Abstract
Mesenchymal stem cells (MSCs) are the main cell source for cell-based therapy. MSCs from articular cavity synovial fluid could potentially be used for cartilage tissue engineering. MSCs from synovial fluid (SF-MSCs) have been considered promising candidates for articular regeneration, and their potential therapeutic benefit has made them an important research topic of late. SF-MSCs from the knee cavity of the New Zealand white rabbit can be employed as an optimized translational model to assess human regenerative medicine. By means of CD90-based magnetic activated cell sorting (MACS) technologies, this protocol successfully obtains rabbit SF-MSCs (rbSF-MSCs) from this rabbit model and further fully demonstrates the MSC phenotype of these cells by inducing them to differentiate to osteoblasts, adipocytes, and chondrocytes. Therefore, this approach can be applied in cell biology research and tissue engineering using simple equipment and procedures.
Keywords: Biology, Issue 138, Rabbit synovial fluid, mesenchymal stem cells, cell isolation, in vitro culture, identification, purification, magnetic activated cell sorting, CD90
Introduction
MSCs have been suggested as a valuable source for regenerative medicine, especially for cartilage lesions. MSCs, including chondrocytes, osteoblasts, adipocytes, skeletal myocytes, and visceral stromal cells, broadly expand the areas for stem cell transplantation due to their high expansion rate and multi-lineage differentiation potential1. MSCs can be isolated from the skeletal muscle, synovium, bone marrow, and adipose tissue2,3,4. Findings have also confirmed the presence of MSCs in synovial fluid, and previous research has identified synovial fluid-derived MSCs (SF-MSCs) as promising candidates for articular regeneration5,6.
However, research and preclinical experimentation on human samples are subject to many ethical issues. Instead, rabbits have been and continue to be the most commonly used animal species to demonstrate that transplantation of MSCs can repair cartilage damage. In recent years, an increasing number of researchers have studied rabbit mesenchymal stem cells (rbMSCs) both in vitro and in vivo, as these cells are similar to human MSCs in their cellular biology and tissue physiology. Similarly, the rbMSCs are capable of adhering to plastic surfaces, displaying spindle-fibroblast morphology as in human MSCs. Furthermore, rabbit mesenchymal samples are simple and easy to obtain7. Additionally, the most crucial points are that rbMSCs express surface markers, such as CD44, CD90, and CD105, and that the multi-lineage differentiation potential is preserved, which is in agreement with the criteria for identification of MSC populations as defined by the International Society for Cellular Therapy8,9. In particular, synovial fluid chondroprogenitors are capable of non-hypertrophic chondrogenesis when induced by TGF-β1, thus making them suitable cell sources for phenotypically articular cartilage regeneration10,11,12.
However, the isolation of SF-MSCs is greatly different from other tissues, including the umbilical cord, adipose tissue, peripheral blood, and bone marrow. Currently, the most common approaches for the purification and sorting of SF-MSCs are flow cytometry and immunomagnetic bead-based sorting, although the flow cytometry method requires a specific environment and highly expensive instruments13.
This article presents a procedure for the simple and minimally invasive collection of samples of synovial fluid from New Zealand white rabbits. During the procedure, the rbSF-MSCs are stably expanded in vitro and then isolated with CD90 positive magnetic bead-based procedures. Finally, the protocol shows how to obtain MSCs with a high purity and viability from the harvested cell sources.
In this protocol, the isolated rbSF-MSCs are characterized based on their morphology, expression of specific markers, and pluripotency for stem cells. Flow cytometry-based immunophenotyping reveals a significant positive expression of CD44 and CD105, whereas the expression of CD45 and CD34 is negative. Finally, an in vitro assay for rbSF-MSCs demonstrates the osteogenic, adipogenic, and chondrogenic differentiation of these cells.
Protocol
All animal experiments were conducted in accordance with the regional Ethics Committee guidelines, and all animal procedures were approved by the Institutional Animal Care and Use Committee of Shenzhen Second People's Hospital, Shenzhen University.
1. Isolate and Culture the rbSF-MSCs
- Preparations for the animal procedure
- Prepare skeletally-mature female New Zealand white rabbits for the collection of rbSF-MSCs. Perform a clinical examination of the rabbits one day prior to the anesthesia and arthrocentesis procedure. NOTE: Physical examinations should include weight (2.0 - 2.5 kg), gender (female), and body temperature, respiratory rate, and heart rate. Parameter intervals for clinically healthy rabbits are 30 - 50 min for respiration rate, 220 - 280 min for heart rate, and 38 - 39 °C for body temperature.
- Fast the animals for 6 h before anesthesia.
- Preparations and procedure for the animal anesthesia
- Restrain the rabbit with a cage (see Table of Materials), and then inject 3% pentobarbital sodium into the marginal ear vein at a dose of 1 mL/kg for general anesthesia.
- Place the rabbit in a comfortable dorsal-recumbent position.
- Monitor the respiratory rate, heart rate, and body temperature of the rabbit during anesthesia.
- Use ophthalmologic ointment on its eyes to prevent dryness during anesthesia.
- Assess the depth of anesthesia following Guedel's classification14.
- Preparation for MSCs isolation and cultivation
- To cultivate rbSF-MSCs, prepare 500 mL of commercial culture medium (see Table of Materials) supplemented with 10% commercial supplement (see Table of Materials), 10% fetal bovine serum (FBS), and 1% Penicillin-Streptomycin.
- Incubate the culture medium at 37 °C in a water bath.
- Prepare 500 mL of phosphate buffer saline (PBS) for cell isolation and cell washing.
- Prepare 100 mL of isotonic saline solution for the knee cavity arthrocentesis procedure.
- Collection of articular synovial fl uid from the knee of the rabbit
- Select an area about 5 x 5 cm in size around the knee and shave the rabbit hair from this area using a safety electric shaver.
- Alternately, disinfect the procedure site 3x with povidone iodine solution and 75% ethanol. Then, apply sterile drapes after the area has thoroughly dried.
- Inject 1 - 2 mL of isotonic saline solution, using a sterile hypodermic syringe (2 mL), into the knee joint cavity from the lateral articular space, move the knee 3 - 4x, and then suck out all the synovial fluid at room temperature.
- Filter the synovial fluid through a 40 µm nylon cell strainer to remove any debris within 4 h.
- Culture of the rbSF-MSCs
- Collect the filtered fluid in 50 mL centrifuge tubes and centrifuge at 1,500 rpm for 10 min at room temperature.
- Discard the supernatant after the centrifugation, wash the pellet with PBS, resuspend the pellet with the complete culture medium, and then plate the medium in 100 mm dishes.
- Incubate the dishes at 37 °C in a humidified atmosphere containing 5% CO2.
- After 48 h, replenish the dishes with fresh medium to remove any non-adherent cells. Replace the medium twice a week for 2 weeks as passage 0 (P0). NOTE: There should be about 1 x 104 adherent cells. Viable cells/ml in SF are about 2 x 102/mL.
- After 14 days following the initial plating, many colonies should have formed in the culture dishes. Select the colonies larger than 2 mm in diameter. Mark where the selected colonies are located by tracing their circumference on the dish bottom.
- Discard the colonies < 2 mm wide, using cell scrapers. Digest the selected colonies with about 5 µL of 0.25% trypsin, using a cloning cylinder, and transfer the colonies to a new dish as passage 1 (P1).
- Post-operative animal care
- Follow standard institutional operating procedures for post-operative monitoring and recovery from anesthesia.
- Monitor the vital signs of the rabbit every 10 min until it has regained consciousness. Finally, transfer the conscious animal to the cage. Do not return a rabbit that has undergone surgery to the company of other animals until it has fully recovered.
- Post-operation, disinfect the site with 0.1% povidone iodine, 2x a day for 3 days.
2. CD90-positive Magnetic Activated Cell Sorting (MACS) of the rbSF-MSCs and Primary Culture
- Sample preparation
- When the cells reach around 80% confluency, aspirate the medium, and then add 1 - 2 mL of 0.25% Trypsin-EDTA to each dish.
- Incubate the dishes for 2 - 3 min to allow cell detachment.
- Once the cells are detached, add an equal amount of the culture medium to inactivate the trypsin.
- Pass the cell suspension through a 40 µm cell strainer, collect the filtrate in a 15 mL tube, and then spin down the cells at 600 × g for 10 min at room temperature.
- Resuspend the cell pellet in MACS running buffer (PBS, pH 7.2, 0.5% bovine serum albumin, and 2 mM EDTA) and then count the cell number.
- Magnetic labeling NOTE: Sort the rbSF-MSCs with a magnetic-activated cell sorting kit containing columns, a stand, and separators (see Table of Materials).
- Determine the cell number using a hemocytometer.
- Centrifuge the cell suspension at 300 × g for 10 min at 4 °C. Completely aspirate the supernatant.
- Add 80 µL of resuspension buffer per 107 total cells.
- For 107 total cells, add 20 µL of microbeads conjugated with a monoclonal anti-rabbit CD90 antibody.
- Mix the magnetic beads and cells evenly in the tubes, then incubate them at 4 °C for 15 min in the dark.
- Add 1 mL of buffer per 107 cells to the tube, and then centrifuge it at 300 × g for 10 min at 4 °C to wash the cells. Discard the supernatant after the centrifugation.
- Resuspend the pellet in 500 µL of buffer per 107 cells.
- Magnetic separation
- Place the column with the column wings to the front into the magnetic field of the magnetic separator.
- Rinse the magnetic separator (MS) column with 500 µL of the buffer per 107 cells.
- Transfer the single cell suspension into the column. Let the negative cells pass through the magnetic field to discard these unlabeled cells.
- Wash the column 1 - 2x with 500 µL of the buffer per 107 cells and discard the flow-through.
- Transfer the column into a centrifuge tube (15 mL).
- Add 1 mL of the buffer per 107 cells to the column, and then immediately push the plunger into the column to flush out the magnetically labeled cells.
- Repeat the aforementioned procedure with a second MS Column to increase the purity of CD90+ cells, which can enrich the eluted fraction.
- Centrifuge the cell suspension at 300 × g for 10 min, aspirate the supernatant, and resuspend it with the culture medium.
- Culture of the CD90+ rbSF-MSCs
- Inoculate the cells in 100 mm dishes after the magnetic activated cell sorting.
- Incubate the dishes at 37 °C in a humidified cell incubator with 5% CO2.
- When 80 - 90% confluence of the primary culture is achieved, at about 7 - 10 days, digest the adherent cells with 0.25% Trypsin-EDTA and passage them at a 1:2 dilution to make passage 2 (P2).
- Use the same method to pass the cells to passage 3 (P3), which can be used for the in vitro assays. NOTE: After the sub-culture and purification, about 1 x 107 rbSF-MSCs are obtained.
3. Identification of rbSF-MSCs
- Surface marker confirmation of the rbSF-MSCs by flow cytometry
- When the MSCs are 80 - 90% confluent at P3, wash the cells with PBS and treat them with 1 mL of 0.25% Trypsin-EDTA. Then, incubate the MSCs at 37 °C for 2 - 3 min until the cells are detached.
- Harvest the cells using 10 mL of PBS, transfer them into a conical tube (15 mL), and centrifuge them at 300 × g for 5 min at room temperature.
- Discard the supernatants. Resuspend the cell pellet in 500 µL of PBS and transfer it into a 1.5 mL tube.
- Incubate a 1:100 dilution of FITC-conjugated and PE-conjugated antibodies (isotype, FITC-CD34/PE-CD45, FITC-CD105/PE-CD44) for 1 h in the dark at 4 °C.
- Centrifuge this mixture at 300 × g for 5 min at room temperature and wash the cells twice with PBS by centrifugation at 300 × g for 5 min each time. Discard the supernatants.
- Resuspend the cells in 500 µL of PBS, transfer the cell suspension into a round-bottom tube (5 mL) and analyze it using a flow cytometer. NOTE: Acquire data on a cytometer equipped with two fluorescence channels: 533/30 and 585/40. For each isotype fluorescence, use the isotype control to adjust the appropriate laser voltage, and set the minimum intensity required to obtain a fluorescence histogram that displays both left and right edges of the peak. Collect a minimum of 10,000 events for the statistical analyses.
- Multidifferentiation of the rbSF-MSCs NOTE: Use P3 rbSF-MSCs for the in vitro multidifferentiation assays.
- Osteogenic differentiation
- Prepare the osteogenic induction medium: DMEM basic (1x) containing 50 mM of L-ascorbic acid-2-phosphate,10 mM of α-glycerophosphate, and 100 nM of dexamethasone.
- Seed the cells at 103 cells/cm2 in a 6-well tissue culture plate and culture them in the osteogenic induction medium.
- Change the induction medium every 3 days for 3 weeks.
- Fix the cells with 4% formaldehyde for 30 min at room temperature after the differentiation is completed, stain the cells with 1% Alizarin red for 5 min, and then wash them 3x with PBS15.
- Adipogenic differentiation
- Prepare the adipogenic induction medium: DMEM basic (1x) consisting of 100 mM of indomethacin, 10 mg/mL of recombinant human insulin, 1 mM of dexamethasone, and 0.5 mM of 3-isobutyl-1-methylxanthine.
- Treat the P3 rbSF-MSCs for 3 weeks in the adipogenic induction medium.
- After 3 weeks, stain the neutral lipid vacuoles using Oil Red O to confirm the adipogenic differentiation. Fix the cells in a 4% formaldehyde solution for 30 min at room temperature, and then wash them 3x with PBS16.
- Chondrogenic differentiation
- Prepare the chondrogenic induction medium: DMEM basic (1x) consisting of 10 ng/mL of TGFβ1, 1% ITS supplement, 0.35 mM of L-proline, 100 nM of dexamethasone, 50 mM of L-ascorbic acid-2-phosphate, and 1 mM of sodium pyruvate.
- Use pellet culturing for the chondrogenic induction. Centrifuge 5×105 P3 rbSF-MSCs at 1500 rpm for 10 min in a polypropylene tube (15 mL) to form a pellet.
- Culture the pellets for 3 weeks with the chondrogenic induction medium.
- Change the medium every 3 days for 3 weeks.
- After 3 weeks induction, embed the cell pellet with paraffin, section it into 4 mm slices, and stain them using 0.1% Toluidine blue for 30 min at room temperature17.
- Total RNA extraction and analysis by quantitative real-time polymerase chain reaction (qRT-PCR)
- Isolate the total RNA of the cells after 3 weeks' differentiation induction using the commercial RNA extraction kit (see Table of Materials).
- Remove the culture medium in the culture dish and then add 1 mL of isolation reagent.
- Lyse the cells by repeated pipetting until the solution is homogeneous. Incubate it at room temperature for 3 min.
- Transfer the cell lysate into a 1.5 mL microcentrifuge tube.
- Add 100 µL of chloroform, shake it by hand 15x, and incubate the mixture at room temperature for 3 min.
- Centrifuge the tube at 12,000 × g for 15 min at 4 °C. Transfer the supernatants into a new 1.5 mL microcentrifuge tube.
- Add 500 µL of isopropanol and precipitate the RNA for 10 min at room temperature.
- Centrifuge at 12,000 × g for 10 minat 4 °C. The RNA pellet should be visible at the bottom of the tube.
- Remove the supernatant and then add 500 µL of 75% ethanol. Briefly spin the tube for several seconds to wash the RNA pellet. Centrifuge it at 7,500 × g for 5 min at 4 °C.
- Remove the residual ethanol.
- Dissolve the RNA pellet in 10 µL of DEPC water and place the tubes on ice.
- Reverse transcribe total RNA into cDNA with a DNA synthesis kit (see Table of Materials). NOTE: Perform all the following procedures on ice.
- Prepare the reverse transcription master mix.
- Add 25 µL of DNase-treated RNA to each tube with 1 µg of RNA.
- Incubate the mixture at the following temperatures using a PCR thermal cycler: 26 °C for 10 min (to allow the random hexamers to anneal), 42 °C for 45 min (reverse transcription) and 75 °C for 10 min (to inactivate the reverse transcriptase).
- Immediately analyze the resulting cDNA by quantitative real-time PCR, or store it at -20 °C.
- Perform the gene expression analysis with a quantitative real-time PCR system.
- Use qRT-PCR Master Mix (see Table of Materials) to perform PCR. The PCR primers for GAPDH, Runx2, Agg, and PPARγ are listed in Table 1.
- Calculate gene expression using the 2−ΔΔCT method.
- Conduct a statistical analysis using standard statistical software. Use an independent sample t-test to compare the group means.
Representative Results
Isolation, Purification, and Culture of the rbSF-MSCs: This protocol uses MACS to isolate rbSF-MSCs, based on the expression of the MSC surface marker CD90. A process flow diagram of rbSF-MSCs' isolation, purification, and characterization and the in vitro culture protocol is shown in Figure 1.

Cell Morphology after Magnetic Activated Cell Sorting (MACS) with CD90: Firstly, for MACS, immunolabel MSCs with CD90 magnetic beads. After centrifugation, resuspend a maximum of 107 cells in 80 µL of a precooled sorting buffer, and then add 20 µL of CD90 magnetic beads, followed by vortexing and incubation at 4 °C for 15 min. After that, wash the cells with 1 mL of the sorting buffer and resuspend them in 500 µL of the sorting buffer. Before proceeding with the magnetic sorting, repeat the washing step. This critical procedure is shown in Figure 2.

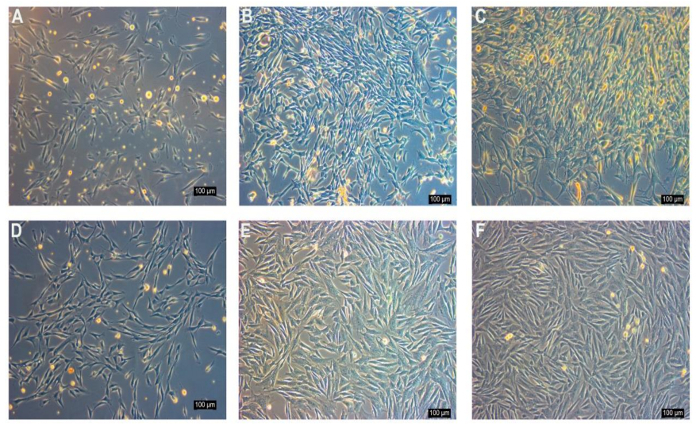
Before sorting, the adherent rabbit synovial fluid cell populations displayed heterogeneous morphology, containing diverse cell types and sizes (Figure 3A-C). Following MACS, the cell populations exhibited homogenous morphology. The cell number was increased with sub-culture (Figure 3D-F).
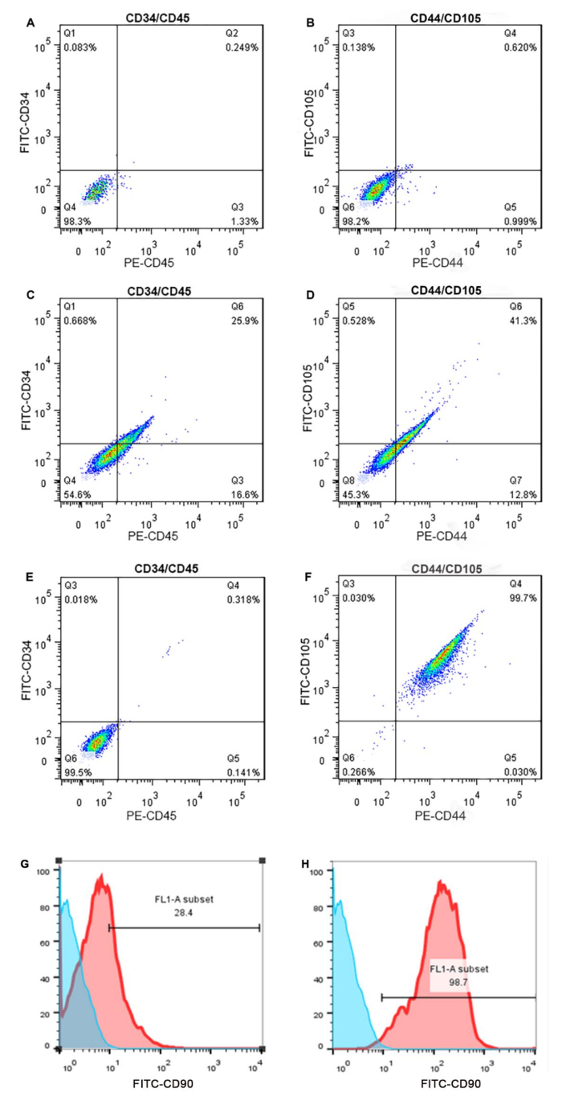
Surface Characteristics of MACS-enriched rbSF-MSCs: Fluorescence-Activated Cell Sorting (FACS) was performed to analyze the enrichment of rbSF-MSCs by MACS with CD90. Prior to MACS, the cell population consisted of approximately 40% MSCs (Figure 4C,D; isotype controls in Figure 4A,B). Following MACS, the enriched population contained approximately > 99% MSCs (Figure 4E,F). The hematopoietic lineage cell markers CD34 and CD45 were, at 0.318%, both rarely expressed after MACS, which is a decline from their initial expression at 25.9%.Before selection, there were about 28% CD90+ cells (Figure 4G); after purification, about 98% CD90+ cells were obtained (Figure 4H).
Multilineage Differentiation Potential of rbSF-MSCs: To characterize the capacity of rbSF-MSCs to differentiate into the various lineages such as osteogenic, adipogenic, and chondrogenic cells, the rbSF-MSCs enriched by MACS were simultaneously cultured in a specific differentiation medium and in a differentiation medium without cytokine to serve as controls. When the induction was completed, lineage-specific markers were analyzed by staining and RT-PCR. Alizarin red staining of calcium compounds demonstrated that mineralized nodules had formed in the rbSF-MSCs after 3 weeks under the osteogenic induction conditions (Figure 5A). After 3 weeks of adipogenic induction, an accumulation of lipid-rich vacuoles could be detected by intracellular Oil Red O staining (Figure 5B). For 21 days of chondrogenic induction, the cell pellet was histologically assayed with toluidine blue staining. Cells positive for staining (to proteoglycan) were regarded as chondrocyte-like cells (Figure 5C). Similar to this result, a quantitative analysis of the gene expression of differentiation potential also proved the differentiation capability of these cells. The expression levels of Agg (a chondrocyte marker), PPARγ (an adipogenic marker), and Runx2 (an osteoblast marker) were upregulated under induced conditions. These data proved the multipotent differentiation capability of rbSF-MSCs into trilineages (Figure 5D).
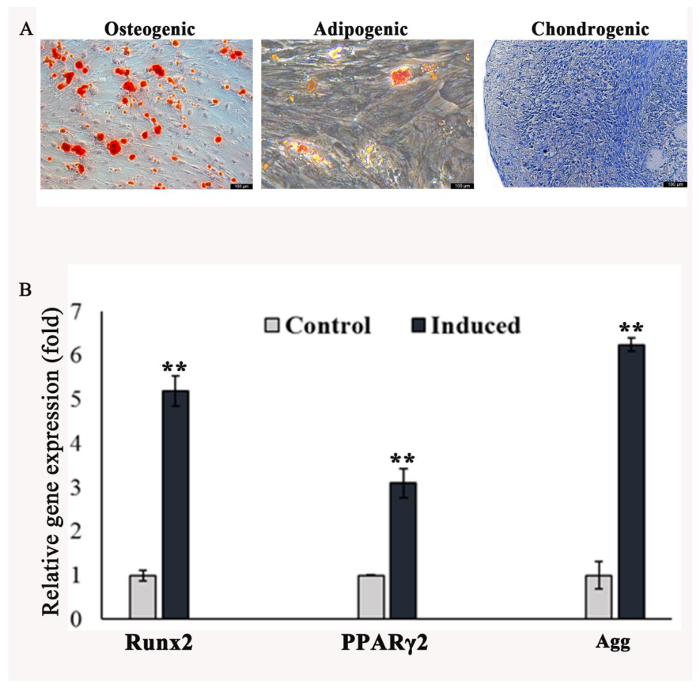
Figure 1: Full schematic of isolation, purification, and characterization of the SF-MSCs of New Zealand white rabbits. Please click here to view a larger version of this figure.
Figure 2: Protocol for magnetic activated cell sorting (MACS) with CD90. Please click here to view a larger version of this figure.
Figure 3: Morphology of the monolayer cultured rbSF-MSCs before and after MACS as examined under ordinary inverted microscopy. A-C) Before sorting, the adherent rabbit synovial fluid cell populations display heterogeneity. They contain diverse cell types and sizes, such as oval, long-spindle, short-spindle, and stellate morphology. The main morphology of P2 and P6 is a spindle morphology (A: P2, B: P3, C: P6). D-F) Following MACS with CD90, the cell populations exhibit a homogenous morphology. With a sub-culture, the cell number increases (D: P2, E: P3, F: P6). Scale bar = 100 µm. Please click here to view a larger version of this figure.
Figure 4: Identification of rbSF-MSC surface markers. Using flow cytometry analysis, the rbSF-MSCs are positive for the MSC markers CD44 and CD105, while negative for the endothelial cell marker CD34 and the hematopoietic cell marker CD45. Prior to MACS, these cells have a low rate of positivity for CD44 and CD105. However, after MACS, the rate of positivity was high. A,B) These top two images show the isotype control data. C,D) These results shows that before MACS the rbSF-MSC population is composed of approximately 40% MSC cells. E,F) After MACS, the enriched population contains > 99% MSC cells. G,H) These images show the flow cytometry analysis of the CD90+ marker, (G) before and (H) after MACS sorting. Please click here to view a larger version of this figure.
Figure 5: Analysis of lineage-specific markers by staining and RT-PCR. A) Alizarin red staining demonstrates that mineralized nodules form under the osteogenic induction for 3 weeks. B) After 3 weeks of adipogenic induction, the accumulation of lipid-rich vacuoles is detected by intracellular Oil Red O staining. C) For 3 weeks of chondrogenic induction, the cell pellet was histologically assayed with toluidine blue staining. Cells positive for staining (to proteoglycan) were regarded as chondrocyte-like cells. Scale bar = 100 µm. D) After culturing for 3 weeks, the relative mRNA expression of osteoblast markers (Runx2), adipogenic markers (PPARγ), and chondrogenic markers (Agg) is detected. For all analyses, p values < 0.01 were considered as statistically significant differences using the Kruskal-Wallis test (shown as **). Please click here to view a larger version of this figure.
| Genes | Forward primer (5’–3’) | Reverse primer (5’–3’) |
| Runx2 | TATGAAAAACCAAGTAGCAAGGTTC | GTAATCTGACTCTGTCCTTGTGGAT |
| AGG | GCTACACCCTAAAGCCACTGCT | CGTAGTGCTCCTCATGGTCATC |
| PPARγ2 | GCAAACCCCTATTCCATGCTG | CACGGAGCTGATCCCAAAGT |
| GAPDH | GGAGAAAGCTGCTAA | ACGACCTGGTCCTCGGTGTA |
Table 1: List of genes and primers used in this study for quantitative real-time PCR.
Discussion
The existence of MSCs in synovial fluid provides an alternative for cell-based therapy. Previous studies have shown that injury sites contain higher amounts of mesenchymal stem cells in their synovial fluid, which may be positively correlated with the post-injury period5. The MSCs in synovial fluid may be beneficial to tissue for enhancing the spontaneous healing after an injury18,19. The clinical application of SF-MSCs has rarely been covered in the literature, mainly because the mechanisms of the SF-MSCs in joints remain undefined20. Jones et al.21 reported that hSF-MSC numbers in knee joints significantly increase 7-fold during the early stages of osteoarthritis (OA). They thought that the increased SF-MSCs could contribute to maintaining the physiological homeostasis of joints.
An ideal animal model is an indispensable tool in the development of therapeutics utilizing regenerative and translational medicine. Although obvious differences exist between humans and rabbits, the rabbit is extensively used as an animal model for the study of tissue regeneration7, thus prompting us to choose it as the model for our study of SF-MSCs. One of the more daunting obstacles in this endeavor is that the isolation of SF-MSCs often results in varied success rates and low colony frequencies, as reported in previous research5. Therefore, numerous researchers have focused on the success rates and optimization of the isolation of MSCs from SF.
This studyeffectively used a MACS procedure for high sorting purity and viability of CD90+ MACSs from rabbit synovial fluid22. This MACS system utilizes magnetic microbeads conjugated to highly specific antibodies coupled to a particular cell surface antigen, CD90 in this case, and allows for the selection of the particular target cell type, namely CD90 expressing cells. Before the purification with CD90 microbeads, we usually culture the primary rbSF-MSCs for 14 days. During this period, many colonies are formed in the culture dishes. This protocol suggests selecting the colonies larger than 2 mm in diameter. When using the cloning cylinder for the colony selection, we carefully observe it under the microscope. The single colony is further propagated for purification.
During the process of separation and purification, the CD90 expressing cells that were magnetically labeled are attracted by the magnetic field of the separator in the column, whereas the unlabeled cells flow through. After the washing process, the column is removed from the magnetic field of the separator, and the target cells are eluted from the column. This specific MACS microbead approach allows the isolation of rbSF-MSCs that specifically expressed the stem cell surface markers CD44 and CD105, demonstrating that these isolated cells are mesenchymal stem cells rather than hematopoietic cells23.These purified rbSF-MSCs can be cultured in vitro over a long time without any significant change of morphological features and marker expression. Moreover, the rbSF-MSCs enriched by MACS exhibit a multipotent differentiation ability into multi-lineage cells, including into chondrogenic, adipogenic, and osteogenic cells.
The operation of MACS is an easy-to-perform protocol and can be done on the bench23. Additionally, the US Food and Drug Administration (FDA) has approved the MACS microbead-based technology, and thus it is easy to perform for clinical applications24. CD90 is a surface marker of mesenchymal stem cells, and researchers have shown that CD90 positive MSCs have a better chondrogenic differentiation ability25,26. The pluripotency of MSCs has been characterized based on morphology and the expression of specific markers for stem cells27.
This study does have several limitations. The first shortcoming of this protocol is related to the surface markers we examined. Based on a statement by the International Society for Cellular Therapy (ISCT), the minimal criteria for defining multipotent MSCs is positive for CD90, CD105, CD44, and CD73, and negative for CD11b, CD14, CD34 or CD45, and CD79a or HLA-DR28. With the goal of mitigating the effort and expense required to test the entire panel, researchers in this study carefully selected two of the negative markers and two of the positive markers recommended. Secondly, it takes a long time to form the colonies that will be selected for further study. In addition, hypertrophy is a major problem during chondrogenic induction of SF-MSCs in vitro. At the later stage, the hypertrophic chondrocytes always express type X collagen (COLX), matrix metalloproteinase 13 (MMP13), alkaline phosphatase (ALP), and runt-related transcription factor 2 (Runx2)29.Research suggests that the three-dimensional (3D) pellet culture, dynamic culture systems, and the coculture with chondrocytes are benefit for MSC stably chondrogenic differentiation30,31. In this study, a pellet culture system was used for chondrogenic induction of MSC to avoid hypertrophy.
In conclusion, we have established a simple method for the isolation and purification of MSCs from the articular cavity flushing fluid and characterized the MSCs obtained therein. This protocol has provided a platform for the exploration and investigation of articular synovial fluid-derived MSCs' potential utility in novel joint regeneration strategies.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This study was financially supported by the following grants: the Natural Science Foundation of China (No. 81572198; No. 81772394); the Fund for High Level Medical Discipline Construction of Shenzhen University (No. 2016031638); the Medical Research Foundation of Guangdong Province, China (No. A2016314); and Shenzhen Science and Technology Projects (No. JCYJ20170306092215436; No. JCYJ20170412150609690; No. JCYJ20170413161800287; No. SGLH20161209105517753; No. JCYJ20160301111338144).
References
- Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Reviews. 2005;1(2):169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- Asakura A, Rudnicki MA, Komaki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68(4-5):245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- De BC, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheumatology. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D, Jones E, English A, Emery P. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis & Rheumatism. 2004;50(3):817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- Jia Z, et al. Isolation and characterisation of human mesenchymal stem cells derived from synovial fluid by magnetic activated cell sorting (MACS) Cell Biology International. 2017. [DOI] [PubMed]
- Bashir M, et al. Isolation, culture and characterization of New Zealand white rabbit mesenchymal stem cells derived from bone marrow. Asian Journal of Animal & Veterinary Advances. 2015;10(8):13–30. [Google Scholar]
- Song X, et al. Differentiation potential of rabbit CD90-positive cells sorted from adipose-derived stem cells in vitro. In Vitro Cellular & Developmental Biology - Animal. 2016;53(1):1–6. doi: 10.1007/s11626-016-0081-6. [DOI] [PubMed] [Google Scholar]
- Lee TC, et al. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLOS One. 2014;9(11):e111390. doi: 10.1371/journal.pone.0111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MC, Chen Y, Bianchessi M, Pondenis H. Phenotypic characterization of equine synovial fluid-derived chondroprogenitor cells. Stem Cell Biology and Research. 2016;3(1) doi: 10.1155/2016/9364974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado AAF, et al. Characterization of mesenchymal stem cells derived from the equine synovial fluid and membrane. BioMed Central Veterinary Research. 2015;11(1):281. doi: 10.1186/s12917-015-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- Wu CC, et al. Intra-articular injection of platelet-rich fibrin releasates in combination with bone marrow-derived mesenchymal stem cells in the treatment of articular cartilage defects: an in vivo study in rabbits. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2017;105(6):1536–1543. doi: 10.1002/jbm.b.33688. [DOI] [PubMed] [Google Scholar]
- John T, Lerche P. Anesthesia and Analgesia for Veterinary Technicians. St. Louis, Missouri: Mosby Elsevier; 2011. [Google Scholar]
- Koyama N, et al. Pluripotency of mesenchymal cells derived from synovial fluid in patients with temporomandibular joint disorder. Life Sciences. 2011;89(19-20):741–747. doi: 10.1016/j.lfs.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kim YS, et al. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. American Journal of Sports Medicine. 2015;43(2):399–406. doi: 10.1177/0363546514559822. [DOI] [PubMed] [Google Scholar]
- Vereb Z, et al. Immunological properties of synovial fluid-derived mesenchymal stem cell-like cells in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2015;74(Suppl 1):A64–A65. [Google Scholar]
- Matsukura Y, Muneta T, Tsuji K, Koga H, Sekija I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clinical Orthopaedics & Related Research. 2014;472(5):1357–1364. doi: 10.1007/s11999-013-3418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T, et al. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology. 2008;47(8):1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- Hegewald AA, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue & Cell. 2004;36(6):431–438. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Jones EA, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single cell level. Arthritis & Rheumatism. 2008;58(6):1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- Makker K, Agarwal A, Sharma RK. Magnetic activated cell sorting (MACS): utility in assisted reproduction. Indian Journal of Experimental Biology. 2008;46(7):491–497. [PubMed] [Google Scholar]
- Schmitz B, et al. Magnetic activated cell sorting (MACS) — a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. European Journal of Haematology. 1994;52(5):267–275. doi: 10.1111/j.1600-0609.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt M, Bader A, Giri S. Devices for stem cell isolation and delivery: current need for drug discovery and cell therapy. Expert Review of Medical Devices. 2015;12(3):353–364. doi: 10.1586/17434440.2015.995094. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino ACW. Prockop DJ, Bunnell BA, Phinney DG, editors. A method to isolate and purify human bone marrow stromal stem cells. Mesenchymal Stem Cells. 2008. pp. 45–57. Vol. 449 of Methods in Molecular Biology. [DOI] [PubMed]
- Krawetz RJ, et al. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLOS One. 2012;7(8):e43616. doi: 10.1371/journal.pone.0043616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y, et al. Purified human synovium mesenchymal stem cells as a good resource for cartilage regeneration. PLOS One. 2015;10(6):e0129096. doi: 10.1371/journal.pone.0129096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Gauci SJ, et al. Modulating chondrocyte hypertrophy in growth plate and osteoarthritic cartilage. Journal of Musculoskeletal & Neuronal Interact. 2008;8(4):308. [PubMed] [Google Scholar]
- Cooke ME, et al. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis and Cartilage. 2011;19(10):1210–1218. doi: 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis and Rheumatism. 2010;62(9):2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]


