Abstract
Cell migration is an important process that influences many aspects of health, such as wound healing and cancer, and it is, therefore, crucial for developing methods to study the migration. The scratch assay has long been the most common in vitro method to test compounds with anti- and pro-migration properties because of its low cost and simple procedure. However, an often-reported problem of the assay is the accumulation of cells across the edge of the scratch. Furthermore, to obtain data from the assay, images of different exposures must be taken over a period of time at the exact same spot to compare the movements of the migration. Different analysis programs can be used to describe the scratch closure, but they are labor intensive, inaccurate, and forces cycles of temperature changes. In this study, we demonstrate an optimized method for testing the migration effect, e.g. with the naturally occurring proteins Human- and Bovine-Lactoferrin and their N-terminal peptide Lactoferricin on the epithelial cell line HaCaT. A crucial optimization is to wash and scratch in PBS, which eliminates the aforementioned accumulation of cells along the edge. This could be explained by the removal of cations, which have been shown to have an effect on keratinocyte cell-cell connection. To ensure true detection of migration, pre-treating with mitomycin C, a DNA synthesis inhibitor, was added to the protocol. Finally, we demonstrate the automated optical camera, which eliminates excessive temperature cycles, manual labor with scratch closure analysis, while improving on reproducibility and ensuring analysis of identical sections of the scratch over time.
Keywords: Retraction, Issue 138, Scratch assay, migration, HaCaT, peptide treatment, optical microscope, epidermal growth factor, Lactoferrin, Lactoferricin
Introduction
Migration is an important process that influences several physiological aspects. In wound healing, migration facilitates re-epithelization of the skin. In non-healing wounds, such as chronic wounds, opportunistic bacteria cause infection of the wound, and open wounds are ideal for bacteria growth and formation of biofilm1,2. Biofilm is one of the causes of the development of resistant bacteria, which is one of the biggest threats to our modern society3. In wound healing, the immune cells cannot penetrate the biofilm. In cancer, migration of the cancer cells is a pivotal step of metastasis, which is the primary cause of death for patients with solid tumors4,5. Therefore, it is crucial to be able to study migration.
The scratch assay is often used to test cell migration because it is cheap and easy to perform on adherent cell lines, such as fibroblast, endothelial, and epithelial cell lines6,7. Today, several methods exist for performing scratches8, and different materials have been reported to be used, including toothpicks9, cell scrapers10, lasers11, and electric currents12. All of the methods have different pros and cons, and the method should be decided upon according to the cell type, budget, and analysis tool. As an example, if the used cell type requires coating, manual pressure removal, e.g. with a toothpick or pipette tip, will influence migration within the area, a different method should be used. In this study, we will focus on manual scraping.
A downside for manual scraping is the variation of the size, shapes of the scratches, and accumulation of cell on the edges of the scratch. Many protocols exist, and a highly cited paper advises increased speed and/or thorough washing after scratching13. Additionally, several methods have been used to minimize proliferation, since the proliferation of the cells cannot be distinguished from migration and therefore may give false-positive results of migration. Some reported methods are serum starvation preceding the scratch14 and decreasing the amount of serum15. However, neither of these methods can ensure that there is no proliferation. Therefore, we report a pre-treatment of the cells with mitomycin C to ensure true results of migration. Mitomycin C is an antibiotic that inhibits DNA synthesis by forming a covalent bond with the DNA, which prevents the DNA from separating16. By pre-treating with mitomycin C and washing with PBS before scratching, the detected closure of the gap demonstrates the true migration of the cells (Figure 1).
A characteristic of all the methods is the need for a system to analyze the process of migration17. A common and an inexpensive method to analyze the progress is to use a light-field microscope to take images of the scratch at pre-determined time points, followed by an analysis of the closure of the gap in an image software18,19. This method is time-consuming, unreliable with regards to taking pictures at the same spot and focus, and the movement from incubator to camera forces cycles of temperature changes while pictures are taken. Other methods have been developed to detect migration by measuring the current flow. The current changes, as cells cover more space on the plate, which is read as an increase in the impedance20. By measuring impedance, the environment of the cells is not affected, and the method is therefore considered non-invasive. This method relies on specialized plates with gold electrodes, which are expensive, but they enable detection of many processes, such as cellular adherence, proliferation, migration, and cell death. A big disadvantage of this method is that the impedance measurement does not directly indicate migration, as factors such as temperature have a great impact on the impedance. Changes in the impedance can be caused by several factors that are not reviewed by the software, making the analysis of the data more difficult. Therefore, the lack of visualization requires a conventional light microscope to verify migration.
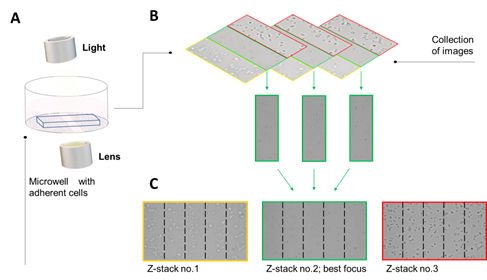
To overcome many of the downsides of the available techniques, we use a real-time automated optical camera. The optical camera is a detection system with a high-throughput microscope in a small platform with a lens, illumination unit, and a digital camera. It has a built-in software, which can test migration and proliferation among other things. To detect migration, two algorithms are used to analyze the growth and the migration kinetics of cells, which are called the proliferation and the wound healing analysis, respectively. The proliferation analysis is based on a two-step image segmentation algorithm that applies texture analysis to detect the difference between cell-covered areas (shown in yellow) and empty background areas by advanced histogram analysis. The wound healing analysis is based on a three-step algorithm that detects the cell monolayer, locates the wound gap, and determines the gap width. These steps are conducted at user-determined time-points, and the data is presented as covered area, gap width, and gap area. One of the great advantages of this machine is that it enables imaging of adherent cells through liquid because of the angle of the camera21. The camera lenstakes tilted images that are projected to a z-stack of a single z-plane generate to ensure that the pictures are in-focus (Figure 2). z-stacks are generated by merging the series of pictures (e.g. 40) taken perpendicular to the wound edge, and across the entire wound. The z-stacks enables capturing of the depth of the cell layer as well as an in-focus plane across the wound. Furthermore, the series of images can be put together to a video collection of the images, with the click of a button.
We demonstrate an optimized protocol for detection of migration in the keratinocyte cell line HaCaT. We tested Lactoferrin and its N-terminal peptide, Lactoferricin, from humans and cows, to analyze their effect on migration as these molecules have been demonstrated to have numerous applications as antimicrobial and immune modulating molecules22,23. The four compounds were compared to untreated HaCaT cells and epidermal growth factor (EGF) was used as a positive control.
Protocol
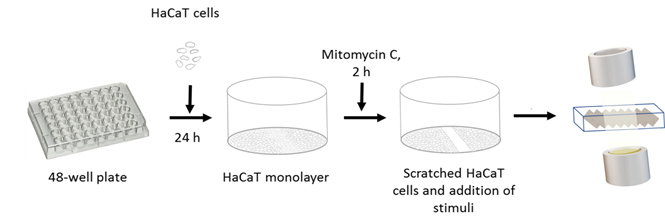
The experimental setup has no ethical and legal restriction and was conducted in agreement with Roskilde University. An overview of the experimental set-up can be seen in Figure 1 and the mechanisms of the optical camera in Figure 2.
1. Preparation
Perform all steps in a sterile laminar flow-bench by cleaning the bench and all equipments with 70% ethanol prior to the start of the experiment.
Place the optical camera in a conventional incubator at 37 °C with 5% CO2 to ensure optimal conditions for the cells.
Grow HaCaT cells in DMEM media with 10% FBS and 1x antibiotics (penicillin and streptomycin) in a T75 flask.
Use 1 mL 1x trypsin to detach the cells and sub-cultivate them every 3-4 days, by dispersing it over the cellular layer. Pre-heat to room temperature before use.
2. Generating a Monolayer of HaCaT Cells in a 48-wells Microtiter Plate
Remove the medium from the T75 flask with a Pasteur pipette.
Wash the adherent cells in the flask by adding 5 mL 1x sterile PBS to the T75 flask. Note: Ensure that the PBS is free of calcium and magnesium.
Circulate the PBS and remove it with a Pasteur pipette.
Repeat step 2.2-2.3.
Add 1 mL 1x trypsin to the flask and disperse it across the cell layer.
Incubate the flask at 37 °C in a conventional incubator until the cells are detached (5-8 min is sufficient for most cell lines).
View under the microscope if the cells are detached. Once the cells are detached, add 9 mL of the regular culture media with 10% FBS to inactivate the 1x trypsin.
Count the cell with either a hemocytometer, a coulter-counter or similar. Note: Trypan blue can be used to view dead cells. However, in 1x trypsin HaCaT cells are perfectly round when alive.
Transfer 7.5 x 104 cells/well in 250 µL 10% FBS media to a round-bottomed 48-well tissue culture plate (standard plate). Note: Assay can also be set up in 6-, 12-, 24-, or 96-well plates, which all can be assayed under the optical microscope.
Gently move the plate from side to side to ensure the cells are equally spread in the wells, or the cells will gather in the center of the wells. Inspect under a light microscope to make sure the cell is evenly spread in the media
Put the plate in a conventional CO2 incubator at 37 °C overnight or until the cells have adhered to the bottom of the wells.
3. Addition of Stimuli for Migration Effects
Check if the wells are 90-95% confluent under a light microscope.
Prepare 10 µg/mL of mitomycin C in an appropriate amount of the medium. Note: In this study, Mitomycin C is kept at 4 °C in a stock solution at 1 mg/mL. Mitomycin C in solution can be stored up to one week in the dark at 2-8 °C.
Remove the spent media with a Pasteur pipette from each well.
Add 250 µL of 5 µg/mL of mitomycin C to each well.
Incubate the plate for 2 h at 37 °C and 5% CO2.
Prepare stimuli of peptides by diluting them in an appropriate amount of the medium. Note: In this study, both the human and bovine Lactoferrin and Lactoferricin were tested at 25 µg/mL in a volume of 250 µL
Remove the medium with mitomycin C with a Pasteur pipette.
Wash the wells 1x with 250 µL of 1x PBS (room temperature) and remove the wash with a Pasteur pipette.
Add 250 µL of 1x PBS and manually scratch the wells vertically with a 200 µL pipette tip. Use a new pipette tip for each well.
Remove the PBS and add 250 µL peptide stimuli.
Mount the plate in optical camera system by adjusting the screws on both sides of the plate. Leave the lid on the microtiter plate. Note: The camera can operate with the lid open if necessary.
4. Set Up the Program for the Optical Camera on the Computer
Click Acquire in the taskbar on the left.
Choose the function Wound healing in the taskbar on the left.
Choose the plate type by clicking below the Plate illustration.
Unselect wells, not in use, by marking them and click Enable at the upper left side on the screen. Note: All wells are selected by default.
Adjust the focus of the lens on the wells to fit the position of the cells by right-clicking on one of the wells. Note: Autofocus can also be adjusted manually by moving the bar to the right of the plate illustration.
Set the time period for images under Picture Interval (e.g. (00:30:00)).
Set the number of repetition to fit the desired period (e.g. 97 repetitions).
Start the run by clicking Start button in the lower right corner.
Representative Results
HaCaT is a keratinocyte cell line, one of the most abundant cell types in the skin. When a wound occurs, the skin cells start to proliferate and migrate over to the wound bed to close the wound (Figure 3). Both processes are, therefore, of utmost importance for proper healing.
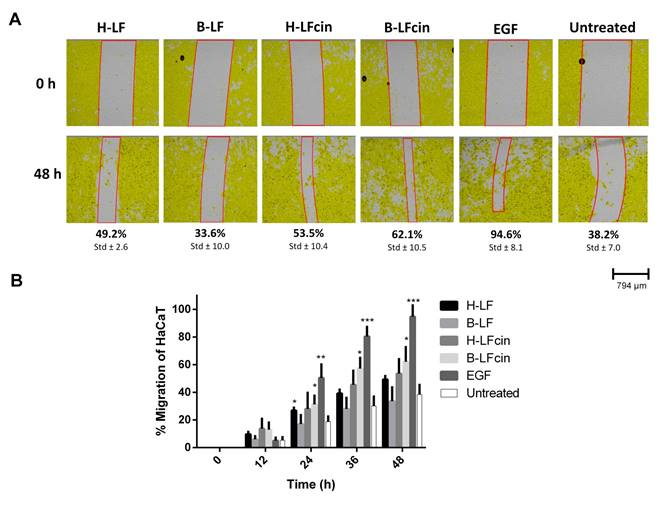
The optical camera can follow both processes in real-time. For migration, the system gives three data sets: cell covered area, gap width, and gap area. When monitoring scratches treated with Lactoferrin, Lactoferricin or EGF, the optical microscope allows us to monitor these three parameters over time. After 48 h incubation, we observe that EGF has resulted in 95% closure of the wound, compared to 38% closure of the untreated control (Figure 3A). The different versions of Lactoferrin and Lactoferricin are placed between 33% and 62% wound closure with the peptides being more potent than the parent proteins. The percentage of wound closure, determined from changes in gap width from the initial scratch, can also be plotted (Figure 3B).
HaCaT cells have a strong cell-cell interaction and when the cells migrate, series of steps are required24. Shortly, the leading cells disrupt their site of adhesion, protrude, and contract the cytoskeleton to move25. The protrusion of the leading cells can easily be viewed with the optical camera (Figure 4).
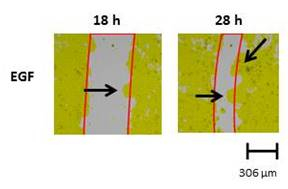
Figure 1: Schematic flowchart of the scratch assay to test migration on an optical camera. HaCaT cells are plated on a 48-well plate and allowed to adhere for 24 h. The cells are pre-treated with mitomycin C for 2 h to inhibit proliferation, and the scratch is performed and followed by the addition of peptide stimuli. The plate is inserted into the unit of the optical camera, and migration is followed for 48 h.
Figure 2: The scanning technology of the optical camera. The optical lens unit of the automated optical camera is angled at 6.25° to the horizontal plane to enable scanning through various solutions such as bacterial and cellular solutions. The acquired series of images contain image stacks of all focuses, both out-of-focus and in-focus, to ensure images with the cells in-focus. Modified from26.
Figure 3: Migration of pre-treated mitomycin C HaCaT cell over 48 hours. (A) Migration is detected on the automated optical camera every half hour for 48 h. The cells are pre-treated with mitomycin C to inhibit proliferation. The cells are treated with 25 µg/mL of either humane- or bovine-Lactoferrin (H-LF or B-LF) or -Lactoferricin (H-LFcin, B-LFcin). Epithelial growth factor (EGF) is used as a positive control at 500 ng/mL. Untreated HaCaT cells are used as a control. (B) Graphic illustration of migration of the treatments. The results are representatives of three independent runs, and results are given as a percentage closure of the initial scratch *p<0.05, **p<0.01, ***p<0.001.
Figure 4: Protruding lamellipodia of treated HaCaT cells. Lamellipodia of the HaCaT cells is marked by the black arrows. The strong cell-cell interaction of the HaCaT results in dynamic movement of the cells as they migrate and can easily be observed over time.
Discussion
The importance of migration in studies of development, wound healing, immunology, and cancer has led to several different methods to study migration. Migration can either be an expression of the expansion or the closure of the gap by the cells. Most often closure of the scratch is tested, as it is easier to grow cells in a monolayer and then make a scratch than growing the cells in a specific form8. Our study demonstrates an optimized method for manual scratching, as the technique is easy to master and low cost. We introduced the DNA synthesis inhibitor mitomycin C, an extra wash to optimize the scratch compared to published methods18, and the use of an optical camera that allows high-throughput studies.
The in vitro scratch assay requires an adherent cell line that binds properly to the bottom of the wells and to detect migration over a scratch, proliferation needs to be inhibited. These two features are essential for the assay. To detect true migration, several approaches have been described with lowering the serum concentration, starving the cell prior to scratching14, DMSO, or various inhibitors 13,27. In general, a serum-free media can be used, however, some cell lines, such as primary cell lines, need serum for the movement and the overall survival. Therefore, the choice should be made according to the cell line. Cell lines that require coating can be problematic in this assay, as manual scratches will disturb the coating as well.
Different cell types require the different number of cells to form a confluent monolayer depending on their size and growth rate. The HaCaT cells adhere after around 4-6 h but are left to settle overnight to make a stable monolayer. One of the major disadvantages of the scratch assay is irregular scratches8. In this study, we show that pre-washing with PBS and making the scratch in new PBS diminish accumulations of wounded and damaged cells along the edges of the scratch. This could plausibly be explained by the desmosomes junction connecting the cells in the skin. The junctions are Ca2+-dependent24 and by using PBS, the cations are removed, which could weaken the strong cell-cell adhesion. Thereby, the use of PBS could allow better removal of the cells individually, leading to less accumulation of the cells along the wound edge. Furthermore, the optical camera measures a wide part of the wells in all plate sizes and adapts to bents in the scratch via its three-step algorithm. Thereby, by pre-washing with PBS and by using the optical camera, the disadvantages of the manual scratches are eliminated. The z-stacking of images makes focusing on the cells more precise, but depending on how different cell types grow, the focus can be a problem. If the cells grow too much, the focus of the camera will shift from in-focus to out-of-focus. Thus, if mitomycin C is not used, it can be advantageous to set the focus a little higher if your experimental setup will run for more than 24 h. The optical camera has an easy set-up that is operated through a standard computer with an intuitive software that can measure plates from 6- to 96-well microtiter plates and therefore enables various test set-ups. When choosing the number of images to be taken, few limitations exist. If a 96-well plate is used, the number of pictures one can obtain is only limited to space on the computer, as data-sets can become large, and by the speed of the camera. If many pictures are required in a quick succession, the movement of the camera can be a limiting factor, as it needs time to scan each well.
The optical system enables simple but powerful monitoring. The pictures of the scratch are analyzed in regard to the process of the closure of the gap but can also be used for morphological characterization of the cells. The software marks the cells yellow and leaves the background color grey as a standard feature, to better visualize the wound edge, though raw images obviously are available should that be needed for e.g. publications. The HaCaT cells have strong cell-cell interactions which make the migration occur in a dynamic sheet28. This feature makes it easy to see lamellipodia of the migrating cells over time (Figure 4).
This study demonstrates the effect of the proteins Lactoferrin and their derived N-terminal peptides Lactoferricin, and EGF as a positive control, but could be modified to test different drugs, peptides/proteins, and combinations, as inhibitors or stimulators of wound healing. The scratch assay can be altered and optimized to fit various cell lines and compounds that can affect migration. Together, with its low cost and easy manageability, this assay is very accessible for most laboratories. The optical camera could be used to test different drugs, inhibitors or stimulators for migration, but also proliferation.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Danish Council for Independent Research, Technology and Production, grant #4005-00029
References
- Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Advances in wound care. 2015;4(9):560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva L, Carvalho E, Cruz MT. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert opinion on biological therapy. 2010;10(10):1427–1439. doi: 10.1517/14712598.2010.515207. [DOI] [PubMed] [Google Scholar]
- Vyas KS, Wong LK. Detection of Biofilm in Wounds as an Early Indicator for Risk for Tissue Infection and Wound Chronicity. Annals of Plastic Surgery. 2016;76(1):127–131. doi: 10.1097/SAP.0000000000000440. [DOI] [PubMed] [Google Scholar]
- Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nature Reviews Cancer. 2016;17(2):131–140. doi: 10.1038/nrc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annual review of medicine. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Monsuur HN, et al. Methods to study differences in cell mobility during skin wound healing in vitro. Journal of Biomechanics. 2016;49(8):1381–1387. doi: 10.1016/j.jbiomech.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Tang L, et al. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. The British journal of dermatology. 2010;163(1):38–47. doi: 10.1111/j.1365-2133.2010.09748.x. [DOI] [PubMed] [Google Scholar]
- Ashby WJ, Zijlstra A. Established and novel methods of interrogating two-dimensional cell migration. Integrative Biology. 2012;4(11):1338–1350. doi: 10.1039/c2ib20154b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klettner A, Tahmaz N, Dithmer M, Richert E, Roider J. Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. British Journal of Ophthalmology. 2014;98(10):1448–1452. doi: 10.1136/bjophthalmol-2014-305105. [DOI] [PubMed] [Google Scholar]
- Doyle W, Shide E, Thapa S, Chandrasekaran V. The effects of energy beverages on cultured cells. Food and Chemical Toxicology. 2012;50(10):3759–3768. doi: 10.1016/j.fct.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Zordan MD, Mill CP, Riese DJ, Leary JF. A high throughput, interactive imaging, bright-field wound healing assay. Cytometry Part A. 2011;79(3):227–232. doi: 10.1002/cyto.a.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proceedings of the National Academy of Sciences. 2004;101(6):1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Räsänen K, Vaheri A. TGF-beta1 causes epithelial-mesenchymal transition in HaCaT derivatives, but induces expression of COX-2 and migration only in benign, not in malignant keratinocytes. Journal of Dermatological Science. 2010;58(2):97–104. doi: 10.1016/j.jdermsci.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Tochio T, Tanaka H, Nakata S, Hosoya H. Fructose-1,6-bisphosphate aldolase A is involved in HaCaT cell migration by inducing lamellipodia formation. Journal of Dermatological Science. 2010;58(2):123–129. doi: 10.1016/j.jdermsci.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chemistry and Biology. 1995;2(9):575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Stamm A, Reimers K, Strauß S, Vogt P, Scheper T, Pepelanova I. In vitro wound healing assays - state of the art. BioNanoMaterials. 2016;17(1-2):79–87. [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro Cell Migration and Invasion Assays. Journal of Visualized Experiments. 2014. pp. 1–8. [DOI] [PMC free article] [PubMed]
- Jonkman JEN, et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adhesion & Migration. 2016;8(5):440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Kandasamy K, Marimuthu M, Choi CS, Kim S. Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. The Analyst. 2011;136(2):237–245. doi: 10.1039/c0an00560f. [DOI] [PubMed] [Google Scholar]
- Fredborg M, et al. Real-time optical antimicrobial susceptibility testing. Journal of Clinical Microbiology. 2013;51(7):2047–2053. doi: 10.1128/JCM.00440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H. Anti herpes simplex virus activity of lactoferrin/lactoferricin - An example of antiviral activity of antimicrobial protein/peptide. Cellular and Molecular Life Sciences. 2005;62(24):3002–3013. doi: 10.1007/s00018-005-5228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H, Hancock REW. Antimicrobial properties of lactoferrin. Biochimie. 2009;91(1):19–29. doi: 10.1016/j.biochi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harbor perspectives in biology. 2009;1(2):1–17. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A. Two Distinct Actin Networks Drive the Protrusion of Migrating Cells. Science. 2004;305(5691):1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Canali C, Spillum E, Valvik M, Agersnap N, Olesen T. Whitepaper a Digital Time-Lapse Bright Field Technology To Drive Faster, Higher Throughput Bioanalysis. 2016.
- Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine. 2013;62(1):1–21. doi: 10.1016/j.cyto.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. Journal of Cell Science. 2009;122(18):3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]


