Abstract
Intralesional therapy of melanoma patients with locally advanced metastatic disease is attracting increasing interest, not least due to its ability to lead to both direct tumor cell killing and the stimulation of both a local and a systemic immune response. An obvious pre-requisite for this type of approach is the presence of accessible metastases that are amenable to direct injection with the therapeutic agent of interest. Patients who present with these characteristics belong to stages IIIB/C or IV of the disease. Surgical resection with intention to cure is the standard of care for patients with limited tumor burden and confined spread of disease (resectable patients). However, this category of patients is at a high risk of further recurrences until the disease becomes inoperable (unresectable) or progresses to a more advanced stage with visceral organ involvement, after which the prognosis is particularly grim. Most of the intralesional treatments tested so far, including the recently approved oncolytic virus talimogene laherparepvec, target the subpopulation of patients with unresectable disease, but the possibility to use the intralesional treatment in a neoadjuvant setting for fully resectable patients is attracting considerable interest. The present article reviews approved products and advanced stage pharmaceutical agents in development for the intralesional treatment of melanoma patients.
Keywords: Intralesional, Immunocytokine, Neoadjuvant, Stage III B/C melanoma, Phase 3, CIMT 2016
Introduction
Melanoma is the deadliest of all skin cancers [1] and its incidence has been steadily increasing over the last 30 years [2].
In stages 0 to II of the disease, only the primary tumor is present and no other metastases to lymph nodes (LNs) or internal organs can be detected either clinically or by imaging [3]. When lesions are discovered at this stage, their surgical removal represents the standard of care and typically results in an excellent prognosis for most patients. However, the risk of relapse is not negligible [4] and this risk increases depending on the disease stage at diagnosis.
In more advanced stages (stage III and IV), the disease may have already progressed to the regional LN or distant organs, respectively [3]. These patients have considerably poorer prognoses with 5-year survival rates ranging from 40 to 78% [3] for stage III and from 9 to 21% [5] for stage IV disease.
In those patients in whom recurrence occurs after resection of a primary tumor, the most common route of first relapse (~50%) is progression to lymph nodes located beyond the draining LN basin [6]. The other recurrences are roughly equally distributed between direct spread to distant organs in various anatomical regions and intralymphatic diffusion to either cutaneous or subcutaneous locations between the primary tumor and the next proximal LN basin [6]. This last type of metastases, often indicated as locoregional disease, can be classified either as satellite metastases if they are located <2 cm from the site of primary tumor or as in-transit metastases if they are located ≥2 cm from the primary tumor [3, 7].
Patients who exhibit a limited number of in-transit/satellite metastases are still candidates for curative surgery. However, when relapses continue to occur even after repeated cycles of surgery, an alternative therapeutic strategy may be considered.
The propensity of melanoma to metastasize to readily accessible cutaneous or subcutaneous locations has prompted increased interest in researching the development of therapeutic approaches based around direct injection of active agents into the metastases, which have collectively been given the name of intralesional approaches.
Patients with limited and often asymptomatic locoregional disease are the ones most likely to benefit from intralesional therapies [8, 9]. In fact, direct injection of a therapeutic agent into the tumor, while maximizing its concentration at the site of disease, is associated with reduced systemic concentrations of the drug and a more favorable tolerability profile, when compared to systemic or regional perfusion-based therapies, which normally lead to considerable toxicity. Furthermore, the local effect exerted directly on treated tumor lesions can in some instances be associated with a systemic bystander effect (termed “abscopal effect”). This effect results in the shrinkage or disappearance of non-treated lesions, some of which may even be located at distant anatomical sites.
Many different agents have been investigated over the years for use in intralesional applications, including Bacille–Calmette–Guerin (BCG), Interferon-alpha (IFNa), Interferon beta (IFNb), Granulocyte macrophage-colony-stimulating factor (GM-CSF) and others have been considered for use as topical agents, such as imiquimod and diphencyprone. There is sporadic evidence of extremely good responses [10], however, neither efficacy nor safety could be consistently demonstrated for any of these products to enable them to receive marketing approval. A short overview of some of these agents and of results obtained during their clinical development is provided in the historical perspective below.
The most interesting results have probably been obtained with the intralesional administration of the cytokine interleukin-2 (IL2) and its targeted forms. This work, pioneered by the Garbe group in Tübingen, Germany exhibited promising responses in the majority of melanoma metastases after intratumoral injection of recombinant IL2 [9, 11–14]. Since the cytokine had to be injected frequently and over a period of several weeks, the use of antibody-based pharmacodelivery strategies has been proposed. Here, the cytokine is recombinantly expressed as a fusion protein with an antibody fragment, specific to an antigen selectively expressed in the tumor extracellular matrix [15]. The targeted form of IL2 produced comparable results in terms of efficacy and allowed a reduction in administration frequency and treatment duration, with the effect being due to the longer residence time in the metastatic lesion as compared to the untargeted drug [16].
Recently, new promising agents for the locoregional treatment of melanoma lesions have been developed. This review will focus on those agents which have either already obtained marketing approval or are currently being investigated in registration phase 3 trials. These include talimogene laherparepvec (T-Vec, Imlygic™), rose bengal (PV-10), and Daromun (L19IL2 + L19TNF). Many other agents are being developed for intratumoral application in several indications including melanoma but they have not been investigated in controlled phase 3 studies yet; these agents and their application have also been described in excellent recent review articles [17–19].
T-Vec was approved in 2015 in the US and Europe for the treatment of unresectable stage IIIB, IIIC or IVM1a metastatic melanoma. Rose Bengal is currently being investigated in a phase 3 trial in a similar indication. Daromun is being studied in a phase 3 trial as a neodjuvant treatment prior to surgery in fully resectable stage IIIB and IIIC patients (see also Table 1).
Table 1.
Clinical trials with novel intralesional agents
| Trial no. | Agent | Sponsor | Phase | Indication | References |
|---|---|---|---|---|---|
| N/A | OncoVEXGM−CSF | Biovex | I | Breast, gastrointestinal adenocarcinoma, malignant melanoma, HNSCC | [37] |
| NCT00289016 | OncoVEXGM−CSF | Biovex | II | Stage IIIC and IV melanoma | [38] |
| NCT00769704 | Talimogene laherparepvec | Amgen | III | Unresected stage IIIB/C and IV melanoma | [8] |
| NCT01740297 | T-Vec + Ipilimumab | Amgen | Ib/II | Unresected stage IIIB/C and IV melanoma | [42] |
| NCT02263508 | T-Vec + Pembrolizumab | Amgen, MSD | III | Unresectable stage IIIB to IVM1c melanoma | [43] |
| NCT02211131 | T-Vec | Amgen | II | Resectable stage IIIB/C and IVM1a melanoma (neoadjuvant) | N/A |
| NCT00219843 | PV-10 (rose bengal) | Provectus | I | Stage III and IV metastatic melanoma | [45] |
| NCT00521053 | PV-10 (rose bengal) | Provectus | II | Stage III and IV metastatic melanoma | [47] |
| NCT02288897 | PV-10 (rose bengal) | Provectus | III | Stage IIIB, IIIC or Stage IV M1a with no active nodal metastases | N/A |
| NCT01253096 | Darleukin (L19IL2) | Philogen | II | Stage IIIB/C melanoma | [15] |
| NCT02076633 | Daromun (L19IL2 + L19TNF) | Philogen | II | Stage IIIB/C and IVM1a melanoma | [56] |
| NCT02938299 | Daromun (L19IL2 + L19TNF) | Philogen | III | Resectable stage IIIB/C melanoma (neoadjuvant) | N/A |
N/A not applicable
Historical perspective
Bacille–Calmette–Guerin
BCG was the first agent to be proposed for intralesional administration in solid tumors [10]. It consists of an attenuated strain of Mycobacterium bovis, which is able to induce an unspecific host immune response and cause a regression in both injected and uninjected melanoma metastases [20]. However, its use is associated with a plethora of serious and potentially lethal adverse events, which have hampered BCG’s general applicability [20, 21].
Notwithstanding earlier reports of clinically outstanding results [10, 22, 23], development of BCG has not been continued after its failure to reach the primary outcome in a phase 3 Eastern Cooperative Oncology Group (ECOG) controlled, randomized trial [24], aimed at investigating efficacy of BCG as an adjuvant treatment in resected melanoma patients of stages I to III, either in monotherapy vs observation or in combination with dacarbazine vs BCG alone. Analysis of results failed to demonstrate an improvement in Recurrence-Free Survival (RFS) and Overall Survival (OS) for BCG-treatment arms.
Topical agents
Topical therapies for the treatment of cutaneous metastases have shown some efficacy and an overall good tolerability profile [25, 26].
Imiquimod
Imiquimod [1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine] is a synthetic small molecule which binds to and activates Toll-like receptor 7 (TLR7). This receptor is part of the innate immune response signaling pathway and is involved in pathogen recognition [27, 28]. The mechanism of action of imiquimod is complex and includes activation of natural killer cells, macrophages and B-lymphocytes [29], secretion of cytokines like IFNa, interleukin-6 and tumor necrosis factor-α (TNF, [30]) by activated cells and activation of Langerhans cells [29].
Imiquimod is currently approved by the Food and Drug Administration (FDA) for the treatment of actinic keratosis and external genital warts. In 2004, the FDA also approved its use for the treatment of superficial basal cell carcinoma.
No controlled studies of the efficacy of Imiquimod in metastatic melanoma have been carried out, nor has a precise administration schedule been established. A retrospective survey of literature [31] identified 57 different studies carried out with Imiquimod, but only 11 of these (with a total of 17 patients) were relative to Imiquimod monotherapy. Mostly, Imiquimod was used as a 5% (w/v) cream and was applied in various regimens, from three times weekly to twice daily. Treatment duration was an average of 22 weeks, ranging from 8 to 72 weeks. In terms of efficacy, a regression of metastases was seen in 82.3% of treated lesions but no data on progression-free survival (PFS) and OS are available. Treatment was generally well tolerated with mild side effects of short duration.
In a small study of the combination of topical imiquimod/fluorouracil in five patients, a response was elicited in 44 of 45 lesions [32]. Imiquimod has also been used in combination with BCG in metastatic melanoma [26].
The lack of carefully conducted, controlled studies has, however, so far limited the use of imiquimod for metastatic melanoma.
Diphencyprone (DPCP)
DPCP (2,3-Diphenylcycloprop-2-en-1-one) is a potent contact sensitizer that is used to treat alopecia areata and genital warts [33, 34].
The largest published study [35] was conducted in 50 patients, who received sensitization to the upper inner arm during 48 h (two drops of 2% DPCP in acetone), followed 2 weeks later by application of an aqueous DPCP cream (0.00001–0.1%) to all cutaneous metastases over 24–48 h. Duration of the treatment averaged 15 months and ranged from 1 to 60 months.
Thirty patients developed blisters after the first treatments, and this was accompanied by generalized dermatitis in four cases. Other less frequent side effects included eczema, urticaria, hyperpigmentation or depigmentation of the skin [35].
Complete responses (CR) were observed in 46% of the treated cases and partial responses (PR) in a further 38% of patients. Nine patients (18%) did not respond to DPCP treatment.
As the therapy is easy to administer and inexpensive, DPCP may represent an option in melanoma patients with cutaneous metastases who are not eligible for other treatments.
Novel agents: intralesional treatment of unresectable advanced metastatic melanoma patients
Talimogene laherparepvec (T-Vec, Imlygic™)
Talimogene laherparepvec (T-Vec) has been approved by the FDA in the US and by the European Medicine Agency (EMA) in Europe at the end of 2015, marketed under the name of Imlygic™. Talimogene laherparepvec is an oncolytic herpes simplex virus type 1, genetically modified to drive expression of GM-CSF in infected cells. Other genetic manipulations have been introduced in the viral genome to delete ICP34.5, a modification that provides tumor-selective replication, and ICP47, which otherwise blocks antigen presentation [36, 37]. The virus infects and selectively replicates in tumors, altering the host cell’s metabolic processes and thereby destroying cancer cells. Furthermore, the released human cytokine GM-CSF initiates a systemic tumor-specific immune response mediated by CD8+ cytotoxic T cells [37].
The clinical development of T-Vec was started in the early 2000’s [38]. Safety was investigated in a phase 1 trial in patients affected by a variety of solid tumors, including melanoma, presenting with cutaneous or subcutaneous metastases. The tolerability profile associated with the intralesional injection of the oncolytic virus was relatively favorable and mostly characterized by low-grade adverse events, which included flu-like symptoms (fever, chills, myalgias) and injection site reactions [38].
A follow-up phase 2 study was run in fifty inoperable stage III and IV patients [39]. Response criteria in solid tumors (RECIST)-based assessment carried out at three months showed an objective response rate (ORR) of 26% (16% CR; 10% PR), while the disease remained stable in 20% of patients [39]. OS at 1 and 2 years was 58 and 52%, respectively [39]. A systemic response in non-injected lesions was also documented. As for the phase 1 trial, the most commonly observed adverse events were flu-like symptoms, with fatigue, chills, nausea, and pyrexia [39].
Based on the results from phase 2, a randomized, controlled phase 3 trial (OPTiM study) was later performed. A total of 436 patients were randomized in a 2:1 ratio to receive either intralesional injections of T-Vec (295 patients) or subcutaneous injections of GM-CSF (141 patients) [8]. The primary objective of the study was an evaluation of durable responses in the two arms of the study, defined as a PR or CR that was observed within 12 months from start of treatment and lasting ≥6 months. In the T-vec arm, durable responses were recorded in 16.3% of patients, as opposed to 2.1% in the GM-CSF arm (P < 0.001), with a more significant benefit for stage IIIB and IIIC patients than for those at more advanced stages of the disease. Efficacy results confirmed those observed in the phase 2 study, with an ORR of 26.4% recorded in the T-Vec group (vs. 5.7% in the control, GM-CSF group) [8]. In terms of OS, the median increase in the T-Vec arm vs the GM-CSF control arm was of about 4 months (23.3 vs 18.9, respectively) but did not reach statistical significance [8].
The results also demonstrated a bystander effect in non-injected, visceral, and nonvisceral lesions, suggesting that a systemic immune response is associated with the intralesional administration of T-Vec. However, the response rate in non-injected lesions was comparably low.
A combination of intralesional treatment modalities and systemically-administered immunomodulatory agents has been proposed as a promising avenue for the treatment of metastatic melanoma [40, 41]. In particular, the combination of an oncolytic virus with checkpoint inhibitors, like the anti-Cytotoxic T lymphocyte antigen 4 (CTLA4) antibody Ipilimumab (Yervoy™) and the anti-Programmed cell death protein 1 (PD1) antibody Pembrolizumab (Keytruda™) has been tested in preclinical studies [42] in murine models of melanoma. The combination of T-Vec with each of these two agents has been recently moved to the clinical setting (NCT01740297 and NCT02263508, Table 1).
An open-label, multicenter, phase 1b trial in 19 previously untreated, unresectable stage IIIB to IV melanoma patients was started in 2013. Intralesional injection of T-Vec was performed at week 1 (106 pfu/mL), week 4, and every 2 weeks thereafter (108 pfu/mL). Starting from week 6, Ipilimumab at a dose of 3 mg/kg was administered i.v. every 3 weeks, for four cycles. The primary end point was incidence of dose-limiting toxicities (DLTs). Secondary end points were ORR by immune-related response criteria (irRC) and safety [43].
The median duration of T-Vec treatment was of 13.3 weeks. No DLTs were recorded during the evaluation window. Treatment-related adverse events (AEs) of any grade were reported by 18 out of the 19 patients, grade 3/4 events occurred in 26.3% of patients. T-Vec and Ipilimumab were attributed 15.8% and 21.1% of the recorded AEs, respectively. One grade 4 elevation of liver enzymes and a grade 5, unrelated AE of neurological involvement due to metastatic disease were recorded. irRC assessment run on 18 patients revealed an ORR of 56%, with 33% CR (6 patients). A phase 2 controlled, randomized extension of the study is ongoing, aiming at enrolling 198 patients to receive T-Vec in combination with ipilimumab compared to ipilimumab alone in the control arm. The primary outcome analysis is expected in the second half of 2016.
The combination of T-Vec with the anti-PD1 antibody Pembrolizumab is also being explored. A phase 1b/3 trial (Masterkey-265) was launched in 2014 to investigate safety and efficacy of this combination in unresectable stage IIIB to IVM1c melanoma patients. The primary endpoint of the phase 1b study is to determine incidence of DLT, while the secondary outcome includes incidence of AEs and of treatment-emergent and treatment-related AEs. The safety and efficacy analysis for the phase 1b portion of the trial have been presented at the ASCO 2016 conference [44].
Patients received intralesional injection of T-Vec at week 1 (106 pfu/mL), then every 2 weeks (108 pfu/mL). Administration of pembrolizumab at 200 mg i.v. was started on day 36 every 2 weeks until CR or PD (according to irRC) or for up to 2 years. Twenty-one patients were enrolled. Treatment-related AEs occurred in all patients, 33% of which were grade 3/4. No grade 5 AEs occurred. Most common AEs were fatigue (62%), pyrexia (52%), and chills (48%). Confirmed ORR was 48%, with a CR rate of 14%. Median time to response was 17 wks. The randomized, double-blind phase 3 portion of the study is presently under way and an update about clinical and biomarker data is expected for next year.
Rose Bengal (PV-10)
Rose Bengal is a small molecule that belongs to the xanthene family and is a derivative of fluorescein. The compound has been in use for decades in the clinic as eye drops to stain damaged conjunctival and corneal cells and thereby more easily identify affected areas. Its potential applicability as an anti-tumor agent has, however, been recognized only much later, during a screening campaign for new photodynamic therapy agents with antineoplastic activity.
A 10% Rose Bengal solution (PV-10) is being developed for intralesional application in melanoma and metastatic breast and liver cancer. The proposed mechanism of action is based on a selective uptake of the compound through the cell membrane of cancer cells because of its higher fluidity as compared to that of normal cells. PV-10 accumulates in the lysosomes [45], triggering lysosomal release and autolysis of the tumor cells, which is normally complete within 30–60 min. Acute exposure of antigenic tumor fragments to APCs is believed to produce the “bystander” effect observed in non-injected tumors.
Results of a phase 1 trial were published in 2008 [46] and showed that treatment was generally well tolerated and elicited minimal side effects, the most common being mild-to-moderate pain at the injection site (≤80%). However, one case of severe systemic phototoxicity was recorded during the study [47]. Other reported AEs included vesicles, edema, skin discoloration, inflammation, headache, and pruritus around the treatment site [46]. ORR were recorded in more than 50% of patients (CR and PR, both 27%) in a dose-dependent fashion. A bystander effect was seen in 27% of the patients and correlated with the response of the injected lesion.
These results were confirmed in a phase 2 study [48]. A multicenter, international trial was conducted in 80 patients with measurable stage III–IV melanoma. PV-10 was administered via intralesional injections in up to ten target and ten non-target cutaneous, subcutaneous, or nodal lesions. Treatment was repeated, when necessary, at weeks 8, 12, or 16. The primary endpoint was ORR for injected lesions at week 52. Response rates were comparable with those seen in the phase 1 study. ORR was achieved in 51% of study patients, including a 26% complete response rate. Among the 38 subjects with bystander lesions, CR of untreated lesions was reported in 24% of patients, and PR in another 13%, with an ORR of 37% and a locoregional control of 55%. Regression of bystander lesions strongly correlated with response in target lesions. However, CR was not seen in any of the study subjects with stage IV disease.
In April 2015, an international multicenter, open-label, randomized controlled phase 3 study was launched to investigate the efficacy of intralesional PV-10 vs systemic chemotherapy or intralesional oncolytic viral therapy, in unresectable patients of stages IIIB, IIIC, and IVM1a without active nodal metastases. Subjects in the comparator arm receive the investigator’s choice between dacarbazine, temozolomide or intralesional T-Vec, also depending on availability in the study country or region. Efficacy is being assessed by comparison of PFS between all intention-to-treat (ITT) subjects in the two study treatment arms. The estimated patient enrollment is 225 patients (randomized 2:1 to the PV-10 arm) and the final data collection for the primary outcome measure is expected in mid-2018.
Novel agents: neoadjuvant intralesional treatment of fully resectable melanoma patients
Daromun (L19IL2 + L19TNF)
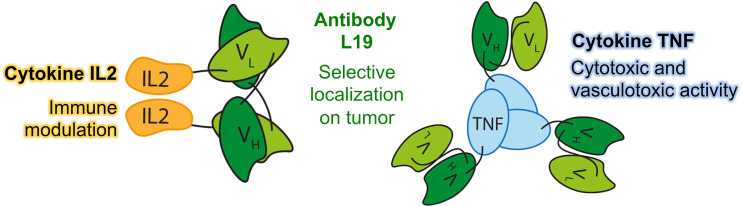
Daromun is the combination of two immunocytokines, L19IL2 and L19TNF. Immunocytokines are biopharmaceuticals that have shown great potential for the therapy of cancer and other serious diseases [49]. These products are recombinant fusion proteins, consisting of a human cytokine linked (at its N- or C-terminus) to a monoclonal antibody or to an antibody fragment, serving as a pharmacodelivery vehicle for the selective localization of the immunostimulatory payload at sites of disease. A schematic representation of the immunocytokine components of Daromun is presented in Fig. 1.
Fig. 1.
The human cytokines IL2 and TNF are conjugated through a flexible linker to the C-terminus of the L19 antibody fragment in single-chain variable format (ScFv). L19IL2 in solution forms non-covalent homodimers, while L19TNF in solution exists as a homotrimer via the trimerization motif present in the TNF sequence
The individual immunocytokines have been extensively characterized in the systemic delivery setting in a number of studies, both as single agents and in combination with chemotherapeutics or biologicals and are still being explored in several indications in oncology.
L19IL2 has been studied as a monotherapy for patients with renal cell carcinoma in a phase 1/2 dose finding study [50] and in a phase 1/2 study in combination with gemcitabine in pancreas cancer patients.
Furthermore, a phase 2 dose finding and activity evaluation study of L19IL2 in combination with DTIC in melanoma patients was carried out [50, 51], (manuscript in preparation).
L19TNF has been studied in a phase 1 clinical trial as monotherapy in patients with confirmed progressive solid tumors, followed by a phase 2 part in 12 patients with colorectal cancer [52], as well as in a phase 1 trial in combination with melphalan using isolated limb perfusion (ILP) for patients presenting with metastatic melanoma in limbs, who were candidates for amputation [53]. Finally, a phase 1b study is ongoing to investigate the combination of L19TNF with doxorubicin in patients with advanced solid tumors, with a particular focus on soft tissue sarcoma.
An international, multicenter phase 2 trial of the intralesional application of L19IL2 as single agent in stage IIIB and IIIC melanoma patients has recently been reported [15]. Twenty-five patients with cutaneous/subcutaneous injectable metastases received weekly intratumoral injections of L19IL2 at a maximum dose of 10 MIU/week for 4 consecutive weeks. Tumor response was evaluated 12 weeks after first treatment. A complete response of all treated metastases was achieved in six patients (25%), and this response was long-lasting in most cases (5 patients ≥ 24 months). Objective responses (OR) were documented in 53.9% of all index lesions. The treatment was very well tolerated, with no grade 4 and only a few grade 3 AEs (injection site reaction, fatigue, pain).
A significant temporary increase of peripheral regulatory T cells and natural killer (NK) cells, a sustained increase of absolute number of CD4+ lymphocytes and a decrease of myeloid-derived suppressor cells were observed upon treatment. Furthermore, encouraging time-to-progression data were recorded in this cohort of patients, suggesting that the locoregional control of in transit/satellite metastases might translate into a slower progression to distant metastases and in an improved overall survival [15].
Preclinical data collected by our group [54] have shown that a combination of L19IL2 with a version of L19TNF featuring a murine tumor necrosis factor alpha (TNF) component induced complete remissions when administered as a single intratumoral injection in a syngeneic immunocompetent mouse model of cancer, whereas the two components did not lead to cures when administered separately. These results have been recently confirmed in two additional mouse models of cancer, the K1735M2 melanoma, and Wehi-164 sarcoma. When used alone, immunocytokines did not exhibit complete cures but only tumor growth retardation. However, combination therapies resulted in complete and long-lasting tumor eradication that cannot be achieved by conventional chemotherapy [16].
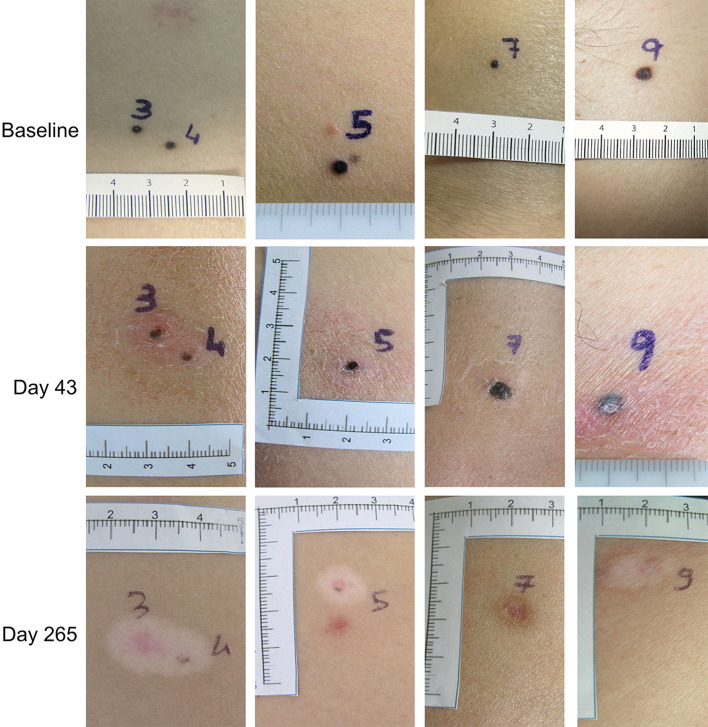
These observations provided the rationale for a phase 2 clinical trial based on the intralesional administration of L19IL2 in combination with L19TNF in patients with unresectable stage IIIB/C and IVM1a metastatic melanoma [55]. Treatment was in general well tolerated, with a limited number of drug-related adverse events, mostly of grade ≤2, which were normally rapidly resolved. In terms of efficacy, according to modified RECIST criteria, PR were recorded in 50% of patients at week 12. Importantly, according to these criteria, no patient was considered to be in PD at the time of tumor assessment. However, three patients showed CR at later tumor assessment (week 36), indicating that the intralesional injection of L19IL2/L19TNF continues to exert a beneficial effect for a long time after the end of treatment (Fig. 2).
Fig. 2.
Evolution of injected lesions in a patient over time. A CR is observed at week 36 after beginning of treatment. In most lesions, a vitiligo-like depigmentation develops over time, as a consequence of a strong antimelanoma immunity that also targets healthy melanocytes, because of shared expression of melanocyte differentiation antigens. Data from the literature have shown that development of a vitiligo-like depigmentation in patients with melanoma may be associated with more favorable clinical outcome [61]
According to the protocol, the investigator could decide to surgically remove remaining lesions or residual scabs to render the patient disease-free after the mandatory tumor assessment at week 12. This neoadjuvant use of the combination was adopted for eight out of the 20 patients evaluable for efficacy. Patients, who had not been considered eligible for surgery, became operable after treatment with the immunocytokine product, underwent surgical ablation of remaining lesions and were therefore withdrawn from the study at that time point.
Importantly, the intralesional administration of L19IL2/L19TNF mediated a systemic anti-tumor effect in 70% of non-injected metastases (bystander effect), including cutaneous/subcutaneous and nodal lesions [55].
These results have prompted Philogen to launch an international, multicenter, randomized phase 3 trial in 2016 of the neoadjuvant efficacy of the intralesional treatment of fully resectable melanoma patients at stage IIIB and IIIC of the disease with L19IL2 + L19TNF followed by surgery, as compared to surgery alone. A pre-requisite for inclusion in the study is the presence of at least one injectable cutaneous, subcutaneous or nodal lesion and complete resectability of all lesions present at baseline, so as to render the patient free of detectable disease after surgery. The primary endpoint of this open-label, controlled study aims at demonstrating that the immunocytokine combination, intralesionally administered prior to surgery, improves the RFS at 1 year after randomization in treated patients as compared to direct surgical removal of all lesions. A hundred and seven patients will be enrolled in each treatment arm resulting in a total sample size of 214. The trial officially started in July 2016 and is actively recruiting.
Conclusions
Over recent years, there has been a radical change in the management of advanced melanoma patients with the approval of many novel therapeutic agents.
Targeted therapies have become the front-line treatment for patients with point mutations in BRAF and MEK genes. Immunotherapeutic antibodies, which block cytotoxic T lymphocyte antigen-4 (CTLA4) or programmed death-1 receptor/ligand (PD-1/PD-L1), have been shown to boost the immune response against the tumor and improve PFS and OS in patients without mutations [56].
The first approved agent for intralesional application in advanced melanoma (Imlygic™) now adds to the arsenal of weapons for pharmacological intervention. All these agents, however, have been developed to treat patients who, because of continuous recurrences, heavy tumor burden or presence of distant metastases in various anatomical regions, are not candidates for surgery.
When considering stage III melanoma patients with resectable disease, however, no progress has been recorded in improving the outcome of surgery, which is still the standard of care for these patients. Adjuvant therapy with IFNa is approved and is performed in some centers, but a consensus about its benefit in terms of RFS has not been reached [57]. In this situation, in which patients receive surgery and often are not submitted to a rigorous follow-up procedure, the use of intralesional immunostimulatory therapies in a neoadjuvant intention may have an important role to play.
Amgen are testing Imlygic™ in a phase 2 trial as neoadjuvant treatment in completely resectable stage IIIB, IIIC, or IVM1a melanoma patients. Philogen have progressed their immunocytokine combination Daromun to phase 3 level in fully resectable stage IIIB/C melanoma (Table 1).
The systemic bystander effect, observed during the phase 2 trial with Daromun, provided a rational basis for the phase 3 trial of the immunocytokine combination in a neoadjuvant setting, followed by surgical resection of all lesions present at baseline. It is known that systemic administration of IL2 in vivo augments the activity of cytotoxic T lymphocytes [58] either alone or in combination with other cytokines [59] and also induces specific T helper cells, natural killer, and lymphokine-activated killer (LAK) cells. On the other hand, systemic administration of TNF may lead to extravasation of erythrocytes and lymphocytes, provoking haemorrhagic necrosis of the tumor (reviewed in [60]). TNF has the ability to kill tumor cells, but may also target the tumor-associated vasculature by inducing hyperpermeability and destruction of the vascular lining. Thanks to the anchoring effect mediated by the antibody moiety, immunocytokines have been shown to exhibit longer residence times in injected lesions with respect to non-targeted cytokines [16]. The results of the phase 2 study indicates that, besides a locoregional control of injected metastases, the combination of L19IL2 and L19TNF is also capable of exerting a beneficial systemic effect on distant, non-injected lesions, an effect which continues even in the absence of additional administrations of the immunocytokines [55].
If the killing of the tumor can stimulate a tumor-specific immune response that persists after the tumor is removed, a positive impact may be expected on non-injected lesions. As the treatment with Daromun is short (4 weeks), the procedure can be performed without an unreasonable delay of surgical intervention.
Many researchers believe that the future of intralesional therapies is linked to their use in combination with the new systemic therapies, in particular immunotherapies [40, 41]. Results of the ongoing trials will tell if, in addition, the use of intralesional therapies as neoadjuvants can play a role in the therapeutic approach for resectable melanoma patients in the years to come.
Acknowledgements
Support from the Swiss Federal Institute of Technology Zurich (ETHZ), the Swiss National Science Foundation (SNF) and from European Research Council (ERC) Advanced Grant (ZAUBERKUGEL) is gratefully acknowledged.
Abbreviations
- AEs
Adverse events
- CR
Complete responses
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- DLT
Dose-limiting toxicity
- DPCP
Diphencyprone
- ECOG
Eastern Cooperative Oncology Group
- EMA
European medicine agency
- FDA
Food and drug administration
- IFNa
Interferon α
- IFNb
Interferon β
- IL2
Interleukin-2
- ILP
Isolated limb perfusion
- irRC
Immune-related response criteria
- ITT
Intention-to-treat
- OR
Objective response
- ORR
Objective response rate
- PFS
Progression-free survival
- PR
Partial response
- PV-10
Rose Bengal (Provectus)
- RFS
Recurrence-free survival
- TLR7
Toll-like receptor 7
- TNF
Tumor necrosis factor α
- T-Vec
Talimogene laherparepvec, Imlygic™ (Amgen)
Compliance with ethical standards
Conflict of interest
B. Weide declares receiving commercial research grants from Bristol-Myers Squibb (BMS) and Merck Sharp and Dohme (MSD), having received travel/accommodation expenses from BMS, MSD, Roche, Amgen, Philogen, Curevac and compensation for advisory services from MSD, BMS, Philogen, and Curevac. D. Neri is co-founder and shareholder of Philogen S.p.A. G. Elia declares no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourteenth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 10th–12th May, 2016. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19(1):2–11. doi: 10.1053/sonu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essner R, Lee JH, Wanek LA, Itakura H, Morton DL. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139(9):961–966. doi: 10.1001/archsurg.139.9.961. [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27(21):3489–3495. doi: 10.1200/JCO.2008.18.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147(1):62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbott AM, Zager JS. Locoregional therapies in melanoma. Surg Clin North Am. 2014;1003–1015:viii. doi: 10.1016/j.suc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 9.Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, Pfohler C, Pawelec G, Garbe C. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 10.Mastrangelo MJ, Bellet RE, Berkelhammer J, Clark WH., Jr Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer. 1975;36(4):1305–1308. doi: 10.1002/1097-0142(197510)36:4<1305::AID-CNCR2820360417>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Boyd KU, Wehrli BM, Temple CL. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J Surg Oncol. 2011;104(7):711–717. doi: 10.1002/jso.21968. [DOI] [PubMed] [Google Scholar]
- 12.Dehesa LA, Vilar-Alejo J, Valeron-Almazan P, Carretero G. [Experience in the treatment of cutaneous in-transit melanoma metastases and satellitosis with intralesional interleukin-2] Actas Dermosifiliogr. 2009;100(7):571–585. doi: 10.1016/S0001-7310(09)71905-2. [DOI] [PubMed] [Google Scholar]
- 13.Gutwald JG, Groth W, Mahrle G. Peritumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br J Dermatol. 1994;130(4):541–542. doi: 10.1111/j.1365-2133.1994.tb03397.x. [DOI] [PubMed] [Google Scholar]
- 14.Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwarz M, Garbe C. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 16.Pretto F, Elia G, Castioni N, Neri D. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother. 2014;63(9):901–910. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20(7):1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28(7):329–338. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakousis CP, Douglass HO, Jr, Yeracaris PM, Holyoke ED. BCG immunotherapy in patients with malignant melanoma. Arch Surg. 1976;111(6):716–718. doi: 10.1001/archsurg.1976.01360240096018. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JC. Risks of BCG intralesional therapy: an experience with melanoma. J Surg Oncol. 1977;9(6):587–593. doi: 10.1002/jso.2930090609. [DOI] [PubMed] [Google Scholar]
- 22.Barth A, Morton DL. The role of adjuvant therapy in melanoma management. Cancer. 1995;75(2 Suppl):726–734. doi: 10.1002/1097-0142(19950115)75:2+<726::AID-CNCR2820751417>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Seigler HF, Shingleton WW, Pickrell KL. Intralesional BCG, intravenous immune lymphocytes, and immunization with neuraminidase-treated tumor cells to manage melanoma; a clinical assessment. Plast Reconstr Surg. 1975;55(3):294–298. doi: 10.1097/00006534-197555030-00004. [DOI] [PubMed] [Google Scholar]
- 24.Agarwala SS, Neuberg D, Park Y, Kirkwood JM. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100(8):1692–1698. doi: 10.1002/cncr.20166. [DOI] [PubMed] [Google Scholar]
- 25.Damian DL, Shannon KF, Saw RP, Thompson JF. Topical diphencyprone immunotherapy for cutaneous metastatic melanoma. Australas J Dermatol. 2009;50(4):266–271. doi: 10.1111/j.1440-0960.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 26.Kidner TB, Morton DL, Lee DJ, Hoban M, Foshag LJ, Turner RR, Faries MB. Combined intralesional Bacille Calmette-Guerin (BCG) and topical imiquimod for in-transit melanoma. J Immunother. 2012;35(9):716–720. doi: 10.1097/CJI.0b013e31827457bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 28.Walter A, Schafer M, Cecconi V, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 29.Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21(1):1–14. doi: 10.1016/S0192-0561(98)00068-X. [DOI] [PubMed] [Google Scholar]
- 30.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149(Suppl 66):5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 31.Sisti A, Sisti G, Oranges CM (2015) Topical treatment of melanoma skin metastases with Imiquimod: a review. Dermatol Online J 21(2) [PubMed]
- 32.Florin V, Desmedt E, Vercambre-Darras S, Mortier L. Topical treatment of cutaneous metastases of malignant melanoma using combined imiquimod and 5-fluorouracil. Invest New Drugs. 2012;30(4):1641–1645. doi: 10.1007/s10637-011-9717-2. [DOI] [PubMed] [Google Scholar]
- 33.Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145(3):385–405. doi: 10.1046/j.1365-2133.2001.04399.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Steen PH, Happle R. Topical immunotherapy of alopecia areata. Dermatol Clin. 1993;11(3):619–622. [PubMed] [Google Scholar]
- 35.Damian DL, Saw RP, Thompson JF. Topical immunotherapy with diphencyprone for in transit and cutaneously metastatic melanoma. J Surg Oncol. 2014;109(4):308–313. doi: 10.1002/jso.23506. [DOI] [PubMed] [Google Scholar]
- 36.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol. 1997;71(8):6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 38.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 39.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte–macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Daud A. A review of novel intralesional therapies for melanoma, with an emphasis on a potential combination approach. Oncology (Williston Park) 2016;30(5):442–443. [PubMed] [Google Scholar]
- 41.Agarwala SS. Intralesional therapy for advanced melanoma: promise and limitation. Curr Opin Oncol. 2015;27(2):151–156. doi: 10.1097/CCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIb-IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long GV, Dummer R, Ribas A et al (2016) Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol 34, (suppl; abstr TPS9598)
- 45.Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P. Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res. 2006;16:S8. doi: 10.1097/00008390-200609001-00012. [DOI] [Google Scholar]
- 46.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18(6):405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 47.Wiener M, Damian DL, Thompson JF. Systemic phototoxicity following intralesional rose bengal for subcutaneous melanoma metastases. Dermatology. 2008;216(4):361–362. doi: 10.1159/000117707. [DOI] [PubMed] [Google Scholar]
- 48.Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 study of intralesional pv-10 in refractory metastatic melanoma. Ann Surg Oncol. 2015;22(7):2135–2142. doi: 10.1245/s10434-014-4169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 50.Johannsen M, Spitaleri G, Curigliano G, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 51.Eigentler TK, Weide B, de Braud F, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 52.Spitaleri G, Berardi R, Pierantoni C, et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139(3):447–455. doi: 10.1007/s00432-012-1327-7. [DOI] [PubMed] [Google Scholar]
- 53.Papadia F, Basso V, Patuzzo R, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–179. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 54.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 55.Danielli R, Patuzzo R, Di Giacomo AM, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DB, Sosman JA. Therapeutic advances and treatment options in metastatic melanoma. JAMA Oncol. 2015;1(3):380–386. doi: 10.1001/jamaoncol.2015.0565. [DOI] [PubMed] [Google Scholar]
- 57.Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dreno B, Kirkwood JM. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer. 2008;112(5):982–994. doi: 10.1002/cncr.23251. [DOI] [PubMed] [Google Scholar]
- 58.Lotze MT. Biologic therapy with interleukin-2: Preclinical studies. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. Philadelphia: Lippincott; 1995. pp. 207–233. [Google Scholar]
- 59.Rosenberg SA, Lotze MT, Yang JC, et al. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol. 1989;7(12):1863–1874. doi: 10.1200/JCO.1989.7.12.1863. [DOI] [PubMed] [Google Scholar]
- 60.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 61.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]