Abstract
Purpose:
To evaluate the prevalence of intermediate-stage age-related macular degeneration (AMD) in patients with the acquired immunodeficiency syndrome (AIDS).
Design:
Cross sectional study of patients with AIDS enrolled in the Longitudinal Study of the Ocular Complications of AIDS
Methods:
Intermediate-stage AMD was determined from enrollment retinal photographs by graders at a centralized Reading Center, using the Age-Related Eye Disease Study grading system. Graders were masked as to clinical data.
Results:
Of 1825 participants with AIDS and no ocular opportunistic infections, 9.9% had intermediate-stage AMD. Risk factors included age, with an odds ratio (OR) of 1.9 (95% confidence interval [CI] 1.6, 2.3, P<0.001) for every decade of age; the prevalence of AMD ranged from 4.0% for participants 30–39 years old to 24.3% for participants ≥60 years old. Other risk factors included the HIV risk groups of injection drug use (OR= 2.4, 95% CI 1.5, 3.9, P<0.001) or heterosexual contact (OR=1.9, 95% CI 1.3, 2.8, P=0.001). Compared with the HIV-uninfected population in the Beaver Dam Offspring Study, there was an approximate 4-fold increased age-adjusted prevalence of intermediate-stage AMD.
Conclusions:
Patients with AIDS have an increased age-adjusted prevalence of intermediate-stage AMD compared with that found in a non-Human Immunodeficiency Virus (HIV)-infected cohort evaluated with similar methods. This increased prevalence is consistent with the increased prevalence of other age-related diseases in antiretroviral-treated, immune-restored, HIV-infected persons when compared to non-HIV-infected persons.
TABLE OF CONTENTS STATEMENT
Compared with an age-adjusted non-HIV-infected cohort, persons with the Acquired Immune Deficiency Syndrome (AIDS) enrolled in the Longitudinal Study of the Ocular Complications of AIDS had an approximate 4-fold increased prevalence of intermediate-stage age-related macular degeneration (AMD). This increased prevalence of AMD is consistent with the increased prevalence of other age-related diseases seen in antiretroviral-treated, immune-restored, HIV-infected persons compared to the non-HIV-infected population.
Age-related macular degeneration (AMD) is the leading cause of late-onset visual impairment and blindness in persons over 65 years of age.1,2 Age-related macular degeneration is staged as early, intermediate, or late. Small drusen are the hallmark of early-stage AMD; intermediate-stage AMD consists of extensive medium-size drusen or any large drusen, with or without pigment changes. Late-stage AMD is defined by either choroidal neovascularization or geographic atrophy.3–6 In the United States, it is estimated that ~1.2 million persons have neovascular AMD; 970,000 have geographic atrophy; and 8 million have intermediate-stage AMD.1
Antiretroviral-treated, immune-restored, human immunodeficiency (HIV)-infected persons have a marked reduction in opportunistic infections and a substantially increased lifespan compared to those from the era before modern combination antiretroviral therapy.7–10 However, despite the improved immune function and increased lifespan, antiretroviral therapy does not fully restore health. Compared to similarly-aged, non-HIV-infected peers, antiretroviral-treated, immune-restored, HIV-infected persons have a substantially shortened lifespan, largely due to an increased risk of non-AIDS diseases associated with aging.11–14 These diseases include cardiovascular disease, non-AIDS cancers, metabolic diseases, and neuro-cognitive decline, and collectively suggest that antiretroviral-treated, immune-restored, HIV-infection is associated with an “accelerated/accentuated aging” phenotype.11 Therefore, we undertook to evaluate whether persons with the acquired immune deficiency (AIDS) had an increased prevalence of AMD, using retinal photographs taken at enrollment in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA) cohort.
Methods
The Longitudinal Study of the Ocular Complications of AIDS was a prospective, observational, cohort study of patients with AIDS in the era of modern combination antiretroviral therapy.15,16 Enrollment began on 1 September 1998 and was completed on 31 July 2011. Eligible persons had AIDS diagnosed according to the 1993 Centers for Disease Control and Prevention revised criteria for the diagnosis of AIDS.17Recruitment occurred at 19 clinical centers throughout the United States, typically located in large urban centers with a large HIV-infected population.15
Participants with and without ocular opportunistic infections were recruited. At enrollment all participants gave a detailed medical and HIV-related disease history; relevant findings were confirmed from the medical record. Wide-field retinal photographs were taken on all participants at enrollment, as previously described.15,16Laboratory testing at enrollment included hematology and blood chemistry, lymphocyte subset analysis for CD4+ T cells, the amount of circulating HIV RNA in the blood (HIV load), and the presence of antibodies to hepatitis C.15,16
Approval for the study and its procedures was obtained from the institutional review boards of the individual participating clinical centers and the three resource centers (chairman’s office, coordinating center, and reading center). Written, informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki.
Grading of retinal photographs.
Photographs were graded by graders at the Studies of the Ocular Complications of AIDS (SOCA) Reading Center at the University of Madison, Wisconsin from stereoscopic color photographs of the macula obtained at the baseline visit. Photographers and camera systems were certified for SOCA photographic procedures by Reading Center personnel. Images were graded either from 35 mm film, mounted in typical slide mounts and viewed on a light box with a Donaldson 5X stereoscopic viewer, or from digital images displayed on calibrated computer monitors and viewed with a stereoscopic viewer (PS Manufacturing, Portland, OR). The Early Treatment Diabetic Retinopathy Grid, measuring 7.2 mm on the retina, was placed on film slides using an acetate overlay sized for the camera type and degree of view.4 Digital image grading employed software tools to calibrate and locate the grid.6 Approximately 10% of the images were digital. Fundus photographs were graded for the features of intermediate-stage AMD, including the presence, size and area of drusen, the presence and area of pigmentary abnormalities. Grading questions and procedures employed the Age Related Eye Disease Study (AREDS)-2 system for classifying AMD from retinal photographs.5,6 Graders were masked as to clinical information. The primary outcome of interest was intermediate-stage AMD, defined as at least one large druse (> 150 μm using modern estimates of the average disc size or >125 μm using the traditional estimate) or extensive medium-sized drusen with or without pigment abnormalities.5 Quality control was provided by a resampling and regrading of 10% of the photographs by the reading center project ophthalmologist (RPD). Because drusen are difficult to evaluate in the face of extensive retinal necrosis and scarring from cytomegalovirus (CMV) retinitis, eyes with ocular opportunistic infections were not graded, and these participants were not included in the study.18 The primary outcome was intermediate-stage AMD.
Statistical methods.
Continuous variables were categorized into clinically meaningful categories or quartiles. Variables with moderate to large (>10%) amounts of missing data included a category for missing. The association of unadjusted participant characteristics with prevalence of intermediate-stage AMD was assessed using the chi-square test (or Fisher’s exact test for small n’s).19 Adjusted independent effects were assessed with a stepwise model selection procedure using Akaike’s Information Criterion logistic regression.20 The candidate set included all unadjusted participant characteristics except individual antiretroviral drugs. Odds ratios and confidence intervals were estimated using Wald’s test.19 All statistical analyses were conducted with SAS/STAT® version 9.3 (Copyright© 2002–2010. SAS Institute, Inc., Cary, NC) and Stata version 12.0 (StataCorp 2011. Stata Statistical Software: Release 12. College Station, Tx: StataCorp LP) software packages.
Comparison with the Beaver Dam Offspring Study.
In order to compare the prevalence of intermediate-stage AMD among patients with AIDS to that seen in the non-HIV infected population, the prevalence of intermediate-stage AMD in LSOCA was compared to the published data from the Beaver Dam Eye Study, which also were obtained from grading retinal photographs at the same institution as the SOCA Reading Center, and used similar grading protocols.21 Comparisons were stratified by age and adjusted for age and gender.22 In addition, because the Beaver Dam offspring Study was a study of Caucasian participants, a comparison also was made with Caucasian participants in the LSOCA study.
Results
Characteristics of the study population.
Of the 2392 participants enrolled in LSOCA, 535 had an intraocular opportunistic infection (primarily CMV retinitis), and 32 did not have enrollment photographs; 1825 had enrollment photographs and no intraocular infections and form the study population. Characteristics of the study population are listed in table 1. The group had a mean age of 43.4 years and was 80.7% male. The plurality of participants were white, non-Hispanic (45.4%), but 37.7% were African American, and 13.3% were Hispanic. The largest transmission category for HIV infection was participants with male to male sexual contact (54.3%); 14.1% had injection drug use, and 26.0% heterosexual transmission as the transmission category for HIV infection. The median nadir CD4+ T cell count prior to enrollment was 44 cells/μL (25th, 75th percentiles, 11 cells/μL, 113 cells/μL), the median enrollment CD4+ T cell count was 198 cells/μL (25th, 75th percentiles, 81 cells/μL, 360 cells/μL). At enrollment 84.9% of participants were receiving combination antiretroviral therapy, and 93.6% had received combination antiretroviral therapy prior to or at enrollment. Comorbidities associated with aging included diabetes in 8.7%, hypertension in 20.9%, and cardiovascular disease in 13.7%.
Table 1.
Baseline Characteristics of the Study Population of Patients with the Acquired Immune Deficiency Syndrome from the Longitudinal Study of the Ocular Complications of AIDS
| Characteristic | Result |
|---|---|
| Number participants | 1825 |
| Demographics | |
| Age (years) | |
| Mean | 43.4 |
| Standard deviation | 8.8 |
| Gender (%) | |
| Men | 80.7 |
| Women | 19.3 |
| Race (%) | |
| White, non-Hispanic | 45.4 |
| African-American | 37.7 |
| Hispanic | 13.3 |
| Other | 3.6 |
| HIV transmission category(%) | |
| Male to male sexual contact | 54.3 |
| Injection drug use | 14.1 |
| Heterosexual contact | 26.0 |
| Other | 5.6 |
| Duration of AIDS at enrollment (years) | |
| Median | 4.3 |
| 25th, 75th percentile | 1.6, 7.3 |
| Smoking (%) | |
| Current | 18.0 |
| Former | 28.4 |
| Non-smoker | 31.3 |
| Missing | 22.3 |
| Immunology and virology | |
| Enrollment CD4+ T cells (cells/μL) | |
| Median | 198 |
| 25th, 75th percentile | 81, 360 |
| Nadir CD4+ T cells (cells/μL) | |
| Median | 44 |
| 25th, 75th percentile | 13, 113 |
| Enrollment HIV load (log10(copies/mL)) | |
| Median | 2.7 |
| 25th, 75th percentile | 1.9, 4.6 |
| Maximum prior HIV load (log10(copies/mL)) | |
| Median | 5.3 |
| 25th, 75th percentile | 4.7, 5.7 |
| HIV treatment | |
| Highly active antiretroviral therapy at enrollment* (%) | |
| Yes | 84.9 |
| No | 15.1 |
| Highly active antiretroviral therapy prior to or at enrollment (%) | |
| Yes | 93.6 |
| No | 6.4 |
| Comorbidities | |
| Diabetes (%) | |
| No | 91.3 |
| Yes | 8.7 |
| Hyperlipidemia (%) | |
| No | 78.4 |
| Yes | 21.6 |
| Renal impairment (elevated serum creatinine) (%) | |
| No | 93.9 |
| Yes | 6.1 |
| Hypertension (%) | |
| No | 79.1 |
| Yes | 13.7 |
| Cardiovascular disease (%) | |
| No | 86.3 |
| Yes | 13.7 |
| Hepatitis C infection (%) | |
| Seronegative | 66.4 |
| Seropositive | 19.0 |
| Missing | 14.6 |
Highly active antiretroviral therapy = combination therapy including at least one potent antiretroviral drug (e.g. a protease inhibitor).
Prevalence of and risk factors for intermediate-stage AMD.
The prevalence of intermediate-stage AMD in the entire study population was 9.9%. The associations of demographic, immunologic and virologic, comorbidity, and antiretroviral treatment risk factors with intermediate-stage AMD in the univariate analysis are listed in table 2. Age was a significant risk factor, with prevalences ranging from 4.0% for participants aged 30 to 39 years to 24.3% for participants 60 year of age and over (P<0.0001). Smoking was associated with intermediate-stage AMD with current smokers having a higher prevalence (13.7%) than former smokers (9.1%) and “never-smokers” (8.2%) (P=0.02). Participants whose risk for HIV infection was either injection drug use (14.4%) or heterosexual contact (12.0%) had a greater prevalence of AMD than did men who had sex with men (7.7%) or other risk groups (5.6%) (P=0.003). Diabetes (14.4% vs 9.4%, P=0.04), hypertension (13.9% vs 8.8%, P=0.003) and cardiovascular disease (14.0% vs 8.9%, P=0.02) all were associated with intermediate-stage AMD.
Table 2.
Univariate Analysis of Baseline Patient Characteristics and Intermediate-stage Age-related Macular Degeneration in Patients with the Acquired Immune Deficiency Syndrome
| Characteristic | Number | % with AMD* | P-value |
|---|---|---|---|
| Overall | 1825 | 9.9 | |
| Age (years) | <0.0001 | ||
| <30 | 98 | 8.2 | |
| 30–39 | 499 | 4.0 | |
| 40–49 | 794 | 9.3 | |
| 50–59 | 364 | 16.8 | |
| ≥60 | 70 | 24.3 | |
| Gender | 0.30 | ||
| Men | 1472 | 9.5 | |
| Women | 353 | 11.3 | |
| Race & ethnicity | 0.15 | ||
| White, non-Hispanic | 828 | 8.2 | |
| African American | 688 | 11.6 | |
| Hispanic | 243 | 9.9 | |
| Other | 66 | 12.1 | |
| Smoking history | 0.02 | ||
| Current | 329 | 13.7 | |
| Former | 518 | 9.1 | |
| Never | 571 | 8.2 | |
| Enrollment cohort (year of enrollment) | 0.36 | ||
| 1998–2000 | 663 | 8.8 | |
| 2001–2004 | 721 | 10.0 | |
| 2005–2012 | 441 | 11.3 | |
| Time since AIDS diagnosis (years) | 0.37 | ||
| Quartile 1 (<1.55) | 445 | 10.1 | |
| Quartile 2 (1.55–4.33) | 457 | 7.9 | |
| Quartile 3 (4.34–7.25) | 448 | 10.0 | |
| Quartile 4 (>7.25) | 450 | 11.3 | |
| HIV transmission category | 0.003 | ||
| Male to male sexual contact | 991 | 7.7 | |
| Injection drug use | 257 | 14.4 | |
| Heterosexual contact | 474 | 12.0 | |
| Other | 103 | 5.6 | |
| Enrollment CD4+ T cells (cells/μL) | 0.98 | ||
| <100 | 519 | 10.2 | |
| 100–199 | 389 | 10.3 | |
| 200–499 | 664 | 9.6 | |
| ≥500 | 238 | 9.7 | |
| Nadir CD4+ T cells (cells/μL) | 0.25 | ||
| <50 | 953 | 9.0 | |
| ≥50 | 845 | 10.7 | |
| Enrollment HIV load (log10(copies/mL) | 0.26 | ||
| <2.6 | 627 | 8.3 | |
| 2.6–3.0 | 305 | 10.8 | |
| ≥3.0 | 786 | 10.7 | |
| Maximum prior HIV load (log10(copies/mL) | 0.98 | ||
| < 2.6 | 47 | 10.6 | |
| 2.6–3.0 | 49 | 10.2 | |
| ≥3.0 | 1645 | 9.8 | |
| HIV Treatment | |||
| Highly active antiretroviral therapy† | 0.21 | ||
| No | 276 | 12.0 | |
| Yes | 1548 | 9.5 | |
| Nucleoside transcriptase inhibitor | 0.27 | ||
| No | 208 | 12.0 | |
| Yes | 1616 | 9.6 | |
| Non-nucleoside transcriptase inhibitor | 0.24 | ||
| No | 1117 | 9.2 | |
| Yes | 707 | 10.9 | |
| Protease inhibitor | 0.09 | ||
| No | 703 | 11.4 | |
| Yes | 1121 | 8.9 | |
| Integrase inhibitor | 0.70 | ||
| No | 1781 | 9.8 | |
| Yes | 43 | 11.6 | |
| Chemokine receptor 5 antagonist | 0.42 | ||
| No | 1818 | 9.9 | |
| Yes | 6 | 0 | |
| Fusion inhibitor | 0.48 | ||
| No | 1795 | 9.8 | |
| Yes | 29 | 13.8 | |
| Comorbidities | |||
| Diabetes | 0.04 | ||
| No | 1666 | 9.4 | |
| Yes | 159 | 14.4 | |
| Hyperlipidemia | 0.87 | ||
| No | 1428 | 9.9 | |
| Yes | 393 | 9.7 | |
| Renal impairment (elevated serum creatinine) | 0.73 | ||
| No | 1714 | 9.8 | |
| Yes | 111 | 10.8 | |
| Hypertension | 0.003 | ||
| No | 1442 | 8.8 | |
| Yes | 382 | 13.9 | |
| Cardiovascular disease | 0.02 | ||
| No | 1258 | 8.9 | |
| Yes | 200 | 14.0 | |
| Hepatitis C infection (sero-positive) | 0.65 | ||
| No | 1211 | 9.8 | |
| Yes | 347 | 10.7 |
AMD =age-related macular degeneration; percent with intermediate-stage AMD reported.
Highly active antiretroviral therapy = combination therapy including at least one potent antiretroviral drug (e.g. a protease inhibitor).
Neither combination antiretroviral therapy nor the use of any class of antiretroviral drugs at enrollment was associated with intermediate-stage AMD (table 2). Details of the relationship of individual antiretroviral drugs to intermediate-stage AMD are listed in supplemental table 1 (online at www.ajo.com). The only individual antiretroviral drug statistically associated with intermediate-stage AMD was adefovir (33.3% in those taking it vs 9.7% in those not taking it, P=0.01); however only 15 participants were taking adefovir, and only 5 participants taking it had intermediate-stage AMD, so adefovir use had a minimal impact on the prevalence of AMD in this population. There was no association of the use of antiretroviral therapy prior to enrollment, the use of any class of antiretroviral drugs, or the use of any specific antiretroviral drugs prior to enrollment with the prevalence of intermediate-stage AMD at enrollment (data not shown).
The multiple regression analysis of risk factors for intermediate-stage AMD is listed in table 3. Age was strongly associated with intermediate-stage AMD with an odds ratio of 1.9 for every decade of age (95% CI 1.6, 2.3, P<0.001). HIV transmission category remained a risk factor with injection drug use (odds ratio 2.4, 95% CI 1.5, 3.9, P=0.01) and heterosexual contact (odds ratio 1.9, 95% CI 1.3, 2.8, P=0.001) associated with intermediate-stage AMD as compared to participants whose HIV risk factor was male to male sexual contact. There was no evidence of effect modification between HIV risk group and gender on the risk of intermediate-stage AMD (interaction P=0.18, data not shown).
Table 3.
Multiple Regression of Risk Factors for Intermediate-stage Age-related Macular Degeneration in Patients with the Acquired Immune Deficiency Syndrome
| Covariate | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Age (decade) | 1.9 | 1.6, 2.3 | <0.001 |
| HIV transmission category | |||
| Male to male sexual contact (reference) | 1.0 | ||
| Injection drug use | 2.4 | 1.5, 3.9 | <0.001 |
| Heterosexual contact | 1.9 | 1.3, 2.8 | 0.001 |
| Other | 1.4 | 0.7, 2.8 | 0.31 |
| Hepatitis C infection | |||
| Seronegative or missing | 1.0 | ||
| Seropositive | 0.7 | 0.4, 1.1 | 0.15 |
| Smoking status | |||
| Never | 1.0 | ||
| Former | 1.1 | 0.7, 1.7 | 0.74 |
| Current | 1.5 | 0.9, 2.3 | 0.09 |
| Missing | 1.4 | 0.9, 2.2 | 0.17 |
Comparison with Beaver Dam Offspring Study.
The prevalence of intermediate-stage AMD compared to that published by the Beaver Dam Offspring Study,21 sub-grouped by age is given as table 4. The crude prevalence of AMD is 3-fold higher in the LSOCA cohort (9.9% vs 3.3%), and the age-adjusted and age- and gender-adjusted prevalences were approximately 4-fold higher in LSOCA (12.1% vs 2.9% and 13.0% vs. 3.2%, respectively).
Table 4.
Age-stratified Prevalence of Intermediate-Stage Age-related Macular Degeneration in Participants in the Longitudinal Study of the Ocular Complications of AIDS vs the Beaver Dam Offspring Study
| LSOCA* | Beaver Dam Offspring Study† | |||
|---|---|---|---|---|
| Age (years) | Number participants | % with AMD‡ | Number participants | % with AMD‡ |
| 21–34 | 244 | 5.3 | 168 | 0.6 |
| 35–44 | 782 | 5.9 | 822 | 1.3 |
| 45–54 | 605 | 12.9 | 1047 | 3.0 |
| 55–64 | 150 | 21.3 | 599 | 5.7 |
| 65–84 | 29 | 34.5 | 174 | 9.2 |
| Total | 1810 | 9.9 | 2810 | 3.3 |
| Adjusted prevalences | ||||
| Age-adjusted | 12.1 | 2.9 | ||
| Age- and gender-adjusted | 13.0 | 3.2 | ||
| Age- and gender-adjusted, whites only | 11.4 | 3.3 | ||
LSOCA = Longitudinal Study of the Ocular Complications of AIDS. Excludes 15 participants age ≤20 years, as this age group was not evaluated in the Beaver Dam Offspring Study.
Data from reference 21, Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors Arch Ophthalmol 2012;128:750–758.
AMD = age-related macular degeneration; % with intermediate-stage AMD reported.
Discussion
Our data suggest an increased age-adjusted prevalence of intermediate-stage AMD when compared to a non-HIV-infected cohort. These results are consistent with the increase in other age-related diseases, such as cardiovascular disease, osteoporosis, metabolic disorders, non-AIDS-related cancers, and neuro-cognitive decline, seen in HIV-infected, antiretroviral-treated, immune-restored persons and with the associations of AMD with the age-related diseases, diabetes, hypertension, and cardiovascular disease, in the LSOCA population. In the non-HIV-infected population, AMD also has been associated with cardiovascular disease and in some studies with diabetes.23–26 Similar to the HIV-uninfected population, in the LSOCA population, age was strongly and significantly associated with the prevalence of AMD with a near-doubling of the risk for each decade of age.
Antiretroviral-treated, immune-restored, HIV-infected persons do not have normal immune systems; instead they have immunologic changes similar to those seen in HIV-uninfected persons over 70 years of age, a phenomenon termed immunosenescence.11,27 In addition, antiretroviral-treated, immune-restored, HIV-infected persons are characterized by a state of chronic immune activation with ongoing systemic inflammation.11,27,28 This increase in age-related diseases in HIV-infected persons is not related to any drug or class of drugs, and it occurs to a greater degree among those with less complete immune recovery, suggesting that it is a consequence of HIV infection itself and/or the state of chronic inflammation it induces.11,27 Studies in non-HIV-infected persons demonstrate that systemic inflammation is a risk factor for AMD.29–31 Therefore, the apparent increased prevalence of intermediate-stage AMD in persons with AIDS may relate to their state of chronic immune activation and systemic inflammation, although immunosenescence also could contribute.
Similar to non-HIV infected populations, several risk factors were associated with AMD in the univariate analysis, including smoking and cardiovascular disease.23–26,32 However, all of these risk factors were not independently associated in the multiple regression model. The only other risk factor, besides age, associated with AMD was the HIV risk group with those participants whose transmission category for HIV acquisition was either injection drug use or heterosexual contact having a greater prevalence than those whose risk was male to male sexual contact. Whether this increased risk represents access to care with less antiretroviral therapy adherence and less complete immune recovery in the past or greater inflammation is uncertain. However, injection drug use itself is associated with immune activation and inflammation, and HIV-infected injection drug users have greater immune activation and inflammation than HIV-infected non-injection drug users,33, again suggesting systemic inflammation as a possible link in the increased prevalence of AMD. In the LSOCA cohort, there was no difference in the prevalence of intermediate-stage for different racial/ethnic groups, whereas in HIV-uninfected persons there is a greater prevalence among European Americans than among African Americans. In the Multi-Ethnic Study of Atherosclerosis (MESA), a cohort study in which AMD also was graded from photographs, and in which the mean age was ~63 years, AMD prevalences were 2.4% among African Americans and 5.4% among whites. 34 One possible explanation for this difference between the LSOCA cohort and non-HIV-infected persons is that the immunologic and inflammatory drivers, which are increasing the overall risk of AMD in persons with AIDS, may be sufficiently strong to overcome any inherent racial differences in AMD risk.
Caution should be taken in interpreting our data. Although the AMD lesions seen in the LSOCA population are clinically and photographically identical to those seen in non-HIV-infected populations, we do not have histology to confirm the nature of the lesions. In order to compare the prevalence of intermediate-stage AMD among persons with AIDS to that in the non-HIV-infected population, we used published data from a cohort evaluated with similar methodology. We were able to adjust the comparison for some recognized non-HIV-related risk factors, but not all, as we were working from published data. As such, the magnitude of the apparent increased risk for AMD among persons with AIDS may be somewhat different from our estimates. Nevertheless, adjustments for those risk factors where we had data, do not alter the conclusion that there appears to be an increased risk of intermediate-stage AMD among persons with AIDS. However, a better method (but one not available to us) would have been a cross sectional study employing concurrently-collected persons with and without HIV infection. Follow-up of HIV-infected persons with intermediate-stage AMD for the occurrence of late-stage AMD (i.e. choroidal neovascularization or geographic atrophy) also would be important, although the potential effect of nucleoside reverse transcriptase inhibitors on inhibiting the progression of late-stage AMD35 might reduce the rate of progression to late stage AMD among antiretroviral-treated, HIV-infected persons with intermediate stage AMD.
Caution also should be taken in extrapolating our data to all HIV-infected persons. The LSOCA cohort enrolled persons with AIDS and not earlier stages of HIV infection.15,16 As such the prevalence of AMD in HIV-infected patients with earlier stage of HIV infection remains uncertain. Nevertheless, because of late diagnosis, as many as 45% of patients diagnosed with HIV infection will progress to AIDS within three years of the diagnosis of HIV infection.36 Furthermore, among patients with HIV infection retained in follow-up care, nearly 30% will not have suppressed HIV replication in the blood.37 As such, there remains a substantial population of HIV-infected patients who will progress to AIDS and for whom these results are relevant.
Participants were enrolled at AIDS ophthalmology clinics, so it is possible that patients with visual symptoms were more likely to enroll and that the prevalence of eye disease is overestimated. However, the LSOCA population is very similar to the AIDS epidemic in terms of demographic and other features, decreasing the likelihood of a referral bias. In that regard, the only HIV risk group under-represented in the LSOCA cohort is injection drug use,15,16 a group with a higher prevalence of AMD, suggesting the possibility of underestimation of the prevalence of AMD in the AIDS epidemic.
In conclusion, persons with AIDS appear to have an approximately 4-fold increased prevalence of intermediate-stage AMD when compared to a similarly-aged HIV-uninfected population. As in the HIV-uninfected population, the prevalence is strongly and significantly related to age. This finding also is consistent with the “aging phenotype” identified in antiretroviral-treated, immune-restored, HIV-infected persons.11,27 Reasons for this increased prevalence are not fully explained, but it may relate to the state of chronic immune activation and systemic inflammation seen in these patients,11,27,28 which would be consistent with the association of AMD with systemic inflammation seen in the HIV-uninfected population.29–31
Supplementary Material
Figure 1.
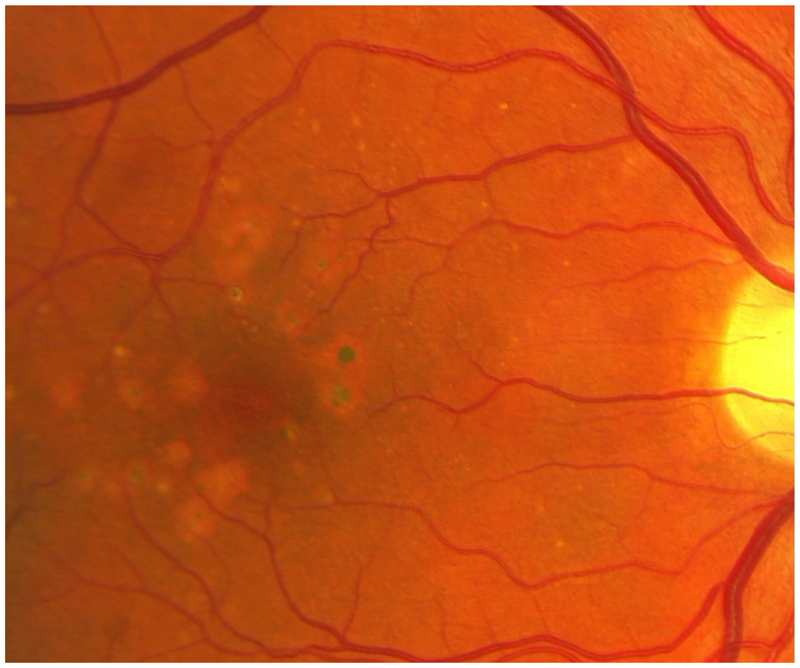
Retinal photograph of an HIV-infected participant in the Longitudinal Study of the Ocular Complications of AIDS, demonstrating large drusen and retinal pigment epithelial changes, characteristic of intermediate-stage age-related macular degeneration.
Acknowledgements
a. Funding/Support: Supported by cooperative agreements from the National Eye Institute, the National Institutes of Health, Bethesda, MD to the Icahn School of Medicine at Mount Sinai, New York, NY (U10 EY 08052); The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (U10 EY 08057); and the University of Wisconsin, Madison School of Medicine, Madison, WI (U10 EY 08067); and in part by the Johns Hopkins Center for AIDS Research (P30 AI 094189), Baltimore, MD, co-funded by the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Institute of Child Health and Development, the National Heart, Lung, and Blood Institute, the National Institute of Drug Abuse, the National Institute of Mental Health, the National Institute of Aging, the Fogarty International Center, the National Institute of General Medical Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Office of AIDS Research, the National Institutes of Health, Bethesda, MD.
b. Financial Disclosures: Douglas A. Jabs: Applied Genetic Technologies Corporation, Alachua, FL (Data and Safety Monitoring Committee), Novartis Pharmaceuticals, Basel, Switzerland (Data and Safety Monitoring Committee), and Santen, Inc., Emeryville, CA (consultant); Mark Van Natta: none; Efe Sezgin: none; Jeong Won Pak: none; Ronald Danis: none.
c. Contributions of authors: design and conduct of the study (Drs. Jabs, Van Natta, and Danis); collection, management, analysis, and interpretation of the data (Drs. Jabs, Van Natta, Sezgin, Pak, and Danis); preparation, review, or approval of the manuscript (Drs. Jabs, Van Natta, Sezgin, Pak, and Danis); and responsibility for the integrity of the entire study and manuscript (Drs. Jabs, Van Natta, and Danis).
d. Other Acknowledgments: none
REFERENCES
- 1.Friedman DS, O’Colmain B, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122(4):564–572. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 3.Davis MD, Gangon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol 2005;123(11):1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: Age-Related Eye Study report no. 6. Am J Ophthalmol 2001;132(5):668–681. [DOI] [PubMed] [Google Scholar]
- 5.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration. Arch Ophthalmol 2005;123(11):1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color photographs in the Age-Related Eye Disease Study 2 (AREDS 2 report number 2). Invest Ophthalmol Vis Sci 2013;54(7):4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Eng J Med 1998;338(13):853–860. [DOI] [PubMed] [Google Scholar]
- 8.Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994–1997. AIDS 1998;12(14):1931–1933. [PubMed] [Google Scholar]
- 9.Sugar EA, Jabs DA, Ahuja A, et al. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 2012;153(6):1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braithwaite RS, Roberts MS, Chang CC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy. Ann Intern Med 2008;148(3):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual Rev Med 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007;146(2):87–95. [DOI] [PubMed] [Google Scholar]
- 13.Hogg R, Lima V, Sterne JA, Grabar S, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008;372(9635):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan RC, Kingsley LA, Sarrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007;45(8):1074–1081. [DOI] [PubMed] [Google Scholar]
- 15.Jabs DA, Van Natta ML, Holbrook JT, et al. The Longitudinal Study of the Ocular Complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology 2007;114(4):780–786. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Van Natta ML, Holbrook JT, et al. The Longitudinal Study of the Ocular Complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology 2007;114(4):787–793. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 18.Jabs DA. Cytomegalovirus retinitis and the acquired immune deficiency syndrome: Bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol 2011;151(2):198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. pp 213–5, 241–56. [Google Scholar]
- 20.Wang Z Model selection using the Akaike information criterion. Stata Technical Bulletin 2000;54:47–49. [Google Scholar]
- 21.Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol 2012;128(6):750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Belle G, Fisher LD, Heargerty PJ, Lumley TS. Biostatistics:A Methodology for the health Sciences. 2nd ed. New York, Wiley & Sons, 2004. Pp. 642–84. [Google Scholar]
- 23.Duan Y, Mo J, Klein R, et al. Age-related macular degeneration is associated with incident myocardial infarction among elderly Americans. Ophthalmology 2007;114(4):732–737. [DOI] [PubMed] [Google Scholar]
- 24.Sun C, Klein R, Wong TY. Age-related macular degeneration and risk of coronary heart disease and stroke:the Cardiovascular Health Study. Ophthalmology 2009;116(10):1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JS, Mitchell, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration The Blue Mountains Eye Study. Ophthalmology 2007;114(6):1143–1150. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Uchino M, Sastry SM, Schaumberg DA.Age-related macular degeneration and the incidence of cardiovascular disease :a systematic review and meta-analysis. PLoS One 2014;9(3):e89600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathai S, Bajillan H, Landay AL, High KP.Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014;69(7):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014;210(8):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitta VP, Christen WG, Glynn RJ, et al. C-reactive protein and the incidence of macular degeneration. JAMA Ophthalmol 2013;131(4):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cousins SW, Espinosa-Heidemann DG, Csaky KG.Monocyte activation in patients with age-related macular degeneration:a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 2004;122(7):1013–1018. [DOI] [PubMed] [Google Scholar]
- 31.Nassar K, Grisanti S, Elfar E, et al. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol (forthcoming). [DOI] [PubMed] [Google Scholar]
- 32.Lechanteur YTE, van de Ven JPH, Samilhodzic D, et al. Genetic, behavioral, and sociademographic risk factors for second eye progression in age-related macular degeneration. Invest Ophthalmol Vis Sci 2012;53(9):5846–5852. [DOI] [PubMed] [Google Scholar]
- 33.Salter ML, Lau B, Mehta SH, Go VF, Leung S, Kirk GD.Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr 2013;64(5):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2006;113(3):373–380. [DOI] [PubMed] [Google Scholar]
- 35.Fowler BJ, Gelfand B, Kim Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014;346(6212):1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Late HIV testing – 34 states, 1996–2005.Morbid Mortal Wkly Rep 2009;58(24):661–665. [PubMed] [Google Scholar]
- 37.Edison L, Hughes D, Drenzek C, Kelly J.Prevalence and indicators of viral suppression among persons diagnosed with HIV infection retained in care – Georgia, 2010. Morb Mortal Week Rep 2014;63(3):55–58. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


