Abstract
Background
Metastatic sarcoma patients have limited options. Nivolumab and ipilimumab are monoclonal antibodies targeting PD-1 and CTLA-4, respectively. We evaluated the efficacy and safety of nivolumab and nivolumab plus ipilimumab separately in sarcoma patients.
Methods
We did an open-label, unblinded, non-comparative multi-center randomized phase II study that enrolled patients from 15 centers in the USA that were members of the Alliance Clinical Trials in Oncology Group (Alliance) and National Clinical Trials Network (NCTN.) Initial study design was a simon stage 2; however due to rapid accrual the study design was changed to Simon single stage design. Patients with central pathology confirmation of sarcoma had to be at least 18 years old to enroll and have evidence of metastatic or unresectable disease and adequate performance status. Patients must have received at least one previous line of systemic therapy and have at least one measurable lesion as per the Response Evaluation Criteria In Solid Tumors version 1.1. Disease progression was not a requirement for enrollment. Patients were assigned to treatment in an unblinded manner, as this trial was conducted as two independent, non-comparative phase II trials. Following registration, the patient was assigned one of the two treatments in a 1:1 ratio utilizing a dynamic allocation algorithm based on the methods by Pocock and Simon. Patients received either nivolumab 3 mg/kg every two weeks or nivolumab 3mg/kg and ipilimumab 1mg/kg every three weeks x four doses followed by nivolumab (3mg/kg) every two weeks thereafter. The primary endpoint was confirmed objective response rate, using a per-protocol analysis for evaluability. Secondary endpoints included safety, duration of response, clinical benefit rate, progression-free and overall survival (PFS, OS). Enrollment is closed and 3 patients remain on treatment as of the data lock on April 24, 2017. ClinicalTrials.gov Identifier: NCT02500797.
Findings
A total of 96 patients from 13 Alliance sites and 2 NCTN sites underwent central pathology review for eligibility between the following dates: August 13 to December 24, 2016 (81 patients); March 16, 2016, to March 17, 2016 (14 patients). Eighty-five patients proceeded to be allocated to one of the two treatment arms. Efficacy was determined in the first 76 evaluable patients, per protocol. Among the 38 patients that received nivolumab monotherapy, the confirmed ORR was 5% [92% CI (1–15%)]. Responses occurred in UPS and sarcoma, NOS. For the 38 patients that received combination therapy, the confirmed ORR was 16%, [92% CI (7–29%)]. Responses occurred in UPS, LMS, myxofibrosarcoma and angiosarcoma. In the monotherapy arm, the most common grade 3 or worse adverse events included anemia (four [10%]), decreased lymphocyte count (three [7%] each) and dehydration, increased lipase, pain, pleural effusion, respiratory failure, secondary benign neoplasm and urinary tract obstruction (two [5%] each.) In the combination arm, the most common grade 3 or worse adverse events included: anemia (seven [17%]), hypotension (four [10%]), pain and urinary tract infection (three [7%.]). Treatment related serious adverse events on the monotherapy arm occurred in eight patients and included anemia, anorexia, dehydration, decreased platelet count, diarrhea, fever, increased creatinine, and pleural effusion (one [2%] each). On the combination arm, treatment related serious adverse events occurred in11 patients. Three [7%] patients had adrenal insufficiency, two [5%] had increased alanine aminotransferase, two [5%] with hyponatremia, one [2%] each experienced anemia, increased aspartate aminotransferase, fatigue, pain and pruritus.
Interpretation
Nivolumab alone does not warrant further study in an unselected sarcoma population given the limited efficacy. Nivolumab combined with ipilimumab demonstrated promising efficacy in certain sarcoma subtypes (UPS, LMS, myxofibrosarcoma and angiosarcoma) with a manageable safety profile comparable to current available treatment options. The combination therapy arm met its pre-defined primary study endpoint; further evaluation of nivolumab plus ipilimumab in a randomized study is warranted.
Funding
Alliance Clinical Trials in Oncology, NCI-CTEP, Bristol-Myers Squibb, Cycle for Survival
INTRODUCTION
Sarcomas are rare, heterogeneous malignant tumors of mesenchymal origin characterized by more than 100 distinct subtypes, accounting for one percent of malignancies in adults.(1) For newly diagnosed metastatic patients that are chemotherapy naïve; efficacy is similar with doxorubicin alone or gemcitabine and docetaxel.(2) In this upfront setting, these agents offer responses rates of about 18% with PFS and OS of 5 months and 16 months, respectively. Beyond the front line setting, there have been approvals by the FDA for systemic agents including pazopanib, trabectedin and eribulin for selected sarcoma subtypes.(3–5) With each of these agents, there were modest improvements in either PFS or OS. Yet the overall response rate (ORR) remains <10% with PFS of about four months and OS <14 months.(3–5) Most recently, a phase II study of doxorubicin + olaratumab versus doxorubicin for patients with soft tissue sarcoma (STS) has demonstrated the superiority of the combination with an overall survival of 26·5 vs 14·7 months, leading to FDA approval.(6) These findings are pending confirmation in a larger, randomized phase III study. Despite these recent FDA approvals, there remains the need for less toxic, more effective therapies that offer prolonged disease control and improved survival.
Modulating the immune system with monoclonal antibodies that block immune checkpoints has emerged as a promising strategy in cancer care leading to durable antitumor activity and prolonged survival in multiple malignancies.(7–13) Immune checkpoints such as the programmed death ligand 1 (PD-L1) can be over-expressed by tumors or in the tumor microenvironment and can inhibit the anti-tumor activity of effector T cells.(14) Some sarcomas express PD-L1, with reported PD-L1 expression ranging from 12% to 65%, depending on the sarcoma histology, timing of sample collection, and assay used for PD-L1 detection.(15, 16) This variability poses a challenge in the utilization of PD-L1 expression as a prognostic biomarker. In addition, clinical data of patients treated with checkpoint inhibitors has suggested that patients can benefit from checkpoint inhibitors regardless of PD-L1 expression.(8, 9, 12, 17) While this highlights the need for a more effective biomarker, it should not preclude exploration of checkpoint inhibitors in sarcoma.
Limited data exist regarding the efficacy of checkpoint inhibitors in sarcoma. In a small phase II study, six patients with synovial sarcoma were treated with ipilimumab 3mg/kg every three weeks without documented responses.(18) A phase II study evaluated the efficacy of pembrolizumab in 80 patients with bone and soft tissue sarcomas.(19) The ORR in the STS arm was 18%, with responses seen only in undifferentiated pleomorphic sarcoma (UPS) and liposarcoma. There were 40 bone sarcoma patients enrolled and only two responses were seen in osteosarcoma and chondrosarcoma. The limited clinical activity in most sarcoma subtypes suggests that pembrolizumab alone cannot adequately activate suppressed effector T cells. UPS is an inflamed tumor, characterized by high tumor infiltrating lymphocytes (TIL)which may explain the clinical activity noted.(20) Most sarcoma subtypes are unlikely to be inflamed tumors, emphasizing the need to explore other, combinatorial immunotherapy strategies. Strategies that enhance anti-tumor effects through different mechanisms such as cytotoxic T-lymphocyte (CTLA-4) and programmed death-1 (PD-1) have proven to be synergistic in pre-clinical models.(21) In addition, clinical data thus far have resulted in higher efficacy in multiple malignancies.(7, 10, 22) We conducted an open-label multi-center, randomized, non-comparative study with nivolumab or nivolumab with ipilimumab for patients with metastatic sarcoma.
METHODS
Study Design and Participants
This is a multi-center, open label, randomized, non-comparative phase II trial (NCT02500797) conducted through the Alliance Clinical Trials in Oncology Group (A091401). Patients at least 18 years old with advanced or metastatic sarcoma and Eastern Cooperative Oncology Group (ECOG) performance status one of 0 or 1 were included with estimated life expectancy of 3 months. All patients required measurable disease per Response Evaluation in Solid Tumors (RECIST v1·1), and at least one systemic therapy for metastatic disease. Disease progression was not an inclusion criteria. Patients required adequate kidney, liver and bone marrow function. Central pathology confirmation of sarcoma diagnosis was required. A minimum period of 28 days was required between any previous systemic therapy and initiation of nivolumab or nivolumab and ipilimumab. Key exclusion criteria included active brain metastases or history of autoimmune diseases. Prior therapy with anti-PD-1 or CTLA-4 therapy was not permitted. (Full eligibility criteria provided in appendix pages 1 and 2) Review boards at each of the participating institutions approved the study. The study was conducted according to the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. This study was monitored for accrual, safety, and the primary endpoint, at least twice annually, by the Data and Safety Monitoring Board (DSMB), a standing committee comprised of individuals outside of the Alliance.
Randomization and masking
Patients were assigned to treatment in an unblinded manner, as this trial was conducted as two independent, non-comparative phase II trials. Following registration, the patient was assigned one of the two treatments in a 1:1 ratio utilizing a dynamic allocation algorithm based on the methods by Pocock and Simon.(23) The assigned treatment arm was automatically generated by systems within the Alliance Statistics & Stat Center, were immediately transferred to the appropriate system for assignment of the patient. A manual list of assignments was not generated. Site of randomization was the only stratification factor used.
Procedures
Patients received either nivolumab 3mg/kg as an intravenous (IV) infusion every two weeks or nivolumab 3mg/kg and ipilimumab 1mg/kg every three weeks for four doses followed by nivolumab 3mg/kg every two weeks thereafter. Treatment was continued until progressive disease (PD) or toxicity for up to two years.
Tumor assessments were performed at baseline, every six weeks for the first 12 weeks and every eight weeks thereafter. Central radiology review was not performed. Patients experiencing PD within the first 12 weeks of treatment were permitted to continue study treatment beyond initial PD if the specific protocol criteria were met including: evidence of clinical benefit, tolerant of study treatment, no more than four new lesions and less than 40% increase in tumor burden. If PD was confirmed at the subsequent 4 week repeat assessment, the date of initial PD was used for analyses. Otherwise, the patient was considered as non-PD and continued study treatment.
Dose reductions were not permitted, however, dose interruptions for up to 6 weeks were allowed. If treatment was discontinued due adverse events, patients were followed until disease progression, initiation of different therapy and 30 days after the last dose of nivolumab or nivolumab and ipilimumab.
Laboratory monitoring and safety was evaluated at baseline and every two to three weeks as per the treatment schedule. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 during treatment and up to 30 days after treatment discontinuation.
Twelve months after initial study start, a protocol amendment was approved that allowed patients receiving nivolumab monotherapy with PD to receive nivolumab and ipilimumab provided that eligibility criteria were met. (Appendix, p 4)
Optional biopsies pre-treatment and during week 6 were obtained in patients who provided informed consent. In addition, blood for correlative analysis was obtained during screening and selected time points during protocol treatment. Twenty-two patients agreed to optional tumor biopsies and blood collections.
Outcomes
The primary endpoint was confirmed objective response rate (ORR) based on RECIST v1·1, during protocol directed treatment. Confirmation of response was required four weeks following the initial response. The confirmed ORR was estimated as the number of patients with a complete response (CR) or partial response (PR), divided by the number of evaluable patients. The clinical benefit rate was defined as best objective status of CR, PR, or stable disease (SD) at a given time point while receiving protocol treatment, divided by the number of patients receiving treatment at the same time point. Duration of response was defined as the time from first CR or PR to date of progressive disease (PD). Progression-free survival (PFS) was defined as the time from randomization to date of PD or death. Patients that discontinued treatment for reasons other than PD (such as adverse events) were censored at the date of their most recent disease evaluation prior to receiving any future systemic treatment regimens. OS was calculated as the time between randomization and date of death. Patients that were lost to follow-up were censored for survival at the date last known to be alive and PD at the date of their most recent disease assessment. Duration of treatment was calculated as the time between randomization and end of treatment date. Patients remaining in active treatment were censored for duration of treatment and on their most recent date of treatment. In addition, all analyses excluded any newly collected data for patients having withdrawn consent future follow-up and beyond the date of withdrawal.
After discontinuation of protocol treatment, survival, disease status, first non-protocol systemic therapy, long term adverse events were captured every six months for three years post-randomization.
Statistical Analyses
The primary endpoint of the study was the confirmed ORR within each treatment arm of nivolumab alone and nivolumab + ipilimumab. Eligible patients having initiated study treatment were considered evaluable for efficacy study endpoints. Within each arm, one confirmed response in 18 evaluable patients in stage 1 would expand enrollment to a total of 38 evaluable patients (stage 1 + stage 2.)(24) Four confirmed responses in 38 patients were required to detect a confirmed ORR rate of at least 20%. This design yielded 95% power at a significance level of 11% (1-sided test) with a 40% chance of stopping early if the true rate was less than 5%. A total of 81 patients were pre-registered by October 6, 2015, and 71 patients subsequently enrolled between August 31, 2015, and December 24, 2015. Given this unexpected, rapid enrollment, a Simon 2 stage design was no longer feasible precluding an interim analysis. With consensus from the Study Team, Alliance DSMB, the Alliance Experimental Therapeutics & Rare Tumors Committee leadership, and the National Cancer Institute Cancer Therapy Evaluation Program, a protocol amendment in December 2015 changed the design to single stage phase 2 design. No efficacy data was reviewed by any of the parties during the process of this design change. Five in 38 evaluable patients achieving a confirmed CR/PR were considered sufficient evidence of promising clinical activity in this setting. This study design yielded 90% power to detect a rate of at least 20% (clinically active) and at 0.04 level of significance (1-sided test) assuming a confirmed ORR of at most 5% represents clinical inactivity.
Ninety-two percent 2-sided confidence intervals are reported for our observed confirmed ORR within each arm. The first 38 eligible patients randomized onto the trial for each arm were used to assess the primary endpoint.
Secondary endpoints included evaluation of toxicity, duration of response, duration of treatment, clinical benefit rate, PFS, and OS. Categorical data analyses and summary statistics were used to report adverse events. All patients initiating study treatment were considered evaluable for the analysis of adverse events, including data to the date of ineligibility for any patients found ineligible after initiating study treatment; whereas, only eligible treated patients were used for the remaining secondary endpoints. Kaplan-Meier methodology was used to estimate the distributions of all time to event endpoints.(25) Ninety-five percent (95%) CIs for statistical estimates were calculated. All patients included in the evaluation of the primary endpoint were also used for the evaluation of the time to event endpoints. All statistical analyses were performed using statistical analysis system (SAS) version 9·4.
This trial is registered with ClinicalTrials.gov, number NCT02500797.
Role of the funding source
The study was investigator-initiated and was performed with support from the Alliance Clinical Trials in Oncology Group, National Cancer Institute-Cancer Therapy Evaluation Program. (Appendix p5) Bristol-Myers Squibb provided support. SPD and MM had full access to all of the data and the ultimate responsibility to draft the initial manuscript. Co-authors provided comments to initial draft which was ultimately modified. The corresponding author submitted the final draft for publication. SPD, MM, GKS, WT and HS led the study design with support from Alliance and NCI-CTEP Data collection was conducted by all the authors. Statistical analyses were performed by the Alliance Statistics and Data Center. BMS did not contribute to study design, data collection, data analysis, data interpretation, or in the writing of this manuscript; but did review this manuscript.
RESULTS
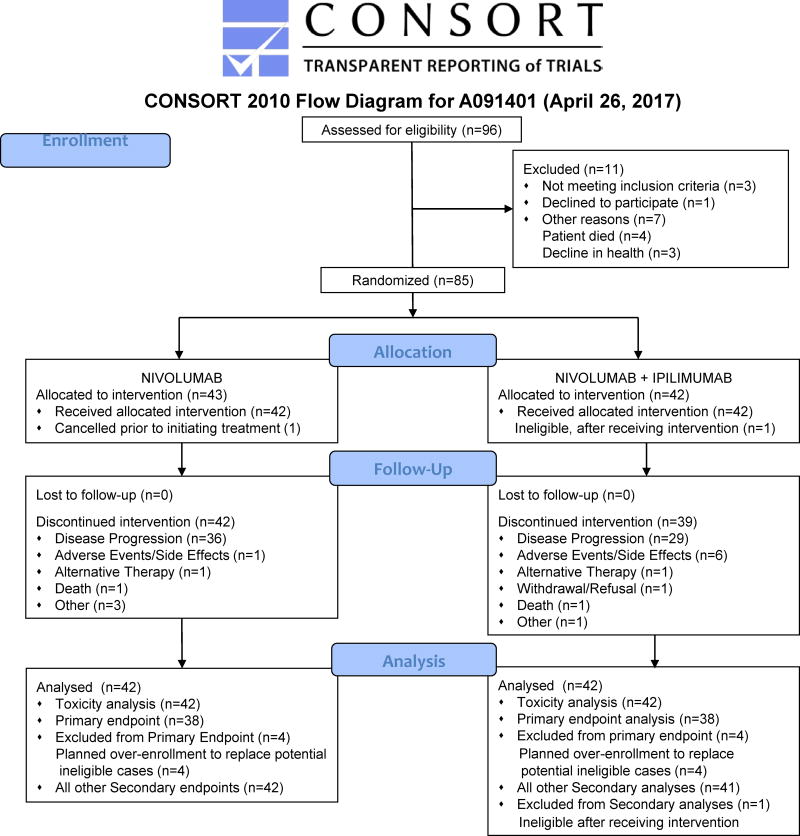
A total of 96 patients underwent central pathology review for eligibility and between the following dates: August 13 to December 24, 2016 (81 patients); March 16, 2016, to March 17, 2016 (1 day, 14 patients). Eighty-five patients proceeded to randomization, including 71 patients from August 31 – December 24, 2015, and a final 14 patients within 1 day (March 16 – 17, 2017), from 13 Alliance Clinical Trials in Oncology sites and two NCTN groups in the United States.(Appendix, page 5) Data collection was locked as of April 24, 2017. Forty-three patients were assigned to nivolumab alone and 42 patients to nivolumab with ipilimumab. One patient refused to initiate monotherapy after randomization and was classified as a cancellation, subsequently excluded from efficacy analyses. Due to non-measurable disease per RECIST, a second patient was determined to be ineligible after starting combination therapy and is considered inevaluable for efficacy analyses, per protocol. (Figure 1) Demographic and safety data are presented for 85 enrolled and 84 treated patients, respectively. Efficacy data for the primary endpoint are presented for the first 38 evaluable patients, as specified in the protocol. Efficacy data also presented for all treated patients in each arm; monotherapy (n=42) and combination (n=41.) Patient demographics and disease characteristics was similar between both treatment arms, although not intentionally balanced on any of these factors at randomization. The average age of patients at study entry was 53 years; with 61% of 85 patients with an ECOG performance status of 0. Fifty-two percent of 85 enrolled patients were female. Patients were heavily pre-treated, with 61% of patients having received at least three lines of prior chemotherapy.(Table 1) The most common enrolled sarcoma types across both arms (of 85 patients) included: bone nine (10·6%), LMS 29 (34·1%), LPS five (5·9%), spindle cell sarcoma 11 (12·9%), UPS/MFH 11 (12·9%), and other 10 (11·7%). (Table 1)
Figure 1.
CONSORT Diagram (Flow of Patients)
Table 1.
Patient & Disease Characteristics
| Randomized | |||
|---|---|---|---|
| Nivolumab | Nivolumab+ Ipilimumab | Total | |
| Number of Patients (N) | 43 | 42 | 85 |
| Age | |||
| Mean (SD) | 52·9 (13·8) | 54·1 (13·3) | 53·5 (13·5) |
| Median (Range) | 56·0 (21·0–76·0) | 57·0 (27·0–81·0) | 57·0 (21·0–81·0) |
| ECOG PS | |||
| 0 | 28 (65·1%) | 24 (57·1%) | 52 (61·2%) |
| 1 | 15 (34·9%) | 18 (42·9%) | 33 (38·8%) |
| Gender | |||
| Female | 21 (48·8%) | 23 (54·8%) | 44 (51·8%) |
| Male | 22 (51·2%) | 19 (45·2%) | 41 (48·2%) |
| # Prior Therapies | |||
| 1 | 5 (11·6%) | 10 (23·8%) | 15 (17·6%) |
| 2 | 12 (27·9%) | 6 (14·3%) | 18 (21·2%) |
| 3 or more | 26 (60·5%) | 26 (61·9%) | 52 (61·2%) |
| Histologic typea | |||
| Angiosarcoma | 0 | 3 (7·1%) | 3 (3·5%) |
| Boneb | 5 (11·6%) | 4 (9·5%) | 9 (10·6%) |
| Leiomyosarcoma | 15 (34·9%) | 14 (33·3%) | 29 (34·1%) |
| LPS (well/dedifferentiated) | 3 (7%) | 2 (4·8%) | 5 (5·9%) |
| Sarcoma, NOS | 2 (4·7%) | 1 (2·4%) | 3 (3·5%) |
| Spindle cell sarcoma | 5 (11·6%) | 6 (14·3%) | 11 (12·9%) |
| Synovial sarcoma | 2 (4·7%) | 2 (4·8%) | 4 (4·7%) |
| UPS/MFH | 5 (11·6%) | 6 (14·3%) | 11 (12·9%) |
| Otherc | 6 (14%) | 4 (9·5%) | 10 (11·7%) |
| Histologic grade (differentiation) | |||
| G1 (Well differentiated) | 3 (7·0%) | 2 (4·8%) | 5 (5·9%) |
| G2 (Moderately differentiated) | 9 (20·9%) | 7 (16·7%) | 16 (18·8%) |
| G3 (Poorly differentiated) | 21 (48·8%) | 20 (47·6%) | 41(48·2%) |
| GX (Grade cannot be assessed) | 10(23·3%) | 13 (31·0%) | 23(27·1%) |
Based on a Central Review of pathology, prior to randomization.
Bone/Other sarcomas in single agent arm: dedifferentiated chondrosarcoma (1), osteogenic sarcoma (1), Ewing’s Sarcoma (3), ASPS (1), epitheloid sarcoma (1), extraskeletal myxoid chondrosarcoma (1), malignant solitary fibrous tumor (1), malignant peripheral nerve sheath tumor (1), PECOMA (1)
Bone/Other sarcomas in dual agent arm: dedifferentiated chondrosarcoma (1), osteosarcoma (1), Ewing’s Sarcoma (2), ASPS (1), (1), extraskeletal myxoid chondrosarcoma (1), malignant peripheral nerve sheath tumor (1), myxofibrosarcoma (1)
At the time of this analysis, all patients have discontinued study treatment on the monotherapy arm. Three patients remain on study treatment on the combination arm. In both treatment arms, the most common reason for discontinuing study treatment was disease progression.(Figure 1) Adverse events lead to discontinuation of treatment in six (14% of 42) and one (2% of 42) patients in the combination and monotherapy arms, respectively. (Figure 1) On the monotherapy arm, nine and 22 patients experienced dose delays and dose omissions, respectively. Median duration of treatment for monotherapy was 2.3 months [n=42, 95% CI (1·5–3·5)]. On the combination arm, 16 and 19 patients experienced dose delays and dose omissions, respectively. Median duration of combination treatment was 3.7 months [n=42, 95% CI (2·1–4·6)].
At the time of the data lock, in there were 27 and 21 total patient deaths on the monotherapy and combination arm, respectively. Deaths occurred during treatment in 5 and 6 patients on the monotherapy and combination arm, respectively. On the nivolumab monotherapy arm, reasons for death included disease progression in 25 patients and unknown in 2 patients. On the combination arm, reasons for death included disease progression (16), unknown (4), and sepsis (1).
Efficacy and Patient Outcome
On the nivolumab monotherapy arm, 15 (31·6%) of 42 evaluable patients are alive with a median of 13·6 months of follow-up (Q1–Q3: 8·9–15 8). The following reasons were provided for the 27 patient deaths: tumor/disease (25) and unknown (two).
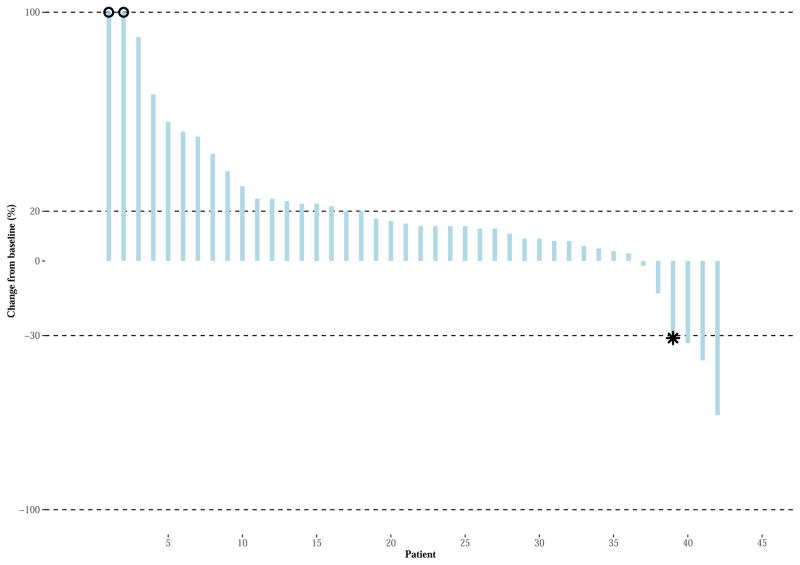
Three of 38 evaluable patients achieved PRs, two of which were confirmed for an ORR of 5% [92% CI (1–15%)]. Responses occurred in the following histological subtypes: alveolar soft part sarcoma (ASPS), non-uterine LMS, and sarcoma NOS. (Figure 2A and appendix page 6) Four additional patients were treated, none of whom had a response resulting in a final adjusted confirmed ORR of 8% [n = 42, 92% CI (1–15%)]. The time to response in three evaluable patients was 1·2, 1·3, and 11·8 months. For the patient with sarcoma NOS, the PR was first observed nearly 12 months after initiating treatment, followed by PD. The median duration of response was 3·2 months [n=3, 95% CI (1·1–3·2)]. (Figure 3A)
Figures 2A–B. Waterfall Plot for Best Response of Target Lesions/Nodes (RECIST v1·1).
Monotherapy (Figure 2A): The Waterfall Plots display the percent changed in the sum of the target lesions/nodes from study entry (vertical axis) for each patient (horizontal axis) during active treatment, for each arm. The two horizontal bars represent the criteria for PD (20% increase in tumor size) and response (30% decrease in tumor size for PR; total disappearance of target lesions/nodes for CR). The two patients noted as having an asterisk (‘*’) achieved PR according to radiographic assessments, yet are classified as PD by unequivocal PD on non-target lesions (as per RECIST v1·1 requirements).
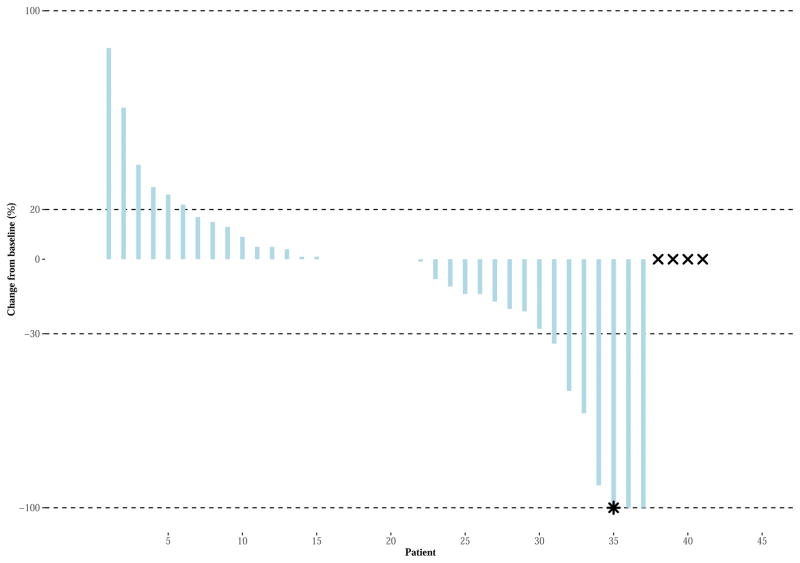
Combination Therapy (Figure 2B): The Waterfall Plots display the percent changed in the sum of the target lesions/nodes from study entry (vertical axis) for each patient (horizontal axis) during active treatment, for each arm. The two horizontal bars represent the criteria for PD (20% increase in tumor size) and response (30% decrease in tumor size for PR; total disappearance of target lesions/nodes for CR). The patient noted as having an asterisk (‘*’) achieved CR according to radiographic assessments, yet classified as PD by unequivocal PD. Four patients (noted by an “x” symbol) received combination therapy and did not have disease assessments following randomization. Three patients died and 1 patient had progressive disease in non-target lesions. Tumor growth was truncated to 100% for 2 patients having an increase of more than 100%, as noted by an “o” symbol.
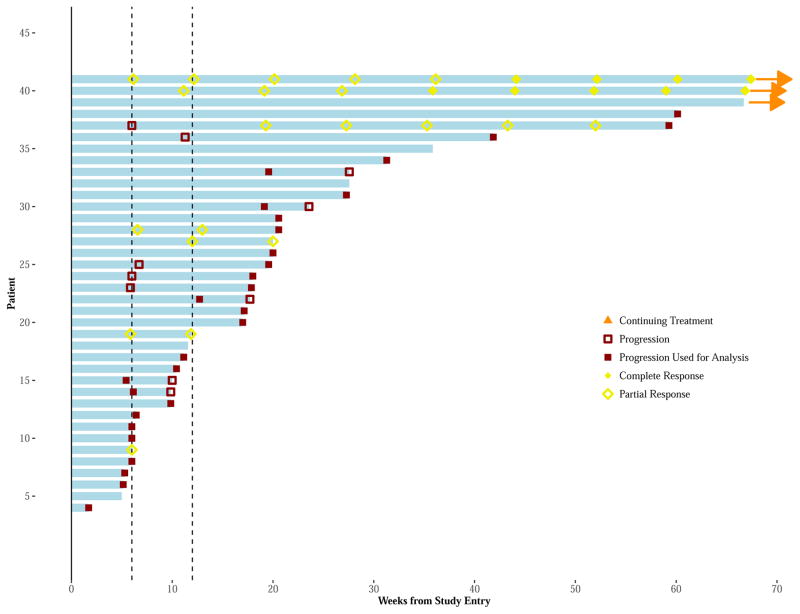
Figure 3A–B. Swimmer Plots for Assessments of Tumor Response, Over Time, by Patient.
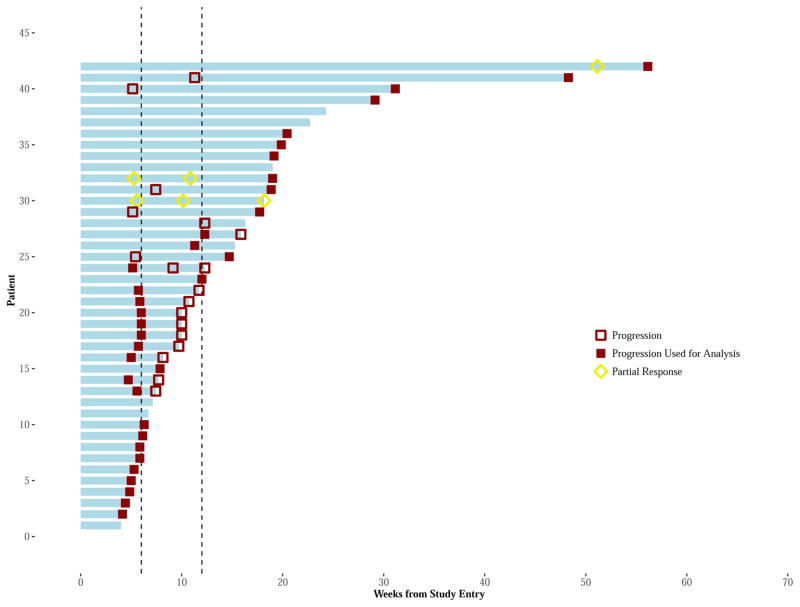
Monotherapy Figure 3A: The Swimmer Plots display a “swim lane” for each patient’s (vertical axis) initial treatment period (horizontal axis) and disease status, during treatment, and for each arm. Vertical lines identify the 6- and 12-week restaging. Red square symbols, either empty or solid, represent suspected and actual PD disease assessments. Patient’s having an initial progression within the first 12 weeks of treatment, may have a confirmatory 4-week evaluation (if meeting criteria for continuation treatment) showing either no PD or PD. In such cases, the patient’s first PD is used for study endpoints (represented by a solid red square symbol). Otherwise, the initial PD for the patient is indicated by an empty red square symbol and the patient may continue treatment. Patient’s having an arrow at the end of their swim lane continue to receive receiving protocol directed therapy at the time of data cut-off. Patient response is further noted by yellow/gold symbols appearing as rhombus (diamond) shape (PR = empty rhombus; CR = solid rhombus). Of note, one patient had 3 disease assessments within the first 12 weeks. This patient had confirmed PD (i.e., 2nd PD), yet continued treatment an additional 3 weeks and subsequently ending due to a 3rd PD.
Combination therapy Figure 3A: The Swimmer Plots display a “swim lane” for each patient’s (vertical axis) initial treatment period (horizontal axis) and disease status, during treatment, and for each arm. Vertical lines identify the 6- and 12-week restaging. Red square symbols, either empty or solid, represent suspected and actual PD disease assessments. Patient’s having an initial progression within the first 12 weeks of treatment, may have a confirmatory 4-week evaluation (if meeting criteria for continuation treatment) showing either no PD or PD. In such cases, the patient’s first PD is used for study endpoints (represented by a solid red square symbol). Otherwise, the initial PD for the patient is indicated by an empty red square symbol and the patient may continue treatment. Patient’s having an arrow at the end of their swim lane continue to receive receiving protocol directed therapy at the time of data cut-off. Patient response is further noted by yellow/gold symbols appearing as rhombus (diamond) shape (PR = empty rhombus; CR = solid rhombus).
Of the 29 (71% of 42) patients with PD at week 12, 18 (62% of 29) were eligible to continue treatment. Eleven (61% of 18) of these patients were confirmed as PD within 1 month and seven (39% of 18) continued receiving treatment for 2 to 8.5 months. It is noted that one of the 11 patients confirmed as PD within 1 month continued treatment in error for an additional 3 weeks, ending treatment at that time due to a third disease status of PD. (Appendix, page 7)
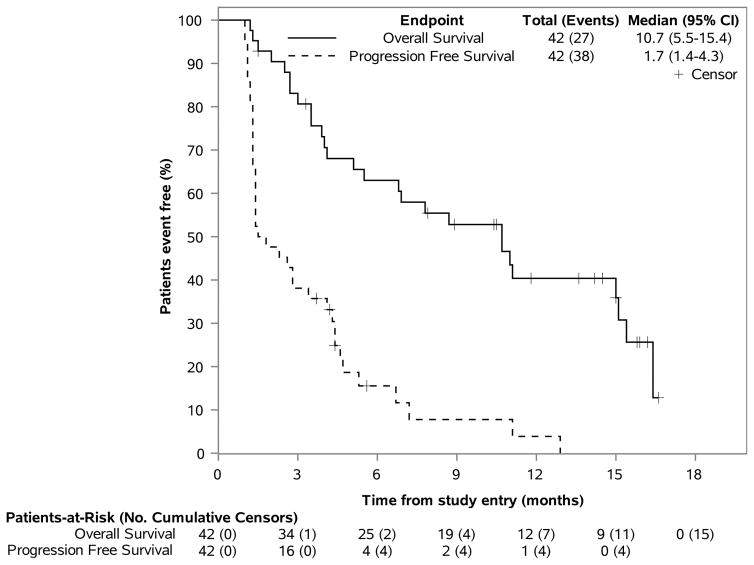
On the monotherapy arm, the clinical benefit rate at 6 and 12 months was 10% [4/42, 95% CI (3–22%)] and 2% [1/42, 95% CI (1–12%)], respectively. Thirty-seven of 42 (88%) evaluable patients have experienced PD. The median PFS was 1·7 months [n=42, 95% CI (1·4–4·3 months). The median OS was 10·7 months [n=42, 95% CI (5·5–15·4)]. The 12 month OS rates was 40·4% [n=12, 95% CI (27·2–59·9%)]. (Figure 4A)
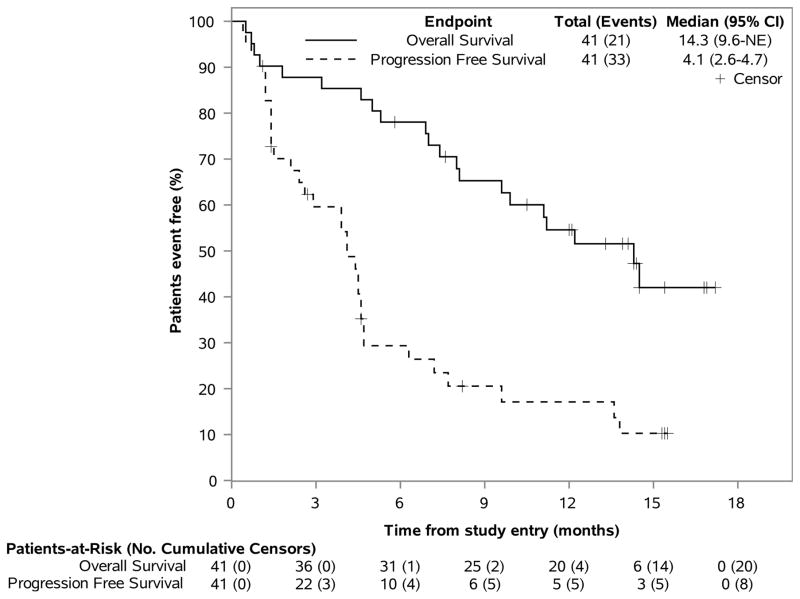
Figures 4A–B. PFS & OS Outcomes, by Treatment Arm.
This figure displays the Kaplan-Meier estimates of the distribution of PFS and OS across time (in months), using one figure for each therapy. Patients who remain alive or without progression, are noted as censors and by a “+” symbol. The total number of patients at risk of an event, as well as the number of patients having an event (eg, deaths for OS) are noted over time and along the horizontal axis at key time points (eg, every 3 months from randomization). The vertical axis represents the percent of patients considered event free (ie, alive for OS) at a time point.
After the cross-over amendment was approved, 4/21 (19%) eligible patients on the nivolumab monotherapy arm went on to combination therapy. All 4 patients had PD within 0·4–2·8 months.
In the combination therapy arm, 20 (48·8%) of 41 evaluable patients are alive with a median of 14·2 months (Q1–Q3: 12·7–16·1) of follow-up. The following reasons were provided for the 21 patient deaths: tumor/disease (16), unknown (4), and sepsis (1).
Six of seven responses were confirmed for an ORR of 16%, [n=41, 92% CI (7–29%)]. Responses occurred in the following histological subtypes: uterine LMS, non-uterine LMS, myxofibrosarcoma, UPS/MFH (3), and angiosarcoma. (Figure 2B and appendix p 6) Three additional patients were treated, none of whom had responses, resulting in a final adjusted confirmed ORR of 15% [n = 41, 92% CI (6–30%)].
The median time to response was 1·5 months (Q1–Q3: 1·4–2·8). The median duration of response cannot be estimated using Kaplan-Meier methods as more than 50% of the responding patients must have had PD to perform this calculation. Thus far, two responses are ongoing, two PDs have occurred and three patients ended protocol treatment due to AEs. (Figure 3B) The clinical benefit rate at 6 and 12 months was 12% [6/42, 95% CI (6–28%)] and 12% [5/42, 95% CI (5–25%]), respectively.
Of the 18 (44% of 42) patients with PD within the first 12 weeks, eight (44% of 18) were eligible to continue treatment. Three (38% of 8) demonstrated PD at confirmation. Five (62% of 8) had SD at confirmation and remained on treatment for another 3–12 months. Of note, one patient converted to PR 3 within months of initial PD, lasting 9.2 months. (Appendix, p 8).
The median PFS was 4·1 months [n=41, 95% CI (2·6–4·7)] and the median OS was 14·3 months [n=41, 95% CI (9.6–not estimable)]. (Figure 4B) The 12 month OS rate for combination therapy was 54·6% [n=41, 95% CI (41–72·7%)].
Treatment Safety
All of the 84 patients (42 on each arm) initiating treatment experienced adverse events of any grade. In the monotherapy arm, the most common grade 3 or worse adverse events included anemia (four [10%]), decreased lymphocyte count (three [7%]) and dehydration, increased lipase, pain, pleural effusion, respiratory failure, secondary benign neoplasm and urinary tract obstruction (two [5%] each.) In the combination arm, the most common grade 3 or worse adverse events included: anemia (seven [17%]), hypotension (four [10%]), pain and urinary tract infection (three [7%] each). (Table 2)
Table 2.
All Adverse Events Regardless of Attribution, by Arm
| Nivolumab (n=42)a | Nivolumab+ Ipilimumab (n=42) | |||||||
|---|---|---|---|---|---|---|---|---|
| CTCAE v4.0 AE Term | Grade 1–2b | Grade 3 | Grade 4 | Grade 5 | Grade 1–2b | Grade 3 | Grade 4 | Grade 5 |
| Adrenal Insufficiency | - | - | - | - | 5 (12%) | 1 (2%) | - | - |
| Alanine Aminotransferase Increased | - | - | - | - | - | 2 (5%) | 1 (2%) | - |
| Alkaline Phosphatase Increased | - | - | - | - | - | 1 (2%) | - | - |
| Anemia | 8 (19%) | 4 (10%) | - | - | 6 (14%) | 7 (17%) | 1 (2%) | - |
| Anorexia | 10 (24%) | 1 (2%) | - | - | 14 (33%) | - | - | - |
| Aspartate Aminotransferase Increased | - | - | - | - | - | 1 (2%) | 1 (2%) | - |
| Cardiac Disorders | - | - | - | - | - | 1 (2%) | - | - |
| Colonic Perforation | - | - | 1 (2%) | - | - | - | - | - |
| Constipation | 5 (12%) | - | 1 (2%) | - | 6 (14%) | - | - | - |
| Cough | 13 (31%) | - | - | - | 8 (19%) | - | - | - |
| Creatinine Increased | - | 1 (2%) | - | - | - | 1 (2%) | - | - |
| Dehydration | - | 2 (5%) | - | - | - | 1 (2%) | - | - |
| Diarrhea | 4 (10%) | 1 (2%) | - | - | 11 (26%) | - | - | - |
| Dyspnea | 10 (24%) | - | - | - | 8 (19%) | 2 (5%) | - | 1 (2%) |
| Edema Limbs | - | - | - | - | - | 1 (2%) | - | - |
| Fatigue | 24 (57%) | 1 (2%) | - | - | 29 (69%) | 2 (5%) | - | - |
| Febrile Neutropenia | - | - | 1 (2%) | - | - | - | - | - |
| Fever | 4 (10%) | - | - | - | - | - | - | - |
| Gastrointestinal Fistula | - | - | - | - | - | 1 (2%) | - | - |
| Generalized Muscle Weakness | - | 1 (2%) | - | - | - | - | - | - |
| Genital Edema | - | - | - | - | - | 1 (2%) | - | - |
| Hyperglycemia | - | - | - | - | - | 3 (7%) | - | - |
| Hyperkalemia | - | - | - | - | - | 2 (5%) | - | - |
| Hypoalbuminemia | - | - | - | - | - | 2 (5%) | - | - |
| Hypocalcemia | - | 1 (2%) | - | - | - | - | - | - |
| Hypokalemia | - | 1 (2%) | - | - | - | 1 (2%) | - | - |
| Hyponatremia | - | 1 (2%) | - | - | - | 3 (7%) | 1 (2%) | - |
| Hypophosphatemia | - | 1 (2%) | - | - | - | - | - | - |
| Hypotension | - | - | - | - | - | 4 (10%) | - | - |
| Hypothyroidism | 6 (14%) | - | - | - | 7 (17%) | - | - | - |
| Hypoxia | - | - | - | - | - | 1 (2%) | - | - |
| Intraoperative Urinary Injury | - | 1 (2%) | - | - | - | - | - | - |
| Jejunal Obstruction | - | 1 (2%) | - | - | - | - | - | - |
| Leukocytosis | - | - | - | - | - | 1 (2%) | - | - |
| Lipase Increased | - | 2 (5%) | - | - | - | 2 (5%) | - | - |
| Localized Edema | - | - | - | - | - | 1 (2%) | - | - |
| Lower Gastrointestinal Hemorrhage | - | 1 (2%) | - | - | - | - | - | - |
| Lung Infection | - | 1 (2%) | - | - | - | 1 (2%) | - | - |
| Lymphocyte Count Decreased | - | 3 (7%) | - | - | - | - | - | - |
| Nausea | 12 (29%) | - | - | - | 10 (24%) | 1 (2%) | - | - |
| Nervous System Disorders | - | - | - | - | - | 1 (2%) | - | - |
| Neoplasms Benign, Mal, Unspecified | - | - | - | 2 (5%) | - | - | - | 2 (5%) |
| Neutrophil Count Decreased | - | - | - | - | - | - | - | - |
| Pain | 23 (55%) | 2 (5%) | - | - | 22 (52%) | 3 (7%) | - | - |
| Platelet count decreased | - | 1 (2%) | - | - | - | - | - | - |
| Pleural Effusion | - | 2 (5%) | - | - | - | - | - | - |
| Pruritus | - | - | - | - | 4 (10%) | - | - | - |
| Pulmonary Edema | - | - | - | - | - | - | 1 (2%) | - |
| Rash | 4 (10%) | - | - | - | 8 (19%) | - | - | - |
| Respiratory Failure | - | - | - | 2 (5%) | - | - | - | 2 (5%) |
| Sepsis | - | - | - | - | - | - | - | 1 (2%) |
| Skin Infection | - | - | - | - | - | 1 (2%) | - | - |
| Small Intestinal Obstruction | - | 1 (2%) | - | - | - | 1 (2%) | - | - |
| Spinal Fracture | - | 1 (2%) | - | - | - | - | - | - |
| Thromboembolic Event | - | - | - | 1 (2%) | - | 1 (2%) | - | - |
| Urinary Tract Infection | - | 1 (2%) | - | - | - | 3 (7%) | - | - |
| Urinary Tract Obstruction | - | 2 (5%) | - | - | - | - | - | - |
| Urine Output Decreased | - | - | 1 (2%) | - | - | - | - | - |
| Vaginal Fistula | - | - | - | - | - | 1 (2%) | - | - |
| Vomiting | - | 1 (2%) | - | - | - | - | - | - |
Patients having initiated study treatment.
The adverse event is reported if at least 10% of patients experienced a maximum severity of grade 1 or 2 of the specified events.
Incidence of treatment related adverse events (TRAE) of any grade was similar within the combination arm 29 (69% of 42) compared to the nivolumab monotherapy cohort 28 (67% of 42). The most common TRAE in the nivolumab monotherapy cohort included (of 42 patients): fatigue 12 (29%), pain nine (21%), anemia and anorexia both occurring in six (14%) patients. For the combination arm, common TRAE included (of 42 patients): fatigue 14 (33%), skin rash eight (19%), adrenal insufficiency, hypothyroidism, pain, and anemia each occurring in six (14% of 42) patients. Grade 3–4 TRAEs occurred with higher frequency with the combination therapy (14% of 42) compared to the monotherapy (7% of 42). (Appendix, p 9)
Treatment related serious adverse events on the monotherapy arm occurred in eight patients and included anemia, anorexia, dehydration, decreased platelet count, diarrhea, fever, increased creatinine, and pleural effusion (one [2%] each). On the combination arm, treatment related serious adverse events occurred in11 patients. Three [7%] patients had adrenal insufficiency, two [5%] had increased alanine aminotransferase, two [5%] with hyponatremia, one [2%] each experienced anemia, increased aspartate aminotransferase, fatigue, pain and pruritus. (Appendix, p 10) No drug-related deaths occurred in either arm.
Eleven patients (5 monotherapy and 6 combination therapy) died of causes secondary to progressive disease during treatment. On the monotherapy arm causes as per CTCAE v4.0 categories included respiratory failure 2, thromboembolic events 1, neoplasm 2. On the combination arm causes as per CTCAE categories included respiratory failure 2, neoplasm 2, infection 1 and dyspnea 1.
DISCUSSION
In this phase II trial of nivolumab +/− ipilimumab, 85 patients were rapidly enrolled in 5 months. Two of 38 sarcoma patients responded to treatment with nivolumab monotherapy. Treatment using combination nivolumab and ipilimumab led to 6 responses in 38 sarcoma patients. For both treatment arms, 61% (52 of 85) of patients had received at least 3 lines of prior chemotherapy. The median PFS was 2·1 and 4·1 months with monotherapy and combination therapy, respectively. These findings are clinically meaningful when placed in context of current available treatment options for metastatic sarcoma patients. Standard treatment for sarcoma remains mostly centered around cytotoxic chemotherapy. Front line response rates are typically about 15–18% with a median PFS of 4–6 months.(2) Efficacy beyond front-line therapy is even worse; ORR is usually <10% and median PFS is about 2–4 months.(3–5)
The clinical benefit with nivolumab monotherapy was not on par with currently available treatment options. In addition, the nivolumab monotherapy arm did not meet its pre-defined primary endpoint, likely precluding further study in an unselected sarcoma population. Six of 38 patients treated with combination therapy responded meeting the pre-defined primary endpoint. The proportion of patients achieving a response with combination therapy in unselected advanced sarcoma is at least similar to treatment with doxorubicin or gemcitabine and docetaxel based chemotherapy. This efficacy in unselected heavily treated sarcoma patients suggests that the combination of nivolumab with ipilimumab has promise as a viable second-line therapy. In addition, the ORR of 16% (of 38 patients) is at least as good as current FDA approved front line systemic chemotherapy options, perhaps supporting further study of nivolumab with ipilimumab as first line therapy as well.
With combination therapy, the median OS was 14·3 months. The usual OS described for similar patient population is approximately 11–13·5 months.(3–5) While the most recent FDA approval with doxorubicin and olaratumab demonstrated a median OS of 24 months, this data is pending confirmation in a randomized phase III clinical trial.(6) The overall survival noted with combination therapy on this clinical trial is encouraging. A future clinical trial is necessary to further explore the potential to improve upon the current standard therapy.
The safety and tolerability of these study drugs were consistent with extensive prior experience and reports.(9, 26, 27) It is clear that nivolumab monotherapy is generally better tolerated with lower incidence of adverse events compared to combination therapy. In our study, the incidence of treatment related grade 3–4 adverse events with combination therapy is 14% (6 of 42 patients). This is in great contrast with the reported incidence of grade 3–4 TRAE of 50% patients, where the dose of ipilimumab is higher at 3mg/kg.(27) The lower dose of ipilimumab 1mg/kg minimized adverse events and made this combinatorial treatment approach tolerable and relatively safe. Of note, with standard cytotoxic chemotherapeutic agents, reported grade 3 treatments related adverse events rates are comparable or even higher.(2–6)
A limitation of this study is that it was not designed to directly compare safety and efficacy among the two treatment arms. Radiographic assessments did not include evaluation by immune related response criteria or immune related-RECIST. The small sample sizes precluded stratification by relevant baseline characteristics. Research biopsies were not mandatory therefore comprehensive analysis of the tumor microenvironment on all patients will not be possible. Further, correlating clinical efficacy with changes in the tumor microenvironment will not possible. Paired tumor samples were collected in 22 patients. In these specimens, correlative analysis will include PD-L1 expression by IHC, mutational burden/neoantigen analysis, T cell receptor clonality and TIL characterization. These analyses are on-going and will be reported in a future publication. In addition, expansions have been approved to better characterize activity in UPS and LPS. Enrollment has not yet begun for these expansions at the time of this report.
Moving forward, there is an obvious need to develop a predictive biomarker to determine which sarcoma patients are most likely to benefit from checkpoint blockade. Studies have shown that high tumor mutational load correlates with benefit from checkpoint blockade agents in melanoma and lung cancers, however outliers exist.(28, 29) Most recently, in urothelial patients treated with atezolizumab specific clinical factors such as the presence of liver metastases as well as low peripheral T cell clonality appear to inversely correlate with survival.(8) In addition, the presence of TILs as well as PD-L1 expression in the TILs has also been described as being potential important biomarkers predictive of benefit in selected malignancies.(30) In sarcoma, there is higher expression of genes related to antigen presentation and T-cell infiltration in UPS and LMS compared to synovial sarcoma and liposarcoma.(20) The responses demonstrated with the combination therapy of nivolumab and ipilimumab on this clinical trial in both UPS and LMS nicely complements these pre-clinical findings. The clinical efficacy demonstrated in LMS with combination therapy is in contrast to what has been described with nivolumab or pembrolizumab monotherapy.(19, 31) Ipilimumab may increase T cell activation thereby allowing nivolumab to augment T cell responses towards the tumor. We hypothesize that up-regulation of pre-existing anti-tumor immunity with combination therapy may maximize benefit of checkpoint inhibitors to some sarcoma patients. Unfortunately, there is limited data in regards to the tumor immune microenvironment in most sarcoma subtypes and this hypothesis will need to be tested prospectively in a larger clinical trial that encompasses robust correlative studies.
The future phase III clinical trial will enrich for specific sarcoma subtypes that have baseline TIL infiltration as well as those subtypes which have demonstrated clinical efficacy to checkpoint inhibitors such as UPS/MFH, LMS, angiosarcoma and myxofibrosarcoma. Selecting these specific sarcoma subtypes may ultimately enhance the efficacy demonstrated on this trial. Including correlative analyses such as PD-L1 testing and calculation of mutational burden will be essential. In addition, enrolling patients earlier in their treatment course may further enhance responses. This phenomenon has been seen in Merkel cell patients treated with avelumab whereby chemotherapy refractory patients had ORR that were lower than chemotherapy naïve patients.(17) In this unselected cohort of heavily treated sarcoma patients who received combination therapy, the confirmed ORR was 16% (6 of 38 patients), which is similar or better than responses obtained with standard chemotherapy agents. The combination cohort in contrast to the monotherapy cohort met its pre-defined statistical endpoint, thereby warranting further study. These clinical results highlight the promise of combined checkpoint inhibition, demonstrating a path forward for future studies in sarcoma.
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed with the terms, “metastatic sarcoma” and “immunotherapy or immune checkpoint and sarcoma” for articles published through June 1, 2017. Review articles and meta-analysis references were excluded. PD-L1 expression has been explored in sarcoma. Results have been variable based on the assay used, type of sarcoma and timing of testing thereby limiting its role as a predictive biomarker of benefit. A recent comprehensive overview of the sarcoma immune microenvironment was performed in 4 sarcoma subtypes, including: undifferentiated pleomorphic sarcoma (UPS), leiomyosarcoma (LMS), synovial sarcoma and liposarcoma. In this analysis, it was determined that UPS and LMS have high expression of genes related to antigen presentation and T-cell infiltration compared to synovial sarcoma and liposarcoma. These analyses may provide rationale for exploration of immunotherapeutic approaches including checkpoint inhibitors in sarcoma. In addition, we identified 4 relevant clinical trials. The first was a single center study of ipilimumab in six patients with synovial sarcoma that did not demonstrate clinical efficacy. The second was a Simon two stage, single center phase II trial of nivolumab for patients with advanced uterine leiomyosarcoma that did not progress to the second stage due to lack of efficacy in the first 12 treated patients. A third clinical trial evaluated pembrolizumab with cyclophosphamide in multiple sarcoma subtypes with 3 patients responding out 50. A fourth clinical trial evaluated pembrolizumab monotherapy in 40 bone and 40 soft tissue sarcoma patient. In the soft tissue arm, responses were seen in UPS and LPS. In the bone arm, there was one response only in osteosarcoma.
Added Value of this study
To our knowledge this is the first report evaluating combination checkpoint inhibition in patients with sarcoma. The Alliance for Clinical Trials in Oncology A091401 clinical trial was a prospective, non- comparative phase II clinical trial of nivolumab with or without ipilimumab across multiple sarcoma histological subtypes. The trial enrolled patients that were heavily pre-treated. Combination treatment with nivolumab and ipilimumab led to promising responses that appear to be clinically meaningful in UPS, LMS, myxofibrosarcoma and angiosarcoma. In addition, the current dose and schedule for the combination tested (3 mg/kg nivolumab plus 1 mg/kg ipilimumab) showed acceptable toxicity with a 14% rate of grade 3/4 treatment related adverse events. Our results are comparable to current existing systemic agents. The combination cohort met its primary endpoint thereby forming the basis for a confirmatory phase III clinical trial that is being planned in selected histologies where responses were seen as indicated above.
Implications of all the available evidence
In this report, nivolumab and ipilimumab showed encouraging objective response rates, progression free survival and overall survival in a cohort of heavily treated, unselected sarcoma patients. Responses were seen in UPS, LMS, myxofibrosarcoma and angiosarcoma. These findings support future studies of nivolumab with ipilimumab for patients with specific metastatic sarcomas subtypes. These findings further support need for identification of a predictive biomarker.
Acknowledgments
Additional funding was obtained from Cycle for Survival. We would like to thank the patients and their families as well as all the investigators and study teams.
Footnotes
Contributors
SPD, MRM, GKS, WT and HS led the study design with support from Alliance and NCI-CTEP. Alliance and MRM performed data management. SPD and MRM performed data monitoring and data reporting. SPD, MRM, WT, JA, MMM and WDT performed the data analysis. SPD, MRM, JA, MMM, CRA, WDT, GKS, did data interpretation and writing. All authors performed data collection. All authors reviewed and approved final manuscript. Additional authors contributed to recruitment, treatment and follow-up of patients as indicated in the appendix (p xx).
Declaration of interests
BAVT Grant funding from Merck and Pfizer, Consultant to Janseen, Lilly, Novartis and Karyppharm and Caris. Speaker for Lilly, Janseen and Caris. EH employee of Astellas 8/2016. WDT Personal fees from Eli Lilly, EMD Serono, Novartis, Eisai, Janseen, Immune design, Adaptimmune, Ariad, Daiichi Sankyo, Plexxikon, Morphotek, Advaxis, Tracon outside from submitted work. Patent for ATRX as a companion diagnostic for CDK4 inhibitors, pending. Patent for Drug discovery pending. Rest of authors have nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Seddon BWJ, Strauss SJ, Gordon Leahy M, Woll PJ, Cowie F, Rothermundt CA, Wood Z, Forsyth S, Khan I, Nash S, Patterson P, Beare S. GeDDiS: A prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29) Journal of Clinical Oncology. 2015:33. [Google Scholar]
- 3.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(8):786–93. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629–37. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 5.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 6.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammers HPE, Infante JR, Ernstoff M, Rini BI, McDermott DF, Razak A, Pal SK, Voss M, Sharma P, Kollmannsberger CK, Heng D, Shen Y, Kurland J, Spratlin J, Gagnier P, Amin A. Phase I Study of Nivolumab in combination with Ipilimumab in metastatic Renal Cell Carcinoma. Annals of Oncology. 2014;25(4) [Google Scholar]
- 8.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The lancet oncology. 2015;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 10.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heery CRCG, Madan RA, Schlom J, Heydebreck A, Cuillerot JM, Sabzevari H, Gulley JL. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. Journal of Clinical Oncology. 32(5) [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. The New England journal of medicine. 2010 doi: 10.1056/NEJMoa1003466. NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Human pathology. 2015;46(3):357–65. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj SBM, Gonzales R, Letson D, Antonia SJ. Impact of PD-L1 Expression on Clinical Outcomes in subtypes of sarcoma. Annals of Oncology. 2014;25(4) [Google Scholar]
- 17.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The lancet oncology. 2016;17(10):1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki RG, Jungbluth AA, Gnjatic S, Schwartz GK, D’Adamo DR, Keohan ML, et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma. 2013;2013:168145. doi: 10.1155/2013/168145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawbi HBM, Crowley J, Van Tine BA, Hu J, Schuetze S, D’Angelo SP, Attia S, Priebat D, Okuno SH, Riedel RF, Davis LE, Movva S, Reed D, Baker LH, Reinke D, Maki R, Patel S. Safety and Efficacy of PD-1 Blockade Using Pembrolizumab in Patients with Advanced Soft Tissue (STS) and Bone Sarcomas (BS): Results of SARC028, a Multicenter Phase II Study. Journal of Clinical Oncology. 2016:34. [Google Scholar]
- 20.Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017 doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The lancet oncology. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 26.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The lancet oncology. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 27.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017 doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.