Significance
The Oklo natural nuclear reactors provide a wealth of information regarding the migration and retention of fission products in nuclear wastes. Radioactive volatile and gaseous elements easily escape from reactor fuel into the environment without proper containment. Cesium, in particular, represents a significant environmental and health hazard. Here, we used an isotope imaging system to identify the location of sequestered fissionogenic Cs and Ba in Oklo. Cesium and Ba were captured in Ru metal/sulfide aggregates shortly after reactor criticality ceased. These elements were otherwise nearly completely lost from the reactor. We have further discovered the most depleted natural U on Earth, indicating that these fission products were retained in the most active region of the reactor.
Keywords: Oklo, SIMS, AMS, isotope imaging, natural fission reactor
Abstract
Understanding the release and sequestration of specific radioactive signatures into the environment is of extreme importance for long-term nuclear waste storage and reactor accident mitigation. Recent accidents at the Fukushima and Chernobyl nuclear reactors released radioactive 137Cs and 134Cs into the environment, the former of which is still live today. We have studied the migration of fission products in the Oklo natural nuclear reactor using an isotope imaging capability, the NAval Ultra-Trace Isotope Laboratory’s Universal Spectrometer (NAUTILUS) at the US Naval Research Laboratory. In Oklo reactor zone (RZ) 13, we have identified the most depleted natural U of any known material with a 235U/238U ratio of 0.3655 ± 0.0007% (2σ). This sample contains the most extreme natural burnup in 149Sm, 151Eu, 155Gd, and 157Gd, which demonstrates that it was sourced from the most active Oklo reactor region. We have discovered that fissionogenic Cs and Ba were captured by Ru metal/sulfide aggregates shortly following reactor shutdown. Isochrons from the Ru aggregates place their closure time at 4.98 ± 0.56 y after the end of criticality. Most fissionogenic 135Ba and 137Ba in the Ru migrated and was incorporated as Cs over this period. Excesses in 134Ba in the Ru point to the burnup of 133Cs. Cesium and Ba were retained in the Ru despite local volcanic activity since the reactor shutdown and the high level of activity during reactor operation.
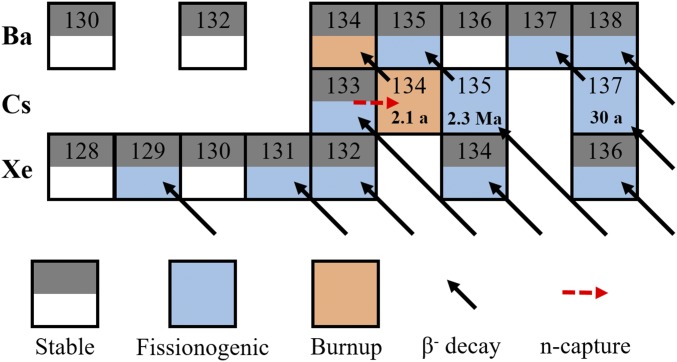
Two billion years ago, an incredible confluence of conditions yielded 17 sites in central Africa where natural nuclear fission reactors operated over periods of 24 ka to 1 Ma, known as the Oklo phenomenon (e.g., refs. 1–5 and references therein). To sustain fission, anthropogenic reactors require uranium fuel to be enriched in its main fissile isotope, 235U, from its present-day abundance of 0.72%. Two billion years ago, the abundance of 235U was naturally higher, ∼3%, due to the shorter half-life of 235U (t1/2 = 0.7 Ga) relative to 238U (t1/2 = 4.5 Ga). This, combined with large deposits of uranium ore and groundwater as a neutron moderator, allowed nuclear fission to occur underground, with the reactors cycling on for 30 min and off for 2.5 h over tens of thousands of years (6). These reactor zones (RZs) provide analogs for the study of long-term nuclear waste storage. Each site experienced a different duration of pulsed fission and was subject to varied postfission processing, such as terrestrial weathering at sites near the surface or local volcanic intrusions. The periodic intrusion of groundwater not only moderated the reactors, but also removed fission products. To enable better nuclear waste storage, it is important to understand how radioactive fission products of different elements migrate from or are retained in the nuclear fuel. For instance, secondary La–Ce–Sr–Ca aluminous hydroxy phosphate (phosphate) minerals in Oklo RZ 13 (7) were found to have captured radiogenic Xe in quantities greater than those found in any other terrestrial mineral, while very little Xe was retained in the uraninite (UO2) fuel (8). Cesium, which is one of the most volatile elements, is also incompatible with the UO2 structure, limiting its retention within the reactor fuel (9). Cesium migrated to grain boundaries and was mostly lost from the Oklo reactors (9, 10); in anthropogenic reactors, it becomes trapped between the nuclear fuel and the reactor cladding, which heightens the risk of instantaneous release if a storage cask were ruptured (11). Several radioactive Cs isotopes result from fission or burnup of 133Cs, including 134Cs (t1/2 = 2.1 y), 135Cs (t1/2 = 2.3 Ma), and 137Cs (t1/2 = 30.2 y) (Fig. 1). These comprise >10% of the total fission yield, creating a problem for both short- and long-term storage. There has been considerable work done mapping the spread and retention of fissionogenic Cs in soils, sediments, plants, and animals following the Fukushima and Chernobyl reactor accidents due to health and environmental concerns (e.g., refs. 12 and 13 and references therein).
Fig. 1.
The fissionogenic and n-capture isotopes of Ba, Cs, and Xe are shown with β− decay pathways. Isotopes with half-lives >2 wk are shown. Barium has three stable fissionogenic isotopes. Its other isotopes are shielded by Xe and have negligible fission yields. Barium-134 is enhanced by n-capture on 133Cs. Adapted from ref. 25, with permission from Elsevier.
Oklo RZ 13 is one of the best-preserved zones, making it a close natural analog to long-term nuclear waste storage and an ideal sample in which to study retention and migration of fission products (3, 7). In addition, large samples (several millimeters) from the different RZs may be easily studied, as they are no longer radioactive, which allows for thorough investigation of heterogeneities across the samples.
Results
We studied the spatial distribution and isotopic abundances of fission and neutron capture products in a sample from drill hole S2 in gallery SD.37 of Oklo RZ 13 (6–8) using the NAval Ultra Trace Isotope Laboratory’s Universal Spectrometer (NAUTILUS) at the US Naval Research Laboratory (NRL). NAUTILUS is a combination secondary ion mass spectrometer (SIMS) and single-stage accelerator mass spectrometer (SSAMS), which enables the micrometer-scale and molecular-isobar-free analysis of samples in situ (14–16). NAUTILUS provides a state-of-the-art, direct, molecule-free isotope imaging capability over regions 10–500 µm in size for the study of heterogeneous materials with high sensitivity and signal-to-noise. Molecular isobars are ordinarily detrimental to mass spectrometry analyses, especially when measuring trace, high-mass elements, such as the actinides and fission products, and in perturbed isotopic systems where corrections cannot be reliably made. Considerable isotopic work on the Oklo and Bangombé reactors has been performed to date [De Laeter and Hidaka (2) and references therein], including bulk studies by isotope dilution (ID) inductively coupled plasma mass spectrometry (ICP-MS) and ID thermal ionization mass spectrometry, in addition to in situ studies by laser ablation ICP-MS and traditional SIMS. We present the results of NAUTILUS measurements which are inaccessible to other mass-spectrometric techniques.
We measured the most depleted natural U on Earth, to our knowledge, in uraninite grains from RZ 13 with 235U/238U ratios as low as 0.3655 ± 0.0007% (2σ) [present-day natural 235U/238U = 0.7255% (17)]. Across the 3- × 4-mm sample, 235U/238U varied grain-to-grain, ranging up to 0.396%. This compares to 235U/238U ratios of, for example, 0.465% in RZ 2, 0.694% in RZ 3, 0.559% in RZ 9, 0.507% in RZ 10, 0.463% in bulk RZ 13 (0.38% in the core), and 0.662% in Bangombé from earlier work (3–5, 18, 19). The gradient in 235U/238U that we observed across our 4-mm sample qualitatively agrees with 235U/238U ranging up to 0.5–0.6% across the ∼30-cm-thick core of RZ 13 (3). This suggests that our sample was located at the most active region of all RZs and that the neutron flux in RZ 13 was heterogeneous and/or not well thermalized (20).
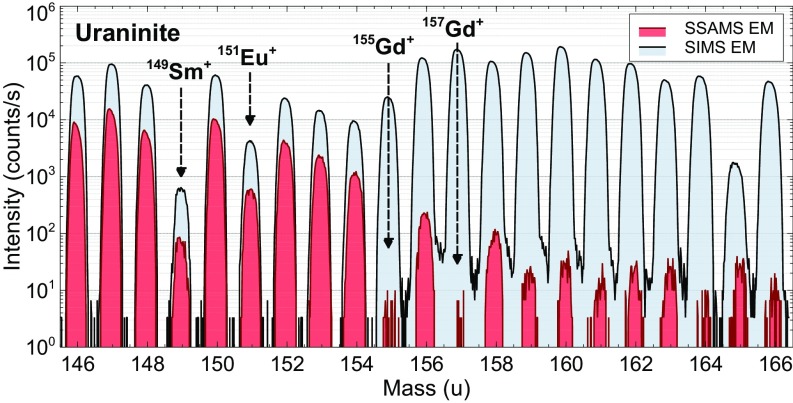
Conventional SIMS cannot measure trace high-mass and rare earth element (REE) isotope abundances in samples with complex and large molecular backgrounds (e.g., any mineral) and with nonnormal isotope ratios. NAUTILUS makes direct measurements of these isotopes by dissociating molecular isobars and forgoing deconvolution (Fig. 2) (14–16, 21–23). Knowledge of fission and n-capture product isotope abundances from the Oklo RZs to this point has predominantly come from bulk ID measurements, with some in situ SIMS work of secondary minerals. Hidaka and Holliger (4) provide a comprehensive account of such abundances; De Laeter and Hidaka (2) provide a review of mass spectrometry applied to Oklo. From direct in situ measurements of uraninites in RZ 13, we found that several isotopes with large n-capture cross-sections were more depleted than those reported by Hidaka and Holliger (4) (SI Appendix, Table S1), with 151Eu/153Eu = 0.2606 ± 0.0020 (2σ) and 149Sm/147Sm = 0.004849 ± 0.000046 (2σ) (normal 151Eu/153Eu = 0.916 and 149Sm/147Sm = 0.922) (see Figs. 2 and 3 for SIMS vs. SIMS+SSAMS comparison). Gadolinium (Gd)-155 and Gd-157 were depleted essentially to zero, with their measured ion signals being equivalent to a 4‰ addition of normal Gd relative to 160Gd, or smaller than the error of the measurement. A small component of spontaneous fission or ingrowth following reactor operation could also be present. Therefore, we could not directly compare 155Gd/156Gd and 157Gd/158Gd ratios to earlier work; however, the 156Gd/160Gd and 158Gd/160Gd ratios we measured were 6.96 ± 0.08 (2σ) and 3.40 ± 0.04 (2σ), respectively, 40% and 20% more enriched than those in Hidaka and Holliger (4) (normal 156Gd/160Gd = 0.936, 158Gd/160Gd = 1.14). This enrichment pattern in burnup products 156Gd and 158Gd relative to 160Gd, which has no fission or n-capture contribution, is consistent with a system where 155Gd and 157Gd were continuously depleted to zero. We expect the enrichment in 156Gd/160Gd to be larger than that in 158Gd/160Gd since 155Gd has several pathways for replenishment during reactor operation—for example, fission yield and the reactions 153Eu(n,γ)154Eu(n,γβ−)155Gd—while 157Gd is only replenished by fission. Europium (Eu)-154 and Eu-155 have half-lives of 8.6 and 4.8 y, respectively, and thermal n-capture cross-sections of >1 kb; 153Eu is stable with a cross-section of 300 b and a resonance integral of 1.4 kb (24). Furthermore, 156Gd has an additional enhancement pathway 155Eu(n, γβ−)156Gd, while 158Gd does not. The phosphate was similarly depleted/enriched in its Gd isotopes, although Gd and other heavy REEs were less abundant than in the uraninite (SI Appendix, Table S1). Dysprosium isotopes 161–164, which all have appreciable n-capture cross-sections and resonance integrals, were measured in uraninite as well, with significant depletions found in the 161,162,164Dy/163Dy ratios of 40%, 22%, and 77% relative to normal. The phosphate composition was 6–9% less depleted in each isotope ratio relative to the uraninite. Since Dy isotopes have negligible fission yield, they act as a neutron fluence monitor, from which we estimated a fluence of 7.7 × 1020 n∙cm−2, in line with earlier work (4).
Fig. 2.
Mass scan comparison (charge, z = +1) over the mid-REEs to heavy REEs measured on the SIMS (blue) and SSAMS (red) EM detectors of NRL’s NAUTILUS. The SSAMS mass scan illustrates the removal of the intense molecular background visible in the SIMS, allowing for direct measurement of fission products and n-capture depletions. The SIMS spectrum cannot be deconvolved by using conventional energy filtering methods because the isotopic abundances are nonnormal.
Fig. 3.
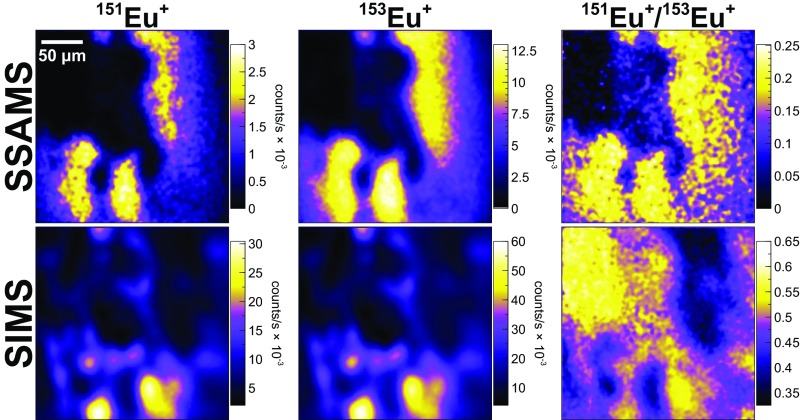
Europium isotope ratio imaging on the SSAMS (Upper; molecule-free) and SIMS (Lower) EMs of the NAUTILUS. Europium is concentrated in uraninite and not in aluminous phosphate. Europium-151 is highly depleted due to n-capture (more than 153Eu), but this is obscured by a large and complex molecular background on the SIMS. The SIMS images contain many false-positive features, and the isotope ratio image is in fact nearly the inverse of the true ratio image (151Eu/153Eunormal = 0.916).
Understanding the behavior of radiogenic Cs in modern reactors is important since two fissionogenic isotopes, 135Cs and 137Cs, each comprise ∼6–6.5% of the total fission yield, with half-lives of 2.3 Ma and 30.2 y, respectively. They decay to the stable isotopes 135Ba and 137Ba, respectively (Fig. 1). Cesium is mobile, and 137Cs accounts for a major portion of reactor waste activity on the timescale of several generations, while 135Cs remains active long after. Earlier bulk isotopic work identified the presence of radiogenic Ba within the RZs, indicating that differentiation between Ba and Cs occurred on the order of 20 y following criticality and that fissionogenic 135Ba and 137Ba chemically behaved as Cs (9, 25, 26).
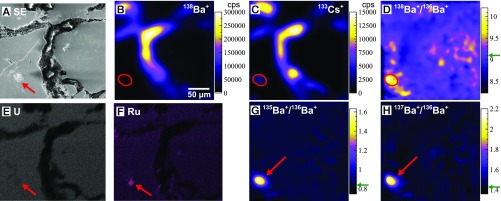
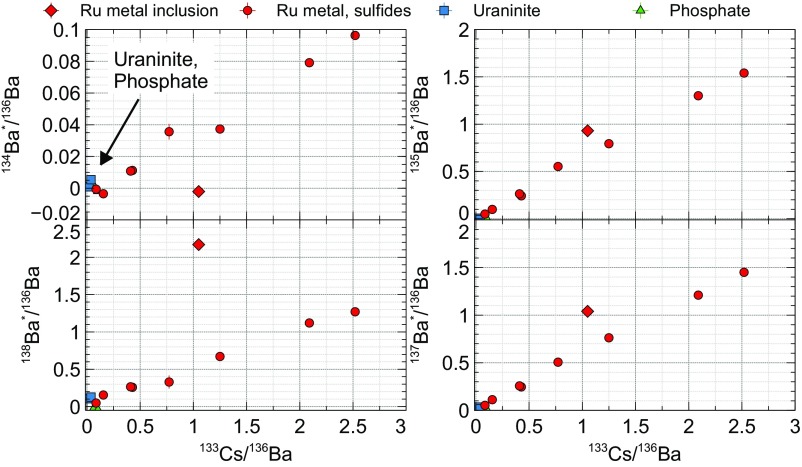
Using raster ion imaging on the NAUTILUS, we discovered several “hotspots” containing large abundances of fissionogenic 134Ba, 135Ba, 137Ba, and 138Ba (denoted with *), correlated with 133Cs (Fig. 4). Barium-134* was from n-capture on 133Cs; 135Ba* and 137Ba* were from the decay of 135Cs* and 137Cs*, respectively; and 138Ba* was from direct fission. The hotspots contain isotope ratios deviating from normal up to δ134Ba = 310‰, δ135Ba = 1830‰, δ137Ba = 1010‰, and δ138Ba = 240‰ (±10‰), with Ba* and Cs abundances generally correlated (Fig. 5 and SI Appendix, Table S1); δiBa = δiBa/136Ba = 1,000 × [(iBa/136Ba) sample/(iBa/136Ba)normal − 1)]. Without molecule-free imaging, these regions would be nearly impossible to locate given the large molecular background at each nominal mass. Barium and Cs in the phosphate had nearly normal isotopic composition, which significantly impacted imaging contrast and necessitated the use of direct isotope ratio imaging (Fig. 4). The uraninite showed Ba* excesses up to 1%, but with vastly lower Cs and Ba abundances than the phosphate. The hotspots of Ba* were found to be localized within Ru metal aggregates and sulfides, one of which was found completely entrained within a uraninite grain (Fig. 4), while others were found on grain perimeters or surrounded by phosphate. Gauthier-Lafaye et al. (3) reported that the metallic aggregates tend to consist of Pb–Ru–As–S and Pb–Te phases with galena (PbS) as a secondary phase, with the majority of aggregates in RZ 13 existing between uraninite grains, as we observed. The Ru aggregate pictured in Fig. 4, which was completely encapsulated within uraninite, showed a remarkable difference in its fissionogenic Ba abundances with respect to the intergrain aggregates (diamond marker in Figs. 5 and 6). In plots of the Ba* isotopes and of 133Cs relative to 136Ba (a shielded and essentially nonfissionogenic isotope; Fig. 1), the intergrain Ru aggregates showed near-perfect correlations of all Ba* isotopes with 133Cs, while the inclusion showed markedly higher 135,137,138Ba* relative to 133Cs and no 134Ba*. Since 133Cs is the only stable Cs isotope, it is not typically feasible to distinguish between fissionogenic and primordial components. Earlier work suggested that the 133Cs in Oklo is nearly all fissionogenic (25, 26). The isochrons in Fig. 5 show a clear difference between the intergrain aggregates and the included Ru grain, indicating fractionation in 133Cs. This is especially apparent in the plot of 134Ba*/136Ba, since 134Ba* is a burnup product of 133Cs. The 133Cs was apparently not retained within the uraninite long enough for 134Cs to be produced by n-capture and subsequently sequestered in the Ru inclusion. For the intergrain aggregates, however, the correlation between 134Ba* and 133Cs indicated that 134Cs (t1/2 = 2.1 y) was live during Ru formation.
Fig. 4.
Molecule-free isotope ratio imaging identified fissionogenic 135,137Ba in a localized spot within a uraninite grain. SEM–energy-dispersive X-ray found this spot to contain Ru metal and sulfides (E and F). The Ru phases formed ∼5 y after criticality and captured 135,137Cs* in similar abundance. The majority of Ba and Cs is concentrated in aluminous phosphate (B and C), although it has normal isotopic composition (indicated by green arrows in D, G, and H scale bars). Secondary electron (A) and SIMS (B–D, G, and H) images are shifted horizontally relative to each other by ∼50 μm.
Fig. 5.
Fissionogenic Ba and Cs relative to 136Ba. Uraninite and aluminous phosphate show almost no retained Ba*, while Ru aggregates between uraninite grains show highly correlated Ba* and Cs (circles). The Ru inclusion within a uraninite grain (diamond) is an outlier, reflecting the fractionation of Cs between uraninite and the interuraninite mineral phases.
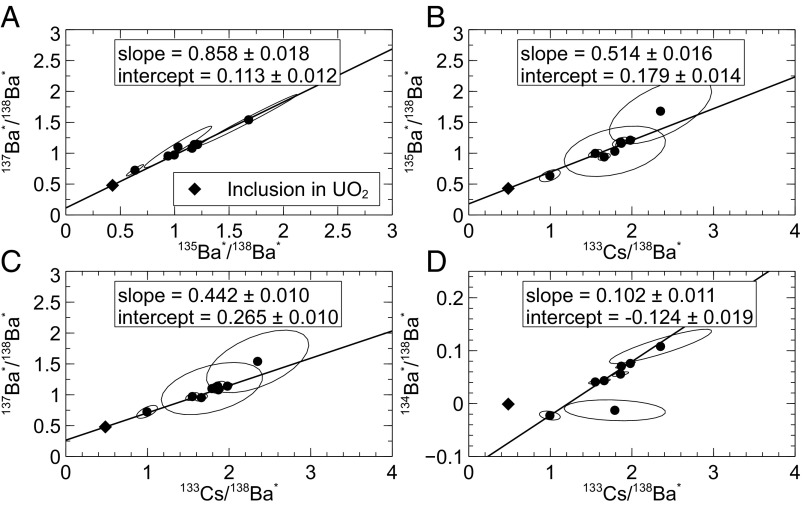
Fig. 6.
Isochrons of fissionogenic Ba and Cs from Ru aggregates. The slope and intercept of the 137Ba*/138Ba* vs. 135Ba*/138Ba* isochron (A) yields a mean formation age of 4.98 ± 0.56 y following reactor shutdown and illustrates that Ba* behaved predominantly as Cs before incorporation. The Ru grain included in uraninite is shown as a diamond; interuraninite Ru aggregates are shown as circles (as in Fig. 5). The 135Ba*/138Ba* intercept (B) indicates that Cs was lost after formation, likely during the intrusion of a dolerite dike ∼1 Ga after shutdown. The 137Ba*/138Ba* intercept (C) is similarly compromised. Excess 134Ba indicates the burnup of 133Cs near the intergrain aggregates, although the abundance appears fractionated (D).
An isochron of 135,137Ba* was constructed relative to 138Ba* to remove uncertainty with respect to the relative Ba/Cs SIMS ion yield and fractionation or loss of 133Cs (Fig. 6A). The Ru metal inclusion was collinear with the other Ru aggregates in this parameterization, so it shared an age relationship. Since the 235U fission yields of 133Cs, 135Cs, 137Cs, and 138Ba were all similar (6.7%, 6.6%, 6.2%, and 6.8%, respectively), and with comparable abundances of 135Ba* and 137Ba* in the Ru grains, we deduced qualitatively that closure occurred within the lifetime of 137Cs following criticality based upon the large difference in 135,137Cs half-lives, in agreement with refs. 25 and 26. Therefore, 135Ba* and 137Ba* must have migrated as Cs before capture. Fig. 6A, relating 137Ba*/138Ba* vs. 135Ba*/138Ba*, depicts a slope of 137Ba*/135Ba* = 0.858 ± 0.018 and an intercept of 137Ba*/138Ba* = 0.113 ± 0.012. The low abundance of 138Ba* in some of the Ru aggregates was responsible for the larger error ellipses. The isochron slope yielded an age of y using Eq. 1:
| [1] |
where Csif is the fission yield for isotope i (Cs137f/Cs135f = 0.943). This agreed with an age estimate of y from the 137Ba*/138Ba* intercept of Fig. 6A using Eq. 2:
| [2] |
where Cs137f/Ba138f = 0.965. From these two estimates, we derived a mean formation age of 4.98 ± 0.56 y for the Ru aggregates following reactor shutdown. We assumed that Cs was being continuously lost from the uraninite during reactor operation since Cs is not compatible with the uraninite matrix (9). Our estimate was shorter than the 20-y timescale previously postulated (25). The isochrons in Fig. 6 B and C show the 135Ba*/138Ba* and 137Ba*/138Ba* ratios relative to 133Cs/138Ba*. Similar to the isochrons in Fig. 5, slopes less than Cs135f/Cs133f = 0.975 and Cs137f/Cs133f = 0.920 indicated an excess of 133Cs relative to the expected fission yield. We assumed that this corresponded to 133Cs produced earlier during reactor operation, since nearly all of the Cs in the Oklo reactor was fissionogenic (25). The 135Ba*/138Ba* intercept in Fig. 6B yielded a nonsensical age of 680,000 y, far longer than the 24,200-y operating time for RZ 13 (3, 4). This was likely due to Cs loss during the intrusion of a dolerite dike ∼850 Ma ago, which would have shifted the isochrons uniformly to the left, increasing the 135Ba*/138Ba* intercept and the apparent age. The intercept in Fig. 6C was similarly compromised by apparent 133Cs loss, so only the isochron in Fig. 6A, with no 133Cs dependence, was useful for dating. Despite the posited loss of 133Cs >1 Ga following reactor shutdown, there was no evidence for loss of fissionogenic Ba, which can form stable BaRuO4 (27) and other Ru oxides via cation substitution (28, 29).
The isochron in Fig. 6D relating 134Ba*/138Ba* to 133Cs/138Ba* was more complicated since 134Ba is not produced from the decay of fission products, but from the burnup of 133Cs to 134Cs. The Ru inclusion was excluded from the linear regression. The positive slope (0.102 ± 0.011) of the isochron indicated that 134Cs was live during Ru formation, which agreed with our estimate of a 5-y formation time. The isochron was not useful, however, for estimating the neutron flux because we could not accurately determine fractionation within the system on this timescale or the addition of 133Cs following reactor shutdown. Using the isochron slope, we estimated the neutron flux immediately before reactor shutdown to be 6.8 × 1020 n∙cm−2∙y−1, a nonsensical value. We assumed a neutron capture cross-section of 28.9 barns and a resonance integral of 446.2 barns (24), a spectral index of 0.241 (4), a temperature of 1,000 K, and the 5-y decay time following reactor shutdown. The large negative intercept on the 134Ba*/138Ba* axis, which would have been more negative before any Cs loss, is also difficult to explain.
Fractionation between Cs and Ba was likely in the uraninite, given the slopes of 135Ba*/136Ba (0.621 ± 0.011) and 138Ba*/136Ba (0.501 ± 0.020) relative to 133Cs/136Ba of the interuraninite Ru, which are less than the expected fission yield (Fig. 5). The overabundance of 135Ba* (as live 135Cs*) relative to 138Ba* indicated that Cs was more efficiently lost from the uraninite and able to be captured by the intergrain Ru. Conversely, the overabundance of 138Ba* in the Ru inclusion relative to both 135Ba* and other Ru aggregates indicated that fissionogenic Ba was retained more efficiently within the uraninite. Fractionation due to Ru formation is difficult to account for without another secondary mineral phase for comparison. We conclude, however, that the fractionation, or relative loss, of Cs/Ba from the uraninite lies in the range of 1.2–2.4. The similar 135Ba*/136Ba, 137Ba*/136Ba, and 133Cs/136Ba ratios in the inclusion, matching fission yields, further show that these isotopes behaved predominantly as Cs before formation, with little fractionation between them.
Discussion
While the Oklo phenomenon has been widely investigated for several decades, the application of new technology has yielded immediate discoveries. The NRL NAUTILUS micrometer-scale, molecule-free imaging capability enabled the identification of trace isotope concentrations in highly heterogeneous materials, which we believe to be the only accelerator mass spectrometry (AMS) imaging capability for electropositive elements. These measurements by conventional SIMS were obscured due to large molecular backgrounds and nonnormal isotopic compositions.
We made direct measurements of U isotopes, fission products, and n-capture products, which were not possible by conventional SIMS. We found the world’s most depleted natural U and identified burnup depletions in Sm, Eu, Gd, and Dy in situ. RZ 13 appears to be a special RZ, being well preserved deep underground and having experienced the highest neutron fluxes over a shorter period of time than other reactors (3, 4). We discovered that Ru metal and sulfides were collocated with fissionogenic Cs and Ba, which was captured 5 y following reactor shutdown. Furthermore, these radioactive fission products were captured in the most active region of all Oklo reactors. Understanding the release and sequestration of specific radiogenic signatures into the environment is important, and this discovery provides insight toward long- and short-term nuclear-waste moderation strategies. Whereas most fissionogenic Ba and Cs were lost from the reactor, Ru captured and held the fission products for 2 Ga following reactor shutdown, with the intrusion of a dolerite dike likely caused loss of 133Cs ∼1 Ga following reactor shutdown. Cesium appears to be more efficiently lost from the reactor fuel than Ba, but at least as efficiently captured by Ru metal and sulfides. It would be of great interest to know whether similar Ru–Cs–Ba correlations are present in Ru aggregates from other RZs and whether the Ru phase (e.g., metal, oxide, or sulfide) affects Cs and Ba capture and retention. Nanoscale transmission electron microscopy could illuminate the bonding differences of Cs relative to Ba in Ru metal/sulfides. The natural nuclear reactor samples from Oklo and Bangombé have provided a wealth of information over the decades regarding the migration and storage of fission products, and based upon recent findings, further investigation is warranted.
Methods
A description of the NAUTILUS instrument is provided in refs. 14–16, but at its most basic level, it can be understood as a SIMS instrument with an additional AMS “detector,” which removes molecular isobars. SIMS and AMS are otherwise well known commercial capabilities. O− and O2− probes between 100 pA and 1 nA were used to perform imaging and spot measurements. See SI Appendix for further details.
Supplementary Material
Acknowledgments
We thank Maurice Pagel (University of Paris) for providing this extraordinary Oklo sample. We thank Kim Knight (Lawrence Livermore National Laboratory) for helpful discussions. This work was supported by Basic Research funding from the Office of Naval Research through NRL.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807267115/-/DCSupplemental.
References
- 1.Bodu R, Bouzigues H, Morin N, Pfiffelmann JP. Sur l’existence d’anomalies isotopiques recontrees dans l’uranium du Gabon. C R Acad Sci Paris. 1972;275:1731–1734. [Google Scholar]
- 2.De Laeter JR, Hidaka H. The role of mass spectrometry to study the Oklo-Bangombé natural reactors. Mass Spectrom Rev. 2007;26:683–712. doi: 10.1002/mas.20141. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier-Lafaye F, Holliger P, Blanc PL. Natural fission reactors in the Franceville basin, Gabon: A review of the conditions and results of a “critical event” in a geologic system. Geochim Cosmochim Acta. 1996;60:4831–4852. [Google Scholar]
- 4.Hidaka H, Holliger P. Geochemical and neutronic characteristics of the natural fossil fission reactors at Oklo and Bangombé, Gabon. Geochim Cosmochim Acta. 1998;62:89–108. [Google Scholar]
- 5.Ruffenach JC, Menes J, Devillers C, Lucas M, Hagemann R. Etudes chimiques et isotopiques de l’uranium, du plomb et de plusieurs produits de fission dans un echantillon de minerai du reacteur naturel d’Oklo. Earth Planet Sci Lett. 1976;30:94–108. [Google Scholar]
- 6.Meshik AP, Hohenberg CM, Pravdivtseva OV. Record of cycling operation of the natural nuclear reactor in the Oklo/Okelobondo area in Gabon. Phys Rev Lett. 2004;93:182302. doi: 10.1103/PhysRevLett.93.182302. [DOI] [PubMed] [Google Scholar]
- 7.Dymkov Y, Holliger P, Pagel M, Gorshkov A, Artyukhina A. Characterization of a La-Ce-Sr-Ca aluminous hydroxy phosphate in nuclear zone 13 in the Oklo uranium deposit (Gabon) Miner Depos. 1997;32:617–620. [Google Scholar]
- 8.Meshik AP, Kehm K, Hohenberg CM. Anomalous xenon in zone 13 Okelobondo. Geochim Cosmochim Acta. 2000;64:1651–1661. [Google Scholar]
- 9.Brookins DG, Lee MJ, Mukhopadhyay B, Bolivar SL. Search for Fission-Produced Rb, Sr, Cs and Ba at Oklo. International Atomic Energy Agency; Vienna: 1975. [Google Scholar]
- 10.Hidaka H, Konishi T, Masuda A. Reconstruction of cumulative fission yield curve and geochemical behaviors of fissiogenic nuclides in the Oklo natural reactors. Geochem J. 1992;26:227–239. [Google Scholar]
- 11.Ewing RC. Long-term storage of spent nuclear fuel. Nat Mater. 2015;14:252–257. doi: 10.1038/nmat4226. [DOI] [PubMed] [Google Scholar]
- 12.Beresford NA, et al. Thirty years after the Chernobyl accident: What lessons have we learnt? J Environ Radioact. 2016;157:77–89. doi: 10.1016/j.jenvrad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Koo Y-H, Yang Y-S, Song K-W. Radioactivity release from the Fukushima accident and its consequences: A review. Prog Nucl Energy. 2014;74:61–70. [Google Scholar]
- 14.Fahey AJ, Groopman EE, Grabowski KS, Fazel KC. Measurement of uranium isotopes in particles of U3O8 by secondary ion mass spectrometry-single-stage accelerator mass spectrometry (SIMS-SSAMS) Anal Chem. 2016;88:7145–7153. doi: 10.1021/acs.analchem.6b01209. [DOI] [PubMed] [Google Scholar]
- 15.Grabowski KS, Groopman EE, Fahey AJ. Enhanced spatially-resolved trace analysis using combined SIMS–single-stage AMS. Nucl Instrum Methods Phys Res Sect B. 2017;410:41–46. [Google Scholar]
- 16.Groopman EE, Grabowski KS, Fahey AJ, Koop L. Rapid, molecule-free, in situ rare earth element abundances by SIMS-SSAMS. J Anal At Spectrom. 2017;32:2153–2163. [Google Scholar]
- 17.Richter S, et al. New average values for the n(238U)/n(235U) isotope ratios of natural uranium standards. Int J Mass Spectrom. 2010;295:94–97. [Google Scholar]
- 18.Loss RD, et al. The Oklo natural reactors: Cumulative fission yields and nuclear characteristics of Reactor Zone 9. Earth Planet Sci Lett. 1988;89:193–206. [Google Scholar]
- 19.Holliger P, Devillers C. Contributionàl’étude de la température dans les réacteurs fossiles d’Oklo par la mesure du rapport isotopique du lutétium. Earth Planet Sci Lett. 1981;52:76–84. [Google Scholar]
- 20.Hidaka H. Isotopic study of natural fission reactors at Oklo and Bangombe, Gabon. J Radioanal Nucl Chem. 1999;239:53–58. [Google Scholar]
- 21.Crozaz G, Zinner E. Ion probe determinations of the rare earth concentrations of individual meteoritic phosphate grains. Earth Planet Sci Lett. 1985;73:41–52. [Google Scholar]
- 22.Fahey AJ. Details of the measurement of rare earth and other trace element abundances by secondary ion mass spectrometry. Int J Mass Spectrom. 1998;176:63–76. [Google Scholar]
- 23.Zinner E, Crozaz G. Ion probe determination of the abundances of all the rare earth elements in single mineral grains. Int J Mass Spectrom Ion Process. 1986;69:17–38. [Google Scholar]
- 24.Shibata K, et al. JENDL-4.0: A new library for nuclear science and engineering. J Nucl Sci Technol. 2011;48:1–30. [Google Scholar]
- 25.Hidaka H, Gauthier-Lafaye F. Ba isotopic signature for early differentiation between Cs and Ba in natural fission reactors. Geochim Cosmochim Acta. 2008;72:4123–4135. [Google Scholar]
- 26.Hidaka H, Holliger P, Masuda A. Evidence of fissiogenic Cs estimated from Ba isotopic deviations in an Oklo natural reactor zone. Earth Planet Sci Lett. 1993;114:391–396. [Google Scholar]
- 27.Donohue PC, Katz L, Ward R. The crystal structure of barium ruthenium oxide and related compounds. Inorg Chem. 1965;4:306–310. [Google Scholar]
- 28.Donohue PC, Katz L, Ward R. The modification of structures of ternary oxides by cation substitution. I. Substitution of strontium for barium in barium ruthenium oxide and in barium iridium oxide. Inorg Chem. 1966;5:335–338. [Google Scholar]
- 29.Donohue PC, Katz L, Ward R. The modification of structures of ternary oxides by cation substitution. II. Substitution of various cations for ruthenium in barium ruthenium oxide. Inorg Chem. 1966;5:339–342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.