Abstract
Fluid retention is the most common risk factor for mortality and cardiovascular complications in patients with volume-overloaded disease states. The extent of diuresis or fluid removal is frequently determined by physical examination which is subject to inaccuracies.
Bedside ultrasound (US) is a portable tool that brings real-time diagnostic imaging to the patient's bedside. This versatile modality makes it possible for the clinician to investigate patients' extravascular and intravascular volume states. The extravascular volume, particularly in the case of pulmonary edema, can be quantitatively assessed by US of the anterior chest. Intravascular volume is estimated by visualizing the inferior vena cava (IVC) caliber. Taken together, the degree of extravascular lung water and the IVC caliber provide objective data that can guide the clinician to determine the level of diuresis needed to effectively yet safely treat pulmonary edema.
The objective of this article is threefold: 1) to summarize the findings of previous studies on the efficacy of portable US to guide fluid management, 2) to describe a proposed ultrasound protocol to help guide fluid management, and 3) to elucidate techniques that address the measurement of intravascular and extravascular volumes using portable US.
Keywords: Medicine, Issue 137, Fluid Removal, Diuresis, Ultrasound, Point-Of Care Ultrasound, Pulmonary Edema, Ultrasound Protocol, Chest Ultrasound, IVC Ultrasound
Introduction
Fluid retention is the most common modifiable risk factor for mortality and cardiovascular events in volume overloaded patients1. Fluid retention leads to poorly controlled hypertension, cardiac dysfunction and pulmonary edema, and has been associated with excess mortality in this population. The clinical estimation of a patient's volume status by combining the patient's symptoms, blood pressure, and weight changes represent a clinical assessment that is prone to imprecision2. This article proposes a protocol that utilizes bedside ultrasound (US) techniques to guide the effective management of pulmonary edema. Chest and inferior vena cava (IVC) US form the cornerstone of this proposed clinical protocol for fluid removal. Both Chest and IVC US have a long track record in the literature for a spectrum of applications including the management of acute respiratory failure, end-stage renal disease (ESRD), and circulatory shock.
Chest US has been shown to provide objective data for the assessment of pulmonary edema in ESRD patients, and accurately reflects the decreasing severity of pulmonary edema as dialysis goes on3. Similarly, US of the inferior vena cava (IVC) has long been utilized for various clinical applications. IVC US is recommend as a routine component of transthoracic echocardiographic examination and serves several purposes, including for the estimation of right atrial pressure (RAP) that is a mark of volume status and pre- load to the heart4. Several studies have elucidated a correlation between IVC caliber and fluid responsivity in shock states5,6. Also notable in the management of shock states, chest ultrasound has been used to detect the onset of new pulmonary edema during fluid resuscitation in circulatory shock, commonly by way of a bedside US application referred to as the Fluid Administration Limited by Lung Sonography (FALLS) protocol7,8. The protocol proposed here, named the Reverse-FALLS protocol, combines well-documented features of chest and IVC ultrasound for the safe and effective management of pulmonary edema.
Portable, or bedside, US is an imaging modality that offers immediate and reliable data that reduces exposure to radiation, eliminates the need for patient transport, and reduces resource utilization. Due to its availability and the essential absence of associated adverse effects, bedside US can be repeated to monitor and tailor diuretic therapy. Finally, bedside US is easy for clinicians to perform and interpret in real-time9.
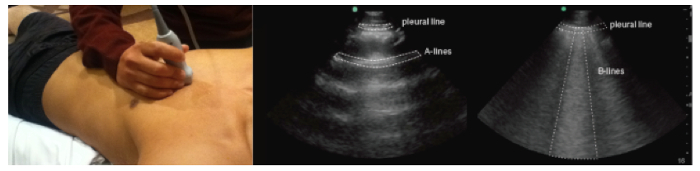
Chest US is a widely-accepted technique that detects specific artifact patterns that represent various pleural and parenchymal pathologies. For instance, "A-lines" are horizontal hyperechoic (bright) reverberation artifacts of the pleural surface and they indicate normal parenchyma that is free of fluid. Alternatively, "B-lines" are hyperechoic vertical artifacts that begin at the inferior aspect of the pleural line and extend to the end of the screen, moving synchronously with respiration. The presence of B-lines in the setting of suspected volume overload indicates extravascular lung water. These B-lines characteristically dissipate during diuresis in a manner that corresponds to volume removal leading to the re-emergence of the dry A-line artifact pattern3. Studies indicate that these US findings are closely associated with elevated pulmonary filling pressures, as assessed by invasive methods10.
US may also be used to measure the IVC vessel diameter and collapsibility index, which is the percentage of diameter reduction during spontaneous breathing. Several studies have shown a correlation between IVC measurements and volume changes during diuresis, demonstrating the feasibility and applicability of this tool for patients with pulmonary edema11.
Here, we propose a sample US protocol, the Reverse-FALLS protocol, which integrates both lung and IVC US to guide fluid removal therapy in patients with pulmonary edema.
Protocol
1. Assess Extravascular Compartment Before Diuresis: Chest Ultrasound
Use a low-frequency, phased array probe.
Select the appropriate machine setting (abdomen or lung) with the screen marker operator-left.
Adjust the depth to 8-12 cm.
- Place the patient in supine or semi-recumbent position and expose the anterior chest. Place the transducer at a 90° angle to the patient's anterior chest with the probe marker facing cephalad.
- Perform this protocol at the midclavicular line in two locations on each side of the chest for a total of four locations: in the 2nd-3rd or the 3rd-4th intercostal space, and in the 4th-5th or 5th-6th intercostal space.
- Visualize B-lines, if present. NOTE: B lines are echogenic artifacts with a narrow origin on the pleural line, and they move synchronously with respiratory movements (Figure 1).
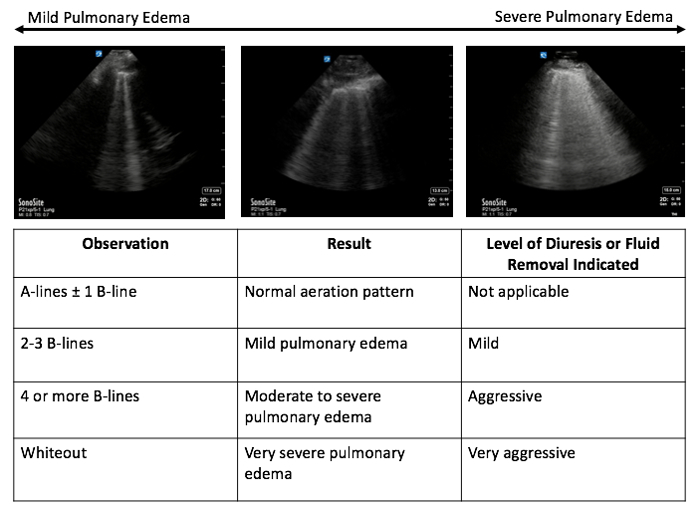
Count the number of B-lines in each scan plane to quantify the extent of pulmonary edema. NOTE: Up to one B-line is normal; two to three B-lines represent mild pulmonary edema, whereas 4 or more B-lines represent moderate to severe edema; a whiteout pattern with no A-lines represents very severe pulmonary edema (Figure 2).
2. Assess Intravascular Compartment Before Diuresis: IVC Ultrasound
Use a low frequency, phased array probe.
Select the cardiac machine setting with the screen marker operator-right and adjust the depth to 12-21 cm for optimal image resolution.
- Place the patient in a supine position and expose the anterior chest. Place the transducer at a 90° angle to the patient's sub-xiphoid region with the probe marker facing cephalad.
- Perform this protocol with the transducer located just right of the midline, tilted slightly right-lateral and cephalad.
- By scanning right-to-left, visualize both the IVC and the aorta.
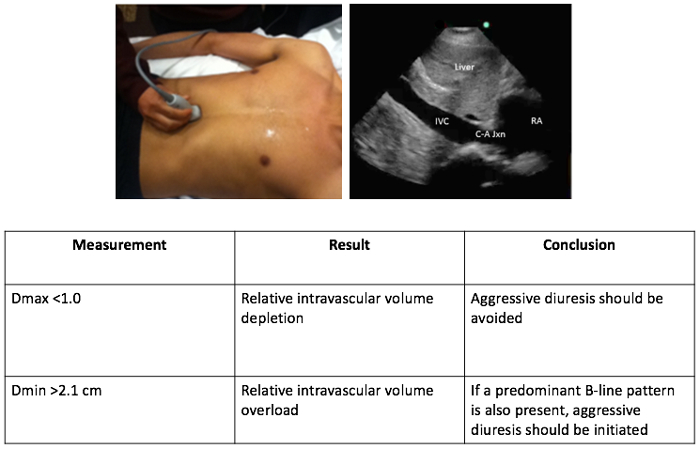
- Note that the objective is to visualize the cavo-atrial junction, diaphragm, liver, hepatic vein, and IVC in its longitudinal plane. The aorta is visualized by angulating the scan plane medially (Figure 3).
- Once the IVC and the cavo-atrial junction have been identified, set the ultrasound to M-Mode to measure the anterior-posterior diameter of the IVC.
- Using the touch pad, select the M-mode beam that corresponds to a near-orthogonal section, 2-4 cm distal to the cavo-atrial junction just beyond the visible hepatic vein.
- Select the M-mode button a second time, and freeze the screen after the M-mode reading is complete through at least 1 respiratory cycle.
- Use machine calipers for all measurements, measuring the vessel from internal edge to internal edge.
Measure D-max and D-min during a normal respiratory cycle.
3. Set Fluid Management Goals Based on Gradation of Pulmonary Edema and Diameter of IVC
4. Reassess Extravascular and Intravascular Compartments Throughout Diuretic Therapy.
Repeat the protocol as outlined above every 6 hours to evaluate the progress of diuretic therapy and to guide fluid management goals.
Representative Results
Lung ultrasound estimates the amount of fluid in the extravascular compartment by visualizing A-line or B-line artifacts, as illustrated in Figure 1. The quantity of B-lines on lung ultrasound helps determine the amount of fluid removal required as illustrated in Figure 2. A mixed A-line and B-line pattern, or an A-line predominant pattern suggests lung parenchyma that is relatively free from extra fluid. Mild diuresis may be necessary for complete resolution of extravascular lung water. A B-line predominant pattern suggests the presence excess extravascular lung water. Moderate to aggressive fluid removal therapy may be necessary for complete resolution with level of diuresis based on the number of B-lines present.
IVC ultrasound estimates the amount of fluid in the intravascular compartment by measuring vessel diameter and observing the degree of collapsibility during the respiratory cycle, as illustrated in Figure 3. A maximum diameter (Dmax) of <1.0 may predict hemodynamic compromise. Do not employ aggressive diuresis in this setting as it may induce hypotension in patients with intravascular volume depletion and may potentially cause early discontinuation of diuretic therapy. Alternatively, a large (minimum diameter [Dmin] >2.1 cm), noncompliant IVC with little respiratory variation indicates a volume overloaded state. In combination with a predominant B-line pattern, an aggressive approach to diuretic therapy should be initiated.
The combination of lung and IVC ultrasound provides objective data for assessing the patient's overall fluid status. Taken together, lung and IVC US techniques can be applied, as proposed in the Reverse-FALLS protocol, to determine the need for diuresis and predict whether the patient can tolerate fluid removal, as illustrated in Figure 4.
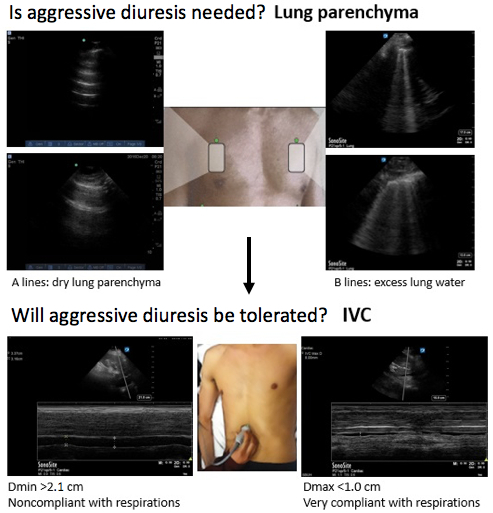
Figure 1.The extravascular compartment: assessment of lung parenchyma. With the patient in supine or semi-recumbent position, use a phased array, low-frequency probe placed on the anterior chest with probe marker facing cephalad. Select the appropriate machine setting (abdomen or lung) with the screen marker operator-left, and adjust the depth to 8-12 cm. Please click here to view a larger version of this figure.
Figure 2.Gradation of pulmonary edema: setting volume goals. The number of B-lines corresponds to the degree of pulmonary edema: up to 1 B-line is normal, 2-3 B-lines represent mild pulmonary edema, and 4 or more B-lines represent moderate to severe pulmonary edema. A whiteout pattern with no A-lines suggests very severe pulmonary edema. The degree of pulmonary edema will guide the level of diuresis necessary for resolution of volume overloaded state.
Figure 3.The intravascular compartment: assessment of the IVC. With the patient in a supine or semi-recumbent position, use a phased array, low-frequency probe placed on the anterior chest, just right lateral to the midline with probe marker facing cephalad. Select the cardiac machine setting, with the screen marker operator-right. Adjust the depth to 12-21 cm for optimal image resolution.
Figure 4.Summary of protocol: utilizing chest and IVC ultrasound to guide fluid management.
| Level of Diuresis Needed | Net Negative Fluid Balance per 24 hours |
| Mild | 0.5-1.0 liters |
| Moderate | 1.0-5.0 liters |
| Aggressive | Great than 3.0-5.0 liters |
| *As determined by lung and IVC ultrasound via the Reverse=FALLS protocol |
Table 1. Goals of Diuretic Therapy Defined
Discussion
Many studies have demonstrated that bedside US is a reliable diagnostic tool that can be used to guide the management of various disease states such as shock and dyspnea5. The correlation of B-lines with extravascular lung water has good sensitivity and specificity in evaluating for pulmonary edema, and studies have shown that lung US can reliably detect even modest variations in extravascular lung water2,10. Similarly, studies have shown that IVC diameter, as measured by US techniques, is a reliable indicator of intravascular volume loss12,13. As it represents intravascular volume status, IVC diameter determines whether a patient can withstand aggressive diuresis. Taken together, chest and IVC US techniques can be used to determine the level of diuresis needed to resolve pulmonary edema, assess the patient's ability to withstand diuresis, and monitor the progress of fluid removal therapy.
Utilization of the Reverse-FALLS protocol allows the clinician to perform an initial evaluation of a patient's intravascular and extravascular volume status, while repetition of the protocol at six-hour intervals provides an objective measurement of treatment progress and allows for augmentation of diuretic therapy as needed. It is important to note that 6 hour intervals are necessary to allow diuretic therapy to reach its maximum effect and to provide adequate time for equilibration of intravascular and extravascular volumes. If IVC caliber is small and collapsible but B-lines persist on lung US, it may be necessary to delay diuretic therapy until the patient's fluid compartments equilibrate further. Clinicians must be cautious to avoid depleting intravascular volume in an attempt to reduce extravascular fluid. Failure to do so may lead to hypotension and a decrease in cardiac output, which could have grave consequences. These measurements must also be considered with the patient's clinical picture, as well as laboratory measurements that provide insight into other disease processes at play, such as serum electrolytes or serum albumin levels.
If IVC US reveals a large and noncompliant vessel, however, then an aggressive approach to fluid removal may be necessary. The approach to diuretic therapy is largely dependent on individual patient factors, as preexisting hemodynamics and comorbidities such as diuretic naivety or chronicity, or preexisting Chronic Kidney Disease may alter the effects of diuretic medications. Standardized therapy with furosemide (IV 10-40 mg/h or boluses) may be initiated and subsequently titrated based on chest and IVC US in combination with urine output. The overall goals of diuretic therapy for each degree of volume overload are defined in Table 114. Further studies are indicated to determine the extent of benefit imparted upon patients by this protocol, and to determine precise goals for diuretic therapy.
Ultrasound provides a non-invasive, reliable method of monitoring a patient's volume status and subsequently augmenting their fluid removal therapy by allowing for real-time evaluation of lung parenchyma and IVC caliber. The current clinically-based methods used to guide this treatment are imprecise. The Reverse- FALLS protocol, named because it is a guide for the removal of fluid rather than a guide for fluid administration as seen in the FALLS protocol, is proposed as a more precise guide for the management of pulmonary edema. Implementation of the lung and IVC ultrasound techniques described in the Reverse-FALLS protocol into standard practices will achieve a level of safe and beneficial diuresis for volume overloaded patients.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors wish to acknowledge Dr. Paul Zamudio, a specialist in Nephrology and Critical Care, for his contributions to this manuscript.
References
- Zoccali C, et al. Pulmonary congestion predicts cardiac events and mortality in ESRD. Journal of the Amerian Society of Nephrology. 2013;24:639–646. doi: 10.1681/ASN.2012100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitturi N, et al. Lung ultrasound during hemodialysis, the role in the assessment of volume status. International Urology and Nephrology. 2014;46:169–174. doi: 10.1007/s11255-013-0500-5. [DOI] [PubMed] [Google Scholar]
- Noble VE, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135:1433–1439. doi: 10.1378/chest.08-1811. [DOI] [PubMed] [Google Scholar]
- Lang RM, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Barbier C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Medicine. 2004;30:1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- Feissel M, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Medicine. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DA. Lung ultrasound in the critically ill. Annals of Intensive Care. 2014;4:1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein DA. BLUE-Protocol and FALLS-Protocol: Two Applications of Lung Ultrasound in the Critically Ill. Chest. 2015;147:1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- Moore CL, Copel JA. Point-of-care ultrasonography. New England Journal of Medicine. 2011;364:749–757. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D, et al. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136 doi: 10.1378/chest.09-0001. [DOI] [PubMed] [Google Scholar]
- Trezzi M, et al. Lung ultrasonography for the assessment of rapid extravascular water variation, evidence from hemodialysis patients. Internal and Emergency Medicine. 2013;8:409–415. doi: 10.1007/s11739-011-0625-4. [DOI] [PubMed] [Google Scholar]
- Lyon M, Blaivas M, Brannam L. Sonographic measurement of the inferior vena cava as a marker of blood loss. American Journal of Emergency Medicine. 2005;23:45–50. doi: 10.1016/j.ajem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Katzarski KS, et al. A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive hemodialysis patients. American Journal of Kidney Disease. 1997;30:459–465. doi: 10.1016/s0272-6386(97)90302-4. [DOI] [PubMed] [Google Scholar]
- Goyfman M, et al. Combined aquaretic and diuretic therapy in acute heart failure. International Journal of Nephrology and Renovascular Disease. 2017;10:129–134. doi: 10.2147/IJNRD.S135660. [DOI] [PMC free article] [PubMed] [Google Scholar]


