Significance
Environmental temperature acclimation is essential to animal survival, yet thermoregulation mechanisms remain poorly understood. In this study, we describe Ca2+-dependent endoribonuclease (EndoU) ENDU-2 located in ADL chemosensory neurons and specific muscle cells as a regulator of multiple pleiotropic phenomena including cold tolerance, life span, and brood size through cell-autonomous and cell-nonautonomous pathways in nematode Caenorhabditis elegans. Ca2+ imaging revealed ADL temperature response to be the result of transient receptor potential (TRP) channel activity and regulated by ENDU-2 via cell-autonomous and cell-nonautonomous pathways. Transcriptome analysis revealed that ENDU-2 influences expression of the caspase gene ced-3. Moreover, ENDU-2 downregulates cold tolerance and synaptic remodeling in the dorsal nerve cord through caspase signaling. We therefore propose a model for cold tolerance regulation that occurs via EndoU action.
Keywords: Caenorhabditis elegans, cold tolerance, temperature tolerance, EndoU, apoptotic pathways
Abstract
Environmental temperature acclimation is essential to animal survival, yet thermoregulation mechanisms remain poorly understood. We demonstrate cold tolerance in Caenorhabditis elegans as regulated by paired ADL chemosensory neurons via Ca2+-dependent endoribonuclease (EndoU) ENDU-2. Loss of ENDU-2 function results in life span, brood size, and synaptic remodeling abnormalities in addition to enhanced cold tolerance. Enzymatic ENDU-2 defects localized in the ADL and certain muscle cells led to increased cold tolerance in endu-2 mutants. Ca2+ imaging revealed ADL neurons were responsive to temperature stimuli through transient receptor potential (TRP) channels, concluding that ADL function requires ENDU-2 action in both cell-autonomous and cell-nonautonomous mechanisms. ENDU-2 is involved in caspase expression, which is central to cold tolerance and synaptic remodeling in dorsal nerve cord. We therefore conclude that ENDU-2 regulates cell type-dependent, cell-autonomous, and cell-nonautonomous cold tolerance.
Organisms adapt to environmental temperature through a number of mechanisms. For instance, many animals regulate their metabolism and body temperature by a network of nervous and muscle tissue. However, these mechanisms are complex and remain elusive. Exploiting well-established genetic approaches (1, 2), the nematode Caenorhabditis elegans has been used as a simple model useful in the study of temperature behaviors and tolerance (3–5). C. elegans is known to exhibit cold tolerance in response to environmental temperature decrease (4, 6), and previous research described cold tolerance as regulated through ASJ sensory neuron, intestine, and sperm tissue networks (4, 6). During the process of cold tolerance, temperature is detected by a pair of ASJ neurons located in the head. Insulin is then released from the ASJ and binds to insulin receptor DAF-2 in the intestine and neurons. Insulin signaling regulates downstream gene expression mediated by DAF-16, a FOXO-type transcription factor. Delta 9-desaturase then initiates cold-induced lipid modulation reactions downstream of DAF-16 (7).
Genes regulating cold tolerance were screened and identified in previous microarray and phenotypic studies (4, 8). ENDU-2 is homologous to human endoribonuclease EndoU and is an ortholog of XendoU in Xenopus. The expression of the endu-2/M60.2 gene significantly increases after lowering ambient temperature from 23 to 17 °C (4, 8), and endu-2 mutants grown at 25 °C display abnormally elevated tolerance to cold (4).
In Xenopus, XendoU is located in the cytosol as well as on the surface of the endoplasmic reticulum (ER). The role of XendoU in living cells is to degrade RNA, generating small nucleolar RNA, which functions to regulate ribosome biosynthesis (9). Humans also possess a form of EndoU that exhibits RNA-binding and cleavage activity (10), referred to as placental protein 11 (PP11). Although the function of XendoU is understood at the cellular level, its systemic role is unclear. ER XendoU is required for catalysis-dependent nuclear envelope assembly and formation of tubular ER networks (9). Although synaptic proteins are synthesized and folded in the ER before being transported to synaptic areas via the Golgi apparatus or other organelles, EndoU-mediated synaptic events remain unknown.
Synapses are continuously formed during animal development. In C. elegans, DD-type GABAergic motor neuron synapses of the ventral nerve cord are eliminated by apoptotic signaling pathways that involve caspase CED-3 (11). L1 larvae possess DD neuronal synapses in the ventral nerve cord, but these synapses are eliminated during L2-to-adult development. Moreover, synaptic elimination in ventral cord DD neurons is accompanied by remodeling of new synapses in the dorsal nerve cord. In ced-3 mutants, synapses remain in the ventral nerve cord until the L4 larval stage because the loss of CED-3 affects cleavage of GSNL-1, which induces F-actin disassembly and synapse pruning (11).
In this report, we propose Ca2+-dependent endoribonuclease ENDU-2 regulates various phenomena, including cold tolerance, life span, brood size, sensory behavior, and synaptic remodeling. Furthermore, we provide evidence of muscle-mediated ENDU-2 function in both cell-autonomous and cell-nonautonomous ADL regulation with regard to cold tolerance, life span, and brood size.
Results and Discussion
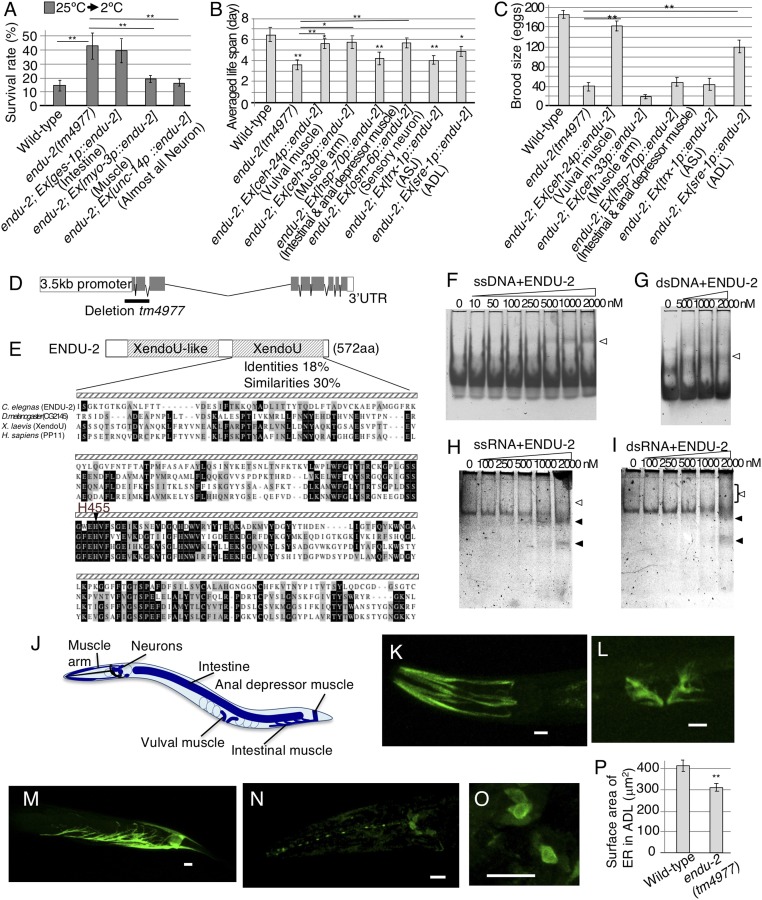
The endu-2 gene encodes a putative endoribonuclease involved in cold tolerance (4). Most wild-type worms grown at 25 °C did not survive at 2 °C cold stimulus (Fig. 1A). In contrast, endu-2 mutants grown at 25 °C survived more at 2 °C (Fig. 1A), in addition to a shorter life span and smaller brood size (Fig. 1 B and C and SI Appendix, Fig. S1 A and B). These results suggest that ENDU-2 is required in multiple biological processes. The endu-2 gene encodes a 572-aa protein with N-terminal signal peptide for translocation into the ER and conserved amino acid sequences containing a poly(U)-specific endoribonuclease (XendoU) domain at the C terminus (Fig. 1 D and E). Additionally, a XendoU-like domain exists within the amino-terminal portion of the molecule, and the catalytic XendoU domain of ENDU-2 is conserved in human poly(U)-specific endoribonuclease placental protein 11 (PP11) (Fig. 1E).
Fig. 1.
Endoribonuclease ENDU-2 involvement in various phenomena. (A–C) Cell-specific rescue of endu-2 mutants. (A) endu-2 abnormal cold tolerance was rescued by expressing ENDU-2 in neurons or muscle (number of assay ≥ 15; F(4,155) = 24.68; **P < 0.01; means ± SEM). We used myo-3p as a muscle promoter, inducing gene expression in body wall, vulval, anal depressor, intestinal, and other muscle cells. (B and C) Rescue experiments of life span and brood size in endu-2 mutant, respectively [C: number of assay ≥ 21; F(9,378) = 12.51; *P < 0.05; **P < 0.01; means ± SEM] [D: number of assay ≥ 10; F(7,140) = 13.09; *P < 0.05; **P < 0.01; means ± SEM]. (D) Genomic endu-2. Exons are boxed (gray). tm4977 mutation is indicated. (E) ENDU-2 contains XendoU domain essential for endoribonuclease activity and XendoU-like domain conserved from nematodes to flies. Amino acid identity (black) and similarity (gray) to XendoU domains for ENDU-2 and EndoU in other animals. (F–I) EMSA titration analyses. Open and solid arrows indicate ENDU-2 bound/degraded nucleic acids, respectively. (F) Single-stranded DNA. (G) Double-stranded DNA. (H) Single-stranded RNA. (I) Double-stranded RNA. Right bracket indicates ladder of various binding products. (J–O) Expression of full-length genomic endu-2::GFP. (J) Schematic diagram of expression pattern (blue). (K–O) ENDU-2::GFP expressions in head muscle arm (K); vulval muscle (L); anal depressor and intestinal muscle (M); neurons (N); and cell body of neurons (O). (Scale bars: 10 μm.) (P) The average ER surface area in the ADL cell body [from Left: n = 17 and n = 8; t(22) = 3.3; **P = 0.003; means ± SEM].
To determine ENDU-2 RNA binding and ribonuclease (RNase) activity, we developed a biochemical assay to test different types of DNA and RNA structures. The ENDU-2 protein was tagged with 6× histidine, FLAG, and T7 (SI Appendix, Fig. S1 C and D) before employing the electrophoretic mobility-shift assay (EMSA). The purified ENDU-2 was incubated with substrate DNA or RNA, then run on native polyacrylamide gel to observe substrate–ENDU-2 binding and/or degradation of the substrate. ENDU-2 bound to the substrate forms a protein–nucleotide complex that runs slower than the free substrate. ENDU-2 bound to all four substrates: ssDNA, dsDNA, ssRNA, and dsRNA (Fig. 1 F–I). ssRNA and dsRNA, however, were degraded by ENDU-2 (Fig. 1 H and I). The degradation products from ssRNA and dsRNA substrates consisted of a few distinctive fragments (shown by solid arrows in Fig. 1 H and I) rather than a ladder of numerous products, suggesting that ENDU-2 is an endoribonuclease.
ENDU-2 Expression in Neurons and Intestine/Muscle Cells.
Cells expressing ENDU-2 were visualized using two different endu-2::gfp fusion genes (SI Appendix, Fig. S1E). Both constructs were expressed in intestine and various muscle cells, including head muscle arm, vulval muscle, intestinal muscle, and anal depressor muscle (Fig. 1 J–M and SI Appendix, Fig. S1F). Additionally, anti-GFP antibodies were used to detect signal strength with greater sensitivity. We observed fluorescence in neurons, including amphid sensory neurons (Fig. 1N), in individuals expressing endu-2::gfp. ENDU-2::GFP was present in the cytosol. Spotty fluorescence was also observed around the nucleus (Fig. 1O), which is characteristic of ER proteins (9). The surface area of the ER in endu-2 mutants was smaller than in the wild type (Fig. 1P and SI Appendix, Results and Discussion). These findings are consistent with the previous report that XendoU was localized on the ER near the nucleus and functions in the ER (9), despite most of XendoU being localized in cytosol (9).
Expressing ENDU-2 in Sensory Neurons and Specific Muscle Cells Is Sufficient to Rescue endu-2 Mutant’s Enhanced Cold Tolerance.
ENDU-2 is expressed in neurons, muscle, and intestine (Fig. 1 J–N). To identify the essential tissue(s) responsible for endu-2-dependent phenomena, we conducted specific tissue rescue experiments in endu-2 mutants (Fig. 1 A–C). The elevated cold tolerance of endu-2 was almost fully rescued by expressing endu-2 cDNA in neurons and/or muscle. However, the trait was not rescued by intestinal expression (Fig. 1A) or by expressing truncated/catalytically impaired ENDU-2 in neurons or muscle (SI Appendix, Fig. S2A). These data suggest that the catalytic activity of ENDU-2 in neurons and/or muscle cells is essential for the rescue of increased cold tolerance. Similarly, ENDU-2 expression in neurons and/or muscle cells rescued endu-2 mutant’s small brood size (SI Appendix, Fig. S2 B–E).
To determine which neuron(s) and muscle(s) are required for endu-2–dependent cold tolerance, we expressed endu-2 cDNA in specific types of neurons and muscle cells. The abnormal increase in cold tolerance was rescued by expressing endu-2 cDNA in sensory neurons, vulval muscle, and head muscle arm (Fig. 2A).
Fig. 2.
Autonomous and nonautonomous cellular ENDU-2 cold tolerance function. (A and B) Cell-specific expression of endu-2 cDNA in endu-2 mutants [A: number of assay ≥ 21; F(9,378) = 12.51; *P < 0.05; **P < 0.01; means ± SEM] [B: number of assay ≥ 10; F(7,140) = 13.09; *P < 0.05; **P < 0.01; means ± SEM]. (C and D) Ca2+ imaging of ADL in 25 °C-grown animals. The graphs indicate YFP/CFP ratio change under temperature stimuli. The bar graph indicates the average ratio change from 110 to 120 s (C) [n ≥ 18; F(3,109) = 2.03; *P < 0.05; means ± SEM]. (D) Wild-type data were partially provided in C given that both experiments were conducted simultaneously [n ≥ 13; t(21) = 3.6; **P = 0.0014; means ± SEM]. The bottom chart indicates the temperature stimuli shared with C. (E) Mutant cold tolerance impaired TPR channels expressed in ADL [from Left: number of assay = 18, 21, 12, 9, 9, 12, 18, and 12; F(7,103) = 7.74; **P < 0.01; means ± SEM]. (F) Cell-specific expression of osm-9 cDNA in osm-9 mutants [from Left: number of assay = 19, 6, 7, and 10; F(3,38) = 8.59; **P < 0.01; means ± SEM]. (G–I) The apoptosis pathway plays an important role in cold tolerance. (G) Cold tolerance phenotype. The upper schematic pathway shows apoptosis pathway in C. elegans, with protein name below indicating mammalian homolog [number of assay ≥ 9; F(4,46) = 31.42; **P < 0.01; means ± SEM]. (H) ced-3 mutation suppressed endu-2 mutation [number of assay ≥ 25; F(3,112) = 26.46; **P < 0.01; means ± SEM]. (I) Cell-specific rescue of ced-3 abnormal cold tolerance [number of assay ≥ 11; F(4,64) = 9.43; **P < 0.01; means ± SEM].
ENDU-2 Has both Cell-Autonomous and Cell-Nonautonomous Function Depending on Cell Type.
We generated transgenic worms expressing endu-2 cDNA in specific sensory neurons to identify which neurons are responsible for endu-2–dependent cold tolerance. As we previously reported, the ASJ neuron, known to sense light and pheromones, is involved in cold tolerance as a temperature sensor (4). However, abnormally elevated cold tolerance in endu-2 mutants was not rescued by expressing endu-2 cDNA in the ASJ (Fig. 2A; endu-2; Ex[trx-1p::endu-2]). Similarly, the trait was not rescued by expressing endu-2 in other neuron sets, either (Fig. 2A; endu-2; Ex[tph-1p::endu-2]). We found that the enhanced cold tolerance in endu-2 mutants was specifically rescued by expressing endu-2 cDNA in the pair of head sensory neurons, ADL (Fig. 2A; endu-2; Ex[sre-1p::endu-2]). endu-2 mutants also displayed abnormal ADL-dependent avoidance behavior toward 1-octanol (SI Appendix, Results and Discussion and Fig. S3 A–C), while no abnormality in AWA-, AWB-, AWC-, ASE-, or ASH-mediated sensory behaviors were seen. These results suggest that the increased cold tolerance in endu-2 mutants is caused by ADL impairment, and that ENDU-2 in the ADL may function cell-autonomously.
Because the abnormal cold tolerance in endu-2 mutant was also rescued by expressing ENDU-2 in vulval muscle and head muscle arm (Fig. 2A), we constructed transgenic endu-2 animals simultaneously expressing the endu-2 cDNA in ADL and in one or both of these two types of muscle (Fig. 2B). Transgenic endu-2 animals expressing ENDU-2 in both ADL and the muscle(s) displayed a similar phenotype to endu-2 mutants expressing ENDU-2 in either of the cell types (Fig. 2B). These results suggest that ENDU-2 expression in either ADL, vulval muscle, or head muscle arm is sufficient to exhibit normal cold tolerance (Table 1). It is probable that ENDU-2 in ADL and muscle cells function within the same cold tolerance regulatory pathway. We therefore propose that, depending on cell type, ENDU-2 exhibits both cell-autonomous and cell-nonautonomous function during the regulation of cold tolerance.
Table 1.
Summary of cells involved in abnormal endu-2 mutant phenotypes
| Abnormal phenotype in endu-2 mutant | Cells responsible for each abnormality of endu-2 mutant |
| Cold tolerance | ADL, vulval muscle, muscle arm |
| Life span | ADL, vulval muscle, muscle arm |
| Brood size | ADL, vulval muscle |
| DD synapses | ND |
Additionally, short endu-2 mutant life span was partially rescued by expressing ENDU-2 in the ADL, vulval muscle, or head muscle arm (Fig. 1B and SI Appendix, Fig. S1 A and B). Likewise, small endu-2 mutant brood size was rescued by expressing ENDU-2 in the ADL or vulval muscle, but not in the muscle arm or ASJ neurons (Fig. 1C). These observations suggest that expression of ENDU-2 in ADL sensory neurons, or in certain muscle cells, is sufficient to rescue short life span and small brood size (Table 1). ENDU-2 therefore plays both cell-nonautonomous and cell-autonomous roles in the regulation of these traits.
ADL Neurons Can Respond to Thermal Stimuli.
We tested ADL sensory neuron temperature response using Ca2+ imaging with the genetically encoded Ca2+ indicator cameleon (Fig. 2 C and D and SI Appendix, Fig. S3D) (12), finding that the Ca2+ concentrations in ADL changed in response to temperature shifts (Fig. 2 C and D). Temperature response of ADL was diminished in endu-2 mutants (Fig. 2C). These observations suggest that ADL neurons can respond to thermal stimuli, and that ENDU-2 is involved in this response. Abnormal ADL temperature response in endu-2 mutants was rescued by expressing endu-2 cDNA in the ADL and partially rescued by expression in the vulval muscle and head muscle arm (Fig. 2C). Ca2+ imaging and the above cold tolerance analysis suggest that wild-type ADL temperature response is regulated by cell-nonautonomous ENDU-2 function in specific muscle cells and cell-autonomous function in the ADL.
Furthermore, we analyzed a single mutant with impaired sensory signal ion channels to confirm the role of ADL temperature sensitivity in cold tolerance. It was previously reported that cGMP-gated channels TAX-4/TAX-2 participate in ASJ sensory neuron temperature signaling (4, 13). ADL neurons do not express TAX-2/4, but instead express TRP channels encoded by osm-9, ocr-1, and ocr-2 genes (14). We found that osm-9 mutants display an abnormal increase in cold tolerance (Fig. 2 E and F), which was rescued by expressing osm-9 cDNA specifically in the ADL (Fig. 2F). Meanwhile, ocr-2 and ocr-1 mutants displayed nearly normal cold tolerance (Fig. 2E). ocr-2 osm-9; ocr-1 triple mutants grown at 25 °C showed similar cold tolerance to that of osm-9 mutants (Fig. 2E). In contrast, ocr-2 osm-9; ocr-1 triple mutants grown at 20 °C showed higher cold tolerance than osm-9 mutants (SI Appendix, Fig. S3E). These results suggest that TRP channel protein OSM-9 negatively regulates cold tolerance in 20 and 25 °C-grown wild-type individuals. The other TRP channels, OCR-1 and OCR-2, played an additive role in cold tolerance for worms grown at 20 °C.
We employed Ca2+ imaging (Fig. 2D) to investigate TRP channel involvement in the ADL temperature response. Ca2+ concentration changes resulting from temperature stimuli were smaller in ocr-2 osm-9; ocr-1 triple mutants than those in wild type, suggesting that these TRP channels are necessary for ADL temperature signaling.
CED-3 Caspase Is Involved in ENDU-2–Dependent Cold Tolerance.
We conducted next-generation sequencing and compared RNA profiling between wild type and endu-2 mutants (SI Appendix, Fig. S4 A–D and Dataset S1) to identify downstream ENDU-2 signaling, finding that expression levels of 3,441 genes were altered in endu-2 mutants. The expression patterns of 1,850 genes have already been described in the database, WormBase (https://wormbase.org/#012-34-5). We then categorized expression patterns of these genes, finding about 33% of the 1,850 genes were expressed in neurons known to play a role in cold tolerance (SI Appendix, Fig. S4A) (4). Of the 3,441 genes isolated in our analysis, ∼100 gene mutants had already been isolated. We then tested the cold tolerance of these mutants, revealing many worms with either an abnormal increase or decrease in tolerance (SI Appendix, Fig. S4 E–G).
Given that ENDU-2 is an endoribonuclease, we speculated that a decrease in cold tolerance could be induced by impairing downstream ENDU-2. We found from RNA profiling that ENDU-2 loss affected ced-3 expression. Moreover, ced-3 mutants did exhibit a decrease in cold tolerance (Fig. 2G), hypothesizing that ced-3 functions downstream of endu-2. In support of our hypothesis, we found the increased cold tolerance of the endu-2 mutant to be strongly suppressed by ced-3 mutation. The ced-3; endu-2 double mutants showed a statistically similar phenotype to that of ced-3 single mutants (Fig. 2H). Such results further suggest that ced-3 acts downstream of endu-2, and that ENDU-2 negatively affects CED-3 signaling.
Cold tolerance decreased in egl-1 (BH3) and in ced-4 (Apaf1) mutants, genes known to code for positive apoptosis regulators (Fig. 2G and SI Appendix, Fig. S5) (15, 16). Conversely, cold tolerance increased in ced-9 mutants (BCL2), which codes for a known negative apoptosis regulator (Fig. 2G) (17). These observations are consistent with the hypothesis that proper caspase signaling is required for adequate cold tolerance. Next, we introduced ced-3 cDNA into vulval muscle and ADL neurons to identify specific cells responsible for decreased ced-3 mutant cold tolerance (Fig. 2I). The phenotype was rescued by expressing CED-3 in vulval muscle and the ADL, but not in ASJ (Fig. 2I). These results suggest that CED-3 and ENDU-2 act in the same cells, ADL and vulval muscle, to regulate cold tolerance.
ENDU-2 Is Involved in Synaptic Remodeling.
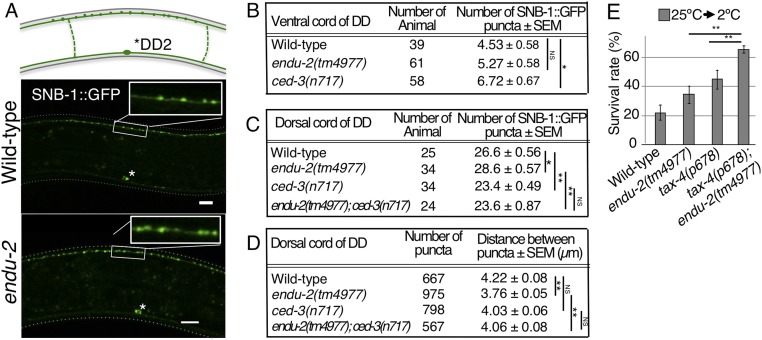
CED-3 regulates synaptic remodeling, neuronal pruning, and apoptosis (11). Since ced-3 gene expression is affected in endu-2 mutants, we examined the morphology of ADL synapses using presynaptic marker synaptobrevin SNB-1::GFP. However, ADL synaptic density was intense, making individual synapses difficult to distinguish. We then analyzed dorsal and ventral nerve cord DD-type GABAergic neuron synapses, which provided a system for observing synaptic remodeling (Fig. 3A). DD neuron synapses in the ventral nerve cord are known to be eliminated by apoptotic signaling molecules. Moreover, this process is accompanied by remodeling of new synapses in the dorsal cord (11). The number of synapses in the DD2 region of the ventral cord remained normal in endu-2 mutants, whereas ced-3 mutant showed an increase in the number of synapses (Fig. 3B) (11). This indicates that ENDU-2 is not involved in synaptic DD pruning of ventral cord. Previous reports and this study both show that ced-3 mutation elicits a decrease in dorsal cord DD synapse number (Fig. 3 C and D) (11). In contrast, endu-2 mutation caused an increase in the number of synapses as well as dorsal cord synaptic density (Fig. 3 C and D), suggesting that ENDU-2 negatively regulates DD synaptic remodeling. ced-3; endu-2 double mutants showed a similar decrease in the number of synapses (Fig. 3 C and D). We determined that endu-2 mutation was suppressed by ced-3 mutation, concluding that ced-3 genetically acts downstream of endu-2. Based on these results, ENDU-2 could function as a negative regulator of CED-3–dependent synaptic remodeling in the DD neurons of the dorsal cord.
Fig. 3.
ENDU-2 affects synaptic remodeling. (A) GABAergic DD motor neuron synapses. The upper diagram indicates synapses between the DD2 and DD3 commissure. The asterisk (*) indicates DD2 cell body. (B and C) Average number of synapses between DD2 and DD3 commissure in ventral cord (B) and dorsal cord (C). (D) Average synaptic distance in dorsal cord. (E) Cold tolerance of 25 °C-grown animals [number of assay ≥ 11; F(3,63) = 21.33; **P < 0.01; means ± SEM].
ENDU-2 Controls Cold Tolerance in Parallel to the Known Temperature-Signaling Cold Tolerance Pathway.
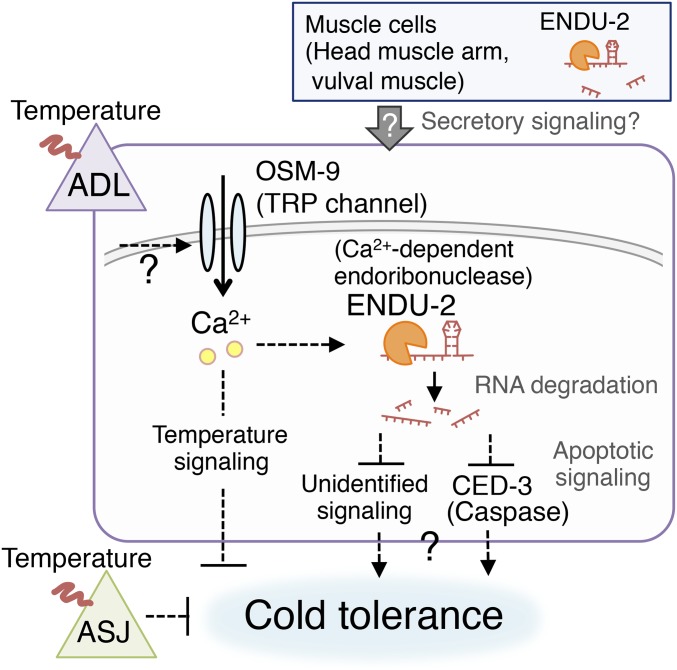
It was previously reported that cold tolerance is regulated by the cGMP-gated channel TAX-4 in ASJ sensory neurons, followed by downstream insulin signaling in the intestine (4). We therefore carried out a genetic epistasis analysis to investigate whether ADL ENDU-2 regulates cold tolerance in parallel with the TAX-4–mediated ASJ signaling pathway (Fig. 3E). The tax-4; endu-2 double mutant showed even higher cold tolerance than that of either tax-4 or endu-2 single mutants (Fig. 3E). This result suggests that ENDU-2–dependent cold tolerance signaling in ADL is independent of TAX-4 signaling in ASJ, and that the two sensory neurons act independently in the cold tolerance neural circuit (Fig. 4).
Fig. 4.
A model for ENDU-2–dependent cold tolerance.
Cold Tolerance Model Mediated by ADL and Muscle Cells.
Ca2+ imaging in this study revealed that mutations in the Ca2+-dependent endoribonuclease ENDU-2, as well as mutations in TRP channel, decreased the ADL temperature response. These results indicate that ENDU-2 and TRP positively regulate ADL neuronal activity, leading us to propose a plausible neural model for the regulation of cold tolerance as mediated by ENDU-2. ENDU-2 may be involved in the expression of yet-unidentified factors (temperature signaling molecules, etc.) involved in ADL neuronal function (Fig. 4). The results provided in Fig. 3 suggest ENDU-2 negatively regulates CED-3–dependent synaptic remodeling in dorsal nerve cord DD neurons. Given the limitation in analyzing ADL synapses, a secondary synaptic abnormality may have been present. Moreover, apoptotic signaling may decrease ADL neuronal function (Fig. 4).
ENDU-2 expression in endu-2 mutant vulval muscle, head muscle arm, and ADL rescued enhanced cold tolerance as well as abnormal ADL response to temperature change. These results suggest cell-nonautonomous ENDU-2 function is required to regulate ADL cold tolerance. Previous work has shown that cell-nonautonomous regulation of the AFD thermosensory neuron is necessary for thermotaxis—a process during which muscle cell heat shock-dependent transcriptional factor (HSF-1) functions cell-nonautonomously to regulate AFD neuronal activity via steroid-hormonal signaling (4, 8). Although HSF-1 is not involved in cold tolerance (4), similar cell-nonautonomous secretory signaling may occur between muscle cells and ADL (Fig. 4). Together, these results and observations suggest that both cell-autonomous and cell-nonautonomous events associated with ENDU-2–dependent signaling are essential for regulating ADL cold tolerance (Fig. 4). Since mammalian EndoU acts as a ribonuclease for many gene transcripts, it is possible that more complex neural and cellular systems are affected by ENDU-2 and/or regulate cold tolerance in C. elegans.
endu-2 mutant’s reduced life span and brood size were also rescued by expressing ENDU-2 in ADL or specific muscle cells. As shown in Table 1, the responsible cells to cause these abnormalities in endu-2 mutants are mostly shared. Still, the mechanism(s) by which ENDU-2 regulates life span, small brood size, and synaptic remodeling may be different from the cold tolerance regulatory mechanism. This is because ENDU-2 catalytic activity was not required to rescue short life span or small brood size (SI Appendix, Fig. S2 A–E). Further research is needed to determine whether ENDU-2 regulates all these traits through the same, or multiple mechanisms.
Although cell-nonautonomous ENDU-2 function could potentially affect other cells that regulate life span and brood size, we surmise that either vulval muscle or the head muscle arm is necessary to determine normal neuronal position. Neurons in question include the HSN, which regulates brood size, and the amphid neurons, which are involved in determining life span. If vulval or head muscle does not function properly, the HSN, amphid neurons, and/or their synapses may become impaired. As such, reduced endu-2 mutant life span and brood size may be the results of abnormal HSN/amphid neuron position or connectivity.
Molecular physiology is largely conserved between C. elegans and humans, and the systems found in this study provide a useful model for understanding temperature-signaling pathways in both humans and other animals. The present study has aimed to elucidate complex temperature response mechanisms that result in tolerance to cold while also exploring other C. elegans traits evidently regulated by this conserved ribonuclease.
Methods
Strains.
The N2 (Bristol) strain was used for wild type in all experiments. The following mutant strains were used: endu-2(tm4977), osm-9(ky10), ocr-1(ok132), ocr-1(ak46), ocr-2(ak47), ocr-2(ak47); ocr-1(ok132), ocr-2(ak47) osm-9(ky10), ocr-2(ak47) osm-9(ky10); ocr-1(ak48), egl-1(n487), ced-4(n1162), ced-3(n717), tax-4(p678). See SI Appendix for further details.
Statistical Analysis.
Error bars in the figures indicate SEMs. All bar graph statistical analyses were performed by unpaired Welch’s t tests for single comparison and one-way ANOVA, followed by Dunnett’s post hoc tests for multiple comparisons. We used the Kaplan–Meier estimate, followed by the generalized Wilcoxon test for life span analysis. Single (*) and double asterisks (**) indicate P < 0.05 and P < 0.01, respectively. See Dataset S2 for further details on raw data and statistical figures.
Cold Tolerance Assay.
Cold tolerance was assayed as described in previous studies (4, 18). We used uncrowded and well-fed young adult worms as they prepared to lay eggs. A few animals were placed on a 3.5-cm plate containing 6 mL of nematode growth medium with 2% (wt/vol) agar, on which Escherichia coli OP50 was seeded. The adult animals were transferred to the outside of the plate after 12–18 h, and their offspring were cultured for either 144–150 h at 15 °C, 85–90 h at 20 °C, or 60–65 h at 25 °C. Plates containing 70–120 animals were transferred to a 2 °C refrigeration cabinet. If the mutant growth rates were slower than wild type, we altered the egg-laying day to synchronize the start of wild-type and mutant cold exposure. After 24 h, the plates were transferred and stored at 15 °C overnight, and surviving as well as deceased animals were counted. See SI Appendix for further details.
In Vivo Ca2+ Imaging.
In vivo Ca2+ imaging was performed in line with previous reports (4, 19). Animals expressing yellow cameleon 3.60 in ASJ were affixed to a 2% (wt/vol) agar pad on glass, immersed in M9 buffer, and then covered by cover glass. Fluorescent images of donor and acceptor fluorescent proteins were simultaneously captured using an EM-CCD camera EVOLVE512 (Photometrics). Relative changes in intracellular Ca2+ concentration were measured as the change in acceptor/donor protein fluorescence ratio. See SI Appendix for further details.
Supplementary Material
Acknowledgments
We thank I. Mori, H. R. Horvitz, C. I. Bargmann, D. Yan, Y. Jin, Y. Iino, T. Nakatani, T. Ii, J. Burkhead, J. M. Kaplan, S. E. Hall, T. G. Kusakabe, and S. Mitani for sharing DNA constructs, strains, and reagents; the National Bioresource Project (Japan) and the Caenorhabditis Genetic Center for strains; and Y. Wataoka, K. Tanaka, M. Higasine, T. Miura, and K. Kanai for supporting experiments and discussion. We thank Eric Odle for English editing and proofreading the manuscript. We further thank the staff of the Comparative Genomics Laboratory at the National Institute of Genetics (NIG) for supporting genome sequencing. A.K. was supported in part by the Naito Foundation, the Uehara Memorial Foundation, the Public Health Research Foundation, the Ono Medical Research Foundation, the Mishima–Kaiun Memorial Foundation, the Sumitomo Foundation, the Daiichi Sankyo Foundation of Life Science, the Hirao Taro Foundation of Konan University, PRIME, Japan Agency for Medical Research and Development Mechano Biology (18gm5810024h0002), Japan Society for the Promotion of Science (JSPS) KAKENHI (Grants 15K21744, 17K19410, and 18H02484); and KAKENHI (Grants 15H05928 and 16H06279) from Ministry of Education, Culture, Sports, Science and Technology of Japan. A.O. was supported in part by the Takeda Science Foundation, the Novartis Foundation, the Astellas Foundation for Research on Metabolic Disorders, the Ono Medical Research Foundation, and JSPS KAKENHI (Grants 16J00123 and 18K06344). T.U. was supported by JSPS KAKENHI (Grant 15J04977). Computations were partially performed by the NIG supercomputer at the Research Organization of Information and Systems, NIG.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data reported in this paper have been deposited in the DNA Data Bank of Japan Sequence Read Archive (accession no. DRA006152).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808634115/-/DCSupplemental.
References
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr MM. Super models. Physiol Genomics. 2003;13:15–24. doi: 10.1152/physiolgenomics.00075.2002. [DOI] [PubMed] [Google Scholar]
- 3.Ohta A, Kuhara A. Molecular mechanism for trimetric G protein-coupled thermosensation and synaptic regulation in the temperature response circuit of Caenorhabditis elegans. Neurosci Res. 2013;76:119–124. doi: 10.1016/j.neures.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Ohta A, Ujisawa T, Sonoda S, Kuhara A. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat Commun. 2014;5:4412. doi: 10.1038/ncomms5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okahata M, et al. Natural variations of cold tolerance and temperature acclimation in Caenorhabditis elegans. J Comp Physiol B. 2016;186:985–998. doi: 10.1007/s00360-016-1011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoda S, Ohta A, Maruo A, Ujisawa T, Kuhara A. Sperm affects head sensory neuron in temperature tolerance of Caenorhabditis elegans. Cell Rep. 2016;16:56–65. doi: 10.1016/j.celrep.2016.05.078. [DOI] [PubMed] [Google Scholar]
- 7.Murray P, Hayward SA, Govan GG, Gracey AY, Cossins AR. An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:5489–5494. doi: 10.1073/pnas.0609590104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011;14:984–992. doi: 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz DS, Blower MD. The calcium-dependent ribonuclease XendoU promotes ER network formation through local RNA degradation. J Cell Biol. 2014;207:41–57. doi: 10.1083/jcb.201406037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laneve P, et al. The tumor marker human placental protein 11 is an endoribonuclease. J Biol Chem. 2008;283:34712–34719. doi: 10.1074/jbc.M805759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L, et al. The cell death pathway regulates synapse elimination through cleavage of gelsolin in Caenorhabditis elegans neurons. Cell Rep. 2015;11:1737–1748. doi: 10.1016/j.celrep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 14.Tobin DM, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 16.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J, Horvitz HR. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- 18.Ujisawa T, Ohta A, Okahata M, Sonoda S, Kuhara A. Cold tolerance assay for studying cultivation-temperature-dependent cold habituation in C. elegans. Protoc Exch. September 8, 2014 doi: 10.1038/protex.2014.032. [DOI] [Google Scholar]
- 19.Kuhara A, Ohnishi N, Shimowada T, Mori I. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat Commun. 2011;2:355. doi: 10.1038/ncomms1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.