula occludens protein 1 (ZO-1) expression.
Notes: Western blotting was performed after ZNF259 knockdown in both MCF-7 and MDA-MB-231 cell lines, and phosphorylated mitogen-activated protein kinase (p-MEK), phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-glycogen synthase kinase 3β (p-GSK3β) (Ser 9), and Snail expressions were downregulated, while E-cadherin and ZO-1 expression was upregulated. The total MEK, ERK, and GSK3β levels did not show obvious changes. The numbers indicated the relative expression ratios of the protein. At least 3 separate experiments were performed. The statistical analysis histogram is shown in the Figure S1.
Abbreviation: ZNF259, zinc finger protein 259.
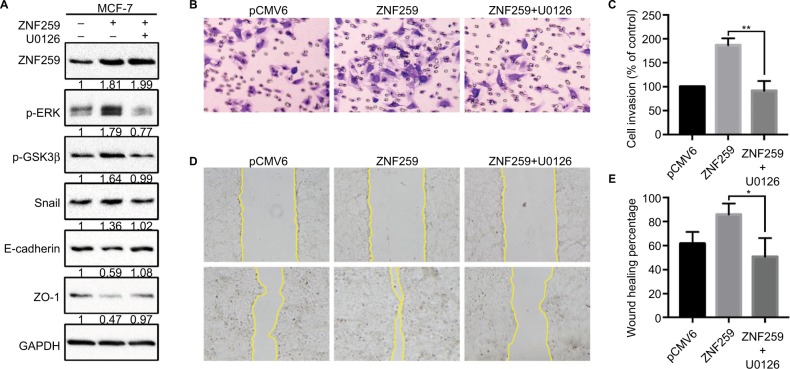
ZNF259 could promote the invasion and migration ability of breast cancer cells by activating the ERK/GSK3β/Snail signaling pathway
Furthermore, we transfected ZNF259 in MCF-7 cells and treated these cells with the ERK inhibitor U0126. The results showed that U0126 could inhibit the increase in p-ERK, p-GSK3β (Ser 9), and Snail levels induced by ZNF259 transfection, and then restore E-cadherin and ZO-1 expression (Figure 5A). Correspondingly, Transwell and wound healing assays showed that the increased cell invasion and migration caused by ZNF259 were also reversed by U0126 (P=0.0028, Figure 5B and C; P=0.0276, Figure 5D and E).
Figure 5.
Invasion and migration promoted by ZNF259 were inhibited by the extracellular signal-regulated kinase (ERK) inhibitor U0126.
Notes: (A) ZNF259 transfection could upregulate p-ERK, phosphorylated-glycogen synthase kinase 3β (p-GSK3β), and Snail expression, and downregulate E-cadherin and zonula occludens protein 1 (ZO-1) expression. All these effects could be restored by the incorporation of the ERK inhibitor U0126 (5 µM). The numbers indicated the relative expression ratios of protein. At least 3 separate experiments were performed. (B, C) Transwell assay showed that U0126 could reverse the ZNF259 transfection-caused increase in cell invasion ability (mean ± standard error of the mean, **P<0.01). (D, E) Wound healing assay also showed that U0126 could reverse the ZNF259 transfection-caused increase in cell migration ability (mean ± standard error of the mean, *P<0.05).
Abbreviation: ZNF259, zinc finger protein 259.
Discussion
The ZNF family members can regulate gene expression and play important roles in multiple biological processes, including proliferation, development, differentiation, autophagy, metabolism, apoptosis, and stemness maintenance.22–29 ZNF259 has essential roles in embryonic development, cell apoptosis, cycle progression, and proliferation,8–12 indicating that ZNF259 may also play a role in the development of malignant tumors. Our data revealed that ZNF259 was obviously upregulated in the cytoplasm of breast cancer cells, was significantly related to breast cancer patients’ TNM stages (P=0.013) and lymph node metastasis (P=0.021), and could promote invasion and migration by breast cancer cells. The data from KM Plotter Online Tool also showed that ZNF259 gene expression significantly correlated with breast cancer patients’ poor survival (relapse-free survival, n=3,951, P=7.1×10−8; overall survival, n=1,402, P=1.5×10−5; distant metastasis-free survival, n=1,746, P=0.00028), which better supported our results.
Furthermore, we confirmed that ZNF259 could upregulate the expression of p-MEK, p-ERK, p-GSK3β, and Snail, and downregulate E-cadherin and ZO-1 expression, which plays a central role in regulating invasion and migration by tumor cells.30,31 Snail is phosphorylated by GSK3β at the Ser/Thr residues and then degraded.15 Active ERK (p-ERK) can inhibit the function of GSK3β by phosphorylating it at Ser 9, leading to increased Snail levels.13,14,16,17 Meanwhile, both E-cadherin and ZO-1 are classic downstream target genes of Snail.18–21 Thus, our hypothesis was that ZNF259 downregulates E-cadherin and ZO-1 expression via activating p-ERK/p-GSK3β/Snail signaling and promotes invasion and migration by breast cancer cells. The ERK inhibitor U0126 could inhibit the increasing expression of p-ERK, p-GSK3β, and Snail caused by ZNF259 transfection, restored the expression of E-cadherin and ZO-1, and subsequently reversed the invasion and migration abilities of breast cancer cells. These results provide strong support for our above hypothesis, although it is still unclear how ZNF259 regulates p-ERK.
ZNF259 could bind the intracellular domain of the inactive EGFR in the cytoplasm,6 suggesting that ZNF259 may play a role in the EGFR signaling pathway activation process. The role of EGFR signaling in various kinds of malignant tumors has been well elucidated, and Ras/Raf/MEK/ERK is one of the classic downstream pathways of EGFR signaling.32–34 Our results showed that p-MEK could also be upregulated by ZNF259. Thus, ZNF259 may participate in the upstream regulation of Ras/Raf/MEK/ERK pathway. In addition, recently, Shan et al35 showed that ZNF259 plays a tumor suppressor role in non-small-cell lung cancer via focal adhesion kinase (FAK)/protein kinase B (AKT) signaling. In our study, we also observed the effect of ZNF259 on p-FAK and p-AKT; however, in breast cancer cells their expression did not show obvious change (data was not shown). This finding indicates that the function of ZNF259 may be tissue-specific, which is worthy of further research in future studies.
Conclusion
ZNF259 levels increased in breast cancer tissues and cell lines, and its high expression was significantly related to breast cancer patients’ TNM stage and lymph node metastasis. ZNF259 could promote invasion and migration by breast cancer cells via activating ERK/GSK3β/Snail signaling.
Supplementary material
Statistical analysis histogram of the Western blotting results for zinc finger protein 259 (ZNF259) knockdown in both MCF-7 and MDA-MB-231 cell lines.
Notes: At least 3 separate experiments were performed. In both the MCF-7 and MDA-MB-231 cell line, ZNF259 knockdown could significantly downregulate phosphorylated mitogen-activated protein kinase (p-MEK), phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-glycogen synthase kinase 3β (p-GSK3β), and Snail expression, and upregulate E-cadherin and zonula occludens protein 1 (ZO-1) expression. The data are shown as mean ± standard deviation (SD), *P<0.05, **P<0.01, ***P<0.001.
Abbreviation: ZNF259, zinc finger protein 259.
Acknowledgments
The authors thank the Department of Pathology of Cancer Hospital of China Medical University and Liaoning Cancer Hospital and Institute for providing tissue samples and technical support. The authors also thank Editage (www.editage.cn) for English language editing.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA. Pitfalls in outcome prediction of breast cancer. J Clin Pathol. 2013;66(6):458–464. doi: 10.1136/jclinpath-2012-201083. [DOI] [PubMed] [Google Scholar]
- 5.Tremont A, Lu J, Cole JT. Endocrine therapy for early breast cancer: updated review. Ochsner J. 2017;17(4):405–411. [PMC free article] [PubMed] [Google Scholar]
- 6.Galcheva-Gargova Z, Konstantinov KN, Wu IH, Klier FG, Barrett T, Davis RJ. Binding of zinc finger protein ZPR1 to the epidermal growth factor receptor. Science. 1996;272(5269):1797–1802. doi: 10.1126/science.272.5269.1797. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz OE, Nikolova LS, Metzstein MM. Drosophila Zpr1 (zinc finger protein 1) is required downstream of both EGFR and FGFR signaling in tracheal subcellular lumen formation. PLoS One. 2012;7(9):e45649. doi: 10.1371/journal.pone.0045649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galcheva-Gargova Z, Gangwani L, Konstantinov KN, et al. The cytoplasmic zinc finger protein ZPR1 accumulates in the nucleolus of proliferating cells. Mol Biol Cell. 1998;9(10):2963–2971. doi: 10.1091/mbc.9.10.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangwani L, Mikrut M, Galcheva-Gargova Z, Davis RJ. Interaction of ZPR1 with translation elongation factor-1α in proliferating cells. J Cell Biol. 1998;143(6):1471–1484. doi: 10.1083/jcb.143.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangwani L, Flavell RA, Davis RJ. ZPR1 is essential for survival and is required for localization of the survival motor neurons (SMN) protein to Cajal bodies. Mol Cell Biol. 2005;25(7):2744–2756. doi: 10.1128/MCB.25.7.2744-2756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyczkowski J, Vingron M. Comparative analysis of cell cycle regulated genes in eukaryotes. Genome Inform. 2005;16(1):125–131. [PubMed] [Google Scholar]
- 12.Gangwani L. Deficiency of the zinc finger protein ZPR1 causes defects in transcription and cell cycle progression. J Biol Chem. 2006;281(52):40330–40340. doi: 10.1074/jbc.M608165200. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Lu J, Chen Y, et al. MCP-1-induced ERK/GSK-3β/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol. 2017;14(7):621–630. doi: 10.1038/cmi.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Zhou GL. CAP1 (Cyclase-Associated Protein 1) exerts distinct functions in the proliferation and metastatic potential of breast cancer cells mediated by ERK. Sci Rep. 2016;6:25933. doi: 10.1038/srep25933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Lee SH, Kim HS, et al. Role of CK1 in GSK3β-mediated phosphorylation and degradation of snail. Oncogene. 2010;29(21):3124–3133. doi: 10.1038/onc.2010.77. [DOI] [PubMed] [Google Scholar]
- 16.Ngo HK, Lee HG, Piao JY, et al. Helicobacter pylori induces Snail expression through ROS-mediated activation of Erk and inactivation of GSK-3β in human gastric cancer cells. Mol Carcinog. 2016;55(12):2236–2246. doi: 10.1002/mc.22464. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Yao Y, Zhao K, et al. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells. Mol Carcinog. 2016;55(12):2121–2134. doi: 10.1002/mc.22456. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Wu Y, Yao J, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122(4):1469–1486. doi: 10.1172/JCI57349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Li M, Xu D, Zhao C, Liu G, Wang F. Overexpression of Snail in retinal pigment epithelial triggered epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;446(1):347–351. doi: 10.1016/j.bbrc.2014.02.119. [DOI] [PubMed] [Google Scholar]
- 20.Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117(Pt 9):1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arenzana TL, Schjerven H, Smale ST. Regulation of gene expression dynamics during developmental transitions by the Ikaros transcription factor. Genes Dev. 2015;29(17):1801–1816. doi: 10.1101/gad.266999.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs CJ, Zhang D, Yin L, Robins DM. The KRAB zinc finger protein RSL1 modulates sex-biased gene expression in liver and adipose tissue to maintain metabolic homeostasis. Mol Cell Biol. 2014;34(2):221–232. doi: 10.1128/MCB.00875-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Zhang W, Li Y, et al. SHP-2-upregulated ZEB1 is important for PDGFRα-driven glioma epithelial-mesenchymal transition and invasion in mice and humans. Oncogene. 2016;35(43):5641–5652. doi: 10.1038/onc.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Wang H, Kornblau SM, et al. Evidence of a role for the novel zinc-finger transcription factor ZKSCAN3 in modulating Cyclin D2 expression in multiple myeloma. Oncogene. 2011;30(11):1329–1340. doi: 10.1038/onc.2010.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai KP, Chen J, He M, et al. Overexpression of ZFX confers self-renewal and chemoresistance properties in hepatocellular carcinoma. Int J Cancer. 2014;135(8):1790–1799. doi: 10.1002/ijc.28819. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan S, Goodwin JG, Chauhan S, et al. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol Cell. 2013;50(1):16–28. doi: 10.1016/j.molcel.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X, Huang M, Wang Z, Liu B, Zhu Z, Li C. ZHX1 inhibits gastric cancer cell growth through inducing cell-cycle arrest and apoptosis. J Cancer. 2016;7(1):60–68. doi: 10.7150/jca.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Deng X, Zhang J, et al. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signalling in oral squamous cell carcinoma. Cell Physiol Biochem. 2017;44(3):920–934. doi: 10.1159/000485360. [DOI] [PubMed] [Google Scholar]
- 30.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 31.Polette M, Mestdagt M, Bindels S, et al. β-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185(1–3):61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 32.Rajaram P, Chandra P, Ticku S, Pallavi BK, Rudresh KB, Mansabdar P. Epidermal growth factor receptor: role in human cancer. Indian J Dent Res. 2017;28(6):687–694. doi: 10.4103/ijdr.IJDR_534_16. [DOI] [PubMed] [Google Scholar]
- 33.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12(1):3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez RH, Valero V, Hortobagyi GN. Emerging targeted therapies for breast cancer. J Clin Oncol. 2010;28(20):3366–3379. doi: 10.1200/JCO.2009.25.4011. [DOI] [PubMed] [Google Scholar]
- 35.Shan Y, Cao W, Wang T, Jiang G, Zhang Y, Yang X. ZNF259 inhibits non-small cell lung cancer cells proliferation and invasion by FAK-AKT signaling. Cancer Manag Res. 2017;9:879–889. doi: 10.2147/CMAR.S150614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis histogram of the Western blotting results for zinc finger protein 259 (ZNF259) knockdown in both MCF-7 and MDA-MB-231 cell lines.
Notes: At least 3 separate experiments were performed. In both the MCF-7 and MDA-MB-231 cell line, ZNF259 knockdown could significantly downregulate phosphorylated mitogen-activated protein kinase (p-MEK), phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-glycogen synthase kinase 3β (p-GSK3β), and Snail expression, and upregulate E-cadherin and zonula occludens protein 1 (ZO-1) expression. The data are shown as mean ± standard deviation (SD), *P<0.05, **P<0.01, ***P<0.001.
Abbreviation: ZNF259, zinc finger protein 259.