Abstract
We correlate chromosome 5 haplotypes and SMN2 copy number with disease expression in 42 Mennonite and 14 Amish patients with spinal muscular atrophy (SMA). A single haplotype (A1) with 1 copy of SMN2 segregated among all Amish patients. SMN1 deletions segregated on four different Mennonite haplotypes that carried 1 (M1a, M1b, M1c) or 2 (M2) copies of SMN2. DNA microsatellite and microarray data revealed structural similarities among A1, M1a, M1b, and M2. Clinical data were parsed according to both SMN1 genotype and SMN2 copy number (2 copies, n = 44; 3 copies, n = 9; or 4 copies, n = 3). No infant with 2 copies of SMN2 sat unassisted. In contrast, all 9 Mennonites with the M1a/M2 genotype (3 copies of SMN2) sat during infancy at a median age of 7 months, and 5 (56%) walked and dressed independently at median ages of 18 and 36 months, respectively. All are alive at a median age of 11 (range 2–31) years without ventilatory support. Among 13 Amish and 26 Mennonite patients with 2 copies of SMN2 who did not receive feeding or ventilatory support, A1/A1 as compared to M1a/M1a genotype was associated with earlier clinical onset (p = 0.0040) and shorter lifespan (median survival 3.9 versus 5.7 months, p = 0.0314). These phenotypic differences were not explained by variation in SMN1 deletion size or SMN2 coding sequence, which were conserved across haplotypes. Distinctive features of SMA within Plain communities provide a population-specific framework to study variations of disease expression and the impact of disease-modifying therapies administered early in life.
Introduction
Biallelic deletions of SMN1 cause spinal muscular atrophy (SMA; MIM# 253300), a common monogenic cause of infant mortality characterized by progressive degeneration of lower motor neurons [1–4]. SMN1 encodes survival motor neuron protein (SMN), which is 294 amino acids in length (32 kDa) and interacts with other proteins to influence ribonucleoprotein assembly, ubiquitin homeostasis, cytoskeletal dynamics, endocytosis, and neuromuscular junction stability [5–7]. SMN is expressed in tissues throughout the body but lower motor neurons are particularly vulnerable to its absence [4, 6].
The SMA locus on human chromosome 5q13 contains telomeric SMN1 and centromeric SMN2, both of which produce SMN protein, and 8 other genes aligned within a 2 Mb vicinity (OCLN, GTF2HCDC, SERF1B, SERF1A, NAIP, GTF2H2, LOC647859, and LINC02197). This region is subject to deletions, duplications, and rearrangements that damage SMN1 and its neighboring genes and also give rise to a variable number of SMN2 copies [7]. Compared to SMN1, SMN2 contains a base difference (c.850C>T) that excludes exon 7 from approximately 90% of mRNA transcripts to produce a truncated protein (SMNΔ7; 282 amino acids, 30.5 kDa) that is non-functional and rapidly degraded [8]. Residual intact SMN translated from each SMN2 copy partially compensates for SMN1 deficiency such that genomic SMN2 copy number correlates inversely with disease severity [9–11]: two SMN2 copies commonly segregate with a severe (type 1) SMA phenotype whereas three or more copies correlate with later disease onset and slower progression [12, 13].
Within ethnically mixed (i.e. outbred) populations, carrier frequencies for pathogenic SMN1 deletions are between 1.4% (African American) and 2.1% (Caucasian)[14, 15], but range from 3 to 13% in certain endogamous populations of Israel [16, 17], Hungary [18], Spain [19], France [20], and the United States [21]. In contrast to the individuals who comprise a multiethnic cohort, patients from a common founder lineage are typically homozygous for the same SMN1 mutation, share a large proportion of background genetic material, experience similar dietary and environmental exposures, live in comparable socioeconomic conditions, and adhere to similar patterns of medical resource utilization. Thus, founder groups naturally control for many genetic and environmental variables that complicate natural history studies in outbred cohorts [22, 23].
Spinal muscular atrophy is relatively common among Old Order Amish and Mennonite (Plain) populations. Contemporary Plain people descended from a small group of Swiss Anabaptist founders who migrated to the New World during the early 18th century and have remained isolated in small demes across North and South America [24]. Here, we investigate ancestral chromosome 5 haplotypes in relation to SMA phenotype among 42 Mennonite and 14 Amish individuals harboring 2 (n = 44), 3 (n = 9), or 4 (n = 3) copies of SMN2. This is the first comprehensive clinical description of SMA within Plain communities and provides a unique lens through which to view the natural history of disease undistorted by technological interventions, genetic variability, and heterogenous approaches to care.
Results
Genotype
SMA haplotype structure and segregation
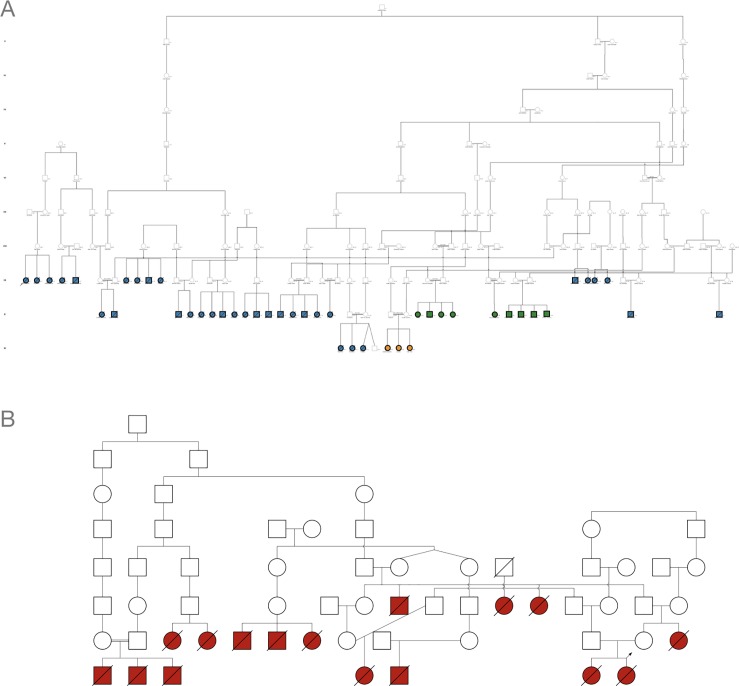
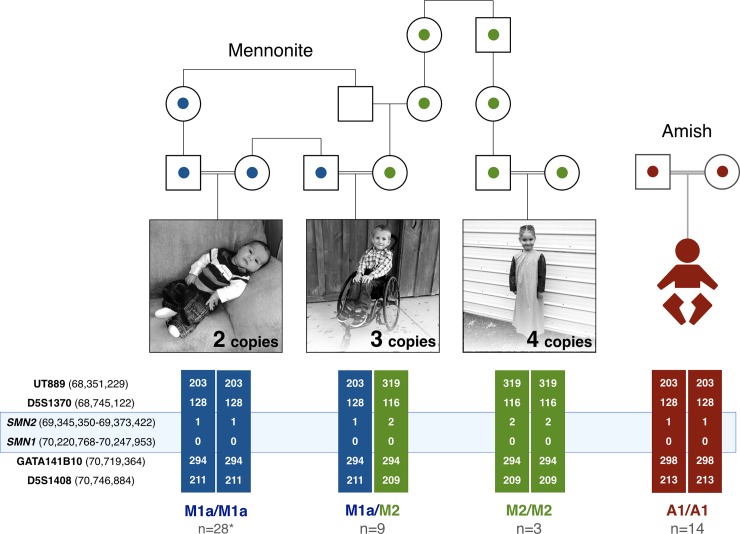
We used DNA microsatellites and 2.6 million-marker single nucleotide polymorphism (SNP) microarrays to study the 5q13 SMA locus of Mennonite SMA patients and their extant family members, all of whom traced back 11 generations to a common ancestor (Fig 1A). A pathogenic SMN1 deletion segregated on two major Mennonite haplotypes (M1a, M2) that encompassed 1 and 2 copies of SMN2, respectively (Fig 2). M1a/M1a (2 copies SMN2, n = 28), M1a/M2 (3 copies SMN2, n = 9), and M2/M2 (4 copies SMN2, n = 3) were the predominant SMA genotypes, segregating predictably with severe (70%), moderate (20%), or mild (10%) disease (Fig 2).
Fig 1. Spinal muscular atrophy in Mennonite and Amish pedigrees.
(A) Forty-two Mennonite SMA patients with homozygous deletions of SMN1 traced to a common founder through 11 generations but had variable numbers of SMN2 copies (blue, 2 copies of SMN2; green, 3 copies of SMN2; orange, 4 copies of SMN2). (B) Although we could not connect 14 Amish SMA patients using extant genealogical records, all were homozygous for the same SMA haplotype (red, 2 copies of SMN2) indicating common ancestry.
Fig 2. Segregation of SMA haplotypes.
(A) To map SMA haplotypes, we used DNA microsatellite markers in close proximity to SMN1 and SMN2 on chromosome 5q13. Among Mennonites, pathogenic SMN1 deletions segregated on four different haplotypes that harbored either 1 (blue; M1a, M1b, M1c) or 2 (green; M2) copies of SMN2 to produce genotypes comprising 2 (M1/M1; n = 30), 3 (M1/M2; n = 9), or 4 (M2/M2; n = 3) copies of SMN2. (B) A single ancestral haplotype (A1) with 1 copy of SMN2 was inferentially shared among all 14 Amish patients homozygous for the A1/A1 genotype (red; 2 copies of SMN2).
Two minor Mennonite SMA haplotypes (M1b and M1c) each contained 1 copy of SMN2 and were compound heterozygous with M1a in a single patient. M1b did not share proximal microsatellite markers with M1a but matched distally (S1 Table, Sample 21358), indicating it might originate from a rare recombination event on M1a. However, deletion mapping revealed M1a and M1b to be altogether different (Fig 3A). Although no patient sample was available, we inferred a potential second minor Mennonite haplotype (M1c) from the mother of an affected child. She carried an SMN1 deletion and 1 copy of SMN2 but microsatellite markers suggested that she carried a rare double recombinant M1a or a novel haplotype (S1A Fig, Individual 4273). Hereafter, and as indicated in Tables and Figures, the simplified designation ‘M1’ refers to any one of three Mennonite genotypes (i.e. M1a/M1a, M1a/M1b, or M1a/M1c) that confers 2 copies of SMN2.
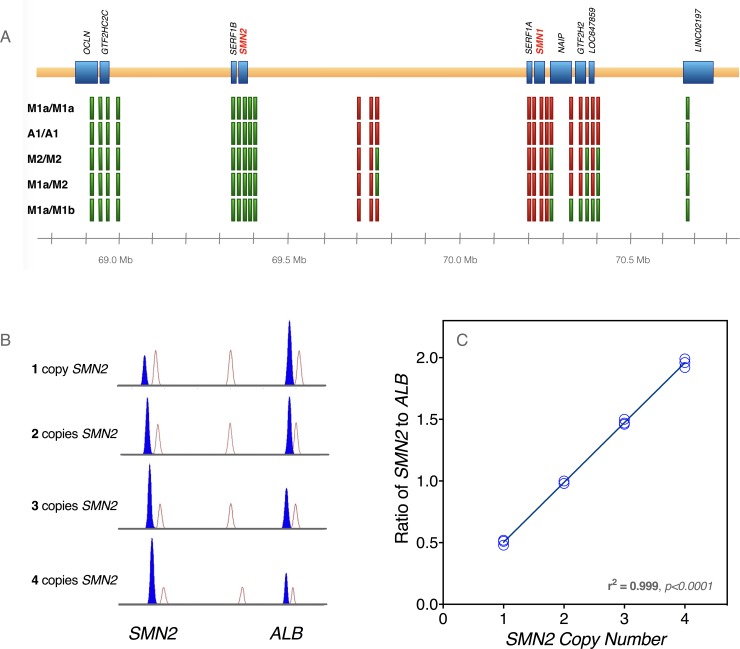
Fig 3. Characterization of SMA haplotypes.
(A) To characterize the extent of SMN1 deletions, we performed PCR on loci that mapped to multiple sites in the region. By selecting amplicons which exhibited sequence changes between loci, we were able to assess the presence or absence of each locus after PCR amplification and Sanger sequencing. Green blocks represent loci that were present, and red blocks denote deleted loci. The samples (i.e. haplotype combinations) are listed on the left side of the figure. (B) Competitive PCR was used to calculate SMN2 copy number in our patient cohort. Samples were subjected to multiplex PCR with limiting deoxynucleotide triphosphates and the resulting amplicons were size-fractionated on an ABI 3130 Genetic Analyzer. Samples with greater SMN2 copy number demonstrated increased generation of SMN2-specific product versus an internal control locus (albumin gene, ALB). (C) The area under the curve for each amplicon, as provided by the Sequencing Analysis software, was used to calculate the ratio of SMN2-specific product to ALB product. For SMN2 copy number from 1–4, three separate samples were PCR amplified and analyzed, and the SMN2/ALB ratios were highly correlated with SMN2 copy number.
A single SMA haplotype (A1) containing 1 copy of SMN2 segregated in Old Order Amish families (Figs 1B and 2). Although we had DNA samples from only two affected children, microsatellite and SMN1 copy number data from additional sibships confirmed segregation of A1 among all 14 Amish patients (Fig 1B and S1 Fig).
Deletion mapping
A1 and M1a haplotypes harbored similar deletions that encompassed all functional transcripts of SMN1, SERF1A, NAIP, and GTF2H2 (Fig 3A). They had remarkably similar SNPs and shared identical proximal microsatellite alleles (S2 Table). Although microsatellite markers differed distally, A1 and M1a showed considerable allele sharing on either side of SMN1 and shared telomeric SNPs with the minor M2 haplotype. Taken together, these data might be explained by (1) a common but ancient origin for all 3 haplotypes which allowed for microsatellite markers to mutate to new alleles over time; (2) independent mutational events that occurred on a permissive (susceptibility) allele, as has been suggested for certain haplotypes associated with Fragile X syndrome [25–27]; or 3) a chance event attributable to a high frequency of the underlying haplotype in the parental population.
SMN2 sequencing and copy number
We performed complete SMN2 sequencing of Amish and Mennonite samples with homozygous A1/A1 and M1a/M1a genotypes, respectively. Primers were designed to amplify all 9 exons of SMN2 as well as intron-exon boundaries and near-intronic regions. SMN2 sequences from A1 and M1a haplotypes were identical. Competitive PCR allowed us to infer SMN2 copy number based on its relationship to copies of the albumin gene (ALB; Fig 3B).
SMA carrier frequency
To estimate SMA carrier frequency among Old Order Mennonites, we interrogated whole exome sequencing (WES) data from 735 Mennonite control samples using the CLAMMS algorithm to detect DNA copy number variations from WES read depth [28]. This allowed us to identify 42 Mennonite controls with deletions of both GTF2H2 and NAIP. These samples were then tested using competitive PCR to confirm co-deletion of SMN1 in 27 individuals. We thus arrived at an empirical SMA carrier frequency of 3.7% (27 of 735) and minor allele frequency of 1.8% among North American Mennonites. Old Order Amish control data were insufficient to reliably calculate an SMA carrier frequency.
Phenotype
Diagnosis
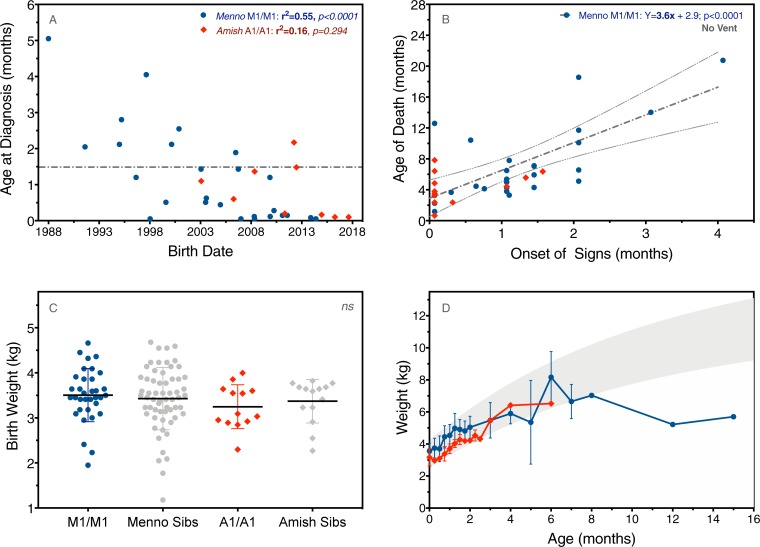
Tables 1 and 2 summarize herald signs, motor milestones, and survival according to genotype and SMN2 copy number. Median age of diagnosis was 0.1 months for Amish infants (A1/A1) with 2 copies of SMN2 and 1.1, 8.5, and 30 months, respectively, for Mennonite children with 2 (M1/M1), 3 (M1a/M2), or 4 (M2/M2) copies (Table 1). Age of diagnosis correlated inversely with birth date among Mennonite (but not Amish) children with 2 SMN2 copies (r = -0.74, p<0.0001)(Fig 4A). Molecular diagnosis preceded clinical onset for 24 (43%) of 56 patients in our cohort. Among A1/A1 (n = 7) and M1a/M1a (n = 9) homozygotes born within the last decade, 15 (94%) were diagnosed before 6 weeks of age (median age 3 days)(Fig 4A).
Table 1. Presenting signs and gross motor development of Amish (n = 14) and Mennonite (n = 42) children with spinal muscular atrophy (SMA) according to haplotype.
| Amish SMA | Mennonite SMA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1/A1, 2 copies SMN2 (n = 14) | M1/M1, 2 copies SMN2 (n = 30)* | M1a/M2, 3 copies SMN2 (n = 9) | M2/M2, 4 copies SMN2 (n = 3) | |||||||
| Percent | Median (range) | Percent | Median (range) | Percent | Median (range) | Percent | Median (range) | P value† | ||
| Age of Diagnosis (months) | 0.1 (0–2.1) | 1.1 (0–5) | 8.5 (0–30) | 30 (8–51) | ||||||
| Presenting Signs | ||||||||||
| Low muscle tone or power | 100% | 97% | - | 100% | >0.999 | |||||
| Decreased fetal movement | 71% | 20% | - | - | 0.0019 | |||||
| Weak cry | 71% | 17% | - | - | 0.0007 | |||||
| Motor Milestones (months)¶ | ||||||||||
| Roll back to front | - | 20% | 1.4 (0.7–4) | 78% | 4 (2.5–5) | 100% | 4 (4–5) | 0.1547 | ||
| Sit independently | - | - | 100% | 7 (6–8) | 100% | 5.5 (5.4–6) | na | |||
| Crawl on all fours | - | - | 75% | 9 (7–10) | 100% | 6.8 (6.8–7) | na | |||
| Walk independently | - | - | 56% | 18 (12–42) | 100% | 10 | na | |||
| Run | - | - | 11% | 16 | 100% | 15 | na | |||
*Includes 28 children with the major homozygous M1a/M1a haplotype as well as 2 compound heterozygotes for M1a/M1b and M1a/M1c, which also confer 2 copies of SMN2.
†Fisher’s exact test comparing Mennonite (haplotype M1/M1, n = 30) to Amish (haplotype A1/A1, n = 14) SMA patients with 2 copies of SMN2.
¶Percent calculated for children old enough to achieve requisite skill.
Abbreviations: A1, Amish SMA haplotype with 1 copy of SMN2; M1, Mennonite SMA haplotype with 1 copy of SMN2; M2, Mennonite SMA haplotype with 2 copies of SMN2; na, not applicable; SMA, spinal muscular atrophy.
Table 2. A1/A1 (n = 14) and M1/M1 (n = 30)* infants with 2 copies of SMN2.
| Mean (SD) | Median (range) | P value† | ||
|---|---|---|---|---|
| Birth Weight (kg) | 0.1470 | |||
| Amish A1/A1 | 3.1 (0.5) | 3.0 (2.3–3.6) | ||
| Mennonite M1/M1 | 3.5 (0.6) | 3.5 (2.0–4.7) | ||
| Onset of Signs (months) | 0.0040 | |||
| Amish A1/A1 | 0.3 (0.6) | 0 (0–1.5) | ||
| Mennonite M1/M1 | 1.1 (0.9) | 1.0 (0–4.0) | ||
| Age of Death (months)¶ | 0.0314 | |||
| Amish A1/A1 | 3.9 (2.1) | 3.9 (0.7–6.5) | ||
| Mennonite M1/M1 | 7.3 (4.9) | 5.7 (1.2–20.8) | ||
*M1/M1 Mennonites include those with M1a/M1a (n = 28), M1a/M1b (n = 1), and M1a/M1c (n = 1) haplotypes; all harbor two copies of SMN2.
¶Age of death statistics only include the subgroup of Amish (A1/A1, n = 13) and Mennonite (M1/M1, n = 26) children who did not receive mechanical ventilatory support.
†Fisher’s exact test comparing children with 2 copies of SMN2 from either the Amish (A1/A1) or Mennonite (M1/M1) haplotype combinations.
Abbreviations: SD, standard deviation; SMA, spinal muscular atrophy.
Fig 4. Diagnosis, prognosis, and growth in patients with 2 copies of SMN2.
(A) Age of diagnosis correlated to birth date (i.e. children born more recently were diagnosed earlier; rs = -0.71, p<0.0001). Among A1/A1 (red diamonds; n = 7) and M1/M1 (blue circles; n = 9) homozygotes born within the last decade, 15 (94%) were diagnosed before 6 weeks of age (gray dashed line). (B) There was a strong linear correlation between clinical onset and age of death among Mennonite but not Amish patients with 2 SMN2 copies (gray dashed line with 95% confidence bands; p<0.0001). (C) M1/M1 (blue circles) and A1/A1 (red diamonds) homozygotes and their unaffected siblings (gray symbols) had similar birth weights. (D) Amish and Mennonite children with 2 copies of SMN2 remained on the growth curve, without gastrostomy, until about 6 months of age (gray shaded area represents World Health Organization reference data, 5th to 95th percentile).
Clinical onset differed by SMN2 copy number and 5q13 genotype (Tables 1 and 2). Weakness was common to all SMA types and noted earliest in children with the A1/A1 genotype (p = 0.0040)(Table 2), which was more commonly associated with reduced fetal movement (71%; p = 0.0019) and a weak perinatal cry (71%; p = 0.0007)(Table 1). Among Mennonite but not Amish patients with 2 SMN2 copies, there was a strong correlation between clinical onset and age of death (r = 0.70, p<0.0001)(Fig 4B). Birth weight was similar for M1/M1 and A1/A1 homozygotes as well as their unaffected siblings (Fig 4C), and both Amish and Mennonite children with 2 copies of SMN2 remained on the growth curve, without gastrostomy, until about 6 months of age (Fig 4D).
Motor development
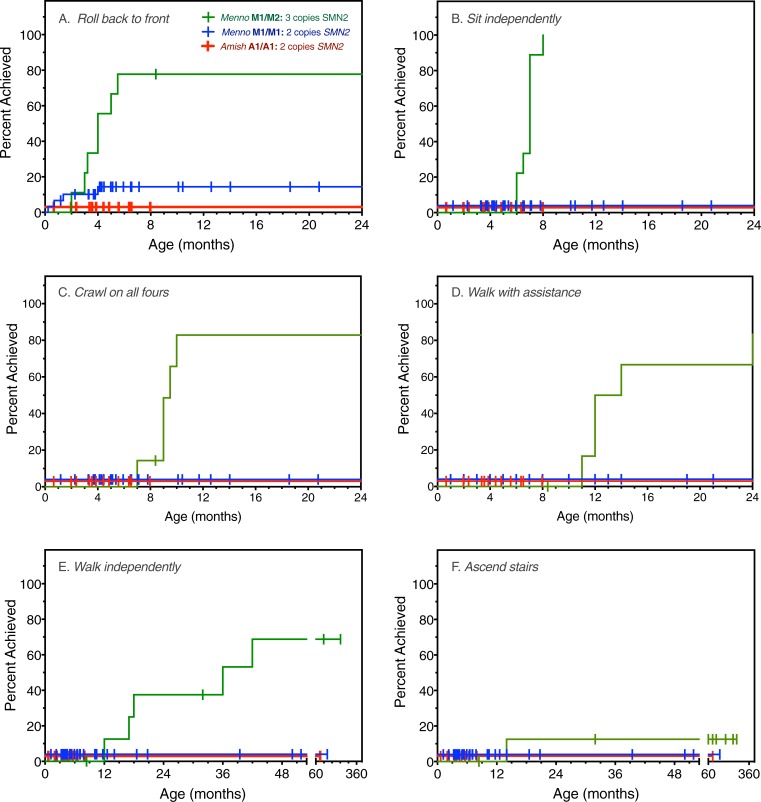
Among 30 Mennonite (M1/M1) and 14 Amish (A1/A1) infants with 2 copies of SMN2, only 6 (all with the M1a/M1a haplotype) rolled back to front; none did so after age 4 months or attained additional motor skills (Table 1 and Fig 5). All 9 individuals with the M1a/M2 genotype (3 copies of SMN2) sat unassisted during infancy at median age 7 months and 5 (56%) walked and dressed independently at median ages of 18 and 36 months, respectively. One M1a/M2 patient even learned to ascend stairs, and did so at the remarkably young age of 14 months (Fig 5). All 3 SMA children with 4 SMN2 copies (M2/M2) achieved motor skills as appropriate for census ages of 0.5, 2.7, and 6.3 years (Table 1).
Fig 5. Motor milestone acquisition and SMN2 copy number.
Time to motor milestone acquisition is plotted for Mennonite (blue; M1/M1, n = 30) and Amish (red; A1/A1, n = 14) patients harboring 2 copies of SMN2 as compared to Mennonites with 3 copies of SMN2 (green; M1/M2, n = 9). Perpendicular symbols indicate subjects unable to achieve a skill at the time of census (or death). Milestones depicted include rolling back to front (A), independent sitting (B), crawling on all fours (C), walking with assistance (D), independent walking (E), and ascending stairs (F). One child with 3 copies of SMN2 walked and ascended stairs at 12 and 14 months of age, respectively. Note the divided X-axis in panels E and F.
Morbidity
Dysphagia (90%) and pulmonary morbidity predominated among children with 2 copies of SMN2. Individuals with 3 SMN2 copies suffered primarily from musculoskeletal complications, including joint contractures (44%) and scoliosis (56%), in 3 cases culminating in surgical spine fusion. Among children with 2 copies of SMN2, only 5 (11%; 1 Amish, 4 Mennonite) were fed by gastrostomy and received daily bilevel positive airway pressure (BiPAP). No patient with the M1a/M2 (3 copies SMN2) or M2/M2 (4 copies SMN2) genotype required gastrostomy or ventilatory support.
Mortality
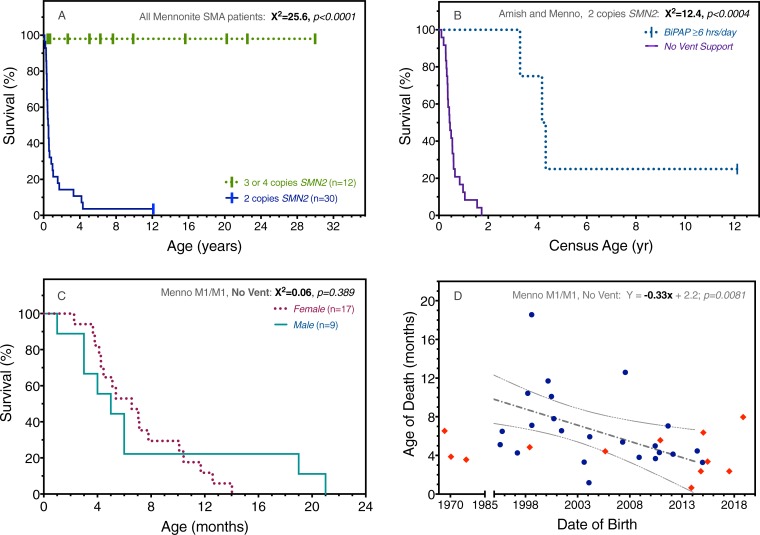
All patients with 3 (M1a/M2) or 4 (M2/M2) copies of SMN2 are alive at a median age of 7.9 (range 1.3–30.9) years (Fig 6A). Overall median survival for children with 2 copies of SMN2 was 6.2 months, but this was strongly influenced by regular use of BiPAP. For 39 children with 2 copies of SMN2 who did not receive routine ventilatory support, median survival was 5.3 (range 0.5–21) months, whereas 5 patients treated with BiPAP survived to a median age of 51 (range 39–92) months (chi-squared 12.4, p = 0.0004)(Fig 6B). When controlled for BiPAP use and genotype, there was no survival difference based on sex (Fig 6C).
Fig 6. Survival in relation to SMN2 copy number, ventilatory support, sex, and birth date.
(A) All SMA patients with 3 or 4 copies of SMN2 (green) are presently living, whereas 29 (97%) of 30 with 2 SMN2 copies (blue) died at a median age of 6.2 months. (B) Among a total of 44 patients with 2 copies of SMN2 (M1/M1, n = 30; A1/A1, n = 14), bilevel positive airway pressure (BiPAP) for ≥6 hours daily (n = 5) increased median survival from 5.3 to 51 months (p<0.0004). (C) When controlled for BiPAP use and genotype (e.g. M1/M1, no BiPAP, as depicted in Panel C), there was no survival difference based on sex. (D) Paradoxically, date of birth and age of death were inversely correlated for Mennonites with the M1/M1 genotype (Pearson r = -0.51, p = 0.0081).
In contrast to historical trends [29], there was no correlation between date of birth and age of death among Amish (A1/A1) patients. Paradoxically, these were inversely correlated (Pearson r = -0.51, p = 0.0081) for Mennonites with the M1/M1 genotype (Fig 6D). This might reflect patterns of palliative care that have emerged in response to the community’s collective experience with type 1 SMA (as discussed below).
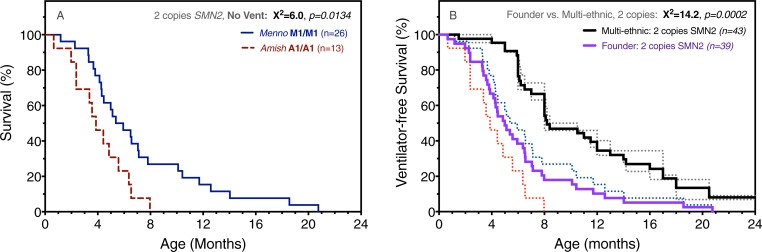
Despite similar SMN1 deletion structure and SMN2 copy number, M1/M1 and A1/A1 genotypes were associated with differential survival (Fig 7A). In the absence of gastrostomy or ventilatory support, median survival for A1/A1 (n = 13) and M1/M1 (n = 26) homozygotes was 3.9 and 5.7 months, respectively (chi-squared 6.0, p = 0.0143).
Fig 7. Survival comparisons among Amish, Mennonite, and outbred cohorts.
(A) For infants who received no gastrostomy or ventilatory support, median survival of A1/A1 (dashed red; n = 13) and M1/M1 (solid blue; n = 26) homozygotes was 3.9 and 5.7 months, respectively (chi-squared 6.0, p = 0.0143). (B) We compared aggregate (solid purple) survival data for A1/A1 and M1/M1 ‘founder’ cohorts to ventilator-free survival from two ‘multi-ethnic’ outbred cohorts (References 29 and 10) that in aggregate (solid black line) encompass 43 SMA infants with 2 copies of SMN2. When permanent ventilatory support (i.e. ≥16 hours daily) is taken as a proxy for death, Amish and Mennonite founder populations had shorter median survival (5.0 versus 8.2 months; chi-squared 14.2, p = 0.0002).
Finally, we compared aggregate A1/A1 and M1/M1 data to ventilator-free survival from two carefully conducted prospective natural history studies published in 2014 (Pediatric Clinical Research [PNCR] Network)[30] and 2017 (NeuroNEXT initiative)[10]. Both defined permanent ventilatory support as a proxy for death and together encompassed 43 SMA infants with 2 copies of SMN2. Compared to heterogenous cohorts with access to modern medical care, Amish (A1/A1) and Mennonite (M1/M1) patients had shorter median survival (5.0 versus 8.2 months; chi-squared 14.2, p = 0.0002)(Fig 7B).
Discussion
SMA in founder populations
Contemporary Plain people descended from just a few hundred Swiss-German Anabaptists and now comprise more than 800,000 individuals living in isolated demes throughout North and South America [31]. Transatlantic migrations during the 18th century reduced genetic diversity within founding settlements and set the stage for genetic drift; over successive generations, some rare alleles became common while others became extinct [24]. Driven by these mechanisms, the Mennonite SMA carrier frequency equilibrated at its present value of 3.7%, which extrapolates to a disease incidence of 1 per 2,800 newborns. Population control data are insufficient to measure carrier frequency among the Amish, but diagnostic rates in our laboratory over the last decade suggest it approximates 3% (Fig 4A).
A high incidence of SMA is not unique to Plain populations. In 1977, Fried and Mundel estimated an SMA carrier frequency of 10% among Egyptian Karaites, a group socially and reproductively isolated for more than 10 centuries. Subsequently, carrier frequencies between 3 and 13% were identified within founder populations worldwide [16–21]. Among these are the Hutterite people, an Anabaptist group descended from 16th century South German founders who migrated east from Austria-Tyrol to settle in Canada, Montana, and the Dakotas [31]. Their unique ancestral roots are reflected in a single extant SMA haplotype that contains 2 copies of SMN2 and segregates with a carrier frequency of 12.5% in colonies of South Dakota [21].
Genotype-phenotype correlations
Jordanova and colleagues first explored the relationship between ancestral haplotypes and SMA phenotype among 32 Romani families living throughout Hungary and Bulgaria [18]. Using 5 microsatellite markers, they delineated three dominant haplotypes (A, B, and C) which segregated in four combinations (A/A, A/B, A/C, and C/C) to cause profoundly different disease patterns. They postulated that rearrangements on a single ancestral chromosome produced haplotypes A, B, and C, and attributed clinical differences to resulting variations of SMN2 copy number. We applied the same principle, with greater resolution, to the study of SMA within Amish and Mennonite populations.
As expected, genotypes with 2 copies of SMN2 (A1/A1, M1a/M1a, M1a/M1b, and M1a/M1c) were associated with earlier disease onset, more restricted motor development, and shorter survival than genotypes with 3 (M1a/M2) or 4 (M2/M2) SMN2 copies. There was a broader developmental spectrum for these latter groups (Table 1) and among 9 patients with 3 SMN2 copies, one even climbed stairs by 14 months of age (Fig 5). Other members of the M1a/M2 cohort, including her 3 siblings, walked between 17 and 42 months of age and never ascended stairs. In our clinical population, such variation is not explained by differences in SMN1 deletion structure [18] or SMN2 mRNA processing [32, 33]. Rather, disease expression in this particular child might have been attenuated by a rare allele outside the 5q13 SMA locus, as has been observed for the PLS3 and NCALD genes [34–37].
It is not uncommon to observe phenotype variability among SMA patients with 3 or 4 copies of SMN2 [12, 21]. More surprising, however, was the variable clinical course of A1/A1 versus M1/M1 homozygotes. The A1/A1 genotype segregated with particularly severe SMA, characterized by prenatal/perinatal onset (Table 1) and median survival of only 3.9 (as compared to 5.7) months (Fig 7A). Microsatellite and SNP analyses of the SMA locus yielded no explanation for this; A1 and M1a were similar with respect to SMN1 deletion size and SMN2 sequence (Fig 3)[31]. One could speculate that Amish and Mennonite families adhere to different norms of medical resource utilization. However, this would not explain the high prevalence of pre- and perinatal signs among A1/A1 (71%) as compared to M1/M1 (20%) infants and the striking uniformity of the A1/A1 phenotype, which seem to indicate a fundamental difference in disease biology.
An allele outside the 5q13 SMA locus might account for such a difference but would need to be relatively fixed within either Amish (deleterious) or Mennonite (protective) populations. More likely, there are structural differences between A1 and M1a that are in linkage disequilibrium with the SMN1 deletion, have a functionally relevant impact on disease expression, and were undetected by our analyses. DNA marker data (Fig 3 and S2 Table) show these haplotypes to be similar but not identical, and we thus plan to use WES data to compare A1 and M1a at higher resolution.
Natural history and implications for clinical trials
Conservative Anabaptists share core beliefs that influence their decisions about end-of-life care, particularly the application of invasive, costly, or ‘heroic’ measures for children who are otherwise helpless [38]. For infants with type 1 SMA, many Old Order Amish and Mennonite parents reject inpatient hospitalization and life-sustaining measures, which they tend to view as compounding their child’s suffering. Accordingly, we managed most type 1 SMA patients with a minimally invasive strategy in the home setting, and thus Fig 7A represents haplotype-specific survival in the absence of supportive technologies or inpatient care.
This is clearly depicted in Fig 6B, which shows a profound survival benefit for the minority of children (11%) who were fed by gastrostomy and used BiPAP. Fig 6D illustrates this principle in a different but striking way: Mennonite patients born more recently were likely to die younger [29], perhaps because the youngest generation of parents, fully informed with a molecular diagnosis and having witnessed the toll of SMA on other community members, were least likely to pursue life-sustaining treatment [39]. Here it is important to emphasize that Plain communities do not eschew modern medical technologies in general. Rather, they tend to view them pragmatically, with a sharp focus upon the potential of any intervention to actually reduce versus prolong suffering, and at what cost.
The advent of safe and effective antisense oligonucleotide (ASO) and adeno-associated viral (AAV)-based gene replacement therapies has shifted this calculus [40–42]. Knowledge of population genetics allows us to conduct targeted SMA carrier testing, identify couples at risk, and collect cord blood from their offspring. We can then use rapid, low-cost methods (Fig 3B) to determine SMN1 mutation status and SMN2 copy number of high-risk newborns within a few hours of life (Fig 4A). This provides a critical therapeutic advantage; data from ASO and AAV-based gene therapy trials consistently show better outcomes with earlier treatment [4, 40, 42, 43].
The opportunity to administer preventative therapies within a few days of life creates a pressing need to use appropriate historical control data to gauge their efficacy [4, 6, 40, 42]. A few prospective studies, carefully conducted in the modern era, have partially filled this gap [10, 30, 44]. For SMA patients with 2 copies of SMN2, data from the PNCR Network (n = 23) and NeuroNEXT (n = 20) projects align closely on the endpoint of median death or permanent ventilation between 8 and 10 months of age. Our data show that type 1 SMA follows a distinctly different trajectory within and between Anabaptist founder cohorts (Fig 7). This observation might extend to members of other founder lineages, who share relatively uniform genetic background and tend to experience common environmental, socioeconomic, and cultural conditions that shape disease expression. These facts give critical context to clinical trial data [22–24, 38, 45] and provide the appropriate framework for evaluating SMA rescue therapies administered to Amish and Mennonite babies within the first weeks of life.
Methods
Ethics and consent
The study was approved by the Penn Medicine-Lancaster General Hospital Institutional Review Board under a protocol entitled “Genetic Medicine and the Plain Communities (LGH IRB00000015; FWA00006038). Parents of all subjects consented to participate on behalf of their children and, where applicable, a separate written consent was obtained for reproduction of photographs (Fig 2). The individuals in this manuscript gave separate written informed consent (as outlined in PLOS consent form) to publish these case details.
Molecular genetic methods
Microsatellite marker analysis
Four microsatellite markers from chromosome 5q13.2 were chosen due to their close proximity to SMN1 and SMN2 and validation in previous studies. Specific oligonucleotide primers were designed for each marker (S3 Table) using Primer3 (http://bioinfo.ut.ee/primer3/), and one primer was end-labeled with a fluorescent dye (either HEX or FAM). PCR was performed under standard conditions (see below) and amplicons were size-fractionated on an Appplied Biosystems (ABI) 3130 Genetic Analyzer. Alleles sizing was performed using GeneMapper software and GeneScan 500 ROX size standard (ThermoFisher Scientific, Waltham, MA).
Chromosomal microarray
A chromosomal microarray containing 740K SNPs (CytoScan HD Array; Affymetrix, San Diego, CA) was performed on several patients to assess haplotype sharing. These data were visualized using Affymetrix Chromosome Analysis Suite software (ChAS 3.1). Data from 5q13 were exported to Excel spreadsheets (Microsoft Corporation, Redmond, WA) for visual examination. Haplotype sharing was assessed using SNP data and 6 microsatellite markers (D5S629, UT889, D5S1370, GATA141B10, D5S1408, D5S1999; amplicon range 130 to 337 base pairs) chosen to be informative and physically close to SMN1 and SMN2 (Fig 2). Genome coordinates for each marker corresponded to the physical location of the forward primer using human genome build GRCh37. Haplotype analysis allowed us to assign a specific SMN1/SMN2 genomic ‘signature’ to each patient (Figs 2 and 3).
Deletion mapping
Loci from the inverted 5q13 duplication were amplified and Sanger sequenced to detect the presence or absence of particular regions, and amplicons were chosen based on their ability to amplify products from multiple regions but also distinguish between the alternate products based on sequence variation contained within the fragment. Amplicons were first chosen to cover telomeric transcripts SERF1A, SMN1, NAIP, GTF2H2, LOC647859, and LINC02197. Targets were selected using BLAT (https://genome.ucsc.edu/) to identify sequence variation between the centromeric and telomeric copies, and further filtered to exclude high frequency (>1%) SNPs. Ten amplicons (S3 Table, amplicons A-J) were chosen to amplify between 2 and 3 separate loci, which could then be distinguished by their unique internal sequence.
Polymerase chain reaction (PCR) primers were designed using Primer3 (http://bioinfo.ut.ee/primer3/). A 25 μL reaction was prepared with 1U of Taq polymerase (New England Biolabs [NEB], Ipswich, MA), 200mM each of dNTP, 2.5μL of 10X PCR buffer (NEB), 1.6 μmol/L of primers, and 100ng of genomic DNA. An ABI Veriti thermocycler was used to amplify products under the following conditions: initial denaturation (3 minutes at 96°C), thermal cycling (30 sec at 96°C, 15 sec at 60°C, 90 sec at 72°C), and terminal incubation (10 minutes at 72°C). PCR products were sequenced with BigDye Terminator v3.1 Cycle Sequencing Protocol (ThermoFisher Scientific, Waltham, MA). Extension products were size-fractionated on an ABI 3130 Genetic Analyzer and analyzed using Sequencing Analysis software (ThermoFisher Scientific, Waltham, MA). Coding exons and adjacent intronic regions from SMN2 were compared to the human reference sequence and dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) to detect sequence variants.
SMN1/SMN2 diagnostic and copy number tests
For rapid diagnostic screening, our laboratory utilized the PCR-based procedure detailed by Dobrowolski et al., 2012 (PMID 22490618) and performed on a LightScanner 32 system (Idaho Technologies, Salt Lake City, UT).
For SMN1 carrier testing and SMN2 copy number analysis, competitive PCR with limiting deoxynucleotide triphosphates was used similar to the procedure outlined in Zhou et al [46]. This procedure was modified to use unique primer sets for SMN1 and SMN2 (S3 Table) and a different reference gene, ALB. An additional modification included labeling the forward primer for each amplicon with FAM to permit detection on an ABI 3130 Genetic Analyzer. Briefly, a 25 μL multiplex PCR reaction was performed using an ABI Veriti thermocycler with limiting deoxynucleotide triphosphates (6.25 μmol/L each), 1U of Taq polymerase (NEB), 2.5 μL of 10X PCR buffer (NEB), and 100 ng of genomic DNA. Primers for the target and reference gene were supplied in 3:1 ratio with a total primer concentration of 1.6 μmol/L. PCR cycling conditions were as described above.
Target and reference gene amplicons were size fractionated on an ABI 3130 Genetic Analyzer using onboard fragment analysis protocols and GeneScan 500 ROX size standard (ThermoFisher Scientific, Waltham, MA). Sample runs were analyzed using GeneMapper software (ThermoFisher Scientific, Waltham, MA). SMN1 and SMN2 copy number was assessed by calculating a ratio of the area under the amplicon peaks (e.g. SMN2/ALB) and comparing these to ratios of known copy number samples run in parallel.
Patients and clinical methods
We studied 56 SMA patients born between 1965 and 2017 (175 aggregate patient-years) who lived in Canada, Pennsylvania, Ohio, Indiana, Wisconsin, Missouri, Colorado, and New York (Fig 1). Forty-two (75%) derived from Old Order Mennonite communities of the Northeastern United States, had homozygous deletions of SMN1, and traced to a common ancestor across 11 generations (Fig 1A). Fourteen Old Order Amish patients shared SMN1 deletions that traced back several generations (Fig 1B).
We used available medical records and structured interviews to collect retrospective information about birth weight, growth, clinical signs, motor development, morbidity, and survival. Interviews were conducted systematically by two investigators and, in most cases, vetted using medical charts and household records.
Statistics
We used Prism7 software (GraphPad, La Jolla) for statistical tests. Continuous variables between two groups (e.g. A1/A1 and M1a/M1a) were tested by unpaired two-way Student’s t-test. Pearson coefficients and simple linear regression were used to test correlations between independent variables. Survival curves were analyzed using a nonparametric log-rank (Mantel-Cox) test.
Supporting information
Chromosome 5q13 microsatellite marker data are listed for 42 Mennonite (M) and 14 Amish (A) patients, sorted by SMN2 copy number (CN).
(DOCX)
Representative single nucleotide polymorphism (SNP) genotypes are shown for spinal muscular atrophy haplotypes M1a/M1a, M2/M2, and A1/A1.
(DOCX)
Unique forward (F) and reverse (R) DNA primers were used for rapid and accurate detection of the SMN1 deletion and SMN2 copy number.
(DOCX)
For several families, one or more children carried a clinical diagnosis of SMA, but our laboratory lacked a DNA sample on the proband to confirm the diagnosis and ascertain SMN2 copy number. In these cases, we collected samples on the parents and surviving children to establish the diagnosis through haplotype analysis. (A) Segregation of SMN1 deletion-bearing chromosomes in a Mennonite family with 3 deceased SMA children. The father harbors the major Mennonite haplotype (M1a, shaded) and the mother carries either a doubly recombinant M1a (lacking common alleles at UT889 and D5S1408) or a novel haplotype (M1c). (B) Segregation of SMN1 deletion-bearing chromosomes in a Mennonite family with 2 deceased SMA children. Both parents are carriers of the major (M1a) haplotype. (C-D) Segregation of SMN1 deletion-bearing chromosomes in an extended Amish kindred with 9 deceased SMA children. All parents harbor the same SMN1 deletion-bearing haplotype identified in two affected Amish boys.
(PDF)
Acknowledgments
The authors are grateful to Dr. Douglas Sproule for his critical insight and guidance during manuscript preparation and thank SMA patients and their families for their creativity, courage, and partnership in this endeavor.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by charitable financial contributions from the Amish and Mennonite communities served by the Clinic for Special Children.
References
- 1.Burglen L, Spiegel R, Ignatius J, Cobben JM, Landrieu P, Lefebvre S, et al. SMN gene deletion in variant of infantile spinal muscular atrophy. Lancet. 1995;346(8970):316–7. Epub 1995/07/29. . [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65. Epub 1995/01/13. . [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16(3):265–9. Epub 1997/07/01. 10.1038/ng0797-265 . [DOI] [PubMed] [Google Scholar]
- 4.Govoni A, Gagliardi D, Comi GP, Corti S. Time Is Motor Neuron: Therapeutic Window and Its Correlation with Pathogenetic Mechanisms in Spinal Muscular Atrophy. Mol Neurobiol. 2018. Epub 2018/01/03. 10.1007/s12035-017-0831-9 . [DOI] [PubMed] [Google Scholar]
- 5.Coady TH, Lorson CL. SMN in spinal muscular atrophy and snRNP biogenesis. Wiley Interdiscip Rev RNA. 2011;2(4):546–64. Epub 2011/10/01. 10.1002/wrna.76 . [DOI] [PubMed] [Google Scholar]
- 6.Groen EJN, Talbot K, Gillingwater TH. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat Rev Neurol. 2018;14(4):214–24. Epub 2018/02/10. 10.1038/nrneurol.2018.4 . [DOI] [PubMed] [Google Scholar]
- 7.Butchbach ME. Copy Number Variations in the Survival Motor Neuron Genes: Implications for Spinal Muscular Atrophy and Other Neurodegenerative Diseases. Front Mol Biosci. 2016;3:7 Epub 2016/03/26. 10.3389/fmolb.2016.00007 ; PubMed Central PMCID: PMCPMC4785180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han KJ, Foster DG, Zhang NY, Kanisha K, Dzieciatkowska M, Sclafani RA, et al. Ubiquitin-specific protease 9x deubiquitinates and stabilizes the spinal muscular atrophy protein-survival motor neuron. J Biol Chem. 2012;287(52):43741–52. Epub 2012/11/01. 10.1074/jbc.M112.372318 ; PubMed Central PMCID: PMCPMC3527959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol. 2016;3(2):132–45. Epub 2016/02/24. 10.1002/acn3.283 ; PubMed Central PMCID: PMCPMC4748311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–91. Epub 2017/11/18. 10.1002/ana.25101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6(8):1205–14. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann P, McDermott MP, Darras BT, Finkel RS, Sproule DM, Kang PB, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79(18):1889–97. Epub 2012/10/19. 10.1212/WNL.0b013e318271f7e4 ; PubMed Central PMCID: PMCPMC3525313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter GT, Abresch RT, Fowler WM Jr., Johnson ER, Kilmer DD, McDonald CM. Profiles of neuromuscular diseases. Spinal muscular atrophy. Am J Phys Med Rehabil. 1995;74(5 Suppl):S150–9. Epub 1995/09/01. . [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson BC, Donohoe C, Akmaev VR, Sugarman EA, Labrousse P, Boguslavskiy L, et al. Differences in SMN1 allele frequencies among ethnic groups within North America. J Med Genet. 2009;46(9):641–4. Epub 2009/07/25. 10.1136/jmg.2009.066969 ; PubMed Central PMCID: PMCPMC2729371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20(1):27–32. Epub 2011/08/04. 10.1038/ejhg.2011.134 ; PubMed Central PMCID: PMCPMC3234503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried K, Mundel G. High incidence of spinal muscular atrophy type I (Werdnig—Hoffmann disease) in the Karaite community in Israel. Clin Genet. 1977;12(4):250–1. Epub 1977/10/01. . [DOI] [PubMed] [Google Scholar]
- 17.Basel-Vanagaite L, Taub E, Drasinover V, Magal N, Brudner A, Zlotogora J, et al. Genetic carrier screening for spinal muscular atrophy and spinal muscular atrophy with respiratory distress 1 in an isolated population in Israel. Genet Test. 2008;12(1):53–6. Epub 2008/02/27. 10.1089/gte.2007.0030 . [DOI] [PubMed] [Google Scholar]
- 18.Jordanova A, Kargaci V, Kremensky I, Litvinenko I, Uzunova M, Turnev I, et al. Spinal muscular atrophy among the Roma (Gypsies) in Bulgaria and Hungary. Neuromuscul Disord. 2002;12(4):378–85. Epub 2002/06/14. . [DOI] [PubMed] [Google Scholar]
- 19.Cusco I, Lopez E, Soler-Botija C, Jesus Barcelo M, Baiget M, Tizzano EF. A genetic and phenotypic analysis in Spanish spinal muscular atrophy patients with c.399_402del AGAG, the most frequently found subtle mutation in the SMN1 gene. Hum Mutat. 2003;22(2):136–43. Epub 2003/07/23. 10.1002/humu.10245 . [DOI] [PubMed] [Google Scholar]
- 20.Pascalet-Guidon MJ, Bois E, Feingold J, Mattei JF, Combes JC, Hamon C. Cluster of acute infantile spinal muscular atrophy (Werdnig-Hoffmann disease) in a limited area of Reunion Island. Clin Genet. 1984;26(1):39–42. Epub 1984/07/01. . [DOI] [PubMed] [Google Scholar]
- 21.Chong JX, Oktay AA, Dai Z, Swoboda KJ, Prior TW, Ober C. A common spinal muscular atrophy deletion mutation is present on a single founder haplotype in the US Hutterites. Eur J Hum Genet. 2011;19(10):1045–51. Epub 2011/05/26. 10.1038/ejhg.2011.85 ; PubMed Central PMCID: PMCPMC3190247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12(1):124 Epub 2017/07/06. 10.1186/s13023-017-0671-8 ; PubMed Central PMCID: PMCPMC5496354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhaart IEC, Robertson A, Leary R, McMacken G, Konig K, Kirschner J, et al. A multi-source approach to determine SMA incidence and research ready population. J Neurol. 2017;264(7):1465–73. Epub 2017/06/22. 10.1007/s00415-017-8549-1 ; PubMed Central PMCID: PMCPMC5502065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss KA, Puffenberger EG. Genetics, medicine, and the Plain people. Annu Rev Genomics Hum Genet. 2009;10:513–36. Epub 2009/07/28. 10.1146/annurev-genom-082908-150040 . [DOI] [PubMed] [Google Scholar]
- 25.Gunter C, Paradee W, Crawford DC, Meadows KA, Newman J, Kunst CB, et al. Re-examination of factors associated with expansion of CGG repeats using a single nucleotide polymorphism in FMR1. Hum Mol Genet. 1998;7(12):1935–46. Epub 1998/11/13. . [DOI] [PubMed] [Google Scholar]
- 26.Curlis Y, Zhang C, Holden JJ, Loesch PK, Mitchell RJ. Haplotype study of intermediate-length alleles at the fragile X (FMR1) gene: ATL1, FMRb, and microsatellite haplotypes differ from those found in common-size FMR1 alleles. Hum Biol. 2005;77(1):137–51. Epub 2005/08/24. . [DOI] [PubMed] [Google Scholar]
- 27.Kunst CB, Warren ST. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994;77(6):853–61. Epub 1994/06/17. . [DOI] [PubMed] [Google Scholar]
- 28.Packer JS, Maxwell EK, O'Dushlaine C, Lopez AE, Dewey FE, Chernomorsky R, et al. CLAMMS: a scalable algorithm for calling common and rare copy number variants from exome sequencing data. Bioinformatics. 2016;32(1):133–5. Epub 2015/09/19. 10.1093/bioinformatics/btv547 ; PubMed Central PMCID: PMCPMC4681995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oskoui M, Levy G, Garland CJ, Gray JM, O'Hagen J, De Vivo DC, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69(20):1931–6. Epub 2007/11/14. 10.1212/01.wnl.0000290830.40544.b9 . [DOI] [PubMed] [Google Scholar]
- 30.Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–7. Epub 2014/08/01. 10.1212/WNL.0000000000000741 ; PubMed Central PMCID: PMCPMC4155049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraybill DB. Concise Encyclopedia of Amish, Brethren, Hutterites, and Mennonites. Baltimore: Johns Hopkins University Press; 2010. [Google Scholar]
- 32.Prior TW, Krainer AR, Hua Y, Swoboda KJ, Snyder PC, Bridgeman SJ, et al. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am J Hum Genet. 2009;85(3):408–13. Epub 2009/09/01. 10.1016/j.ajhg.2009.08.002 ; PubMed Central PMCID: PMCPMC2771537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, Wang SH, Sun J, Krainer AR, Hua Y, Prior TW. A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum Mol Genet. 2017;26(14):2768–80. Epub 2017/05/02. 10.1093/hmg/ddx166 ; PubMed Central PMCID: PMCPMC5886194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oprea GE, Krober S, McWhorter ML, Rossoll W, Muller S, Krawczak M, et al. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320(5875):524–7. Epub 2008/04/29. 10.1126/science.1155085 ; PubMed Central PMCID: PMCPMC4908855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackermann B, Krober S, Torres-Benito L, Borgmann A, Peters M, Hosseini Barkooie SM, et al. Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Hum Mol Genet. 2013;22(7):1328–47. Epub 2012/12/25. 10.1093/hmg/dds540 . [DOI] [PubMed] [Google Scholar]
- 36.Hosseinibarkooie S, Peters M, Torres-Benito L, Rastetter RH, Hupperich K, Hoffmann A, et al. The Power of Human Protective Modifiers: PLS3 and CORO1C Unravel Impaired Endocytosis in Spinal Muscular Atrophy and Rescue SMA Phenotype. Am J Hum Genet. 2016;99(3):647–65. Epub 2016/08/09. 10.1016/j.ajhg.2016.07.014 ; PubMed Central PMCID: PMCPMC5011078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseinibarkooie S, Schneider S, Wirth B. Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev Proteomics. 2017;14(7):581–92. Epub 2017/06/22. 10.1080/14789450.2017.1345631 . [DOI] [PubMed] [Google Scholar]
- 38.Strauss KA, Puffenberger EG, Morton DH. One community's effort to control genetic disease. Am J Public Health. 2012;102(7):1300–6. 10.2105/AJPH.2011.300569 ; PubMed Central PMCID: PMCPMC3477994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss KA. Genomics for the People. Scientific American. 2015:66–73.26336688 [Google Scholar]
- 40.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Engl J Med. 2017;377(18):1723–32. 10.1056/NEJMoa1702752 . [DOI] [PubMed] [Google Scholar]
- 41.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. 2018;378(7):625–35. Epub 2018/02/15. 10.1056/NEJMoa1710504 . [DOI] [PubMed] [Google Scholar]
- 42.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. 2017;377(18):1713–22. 10.1056/NEJMoa1706198 . [DOI] [PubMed] [Google Scholar]
- 43.van der Ploeg AT. The Dilemma of Two Innovative Therapies for Spinal Muscular Atrophy. N Engl J Med. 2017;377(18):1786–7. 10.1056/NEJMe1712106 . [DOI] [PubMed] [Google Scholar]
- 44.De Sanctis R, Pane M, Coratti G, Palermo C, Leone D, Pera MC, et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord. 2018;28(1):24–8. Epub 2017/11/28. 10.1016/j.nmd.2017.09.015 . [DOI] [PubMed] [Google Scholar]
- 45.Strauss KA, Markx S, Georgi B, Paul SM, Jinks RN, Hoshi T, et al. A population-based study of KCNH7 p.Arg394His and bipolar spectrum disorder. Hum Mol Genet. 2014;23(23):6395–406. Epub 2014/07/06. 10.1093/hmg/ddu335 ; PubMed Central PMCID: PMCPMC4222358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Palais RA, Paxton CN, Geiersbach KB, Wittwer CT. Copy number assessment by competitive PCR with limiting deoxynucleotide triphosphates and high-resolution melting. Clin Chem. 2015;61(5):724–33. Epub 2015/03/12. 10.1373/clinchem.2014.236208 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chromosome 5q13 microsatellite marker data are listed for 42 Mennonite (M) and 14 Amish (A) patients, sorted by SMN2 copy number (CN).
(DOCX)
Representative single nucleotide polymorphism (SNP) genotypes are shown for spinal muscular atrophy haplotypes M1a/M1a, M2/M2, and A1/A1.
(DOCX)
Unique forward (F) and reverse (R) DNA primers were used for rapid and accurate detection of the SMN1 deletion and SMN2 copy number.
(DOCX)
For several families, one or more children carried a clinical diagnosis of SMA, but our laboratory lacked a DNA sample on the proband to confirm the diagnosis and ascertain SMN2 copy number. In these cases, we collected samples on the parents and surviving children to establish the diagnosis through haplotype analysis. (A) Segregation of SMN1 deletion-bearing chromosomes in a Mennonite family with 3 deceased SMA children. The father harbors the major Mennonite haplotype (M1a, shaded) and the mother carries either a doubly recombinant M1a (lacking common alleles at UT889 and D5S1408) or a novel haplotype (M1c). (B) Segregation of SMN1 deletion-bearing chromosomes in a Mennonite family with 2 deceased SMA children. Both parents are carriers of the major (M1a) haplotype. (C-D) Segregation of SMN1 deletion-bearing chromosomes in an extended Amish kindred with 9 deceased SMA children. All parents harbor the same SMN1 deletion-bearing haplotype identified in two affected Amish boys.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.