Abstract
Activated neutrophils release neutrophil extracellular traps (NETs), which can capture and destroy microbes. Recent studies suggest that NETs are involved in various disease processes, such as autoimmune disease, thrombosis, and tumor metastases. Here, we show a detailed in vitro technique to detect NET activity during the trapping of free tumor cells, which grow after attachment to NETs. First, we collected low density neutrophils (LDN) from postoperative peritoneal lavage fluid from patients who underwent laparotomies. Short-term culturing of LDN resulted in massive NET formation that was visualized with green fluorescent nuclear and chromosome counterstain. After co-incubation of human gastric cancer cell lines MKN45, OCUM-1, and NUGC-4 with the NETs, many tumor cells were trapped by the NETs. Subsequently, the attachment was completely abrogated by the degradation of NETs with DNase I. Time-lapse video revealed that tumor cells trapped by the NETs did not die but instead grew vigorously in a continuous culture. These methods may be applied to the detection of adhesive interactions between NETs and various types of cells and materials.
Keywords: Cancer Research, Issue 138, Low density neutrophils (LDN), neutrophil extracellular traps (NETs), DNase, peritoneal metastases, adhesion, microenvironment
Introduction
Polymorph nuclear neutrophils in circulating blood are typically separated from mononuclear cells through the density gradient preparation method. However, some neutrophils known as low-density neutrophils (LDN), with CD11b(+), CD15(+), CD16(+), and CD14(-) phenotypes, are co-purified with mononuclear cells. The relative number of LDN significantly increases in various pathological conditions including autoimmune diseases1,2, sepsis3, and cancer4,5. Previous studies have shown that LDN are a phenotypically and functionally distinct class of neutrophils6. It should be noted that LDN in circulating blood are more likely to produce neutrophil extracellular traps (NETs) than normal density neutrophils2,7. NETs are web-like structures composed of nucleic acids, histones, proteases, and granular and cytosolic proteins, and they can efficiently entrap and destroy pathogens8.
Recently, NETs have been shown to capture not only microbes, but also platelets and circulating tumor cells which can assist in thrombus formation9 and tumor metastases10,11. However, the molecular mechanisms behind the adhesive interactions between NETs and platelets or tumor cells are still unclear. More recently, an in vitro adhesion assay revealed that myeloid leukemia cells (K56212) and lung carcinoma cells (A54913) attach to NETs via β1 and β3 integrins. The authors used NET stock isolated from neutrophils and activated by phorbol 12-myristate 13-acetate (PMA) as the adhesion substrate14. Although this assay allows detection of real interactions with NET components in the absence of neutrophils, it is arguable whether the "cell-free NET stock" isolated by high-speed centrifugation retains the molecular structure identical to NETs produced in vivo. Recently, we found that peritoneal lavage fluid after abdominal surgery contained many mature LDN, which generated massive NETs and attached to tumor cells causing peritoneal metastases15. In this study, we successfully examined the adhesion of tumor cells to intact NETs without any physical manipulation. Here, we show details of a technique to detect adhesive interactions between NETs and free tumor cells.
Protocol
LDN were obtained from patients enrolled in this study and were approved by the Institutional Review Board of Jichi Medical University.
1. Isolation of LDN from Abdominal Cavity Lavages and NET Detection
- Sample acquisition
- Infuse 1,000 mL of normal sterile saline directly into the abdominal cavity before wound closure in patients who have undergone abdominal surgery due to gastrointestinal malignancy. NOTE: Samples were obtained from patients who underwent a gastrectomy, colectomy, or esophagectomy without bias based on age or sex. Saline was transferred into a container and poured into the whole abdomen within one minute. This is routinely performed as a postoperative peritoneal washing without significant effects on patients.
- Lavage the abdominal cavity extensively for at least 1 min. NOTE: It is recommended that the infused fluid is slowly stirred with the surgeon's hands so that the samples are uniform.
- Recover 200 mL of lavage fluid with four 50 mL syringes. NOTE: Sometimes a rubber connector is used to take up the fluids.
- Perform purification of peritoneal LDN using granulocyte specific mAb, CD66b16. NOTE: Because the intermediate layer after density gradient centrifugation contains many mononuclear cells, positive selection of polymorph neutrophils using CD66b mAb was performed.
- Transfer the peritoneal lavage fluid to a 50 mL tube. NOTE: In this step, pass the fluids through a 100 µm nylon filter to remove impurities.
- Centrifuge the peritoneal fluid at 270 x g for 7 min at RT.
- Resuspend the pellets in 5 mL of PBS with 0.02% EDTA.
- Carefully overlay the 5 mL of cell suspension on a 3 mL density gradient solution.
- Centrifuge at 1,700 x g for 15 min at RT without any breaks.
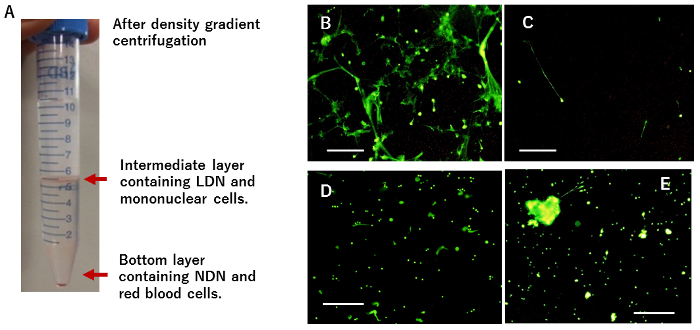
- Harvest the ~2 - 3 mL of solution containing the intermediate layers (Figure 1A) using a pipette and mix it with 10 mL of PBS with 0.02% EDTA.
- Centrifuge the peritoneal fluid at 400 x g for 7 min at RT and discard the supernatant.
- Add another 10 mL of PBS with 0.02% EDTA, and centrifuge at 270 x g for 7 min at RT. Discard the supernatant.
- Dissolve the cell pellet (1 x 107) in 60 µL of buffer for the magnetic cell separation kit.
- Add 20 µL of Fc block to the pellets and incubate for 10 min at 4 °C.
- Add 20 µL of the anti-CD66b conjugated with microbeads and incubate for an additional 10 min at 4 °C.
- Wash and re-suspend the pellets in 500 µL of MACS buffer.
- Apply the cell suspension to a magnetic column in the magnetic field of a suitable magnetic separator and collect the flow-through containing CD66b(-) cells by washing the column with 15 mL of buffer.
- Remove the column from the magnetic separator and immediately flush out the magnetically labeled CD66b(+) cells by firmly pushing the plunger into the column. NOTE: The CD66b(+) cell population consists mostly of neutrophils as determined by FACS analysis indicating that >95% of the CD66(+) fraction are positive for CD11b, CD15, and CD16, but negative for CD14.
- Generation of NETs
- After centrifugation at 270 x g for 7 min at RT, re-suspend the isolated LDN (5 x 106) in 1 mL of RPMI1640 with 10% FCS.
- Culture the LDN on a 6-well poly-L-lysine coated plate (6 cm in diameter) for 2 h at 37 ˚C in 5% CO2.
- Add the green fluorescent counterstain (a membrane-impermeable dye to stain the nucleus and chromosomes) at the final concentration of 5 µM.
- Immediately observe the morphology of NETs (the extracellular DNA components expelled from the LDN under fluorescence microscopy).
2. Staining of Tumor Cells with Red Fluorescent Cell Linker Dye
Prepare human cancer cell lines MKN45, NUGC, and OCUM-1.
Wash 1 x 107 cells with PBS + 0.02% EDTA in a 15 mL tube and centrifuge at 270 x g for 7 min at RT.
Add 1 mL of solution for dye staining to the pellets, and pipette gently.
Dissolve 4 µL of Red Fluorescent Cell Linker dye in 1 mL of solution for staining and mix with the cell suspension from step 2.2.
Incubate the pellets for 4 min at RT.
Add 4 mL of DMEM (10% FBS) to stop the staining reaction.
Centrifuge 270 x g for 7 min and discard the supernatant.
Repeat steps 2.6 and 2.7 twice.
Check with a fluorescence microscope (excitation = 551 nm, emitting = 567 nm) that the tumor cells are stained red.
3. Analysis of Tumor Cell Adhesion to NETs
Re-suspend the red fluorescent stained tumor cells (1 x 106) in 1 mL of RPMI 1640 supplemented with 0.1% BSA.
Add the tumor cells to the LDN culture that produced the NETs as described in step 1.3.
Incubate for 5 min at 37 ˚C, which enables the tumor cells to contact the NETs. NOTE: Long incubation induces tumor cell adhesion to LDN or to the plates.
Remove the medium and gently wash the wells by adding 2 mL of prewarmed media (0.1% BSA + RPMI 1640) and swirling the dish.
Repeat the washing procedure in step 3.4 twice. NOTE: Since NETs weakly attach to the plate, washing should be done as gently as possible to avoid removal of the NETs themselves.
Add the green fluorescent dye for staining the nucleus and chromosomes at a final concentration of 5 µg/mL for visualization of the NETs.
Observe the NETs and attached tumor cells using the appropriate filters (green, excitation = 504 nm, emitting = 523 nm; red, excitation = 551 nm, emitting = 567 nm).
Merge the figures to show the tumor cells that are trapped by the NETs. NOTE: In some experiments, DNA degradation enzyme was added to the LDN culture (final concentration of 100 U/mL) 5 min before co-incubation for 5 min.
4. Time Lapse Video Analysis of the Trapped Tumor Cells
Culture the peritoneal LDN in a 35 mm round dish coated with poly-L-lysine as described in step 1.3.
Add the green fluorescent dye for staining the nucleus and chromosomes to visualize NETs as described in step 1.3.
After 1 min of incubation, remove the media and add 2 mL of 0.1% BSA + RPMI 1640.
Remove the media and add unstained 1 x 106 MKN45 cells suspended in 0.1% BSA + RPMI 1640. Incubate for 5 min at RT.
Remove any unattached tumor cells by gently washing as described in step 3.4 and add DMEM with 10% FCS supplemented with 100 u/mL Penicillin and 100 µg/mL streptomycin.
Mount the culture dish in a whole-view cell observation system and select the appropriate field in which tumor cells are trapped by the NETs.
Continue to co-culture for an additional 2 days and take continuous photos of the field every 6 min under normal light and fluorescence, which detects MKN45 cells and NETs, respectively.
Superimpose the images at each timepoint and construct time-lapse videos using Image Viewer software.
Representative Results
In the 2-hour culture, CD66b(+) LDN derived from peritoneal lavage fluid showed string structures stained with green-fluorescent dye for nuclear and chromosome (Figure 1B), while CD66b(-) mononuclear cells did not (Figure 1C). However, when the LDN cultures were pretreated with 100 U/mL DNase I, the characteristic structure was destroyed (Figure 1D), indicating that they were composed of extracellular DNA expelled from neutrophils. When LDN were cultured on plastic plates without coating, many NET clusters floating in media were observed, with few NETs on the bottom (Figure 1E).
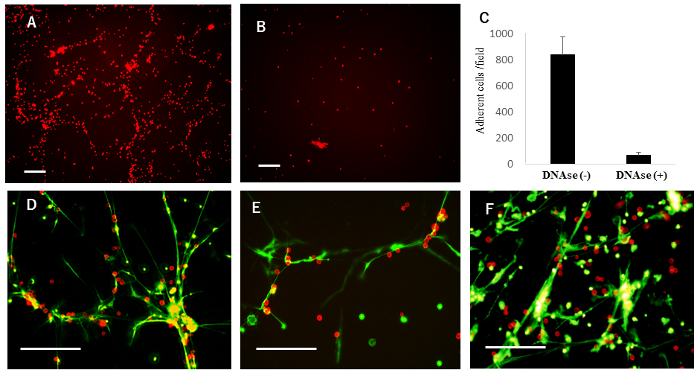
Next, to examine adhesion of the tumor cell lines to NETs, MKN45 cells labelled with red fluorescent dye were added to the NETs and co-incubated for 5 minutes. After gentle washing, many MKN45 cells were found attached (Figure 2A and 2C). In contrast, few MKN45 cells attached when LDN were pretreated with DNase I (Figure 2B and 2C). The merged figure clearly showed that a majority of the attached MKN45 cells were co-localized with NETs, seen as structures formed on the bottom of the plate (Figure 2D). Similar adhesion patterns were observed with OCUM-1 and NUGC-4 (Figure 2E and 2F).
Since NETs have been shown to be cytotoxic to bacteria, we then examined whether tumor cells trapped by NETs die or survive in a continuous co-culture using time-lapse video. Interestingly, while NETs gradually disappeared within several hours, the MKN45 cells trapped by NETs started to proliferate vigorously afterwards (Video 1).
Figure 1: NET formation after the culture of peritoneal LDN. (A) After density gradient centrifugation of postoperative lavage fluid, cells recovered from the intermediate layer are shown. (B) Then, CD66b(+) LDN were separated from CD66b(-) mononuclear cells and cultured on poly-L-lysine coated 6-well plates, followed by nuclear staining dye addition and immediate observation with fluorescence microscopy. (C) The same number of CD66b(-) mononuclear cells were cultured under the same conditions. (D) The LDN monolayer was incubated with DNase I for 5 minutes before nuclear staining. (E) CD66b(+) LDN were cultured on plastic plates without coating. White bars represent 100 µm. This figure was modified from a previous publication15. Please click here to view a larger version of this figure.
Figure 2: Adhesion of tumor cells to NETs. (A) Peritoneal LDN were cultured on poly-L-lysine coated 6-well plates for 2 h, which produced massive NETs. Red fluorescein-labelled MKN45 were then added. After a 5-minute incubation, unattached cells were removed by gentle washing, and remaining tumor cells were observed under a fluorescence microscope. (B) Before the addition of MKN45, the LDN monolayer was pretreated with DNase I for 5 min. (C) In experiments (A) and (B), the number of attached MKN45 cells were counted in 3 random fields under the fluorescence microscope and mean ± SD was expressed. (D) After gentle washing, green fluorescent dye for nuclear and chromosome was added to the co-culture. In representative fields, photos of attached MKN45 and NET structures were taken using appropriate optical wavelength filters, and the two figures were merged. The same experiments performed in (D) were also performed for OCUM-1 (E) and NUGC-4 (F). White bars represent 100 µm. This figure was modified from a previous publication15. Please click here to view a larger version of this figure.
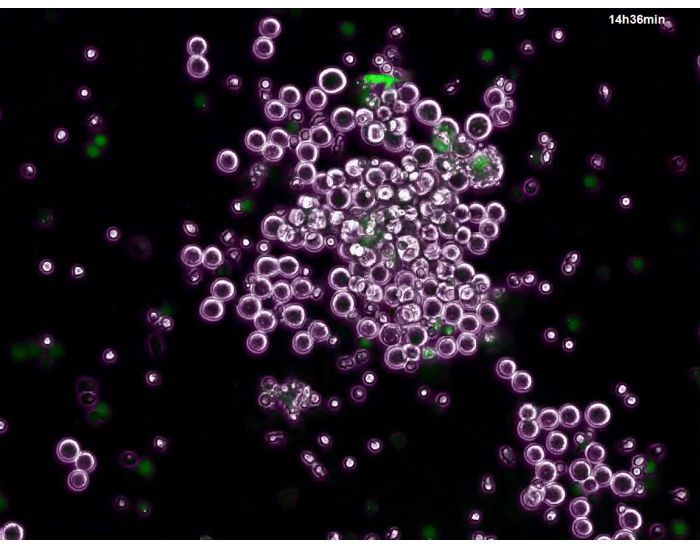 Video 1: Post-adhesion behavior of the trapped tumor cells. LDN were cultured on poly-L-lysine coated 6-well plates for 2 hours, and NET formation was confirmed by green fluorescent dye for nuclear and chromosome. Then, unstained MKN45 cells were added and co-incubated for 5 minutes, and unattached cells were removed by gentle washing and continued to be co-cultured in 10% FCS + DMEM. Using a whole-view cell observation system, areas where tumor cells were trapped by NETs were searched for. Photographs of the field were then taken every 6 minutes under normal light and fluorescence, which enabled clear figures of tumor cells and NETs, respectively. Finally, the images were superimposed with Image Viewer software, and a time lapse video was created at a 3600x higher speed than real-time. Please click here to view this video. (Right-click to download.)
Video 1: Post-adhesion behavior of the trapped tumor cells. LDN were cultured on poly-L-lysine coated 6-well plates for 2 hours, and NET formation was confirmed by green fluorescent dye for nuclear and chromosome. Then, unstained MKN45 cells were added and co-incubated for 5 minutes, and unattached cells were removed by gentle washing and continued to be co-cultured in 10% FCS + DMEM. Using a whole-view cell observation system, areas where tumor cells were trapped by NETs were searched for. Photographs of the field were then taken every 6 minutes under normal light and fluorescence, which enabled clear figures of tumor cells and NETs, respectively. Finally, the images were superimposed with Image Viewer software, and a time lapse video was created at a 3600x higher speed than real-time. Please click here to view this video. (Right-click to download.)
Discussion
Previous studies have reported that circulating tumor cells can be trapped by NET substrates in vivo10,11. Metastatic breast cancer cells have been shown to stimulate neutrophils and induce formation of NETs, which assists in tumor cell growth in the target organ17. In addition, we found that short-term cultures of LDN from postoperative lavage fluid produced massive NETs that could efficiently entrap tumor cells without further stimulation15. These observations suggest a possible role for mechanical involvement of NETs in the metastatic process. However, the detailed molecular mechanisms that mediate adhesive interactions between the NETs and tumor cells is still unclear. Recent studies have implicated the involvement of integrin in this process through the use of NET stock isolated from activated neutrophils that serve as the adhesion substrate12,13. However, purified and concentrated NETs components are supposed to have largely different molecular structures from original NETs. In comparison, our method uses intact NETs without physical manipulation, which is a strong advantage when examining the detailed mechanisms of adhesive interactions with NETs. With this simple technique, researchers can detect the specific attachment of many tumor cells to NETs quickly (within 5 min).
The most important feature of the protocol is the use of a poly-L-lysine coated plate to fix the NET structures on the bottom of the plate. When intact plastic plates were used for the LDN culture, only a few NETs were detected at the bottom. Furthermore, many green-positive clusters were observed as freely floating in the culture media, when they should have been detached from the plate (Figure 1D). We also examined NET formation on plates coated with collagen, fibronectin, and laminin, which enabled the detection of web-like structures in the bottom layer. However, the NETs appeared to attach to the substrates less tightly than to poly-L-lysine, and they were easily detached by the washing procedure. Therefore, these other options are insufficient for adhesion assays. In fact, if we use plates without poly-L-lysine coatings, NETs and any attached tumor cells can become easily removed from the plate by simply adding media to the wells.
The critical step in the adhesion assay is the incubation period of the co-culture with the tumor cells. Five minutes is sufficient for tumor cells to bind to the NETs. Under the experimental conditions, when adding the solution containing tumor cells to the 6-well plate, all tumor cells make physical contact with the NET structures formed at the bottom of the plate. However, co-culturing for a longer period (e.g., longer than 10 minutes) results in the binding of tumor cells to LDN or poly-L-lysine substrates. Therefore, the incubation time should be short to detect specific binding to the NETs. The maneuvering of gentle washing is another essential point. We made sure to slowly add the warmed media from the side-wall of each well, gently swing the plate, and remove the supernatant with an aspirator. Since NETs stick to the plate weakly, this process should be performed in an especially cautious manner.
This technique highlights the fact that a different molecular mechanism is needed for tumor cells binding to NETs within minutes, than that shown in an adhesion assay with a ~30 - 60 minute incubation to NET stocks12,13. Blocking experiments using this method can elucidate the detailed molecular mechanisms in which real NETs trap various materials. However, a limitation of this technique is that the washing process can differ when the adhesive interactions occur between NETs and tumor cells in vivo. In the clinical setting, the density of tumor cells is much lower than that used in this protocol, which may present another limitation.
Time-lapse video analysis is highly useful for monitoring the outcomes of various cellular events over a period of days. Using the whole-cell view system, we show that NETs are gradually degraded, presumably by DNAse contained in serum, whereas MKN45 trapped by NETs start to proliferate vigorously thereafter. This video only showed the division of tumor cells attached to NETs, with no comparison to MKN45 attached to the plate. Contaminated neutrophils and the added nuclear staining dye may have also affected the growth of the tumor cells. Therefore, we could not draw a definite conclusion regarding the net effects of NETs on tumor cell growth. However, this analysis does indicate that tumor cells are not killed by the NETs and can in fact survive following entrapment by NETs.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We thank Ms. J. Shinohara and I. Nieda for technical and clerical work. Also, we thank Drs. Shiro Matsumoto, Hidenori Haruta, Kentaro Kurashina, and Kazuya Takahashi for their cooperation for sample acquisition in operating room. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and the Japan Society for the Promotion of Science (17K10606).
References
- Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis and Rheumatology. 1986;29(11):1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. The Journal of Immunology. 2010;184(6):3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T, Goya T, Ishimitsu T, Torisu M. The increase of low density subpopulations and CD10 (CALLA) negative neutrophils in severely infected patients. Surgery Today. 1992;22(4):322–327. doi: 10.1007/BF00308740. [DOI] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Research. 2001;61(12):4756–4760. [PubMed] [Google Scholar]
- Brandau S, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. Journal of Leukocyte Biology. 2011;89(2):311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Seminars in Immunopathology. 2013;35(4):455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. The Journal of Immunology. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Demers M, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. Journal of Clinical Investigation. 2013. [DOI] [PMC free article] [PubMed]
- Tohme S, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Research. 2016;76(6):1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M, et al. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. Public Library of Science One. 2017;12(2):e0171362. doi: 10.1371/journal.pone.0171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmeh S, et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. International Journal of Cancer. 2017;140(10):2321–2330. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- Najmeh S, Cools-Lartigue J, Giannias B, Spicer J, Ferri LE. Simplified Human Neutrophil Extracellular Traps (NETs) Isolation and Handling. Journal of Visualized Experiments. 2015. [DOI] [PMC free article] [PubMed]
- Kanamaru R, et al. Low density neutrophils (LDN) in postoperative abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular traps (NETs) Scientific Reports. 2018;8(1):632. doi: 10.1038/s41598-017-19091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eades-Perner AM, Thompson J, van der Putten H, Zimmermann W. Mice transgenic for the human CGM6 gene express its product, the granulocyte marker CD66b, exclusively in granulocytes. Blood. 1998;91(2):663–672. [PubMed] [Google Scholar]
- Park J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science Translational Medicine. 2016;8(361):361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]


