Abstract
In this article, we introduced a rat model of central fatigue using the modified multiple platform method (MMPM). The Multiple Platform box was designed as a water tank with narrow platforms on the bottom. The model rats were put into the tank and stood on the platforms for 14 h (18:00 - 8:00) per day for a consecutive 21 days, with a blank control group set for contrast. At the end of modeling, rats in the model group showed an obvious fatigued appearance. To assess the model, we performed several behavioral tests, including the open field test (OFT), the elevated plus maze (EPM) test, and the exhaustive swimming (ES) test. The results showed that anxiety, spatial cognition impairment, poor muscle performance, and declined voluntary activity presented in model rats confirm the diagnosis of central fatigue. Changes of the central neurotransmitters also verified the result. In conclusion, the model successfully simulated central fatigue, and future study with the model may help reveal the pathological mechanism of the disease.
Keywords: Neuroscience, Issue 138, Modified multiple platform method (MMPM), rat model, central fatigue, behavioral test
Introduction
Fatigue is one of the main factors that threaten human health1. In the past decades, various researches have proved that fatigue is peripherally-triggered but centrally-driven, and always accompanied with emotional and cognitive disorders. Italian physiologist A. Mosso first proposed the word Central Fatigue2. It is generally defined as limited voluntary activity and cognition impairment due to dysfunction of impulse transmission in the central nervous system (CNS)3. Compared with peripheral muscle fatigue, central fatigue emphasizes changes in the CNS, as well as the consequent emotional/behavioral disturbances, including depression, anxiety, cognition impairment, and memory loss. One study shows that many factors can induce central fatigue, among which excessive physical activity and mental stress are quite indispensable4. As for the pathogenesis, theories like the tryptophan-kynurenine pathway hypothesis5 explain changes in certain pathways; however, more in-depth studies are still required to reveal the central-peripheral correlations of central fatigue.
As the underlying mechanism of central fatigue is still unclear, an effective animal model is quite important for further research. The existing fatigue models are mostly induced by excessive exercise, like treadmills6 and weight-loaded swimming7, with little concern on mental factors. To better simulate the development of central fatigue, our group developed a rat model with the MMPM. During the modeling process, rats remain standing on the narrow platforms in the Multiple Platform box for long hours including part of the sleep time. Different from excessive exercise models, the MMPM model uses partial sleep deprivation as a mental factor in consideration of the complex pathogenesis of central fatigue.
For model evaluation, we use the OFT and EPM tests to determine anxiety mood and voluntary activity. The ES test is performed to measure the peripheral muscle performance. In addition, we take the rat's brain and detect dopamine (DA)/serotonin (5-HT) content in both hypothalamuses to observe the central neurotransmitter differences.
The protocol presented below is designed to model central fatigue induced by repeated physical activity and lack of sleep, mimicking a common condition in human life. However, by adjusting the model duration, it can be used in many other fields, like in sleep observation and stress studies. In future research, we hope that this model will help discover more CNS changes and their connection with the peripheral system, to reveal the pathogenesis mechanism of central fatigue.
Protocol
All the animals were maintained in accordance with the guidelines by the Chinese legislation on the ethical use and care of laboratory animals.
1. Pre-modeling Preparation
- Laboratory preparation
- Run the UV lamp for at least 30 min before the experiment.
- Control the lab temperature at 25 ± 3 °C, and relative humidity around 30%.
- Turn on the lab light at 6:00 and turn it off at 18:00 to establish a 12 h/12 h light/dark cycle.
- Multiple Platform box construction
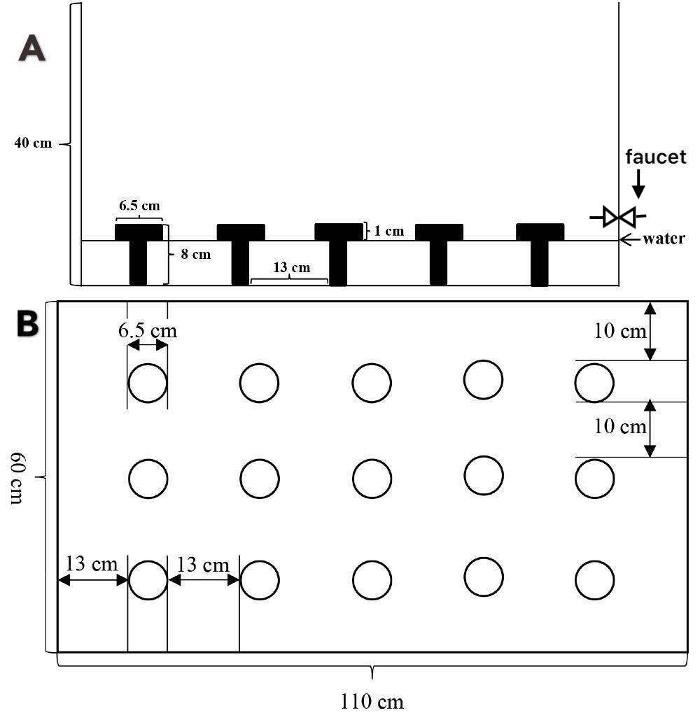
- Construct an opaque plastic tank without a cover of 110 × 60 × 40 cm3.
- Fix fifteen circular platforms (h = 8 cm, d = 6.5 cm) on the bottom of the tank, which orderly distribute in three rows and five columns. Leave enough space between each platform, roughly 10 cm between columns and 13 cm between rows.
- Set a water outlet on the lateral side of the tank and install a faucet.
- Make an iron-wire netting cover for the tank with a food box hanging on it.
- Grouping and housing rats NOTE: Wistar male rats of 8 weeks old, weighing approximately 200 - 210 g, are used in the experiment. The rats live in groups during the modeling process.
- Number the rats' tail roots with a marker pen.
- Weigh the rats, exclude the extremely light or heavy ones, and divide the rest randomly into the model and control groups.
- Put the rats gently into the clean cages and allow them to acclimate to the lab for at least 3 days. Provide sufficient water and food supply.
2. Modeling with MMPM
NOTE: The process starts at 18:00 and ends at 8:00 the next day, for a total of 14 h per day, over 21 days. To avoid interference factors, the same person is required to conduct the entire experiment, while wearing the same lab coat. 10 Wistar rats are used in the experiment.
Place the tank on a flat surface, e.g., the floor. Then fill the tank with roughly 7 cm of warm (25 ± 3 °C) water, approximately 1 cm below the platform flat.
Prepare enough food and drink for all the rats in the tank for 1 day. Put fodder and water into the food box and hang it on the cover. NOTE: Some smart rats learn to rest on the food box. If so, drive them back into the tank.
Take the model group rats out of the cage, grab them by the tail, and put them gently into the tank. Launch all the rats in the water instead of the platforms to motivate their fear of water. Make sure that every rat gets a platform to stand on, while rats of the control group stay in their original cages with sufficient food and water.
Cover the tank. Monitor the rats to avoid accidental injury. If a rat stays in water for more than 1 h without climbing onto the platform, pick it out of the tank and remove it from the test.
After 14 h, take the model rats out of the tank and dry their hair with a dryer. Re-mark the rats' tails if it fades. Return the rats to their original cages and provide them with sufficient food and water.
Flush every corner of the tank. Elevate one side of the tank and open the faucet to outflow the sewage.
Sterilize the tank with a 75% ethanol spray and expose it to the UV light.
3. Model Assessment: Behavioral Test
NOTE: All the tests are performed in the behavioral lab. Noise and extra light are not allowed during the test to avoid disturbance. If possible, use the same person(s) to conduct each test. A dark coat and gloves are required for grey scale recognition in image processing. Perform the OFT first as it has the least effect on rat behavior.
- OFT
- Check the recorder over the Open Field Box to make sure it is properly connected to the workstation and covers every corner of the box. Adjust the lighting to eliminate shadows in the box.
- Move the rats into the behavioral lab in their original cages. Allow them to acclimate for at least 1 h before the test.
- Clean and sanitize the box with 75% ethanol to ensure that there is no excrement or smell left from previous experiment.
- Remove a rat from the cage by its back and put it gently into the central area of the box. Quickly retreat arms from the box so as not to block the shot.
- Input the rat's number and start recording. Count and record the frequency of the rat's vertical activities, including rearing and climbing.
- After 5 min, stop recording, take the rat out of the box, and return it to the cage.
- Repeat steps 3.1.3 - 3.1.6 until all the rats have finished the test.
- EPM
- Perform the pre-check and acclimation steps as for the OFT (steps 3.1.1 - 3.1.2).
- Remove a rat from the cage by its back and put it gently onto the junction part of the two arms. Land the rat towards the left open arm and leave quickly so as not to block the shot.
- Input the rat's number and start recording. Count and record the frequency of different arm entrances. If the rat drops off the maze in the test, pick it up and send it back to the maze. Record detailed information for data analysis.
- After 5 min, stop recording, take the rat out, and return it to the cage.
- Remove the excrement and wipe the maze with 75% ethanol to eliminate the former rat's smell.
- Repeat steps 3.2.2 - 3.2.5 until all the rats have finished the test.
- ES test
- Fill the swimming tank (70 × 30 × 110 cm3) with 80 cm of warm (25 ± 3 °C) water. NOTE: If there is a thermostat in the tank, the water temperature should be set around 37 °C, which is similar to the rat's body temperature. If not, set it to room temperature to keep it constant.
- Make a load for each rat with pin bunches and tie it gently on its tail root. The load weighs 10% of the rat's weight.
- Grab a rat by the tail and throw it into the swimming tank. If the rats huddle or cling to the wall, set them apart and drive them back into water.
- Start timing at the moment when the rat is put into water and stop timing when it's exhausted, which is demonstrated as the failure to struggle out of water with the mouth and nose beneath the water for more than 10 s. NOTE: Sometimes, exhaustion and drowning occur suddenly. Be sure to have enough experimenters to record and save the animal at the same time.
- Remove the exhausted rats out of the water without interrupting others. Dry their hair, re-mark their numbers, and send them back to cage.
- Change the water in the tank after one group finishes. After all the rats are done, empty the swimming tank, and clean and sterilize it with ethanol and UV light.
4. Model Assessment: Central Neurotransmitter Detection
Anesthetize the rat with intra-peritoneal injection of 10% chloral hydrate (3 mL/kg) until it is unconscious.
Decapitate the rat.
Make a longitudinal incision along the post-medial line, open the cranium to both sides, and expose the brain. Turn the cranium over, remove the brain, and put the brain on an ice bag.
Separate and remove the hypothalamus, which is the diamond-shaped area in the central part of the base of the brain that has a clear boundary with surrounding tissues. Place it in a sterile tube and freeze it with liquid nitrogen. Store all the samples in a -80 °C refrigerator.
Detect the content of DA and 5-HT in the hypothalamus using high-performance liquid chromatography (HPLC)8.
Representative Results
We describe a rat model of central fatigue using MMPM. 24 Wistar rats are randomly divided into the control group and the model group, with 12 rats in each group. The model apparatus is designed as a water tank with narrow platforms on the bottom (Figure 1). Model Rats stand on the platforms for 14 h per day, including partial sleep time, for 21 days (Figure 2).

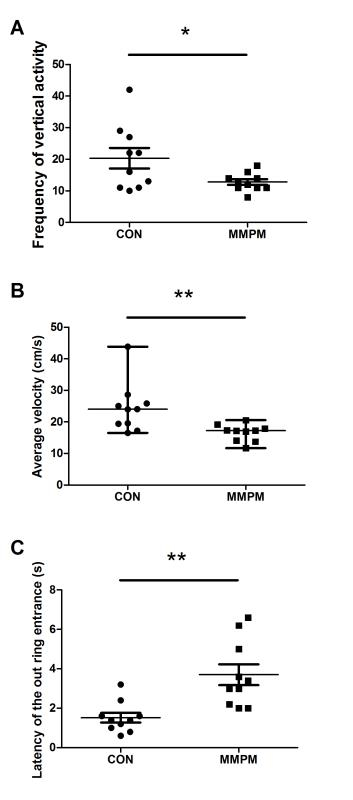
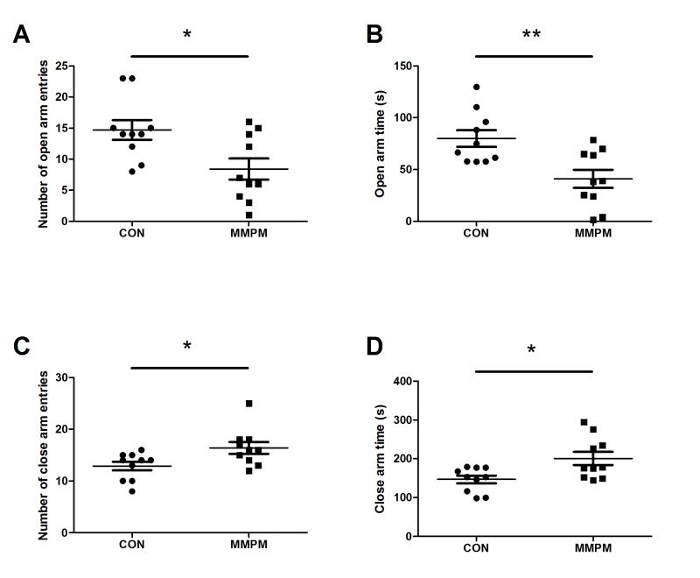
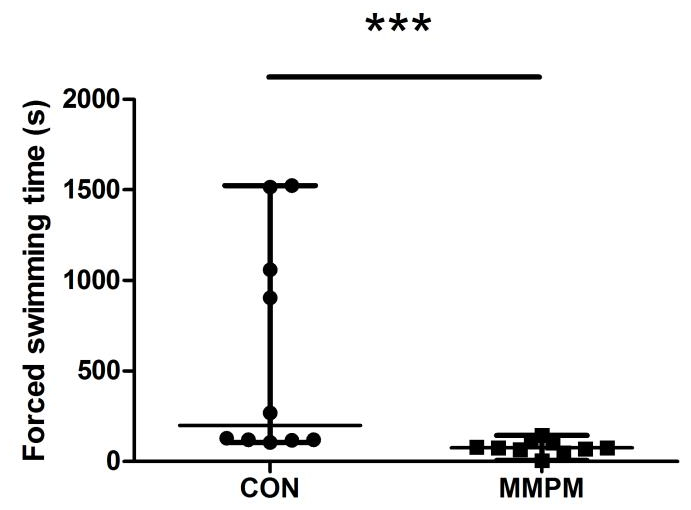
Behavioral tests are performed after modeling to assess the emotional and physical changes in the rats. The OFT result (Figure 3) shows that, compared with the control group (n = 10), there is a significant decrease in both rearing movement and average velocity of voluntary activity (p <0.05, p <0.01) in model rats (n = 10), and an obvious increase in latency of the out ring entrance (p <0.01). The EPM test (Figure 4) shows that 21 days of modeling decreased both frequency of open arm entries and duration in open arm significantly compared to the control group (n = 10) (p <0.05, p <0.01), while there was an increase in both frequency of close arm entries and duration in close arm (p <0.05). The result of the ES test (Figure 5) shows that the swimming duration of the model group (n = 10) is significantly shorter than the control group (n = 10) (p <0.001).
Next, we detect DA and 5-HT content in both hypothalamuses to observe the central neurotransmitter differences. Results (Figure 6) show that DA in the hypothalamus and the ratio of DA to 5-HT significantly decreases in the model group (n = 10) compared to the control group (n = 10) (p <0.05, p <0.01), while the 5-HT content increases significantly (p <0.05).
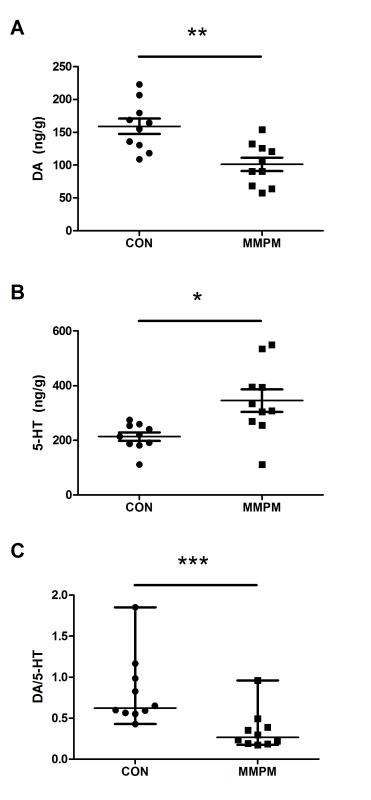
Figure 1: Schematic of the Multiple Platform box. (A) Front view. (B) Top view. The Multiple Platform box is a coverless plastic tank (110 × 60 × 40 cm3) with fifteen acrylic platforms fixed on the bottom and a faucet on the lateral side. Each platform consists of a pillar and a circular flat (d = 6.5 cm) platform bigger than the pillar top. The platforms (h = 8 cm) distribute in three rows and five columns. The adjacent platforms are 10 cm apart in the columns and 13 cm in the rows. The tank can hold a maximum of 15 rats. Please click here to view a larger version of this figure.
Figure 2: A photo of modeling. The rat standing on the platform was on the 15th day of modeling. Its dry hair and dim eyes suggest an obvious fatigue state.
Figure 3: Analysis of OFT. (A) Comparison of the frequency of vertical activities. Data are presented as mean ± SEM (n = 10). With unequal variance (F = 9.877, p = 0.006 <0.05), significance was determined by independent-sample t-test, t = 2.226, p = 0.049 <0.05. The frequency of the vertical activity (rearing) decreases in model rats (n = 10) compared with control rats (n = 10). (B) A comparison on the average velocity of the voluntary activity. Data are presented as median ± IQR (n = 10). Significance was determined by Mann-Whitney U test, z = -2.685, p = 0.007 <0.01. The average velocity of the voluntary activity in the model rats decreases in comparison with control rats. (C) Comparison on the latency of the out ring entrance. Data are presented as mean ± SEM (n = 10). With unequal variance (F = 5.748, p = 0.028 <0.05), significance was determined by t-test, t = -3.724, p = 0.03 <0.01. The latency of the out ring entrance increases in model rats, which means they spend more time before entering the out ring compared to control rats. Note: p<0.05 (*); p <0.01 (**); p <0.001 (***).
Figure 4: Analysis of EPM test. (A) Comparison on frequency of open arm entries. Data are presented as mean ± SEM (n = 10). With equal variance (F = 0.982, p = 0.348 >0.05), Significance was determined by t-test, t = 2.710, p = 0.014 <0.05. The frequency of open arm entries in model rats (n = 10) decreases compared with control rats (n = 10). (B) Comparison on duration in open arm. Data are presented as mean ± SEM (n = 10). With equal variance (F = 0.100, p = 0.755 >0.05), significance was determined by t-test, t = 3.304, p = 0.004 <0.01. The open arm duration in model rats decreases compared with control rats, which means the model rats spend less time in the open arm. (C) Comparison on frequency of close arm entries. Data are presented as mean ± SEM (n = 10). With equal variance (F = 0.141, p = 0.712 >0.05), significance was determined by t-test, t = -2.466, p = 0.024 <0.05. The frequency of the close arm entries in model rats increases compared with control rats.(D) Comparison on duration in close arm. Data are presented as mean ± SEM (n = 10). With unequal variance (F = 4.796, p = 0.042 <0.05), Significance was determined by t-test, t = -2.736, p = 0.0016 <0.05. The close arm duration in model rats increases compared with control rats, which means the model rats spend more time in the close arm. Note: p <0.05 (*); p <0.01 (**); p <0.001 (***). Please click here to view a larger version of this figure.
Figure 5: Analysis of Exhaustive Swimming test. Data are presented as median ± IQR (n = 10), and are reported as p <0.05 (*), p <0.01 (**), p <0.001 (***). Significance was determined by Mann-Whitney U test, z = -3.326, p = 0.001. The swimming time of the model rats (n = 10) is significantly shorter than the control rats (n = 10).
Figure 6: Analysis of central neurotransmitter content. (A) Comparison on DA content. Data are presented as mean ± SEM (n = 10). With equal variance (F = 0.088, p = 0.771 >0.05), significance was determined by t-test, t = 3.717, p = 0.002 <0.01. The DA content in both hypothalamuses decreases in model rats (n = 10), compared with the control rats (n = 10). (B) Comparison on 5-HT content. Data are presented as mean ± SEM (n = 10). With unequal variance (F = 5.282, p = 0.034 <0.05), significance was determined by t-test, t = -2.997, p = 0.012 <0.05. The 5-HT content in both hypothalamuses decreases in model rats, compared with the control rats. (C) Ratio Comparison. Data are presented as median ± IQR (n = 10). Significance was determined by Mann-Whitney U test, z = -3.175, p = 0.001. The ratio of DA to 5-HT significantly decreases in the model rats, compared with the control rats. Note: p <0.05 (*); p <0.01 (**); p <0.001 (***).
Discussion
The MMPM is originally designed for sleep deprivation9. Rats are launched into a water tank with platforms fixed on the bottom. Driven by the instinctive fear of water, rats remain standing on the platforms and no sleep occurs. The study shows that different hours of sleep deprivation lead to various changes in rat behavior and mood, including recognition impairment10, negative emotions11, and central fatigue. Some researchers prove that chronic sleep deprivation with the single platform method (SPM) can induce central fatigue, with recognition impairment and social disorders12. Other research shows that intermittent deprivation on consecutive days can cause emotional disorders and central fatigue, which can be treated with endorphins13. Our previous study proves that compared with 5 days and 14 days, 21 days deprivation induces more central fatigue rather than stress-related emotion disorders14. Many factors of MMPM may cause central fatigue, including the long hours of standing, narrow shelter space, tedious and repeated environment, as well as the lack of sleep. The underlying correlation between sleep deprivation and central fatigue may associate with the hypothalamic-pituitary-adrenal (HPA) axis in different levels, among which monoamine neurotransmitters changes may play a key role.
In this protocol, we develop a central fatigue model using MMPM and assess it with behavioral tests and neurotransmitter detection. First, we create a 12 h/12 h light/dark cycle (6:00 - 18:00) in the lab to imitate the natural circadian rhythm of the Wistar rat, whose average sleep time is 12.6 h, approximately 2.4 - 4.2 h at night, and 8.2 - 9.6 h at daytime15. Then the model rats are put into the Multiple Platform box to stand for 14 h (18:00 - 8:00) per day for 21 consecutive days, with a control group set for contrast. At the end of the experiment, rats in the model group show an obvious fatigue appearance, including dull hair, faint tail color, dim eyes, and decreased activity in the cage.
The results of the behavioral tests show changes in both the physical and emotional aspects. OFT is widely used in rodent model assessment to evaluate exploration behavior and voluntary activity16. Rodents have the instinct of thigmotaxis, that is, once they are put into an open field, they tend to move quickly into the out ring near the wall. At the same time, they are curious about the new environment and are eager to explore the central area by both vertical rearing and horizontal movements. The conflict of the two motivations reflects the anxiety mood17. The result of the OFT suggests declined voluntary activity in the model rats based on their decreased average velocity. Additionally, the frequency of rearing in the model rats significantly decreases compared to the control rats, which may imply anxiety emotion. Furthermore, the model rats tend to spend more time before entering the out ring with no obvious preference in exploration, suggesting spatial cognition disorder in the model rats. The EPM test is a classical test to assess anxiety. Rats with anxiety tend to stay in the close arm for safety instead of exploring the open arm18. The result shows that compared with the control rats, the model rats spend more time in the close arms and less time in the open arms, and this parallels the entrance frequency of the different arms, and overall verifies the anxiety mood in the model rats. In the ES test, the swimming duration of the model rats is much shorter than the control rats, suggesting poor muscle performance caused by fatigue. In conclusion, anxiety, cognition impairment, poor muscle performance, and limited voluntary activity appear in model rats, all indicating central fatigue.
As for the CNS, all the changes in the central neurotransmitters suggest central fatigue. We find a significant decrease in DA content and an increase in 5-HT of the hypothalamus. 5-HT is a monoamine neurotransmitter synthesized from the amino acid tryptophan (TRP). Intense activity will increase 5-HT generation by releasing more free TRP into the blood; the accumulated 5-HT in return, restrains the central control of the loco-motor system, leading to poor muscle porformance19. DA is an excitable neurotransmitter, which increases at the beginning of loco-motor activity and drops by the appearance of fatigue20. DA and 5-HT correlate and interact as an excitation-inhibition system that effects the central control of the loco-motor system21. Thus, the drop in the ratio of DA to 5-HT is an important indicator of central fatigue.
There are some notes in the protocol that are critical to success. First, the model duration and conditions are tested with male Wistar rats. The temperature preference and sleep duration differ among strains and genders14. Secondly, the rats should live in groups, with at most 6 rats in a cage, and provided with sufficient food and water throughout the experiment. During the first two weeks of modeling, the rats are quite irritated and may fight both in the tank and in the cages. Keep monitoring them and prevent the injured rats from death. Also, remember to dry the rats' hair after they are removed from the tank, especially in winter to avoid cold conditions.
Though the model is designed for central fatigue, it is feasible to add complex factors to enlarge its use. For example, we install vibration motors and springs to the platform to imitate the waves in sea and change the deprivation pattern in attempt to establish a navigation fatigue model. By adjusting the model duration, it can be used in many other fields. As an animal model study, the research has its limitation. First, there is no proof in predictive validity of the model. In a future study, we should perform anti-fatigue treatment on rats and assess their recovery to prove the model's validity. Besides, current assessment of the model focuses more on negative emotion and CNS changes; however, central fatigue also manifests as a learning difficulty and social avoidance12. Behavioral tests like the Morris Water maze and social interaction test can be conducted in the future to obtain a more comprehensive understanding of the disease. We hope the central fatigue model introduced here may help to explore the pathological mechanism of central fatigue.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by Natural Science Foundation of Beijing (No.7162124), and Xin-ao Foundation for Beijing University of Chinese Medicine.
References
- Ishii A, Tanaka M, Yamano E, Watanabe Y. The neural substrates of physical fatigue sensation to evaluate ourselves: a magnetoencephalography study. Neuroscience. 2014;261:60–67. doi: 10.1016/j.neuroscience.2013.12.049. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Secher NH. The Brain at Work: A Cerebral Metabolic Manifestation of Central Fatigue? Journal of Neuroscience Research. 2007;85(15):3334–3339. doi: 10.1002/jnr.21274. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue in neurological disorders. The Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Baston G. Exercise-induced central fatigue: a review of the literature with implications for dance science research. Journal of Dance Medicine & Science. 2013;17(2):53–62. doi: 10.12678/1089-313x.17.2.53. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Yamamoto T. Tryptophan and Kynurenic Acid May Produce an Amplified Effect in Central Fatigue Induced by Chronic Sleep Disorder. International Journal of Tryptophan Research. 2014;7:9–14. doi: 10.4137/IJTR.S14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, et al. The impact of duration of one bout treadmill exercise on cell proliferation and central fatigue in rats. Journal of Exercise Rehabilitation. 2013;9(5):463–469. doi: 10.12965/jer.130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su kY, et al. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. International Journal of Medical Sciences. 2014;11(5):528–537. doi: 10.7150/ijms.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi F, Laufer R, Szegi P, Csomor V, Kal ász H, Tekes K. HPLC determination of brain biogenic amines following treatment with bispyridinium aldoxime K203. Acta Physiologica Hungarica. 2014;101(1):40–46. doi: 10.1556/APhysiol.101.2014.1.5. [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipo'lide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Research. 2004;1004(1-2):45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Tashtoush NH, AI-Azzam SI, Mhaidat NM. Evaluation of the Effect of Pentoxifylline on Sleep-Deprivation Induced Memory Impairment. Hippocampus. 2013;23(9):812–819. doi: 10.1002/hipo.22135. [DOI] [PubMed] [Google Scholar]
- Pires GN, Tufik S, Andersen ML. Grooming analysis algorithm: Use in the relationship between sleep deprivation and anxiety-like behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;41:6–10. doi: 10.1016/j.pnpbp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Yamamoto T. Establishment of a rat model of central fatigue induced by chronic sleep disorder and excessive brain tryptophan. Japanese Journal of Cognitive Neuroscience. 2013;15:67–74. [Google Scholar]
- Arai M, Yamazaki M, Inoue K, Fushiki T. Effects of intracranial injection of transforming growth factor-beta relevant to central fatigue on the waking electroencephalogram of rats Comparison with effects of exercise. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(2):307–312. doi: 10.1016/s0278-5846(01)00272-x. [DOI] [PubMed] [Google Scholar]
- Han CX, et al. Distinct behavioral and brain changes after different durations of the modified multiple platform method on rats: An animal model of central fatigue. PloS One. 2017;12(5):e0176850. doi: 10.1371/journal.pone.0176850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yang L, Sanford LD. Individual variation in sleep and motor activity in rats. Behavioural Brain Research. 2007;180(1):62–68. doi: 10.1016/j.bbr.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SC. The Open Field Test: reinventing the wheel. Journal of Psychopharmacology. 2007;21(2):134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- Ahn SH, et al. Basal anxiety during an open field test is correlated with individual differences in contextually conditioned fear in mice. Animal Cells and Systems. 2013;17(3):154. [Google Scholar]
- Costa AA, Morato S, Roque AC, Tin ós R. A computational model for exploratory activity of rats with different anxiety levels in elevated plus-maze. Journal of Neuroscience Methods. 2014;236:44–50. doi: 10.1016/j.jneumeth.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wu Y, Liu T, Li R, Xie M. Serotonin regulation in a rat model of exercise-induced chronic fatigue. Neuroscience. 2017;349:27–34. doi: 10.1016/j.neuroscience.2017.02.037. [DOI] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. NeuroMolecular Medicine. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Leite LH, Rodrigues AG, Soares DD, Marubayashi U, Coimbra CC. Central fatigue induced by losartan involves brain serotonin and dopamine content. Medicine & Science in Sports & Exercise. 2010;42(8):1469–1476. doi: 10.1249/MSS.0b013e3181d03d36. [DOI] [PubMed] [Google Scholar]


