Lifestyle exposes filamentous fungi to antagonists
Filamentous fungi arrange their cells in linear, coenocytic arrays, referred to as hyphae, that extend at their tips and are able to branch and fuse, leading to a loose, three-dimensional network referred to as mycelium [1]. This architecture represents an optimal adaptation to the osmotrophic lifestyle of fungi in that it maximizes the surface for nutrient absorption and enables the fungus to efficiently reach and colonize its substrates. Some hyphae of the long-lived and constantly renewed vegetative mycelium may differentiate in other, more compact tissues, e.g., the (usually) short-lived and spore-producing fruiting bodies formed by dikaryotic fungi during their sexual reproduction. The different fungal tissues are exposed to different types of antagonists dependent on the ecological niche of the fungus. The vegetative mycelium of a saprophytic fungus, e.g., is exposed to other microorganisms that compete for the same nutrients and may feed on the degradation products released by the action of the hydrolytic enzymes secreted by the fungus. Accordingly, nutrient-rich substrates, such as the dung of herbivores, are battlefields of competing saprophytic bacteria and fungi [2]. On the other hand, the lack of motility and high content of nutrients make both the fungal vegetative mycelium and the fruiting bodies attractive dietary resources for animal predators. Accordingly, soil-inhabiting fungi are an important dietary resource for soil arthropods and nematodes [3].
The main defense strategy of fungi is chemical defense
Fungi have evolved different strategies to increase their competitiveness for nutrient acquisition toward other microorganisms and to protect themselves from predation by animals. Similar to plants, the main defense strategy of fungi is chemical defense, i.e., the production of toxins impairing the growth, development, or viability of the antagonists by the fungus [4]. These defense effectors include secondary metabolites [5], peptides (ribosomally or nonribosomally synthesized) [6, 7], and proteins [8] and usually act by binding to specific target molecules of the antagonists (Table 1). It has been hypothesized that effectors against microbial competitors are secreted, whereas effectors against metazoan predators are usually stored within the fungal cells and are taken up during predation (Fig 1) [9]. Examples of fungal defense effectors in accordance with this hypothesis are the β-lactam antibiotic penicillin produced by some Penicillium species [10], the antifungal lipopeptide pneumocandin B0 produced by Glarea lozoyensis [11], and the cytotoxic, ribosomally synthesized octapeptide α-amanitin produced by some Amanita, Galerina, Conocybe, and Lepiota species [12]. Penicillin is secreted and binds and inhibits extracellular enzymes involved in peptidoglycan biosynthesis, an essential and conserved process in all bacteria [13]. Similarly, pneumocandin B0 is secreted and inhibits 1,3-β-D-glucan synthase, one of the main enzymes involved in fungal cell wall biosynthesis and is therefore called “penicillin of the antifungals” [11]. In contrast, α-amanitin is taken up from the fungal cell upon predation and enters epithelial cells of the digestive tract of animal predators where it binds and inactivates the essential and conserved nuclear enzyme RNA polymerase II [14]. Exceptions to the hypothesis are a number of secreted insecticidal and nematicidal secondary metabolites [15]. In addition to the action of toxins, fungi have more subtle ways of chemical defense, e.g., by the production of molecules interfering with bacterial and animal communication. Examples are intracellular lactonases of the coprophilous ink cap mushroom Coprinopsis cinerea acting as a sink for quorum sensing signals of gram-negative bacteria [16] and the production of insect juvenile hormones by the mold Aspergillus nidulans [17].
Table 1. Examples of fungal toxins and their targets.
| Toxin | Producing fungus | Regulation of production | Subcellular localization | Target organism | Toxin class | Target molecule | Reference |
|---|---|---|---|---|---|---|---|
| Gliotoxin | Aspergillus spp. | Autonomous | Extracellular | Fungi | Secondary metabolite | Proteasome | [65] |
| Lovastatin | Aspergillus terreus | Autonomous | Extracellular | Fungi | Secondary metabolite | HMG-CoA-reductase | [66] |
| Strobilurin A | Oudemansiella mucida | Fungus-induced | Extracellular | Fungi | Secondary metabolite | Cytochrome b | [24] |
| Pneumocandin B0 | Glarea lozoyensis | Autonomous | Extracellular | Fungi | Peptide | 1,3-β-D-glucan synthase | [11] |
| Copsin/Plectasin/Micasin | Coprinopsis cinerea/Pseudoplectania nigrella/Microsporum canis | Autonomous | Extracellular | Bacteria | Peptide | Lipid II | [22, 67, 68]/[69]/[70] |
| Penicillin | Penicillium spp. | Autonomous | Extracellular | Bacteria | Secondary metabolite | Peptidoglycan transpeptidases | [10] |
| Enniatin A1 and B1 | Fusarium tricinctum | Bacterium-induced | Extracellular | Bacteria | Peptide | Membrane (ionophor) | [28] |
| Aflatoxin B1 | Aspergillus flavus | Autonomous and damage-induced | Extracellular | Insects | Secondary metabolite | DNA | [29] |
| α-Amanitin | Amanita, Galerina, Conocybe, and Lepiota spp. | Autonomous | Intracellular | Insects/Nematodes | Peptide | RNA polymerase II/III | [12] |
| Omphalotin A | Omphalotus olearius | Autonomous | Extracellular | Insects/Nematodes | Peptide | unknown | [71] |
| Cyclosporin A | Tolypocladium inflatum | Autonomous | Extracellular | Insects/Nematodes | Peptide | Cyclophilin/Calcineurin | [72] |
| CGL2 | Coprinopsis cinerea | Autonomous and nematode-induced | Intracellular | Insects/Nematodes | Protein | N-glycoproteins | [23, 73] |
| MOA | Marasmius oreades | Autonomous | Intracellular | Nematodes | Protein | Glycosphingolipids | [74] |
| Clitocypin | Clitocybe nebularis | Autonomous | Intracellular | Insects | Protein | Cysteine proteases | [75] |
| α-Sarcin | Aspergillus giganteus | Autonomous | Intracellular | Insects | Protein | 28S rRNA | [76] |
Abbreviations: CGL2, Coprinopsis cinereal Galectin 2; CoA, Coenzyme A; HMG, β-Hydroxy β-methylglutaryl; MOA, Marasmius oreades agglutinin.
Fig 1. Regulation of the chemical defense of filamentous fungi (on the example of a mushroom) against microbial competitors and animal predators, exemplified by bacteria and fungivorous nematodes (adapted from Fig 1 in [9]).
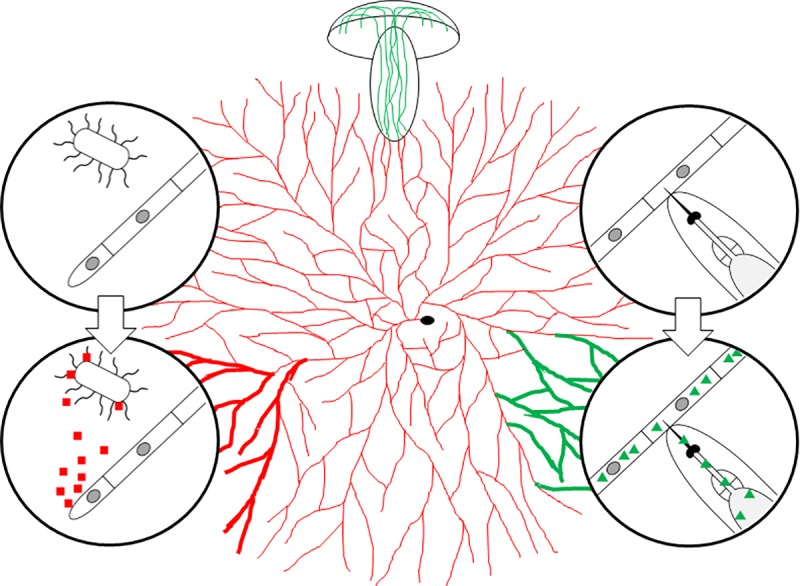
The fungus is represented by its vegetative mycelial network originating from a spore (black oval) and a fruiting body (mushroom) arising from that network. The circles show close ups on the competition between the fungal hyphae and bacteria (left) and predation by fungivorous nematodes (right) and the induction of respective fungal defense effectors; fungal nuclei are represented by grey ovals, extracellular antibacterial defense effectors by red squares, and intracellular defense effectors against nematodes by green triangles. Specific examples of antibacterial and antinematode effectors and their properties are listed in Table 1. Fungal hyphae producing the two types of defense effectors are colored respectively. Autonomous and antagonist-dependent production of defense effectors is indicated by thin and thick hyphae, respectively. The indicated spatial restriction of antagonist-dependent defense effector production in the fungal mycelium is hypothetical.
Fungal defense can be autonomous and/or antagonist-dependent
The biosynthesis of chemical defense effectors is usually tightly regulated because these molecules are not essential for the viability of an organism, and their biosynthesis requires resources that may be limited [18]. This regulation can be autonomous, i.e., independent of the antagonist and/or antagonist-dependent. Accordingly, it has been shown that the regulation of secondary metabolism and sexual development are coordinated in A. nidulans [19]; some of the secondary metabolites, whose biosynthesis is restricted to the fruiting body, exert toxicity toward arthropods suggesting that these organs are prey of and therefore require protection from animal predators [20]. Similarly, the concentration of amatoxins of the mushroom Amanita phalloides, including α-amanitin, is lowest in the vegetative mycelium and highest in the fruiting body [21]. Analogously, genome-wide gene expression analysis of the vegetative mycelium and young fruiting bodies of the model mushroom C. cinerea revealed that the secreted antibacterial peptide Copsin is almost exclusively produced in the vegetative mycelium, whereas most of the C. cinerea genes coding for intracellular insecticidal and nematicidal lectins are specifically expressed in the fruiting body (Fig 1) [22]. This spatiotemporal, autonomous regulation results in an efficient constitutive protection of specific fungal tissues against the most relevant antagonists because some of the defense effectors are already in place when the antagonist attacks the fungus. On the other hand, at least some of the lectin-encoding genes directed against animal predators were induced in the C. cinerea vegetative mycelium when this tissue was challenged with a fungivorous nematode [23]. Similarly, challenge of the vegetative mycelium of the basidiomycete Oudemansiella murata with two different Penicillium spp. induced the production of the antifungal strobilurin A [24], and challenge of various ascomycetous molds with bacteria and arthropods led to the induction of various gene clusters coding for the biosynthetic machineries of antimicrobial and cytotoxic secondary metabolites, respectively [25–29]. These results suggest that fungi possess, in addition to an autonomous, tissue-specific defense, also an inducible defense (Fig 1). This type of regulation is also known from the innate defense systems of plants and animals [30].
Open questions
The presence of innate defense systems in multicellular fungi, plants, and animals suggests that such systems are a universal requirement of multicellular organisms. In order to clarify whether these defense systems are the result of divergent or convergent evolution, the fungal defense system has to be better characterized with regard to three key issues of innate defense.
What is the plasticity and specificity of the induced chemical defense?
Despite above mentioned reports about the induction of fungal defense effector genes upon challenge with bacterial competitors and animal predators, it is not clear how specific these responses are, since almost no signals, receptors, and signaling pathways responsible for these responses are known. This is in contrast to plants and animals, in which sophisticated and multilayered systems of receptors and signaling pathways responsible for the recognition of antagonist-associated molecular patterns or effectors and the induction of antagonist-specific innate defense responses have been identified [31].
While mere wounding triggers the production of fungal defense effectors in some cases [5, 29], there are a few reports about antagonist-associated molecular patterns perceived by fungi. These patterns include cell wall fragments [32] and quorum-sensing signal molecules [33] in the case of bacteria and nematode developmental signal molecules in the case of animals [34]. Besides these soluble signal molecules or patterns, physical contact between the fungus and the antagonist appears to be required for induction of defense [23, 25]. With regard to pattern recognition receptors, plants and animals use two related sets of receptors, namely Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs) for the extracellular and intracellular perception of signals, respectively [30, 35]. Binding of the molecular patterns by these receptors is often mediated by leucine-rich repeat (LRR) domains. Besides the well-characterized G-protein coupled receptors for endogenous sex pheromones, only a few reports of fungal receptors for specific biotic signals exist. Interestingly, the chemotropic sensing of host plant signals by the plant pathogenic fungus Fusarium oxysporum was recently demonstrated to be mediated by the sex pheromone receptor [36], suggesting that these receptors may have a broader specificity. None of the over 600 presently known fungal genome sequences, however, appear to encode TLRs. Hyphal growth of the animal-pathogenic yeast Candida albicans was shown to be triggered by direct interaction of bacterial muramyl dipeptides (MDPs) with the LRRs of an intracellular fungal receptor protein containing, in addition, a protein phosphatase and an adenylate cyclase domain [32]. This recognition mechanism is similar to the binding of MDP to the mammalian NLR-type receptor NOD2, which triggers inflammation in response to bacterial infections and whose genetic variation has been implicated in susceptibility to Crohn's disease [37]. NLRs are involved in the hetero-incompatibility reaction between different strains of the same fungal species, and it was hypothesized that these proteins, which are widely spread among fungi [38], might also play a role in the perception of fungal antagonists similar to plants and animals [39]. To our knowledge, there is no experimental evidence for this hypothesis so far.
One of the earliest responses of the plant to herbivory (as well as to pathogen and parasite attack) is the production of reactive oxygen species (ROS) and a rapid increase in intracellular calcium (Ca2+) [40]. Analogously, fungi react to biotic and abiotic stress with ROS formation and Ca2+ influx into cells, and the formation of ROS is dependent on NADPH-dependent oxidases (Nox) [41]. Since Nox's have also been implicated in fungal differentiation [42], these enzymes may play a dual role in development and defense, as demonstrated for other multicellular organisms. Similar to downstream signaling pathways of plant and animal defense responses [31], above mentioned MDP-receptor in C. albicans suggests downstream signaling via protein phosphorylation/dephosphorylation and cAMP [32]. Accordingly, mitogen-activated protein kinase high osmolarity glycerol (Hog1p) of the yeast Saccharomyces cerevisiae was shown to be phosphorylated in response to bacterial lipopolysaccharide (LPS) [43]. In terms of transcription factors involved in the response of fungi to antagonists, to our knowledge, only one example has been reported so far. Overexpression of the transcription factor remediation of secondary metabolism (RsmA) in A. nidulans lead to induction of transcription factor Aflatoxin biosynthesis regulator (AflR), which in turn resulted in increased expression of an antipredation secondary metabolite gene cluster and avoidance of the transformed mycelium by Folsomia candida [44]. In addition, the velvet family of fungal regulators, involved in the above-mentioned coordination of secondary metabolism and sexual development in the same fungus, contains a DNA-binding domain that is structurally related to the main transcription factor NF-κB at the end of the animal TLR-signaling cascade [45].
Is there a systemic defense response and priming?
In plants, the induction of plant defense effector proteins is not restricted to sites of herbivory but can be propagated to other parts of the same plant or even neighboring plants that have not yet been in contact with the antagonist [46, 47]. This propagation of the defense response relies on amplification of the originally perceived signal by the generation of endogenous signal molecules and the transmission of these molecules within the plant and even to other plants. Endogenous signal molecules implicated in local and systemic plant defense are some plant hormones (salicylic acid, abscisic acid, ethylene) [48], plant oxylipins [49], green leaf volatiles (GLVs) [50], peptides [51], and above-mentioned ROSs [52]. In addition to this chemical signal transmission, this systemic defense response of plants may also be mediated by membrane depolarization [53]. Signaling within fungal mycelia has been studied in some ascomycetes, and a variety of volatile and nonvolatile endogenous signal molecules have been identified [54]. As examples, conidiation of the ascomycetous mold Trichoderma is dependent on the 1-octen-3-ol [55], a volatile compound that is also produced by basidiomycetes [56]; oxylipins, which are known endogenous signal molecules in plants and animals, are also known as endogenous signal molecules modulating sexual development and the host interaction of pathogenic fungi [57]. Little is known, however, about the role of such molecules in fungal defense. Interestingly, two genes coding for fatty acid oxygenases involved in the biosynthesis of oxylipins in A. nidulans are induced upon grazing of the mycelium by larvae of the fruitfly Drosophila melanogaster [58], suggesting a possible dual function of these signal molecules in development and defense, as suggested for mosses [59]. The spatial distribution and the propagation of the induced defense response within a fungal mycelium have not been studied so far.
The original dogma that innate immune systems are not able to build up an immunological memory has recently been reconsidered both for plants and animals, and epigenetic histone modifications have been implicated in this process [47, 60]. To our knowledge, the only reports about persistence of an up-regulated defense response in mycelia in the absence of the antagonist, a phenomenon referred to in plants and animals as priming, are two studies by Rohlfs and coworkers [26, 58]. In these studies, the authors show that grazing by D. melanogaster larvae and the soil arthropod F. candida induces resistance of the mycelium toward grazing by these predators even after a period of 6 hours without grazing. These results suggest that the induction of the chemical defense in fungi is preserved for some time to protect the mycelium from further damage by predation. There is no data on the spatial distribution of this defense response in the mycelium, however. Interestingly, the induction is dependent on the master regulator of secondary metabolism, the LaeA-velvet system known to act via histone methylation [19], suggesting an epigenetic mechanism for defense gene induction and priming. Accordingly, induction of defense-related metabolic gene clusters in A. nidulans by bacteria was shown to involve histone acetylation [61].
What is the ecological significance of fungal defense?
Only a few studies, amongst the two above-mentioned studies of A. nidulans [26, 58], have addressed the ecological significance of fungal defense in terms of fungal resistance toward grazing. Previous to these studies, it had been shown that A. nidulans mutants lacking the master regulator of secondary metabolism LaeA and transformants overexpressing the transcription factor RsmA are more susceptible to grazing by D. melanogaster larvae and more resistant to grazing by F. candida, respectively [44, 62]. Accordingly, aflatoxin production correlated with the fitness of different Aspergillus flavus isolates with regard to grazing by D. melanogaster larvae [29].
Relevance and impact
Future in-depth characterization of the fungal innate defense against microbial competitors and animal predators is not only important in terms of basic research, e.g., the evolution of innate defense in eukaryotes, but also in terms of applied research. Fungi are a rich source of chemically diverse natural products, many of which are used for antagonistic interactions. These compounds have a high potential to be used as drugs in management of relevant human or animal diseases and pests. As many of these compounds are produced only in response to an antagonist, investigations of fungal antagonistic interactions are key to exploitation of this “treasure of nature” [63, 64].
Acknowledgments
I apologize to all colleagues whose work could not be cited in this review due to space limitations.
Funding Statement
The work of the author was supported by the Swiss National Science Foundation (Grant no. 31003A_173097), the Swiss Commission for Technology and Innovation (Grant no. 25951.2), and ETH Zürich (Grant no. ETH-45 16-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stajich JE, Berbee ML, Blackwell M, Hibbett DS, James TY, Spatafora JW, et al. The fungi. Curr Biol. 2009;19(18):R840–5. 10.1016/j.cub.2009.07.004 ; PubMed Central PMCID: PMC2913116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bills GF, Gloer JB, An Z. Coprophilous fungi: antibiotic discovery and functions in an underexplored arena of microbial defensive mutualism. Curr Opin Microbiol. 2013;16(5):549–65. Epub 2013/08/28. doi: S1369-5274(13)00142-2 [pii] 10.1016/j.mib.2013.08.001 . [DOI] [PubMed] [Google Scholar]
- 3.Ruess L, Lussenhop J. Trophic interactions of Fungi and Animals In: J. D, White JF, Oudemans P, editors. The Fungal Community: Its Organization and Role in the Ecosystems. Boca Raton: CRC Press; 2005. p. 581–98. [Google Scholar]
- 4.Kempken F, Rohlfs M. Fungal secondary metabolite biosynthesis—a chemical defence strategy against antagonistic animals? Fungal Ecology. 2009;3:107–14. 10.1016/j.funeco.2009.08.001 [DOI] [Google Scholar]
- 5.Spiteller P. Chemical ecology of fungi. Nat Prod Rep. 2015;32(7):971–93. 10.1039/c4np00166d . [DOI] [PubMed] [Google Scholar]
- 6.Ding W, Liu WQ, Jia Y, Li Y, van der Donk WA, Zhang Q. Biosynthetic investigation of phomopsins reveals a widespread pathway for ribosomal natural products in Ascomycetes. Proc Natl Acad Sci U S A. 2016;113(13):3521–6. 10.1073/pnas.1522907113 ; PubMed Central PMCID: PMC4822579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bills G, Li Y, Chen L, Yue Q, Niu XM, An Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat Prod Rep. 2014;31(10):1348–75. 10.1039/c4np00046c . [DOI] [PubMed] [Google Scholar]
- 8.Sabotic J, Ohm RA, Kunzler M. Entomotoxic and nematotoxic lectins and protease inhibitors from fungal fruiting bodies. Appl Microbiol Biotechnol. 2016;100(1):91–111. 10.1007/s00253-015-7075-2 . [DOI] [PubMed] [Google Scholar]
- 9.Kunzler M. Hitting the Sweet Spot: Glycans as Targets of Fungal Defense Effector Proteins. Molecules. 2015;20(5):8144–67. 10.3390/molecules20058144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg MA, Westerlaken I, Leeflang C, Kerkman R, Bovenberg RA. Functional characterization of the penicillin biosynthetic gene cluster of Penicillium chrysogenum Wisconsin54-1255. Fungal Genet Biol. 2007;44(9):830–44. 10.1016/j.fgb.2007.03.008 . [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen L, Yue Q, Liu X, An Z, Bills GF. Genetic Manipulation of the Pneumocandin Biosynthetic Pathway for Generation of Analogues and Evaluation of Their Antifungal Activity. ACS Chem Biol. 2015;10(7):1702–10. 10.1021/acschembio.5b00013 . [DOI] [PubMed] [Google Scholar]
- 12.Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc Natl Acad Sci U S A. 2007;104(48):19097–101. Epub 2007/11/21. doi: 0707340104 [pii] 10.1073/pnas.0707340104 ; PubMed Central PMCID: PMC2141914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159(6):1300–11. 10.1016/j.cell.2014.11.017 ; PubMed Central PMCID: PMC4258230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushnell DA, Cramer P, Kornberg RD. Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution. Proc Natl Acad Sci U S A. 2002;99(3):1218–22. 10.1073/pnas.251664698 ; PubMed Central PMCID: PMC122170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anke H. Insecticidal and Nematicidal Metabolites from Fungi In: Hofrichter M, editor. The Mycota X: Industrial Applications, 2nd Edition The Mycota. Industrial Applications. Berlin Heidelberg: Springer-Verlag; 2010. p. 151–63. [Google Scholar]
- 16.Stockli M, Lin CW, Sieber R, Plaza DF, Ohm RA, Kunzler M. Coprinopsis cinerea intracellular lactonases hydrolyze quorum sensing molecules of Gram-negative bacteria. Fungal Genet Biol. 2016;102:49–62. 10.1016/j.fgb.2016.07.009 . [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MT, Klejnstrup ML, Rohlfs M, Anyaogu DC, Nielsen JB, Gotfredsen CH, et al. Aspergillus nidulans synthesize insect juvenile hormones upon expression of a heterologous regulatory protein and in response to grazing by Drosophila melanogaster larvae. PLoS One. 2013;8(8):e73369 10.1371/journal.pone.0073369 ; PubMed Central PMCID: PMC3753258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meldau S, Erb M, Baldwin IT. Defence on demand: mechanisms behind optimal defence patterns. Ann Bot. 2012;110(8):1503–14. Epub 2012/10/02. doi: mcs212 [pii] 10.1093/aob/mcs212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayram O, Braus GH. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev. 2012;36(1):1–24. Epub 2011/06/11. 10.1111/j.1574-6976.2011.00285.x . [DOI] [PubMed] [Google Scholar]
- 20.Stotefeld L, Scheu S, Rohlfs M. Fungal chemical defence alters density-dependent foraging behaviour and success in a fungivorous soil arthropod. Ecological Entomology. 2012;37(5):323–9. 10.1111/j.1365-2311.2012.01373.x PubMed PMID: WOS:000308636400001. [DOI] [Google Scholar]
- 21.Kaya E, Karahan S, Bayram R, Yaykasli KO, Colakoglu S, Saritas A. Amatoxin and phallotoxin concentration in Amanita phalloides spores and tissues. Toxicol Ind Health. 2013. 10.1177/0748233713491809 . [DOI] [PubMed] [Google Scholar]
- 22.Plaza DF, Lin CW, van der Velden NS, Aebi M, Kunzler M. Comparative transcriptomics of the model mushroom Coprinopsis cinerea reveals tissue-specific armories and a conserved circuitry for sexual development. BMC Genomics. 2014;15:492 10.1186/1471-2164-15-492 ; PubMed Central PMCID: PMC4082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleuler-Martinez S, Butschi A, Garbani M, Walti MA, Wohlschlager T, Potthoff E, et al. A lectin-mediated resistance of higher fungi against predators and parasites. Mol Ecol. 2011;20(14):3056–70. Epub 2011/04/14. 10.1111/j.1365-294X.2011.05093.x . [DOI] [PubMed] [Google Scholar]
- 24.Kettering M, Sterner O, Anke T. Antibiotics in the chemical communication of fungi. Z Naturforsch C. 2004;59(11–12):816–23. . [DOI] [PubMed] [Google Scholar]
- 25.Schroeckh V, Scherlach K, Nutzmann HW, Shelest E, Schmidt-Heck W, Schuemann J, et al. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci U S A. 2009;106(34):14558–63. Epub 2009/08/12. doi: 0901870106 [pii] 10.1073/pnas.0901870106 ; PubMed Central PMCID: PMC2732885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doll K, Chatterjee S, Scheu S, Karlovsky P, Rohlfs M. Fungal metabolic plasticity and sexual development mediate induced resistance to arthropod fungivory. Proc Biol Sci. 2013;280(1771):20131219 Epub 2013/09/27. 10.1098/rspb.2013.1219 rspb.2013.1219 [pii]. ; PubMed Central PMCID: PMC3790476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konig CC, Scherlach K, Schroeckh V, Horn F, Nietzsche S, Brakhage AA, et al. Bacterium Induces Cryptic Meroterpenoid Pathway in the Pathogenic Fungus Aspergillus fumigatus. Chembiochem. 2013;14(8):938–42. Epub 2013/05/08. 10.1002/cbic.201300070 . [DOI] [PubMed] [Google Scholar]
- 28.Ola AR, Thomy D, Lai D, Brotz-Oesterhelt H, Proksch P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J Nat Prod. 2013;76(11):2094–9. 10.1021/np400589h . [DOI] [PubMed] [Google Scholar]
- 29.Drott MT, Lazzaro BP, Brown DL, Carbone I, Milgroom MG. Balancing selection for aflatoxin in Aspergillus flavus is maintained through interference competition with, and fungivory by insects. Proc Biol Sci. 2017;284(1869). 10.1098/rspb.2017.2408 ; PubMed Central PMCID: PMCPMC5745424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–66. Epub 2004/06/18. . [DOI] [PubMed] [Google Scholar]
- 31.Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330(6007):1061–4. Epub 2010/11/26. doi: 330/6007/1061 [pii] 10.1126/science.1189468 . [DOI] [PubMed] [Google Scholar]
- 32.Xu XL, Lee RT, Fang HM, Wang YM, Li R, Zou H, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4(1):28–39. Epub 2008/07/16. doi: S1931-3128(08)00174-1 [pii] 10.1016/j.chom.2008.05.014 . [DOI] [PubMed] [Google Scholar]
- 33.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54(5):1212–23. 10.1111/j.1365-2958.2004.04349.x . [DOI] [PubMed] [Google Scholar]
- 34.Hsueh YP, Mahanti P, Schroeder FC, Sternberg PW. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol. 2013;23(1):83–6. Epub 2012/12/19. 10.1016/j.cub.2012.11.035 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duxbury Z, Ma Y, Furzer OJ, Huh SU, Cevik V, Jones JD, et al. Pathogen perception by NLRs in plants and animals: Parallel worlds. Bioessays. 2016. 10.1002/bies.201600046 . [DOI] [PubMed] [Google Scholar]
- 36.Turra D, El Ghalid M, Rossi F, Di Pietro A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature. 2015;527(7579):521–4. 10.1038/nature15516 . [DOI] [PubMed] [Google Scholar]
- 37.Boyle JP, Parkhouse R, Monie TP. Insights into the molecular basis of the NOD2 signalling pathway. Open Biology. 2014;4(12). doi: UNSP 140178 10.1098/rsob.140178 PubMed PMID: WOS:000347901500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyrka W, Lamacchia M, Durrens P, Kobe B, Daskalov A, Paoletti M, et al. Diversity and Variability of NOD-Like Receptors in Fungi. Genome Biol Evol. 2014;6(12):3137–58. 10.1093/gbe/evu251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paoletti M, Saupe SJ. Fungal incompatibility: evolutionary origin in pathogen defense? Bioessays. 2009;31(11):1201–10. Epub 2009/10/02. 10.1002/bies.200900085 . [DOI] [PubMed] [Google Scholar]
- 40.Furstenberg-Hagg J, Zagrobelny M, Bak S. Plant defense against insect herbivores. Int J Mol Sci. 2013;14(5):10242–97. 10.3390/ijms140510242 ; PubMed Central PMCID: PMC3676838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez-Onate MA, Esquivel-Naranjo EU, Mendoza-Mendoza A, Stewart A, Herrera-Estrella AH. An injury-response mechanism conserved across kingdoms determines entry of the fungus Trichoderma atroviride into development. Proc Natl Acad Sci U S A. 2012. Epub 2012/08/29. doi: 1209396109 [pii] 10.1073/pnas.1209396109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemoto D, Tanaka A, Scott B. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet Biol. 2007;44(11):1065–76. Epub 2007/06/15. doi: S1087-1845(07)00084-9 [pii] 10.1016/j.fgb.2007.04.011 . [DOI] [PubMed] [Google Scholar]
- 43.Marques JM, Rodrigues RJ, de Magalhaes-Sant'ana AC, Goncalves T. Saccharomyces cerevisiae Hog1 protein phosphorylation upon exposure to bacterial endotoxin. J Biol Chem. 2006;281(34):24687–94. 10.1074/jbc.M603753200 . [DOI] [PubMed] [Google Scholar]
- 44.Yin WB, Amaike S, Wohlbach DJ, Gasch AP, Chiang YM, Wang CC, et al. An Aspergillus nidulans bZIP response pathway hardwired for defensive secondary metabolism operates through aflR. Mol Microbiol. 2012;83(5):1024–34. Epub 2012/01/31. 10.1111/j.1365-2958.2012.07986.x ; PubMed Central PMCID: PMC3288630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed YL, Gerke J, Park HS, Bayram O, Neumann P, Ni M, et al. The Velvet Family of Fungal Regulators Contains a DNA-Binding Domain Structurally Similar to NF-kappaB. PLoS Biol. 2013;11(12):e1001750 10.1371/journal.pbio.1001750 ; PubMed Central PMCID: PMC3876986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–63. 10.1146/annurev-arplant-042811-105606 . [DOI] [PubMed] [Google Scholar]
- 47.Espinas NA, Saze H, Saijo Y. Epigenetic Control of Defense Signaling and Priming in Plants. Front Plant Sci. 2016;7:1201 10.3389/fpls.2016.01201 ; PubMed Central PMCID: PMC4980392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodenhausen N, Reymond P. Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant Microbe Interact. 2007;20(11):1406–20. Epub 2007/11/06. 10.1094/MPMI-20-11-1406 . [DOI] [PubMed] [Google Scholar]
- 49.Blee E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002;7(7):315–22. Epub 2002/07/18. doi: S1360138502022902 [pii]. . [DOI] [PubMed] [Google Scholar]
- 50.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A. 2004;101(6):1781–5. Epub 2004/01/30. 10.1073/pnas.0308037100 [pii]. ; PubMed Central PMCID: PMC341853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi Y, Huffaker A. Endogenous peptide elicitors in higher plants. Curr Opin Plant Biol. 2011;14(4):351–7. 10.1016/j.pbi.2011.05.001 . [DOI] [PubMed] [Google Scholar]
- 52.Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13(1):179–91. Epub 2001/02/07. ; PubMed Central PMCID: PMC102208. [PMC free article] [PubMed] [Google Scholar]
- 53.Salvador-Recatala V, Tjallingii WF, Farmer EE. Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol. 2014;203(2):674–84. 10.1111/nph.12807 . [DOI] [PubMed] [Google Scholar]
- 54.Leeder AC, Palma-Guerrero J, Glass NL. The social network: deciphering fungal language. Nat Rev Microbiol. 2011;9(6):440–51. Epub 2011/05/17. doi: nrmicro2580 [pii] 10.1038/nrmicro2580 . [DOI] [PubMed] [Google Scholar]
- 55.Nemcovic M, Jakubikova L, Viden I, Farkas V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol Lett. 2008;284(2):231–6. 10.1111/j.1574-6968.2008.01202.x . [DOI] [PubMed] [Google Scholar]
- 56.Berendsen RL, Kalkhove SI, Lugones LG, Baars JJ, Wosten HA, Bakker PA. Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl Microbiol Biotechnol. 2013;97(12):5535–43. 10.1007/s00253-013-4793-1 . [DOI] [PubMed] [Google Scholar]
- 57.Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15(3):109–18. Epub 2007/02/06. doi: S0966-842X(07)00006-6 [pii] 10.1016/j.tim.2007.01.005 . [DOI] [PubMed] [Google Scholar]
- 58.Caballero Ortiz S, Trienens M, Rohlfs M. Induced fungal resistance to insect grazing: reciprocal fitness consequences and fungal gene expression in the Drosophila-Aspergillus model system. PLoS ONE. 2013;8(8):e74951 Epub 2013/09/12. 10.1371/journal.pone.0074951 PONE-D-13-22162 [pii]. ; PubMed Central PMCID: PMC3758311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponce de Leon I, Hamberg M, Castresana C. Oxylipins in moss development and defense. Front Plant Sci. 2015;6:483 10.3389/fpls.2015.00483 ; PubMed Central PMCID: PMC4490225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098 10.1126/science.aaf1098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nutzmann HW, Reyes-Dominguez Y, Scherlach K, Schroeckh V, Horn F, Gacek A, et al. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc Natl Acad Sci U S A. 2011;108(34):14282–7. Epub 2011/08/10. doi: 1103523108 [pii] 10.1073/pnas.1103523108 ; PubMed Central PMCID: PMC3161617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohlfs M, Albert M, Keller NP, Kempken F. Secondary chemicals protect mould from fungivory. Biol Lett. 2007;3(5):523–5. Epub 2007/08/10. doi: 2731164232160636 [pii] 10.1098/rsbl.2007.0338 ; PubMed Central PMCID: PMC2391202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Netzker T, Flak M, Krespach MK, Stroe MC, Weber J, Schroeckh V, et al. Microbial interactions trigger the production of antibiotics. Curr Opin Microbiol. 2018;45:117–23. 10.1016/j.mib.2018.04.002 . [DOI] [PubMed] [Google Scholar]
- 64.Adnani N, Rajski SR, Bugni TS. Symbiosis-inspired approaches to antibiotic discovery. Nat Prod Rep. 2017;34(7):784–814. 10.1039/c7np00009j ; PubMed Central PMCID: PMCPMC5555300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scharf DH, Brakhage AA, Mukherjee PK. Gliotoxin—bane or boon? Environ Microbiol. 2016;18(4):1096–109. 10.1111/1462-2920.13080 . [DOI] [PubMed] [Google Scholar]
- 66.Hasan H, Abd Rahim MH, Campbell L, Carter D, Abbas A, Montoya A. Overexpression of acetyl-CoA carboxylase in Aspergillus terreus to increase lovastatin production. N Biotechnol. 2018;44:64–71. 10.1016/j.nbt.2018.04.008 . [DOI] [PubMed] [Google Scholar]
- 67.Essig A, Hofmann D, Munch D, Gayathri S, Kunzler M, Kallio PT, et al. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J Biol Chem. 2014;289(50):34953–64. 10.1074/jbc.M114.599878 ; PubMed Central PMCID: PMC4263892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franzoi M, van Heuvel Y, Thomann S, Schurch N, Kallio PT, Venier P, et al. Structural Insights into the Mode of Action of the Peptide Antibiotic Copsin. Biochemistry. 2017;56(37):4992–5001. 10.1021/acs.biochem.7b00697 . [DOI] [PubMed] [Google Scholar]
- 69.Schneider T, Kruse T, Wimmer R, Wiedemann I, Sass V, Pag U, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328(5982):1168–72. Epub 2010/05/29. doi: 328/5982/1168 [pii] 10.1126/science.1185723 . [DOI] [PubMed] [Google Scholar]
- 70.Zhu S, Gao B, Harvey PJ, Craik DJ. Dermatophytic defensin with antiinfective potential. Proc Natl Acad Sci U S A. 2012;109(22):8495–500. 10.1073/pnas.1201263109 ; PubMed Central PMCID: PMC3365176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Velden NS, Kalin N, Helf MJ, Piel J, Freeman MF, Kunzler M. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nat Chem Biol. 2017;13(8):833–5. 10.1038/nchembio.2393 . [DOI] [PubMed] [Google Scholar]
- 72.Bushley KE, Raja R, Jaiswal P, Cumbie JS, Nonogaki M, Boyd AE, et al. The genome of Tolypocladium inflatum: evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet. 2013;9(6):e1003496 10.1371/journal.pgen.1003496 ; PubMed Central PMCID: PMC3688495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plaza DF, Schmieder SS, Lipzen A, Lindquist E, Kunzler M. Identification of a Novel Nematotoxic Protein by Challenging the Model Mushroom Coprinopsis cinerea with a Fungivorous Nematode. G3 (Bethesda). 2015;6(1):87–98. 10.1534/g3.115.023069 ; PubMed Central PMCID: PMC4704728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wohlschlager T, Butschi A, Zurfluh K, Vonesch SC, Auf dem Keller U, Gehrig P, et al. Nematotoxicity of Marasmius Oreades Agglutinin (Moa) Depends on Glycolipid-Binding and Cysteine Protease Activity. J Biol Chem. 2011;286:30337–43. Epub 2011/07/16. doi: M111.258202 [pii] 10.1074/jbc.M111.258202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smid I, Rotter A, Gruden K, Brzin J, Buh Gasparic M, Kos J, et al. Clitocypin, a fungal cysteine protease inhibitor, exerts its insecticidal effect on Colorado potato beetle larvae by inhibiting their digestive cysteine proteases. Pestic Biochem Physiol. 2015;122:59–66. 10.1016/j.pestbp.2014.12.022 . [DOI] [PubMed] [Google Scholar]
- 76.Olombrada M, Martinez-Del-Pozo A, Medina P, Budia F, Gavilanes JG, Garcia-Ortega L. Fungal ribotoxins: Natural protein-based weapons against insects. Toxicon. 2014;83C:69–74. 10.1016/j.toxicon.2014.02.022 . [DOI] [PubMed] [Google Scholar]


