Introduction
Human norovirus (HNoV) is the leading cause of epidemic nonbacterial gastroenteritis worldwide, causing an acute diarrheal infection and occasionally chronic infection in immunocompromised individuals. Mouse and tissue culture models utilizing murine norovirus (MNoV) have allowed for interrogation of viral mechanisms of infection and pathogenesis. Here, we outline the interactions between the commensal microbiota of the intestine and norovirus and their implications (Fig 1).
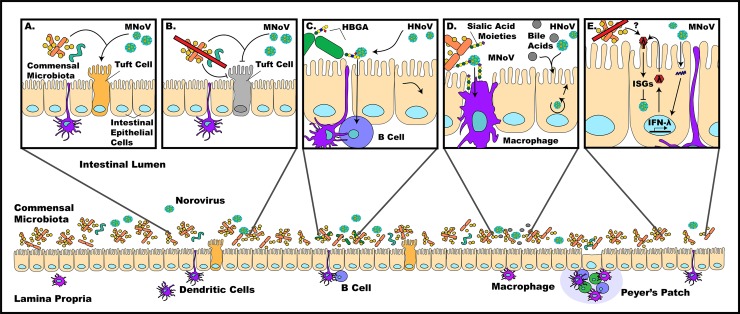
Fig 1. Norovirus pathogenesis is affected by many factors in the enteric environment.
a) The presence of commensal bacteria allows for efficient MNoV infection, with tuft cells being one rare cell population infected. b) Absence of commensal bacteria reduces MNoV titers by depleting tuft cell populations and potentially altering innate immune responses during persistent MNoV infection. c) Binding of HNoV to HBGA-positive enteric bacteria has been found to facilitate infection of B cells. d) Sialic acid moieties on the cell surface have been found to act as coreceptors for MNoV infection of macrophages, while bile acids have been found to be important for the establishment of HNoV infection of enterocytes. e) MNoV infection has been found to trigger the expression of IFN-λ in infected cells, up-regulating interferon simulated genes that restrict viral replication and subsequent spread of infection—the mechanism of this process has yet to be characterized, but commensals are believed to play a major regulatory role. HBGA, histo-blood group antigen; HNoV, human norovirus; IFN-λ, interferon lambda; MNoV, murine norovirus.
Question 1: Is norovirus infection in mice a good model for norovirus infection in humans?
Due to the fact that HNoV cannot readily grow in mice and, until recently, has not been culturable in vitro, the use of MNoV has provided robust animal and tissue culture model systems, which allow for mechanistic studies of an orthologous pathogen [1–4]. The MNoV model system allows for the merging of basic mechanistic principles of infection and replication from cell culture systems to pathogenesis in a host system that is both genetically malleable and affordable.
Thus far, MNoV studies have allowed for the elucidation of a species-specific proteinaceous receptor (CD300LF) and viral tropism for a rare intestinal epithelial cell population called tuft cells during persistent infection as well as macrophages, dendritic cells, and lymphocytes during acute infection in vivo [5–7]. Many external factors outside of the narrow window of host cell–virus interactions that affect NoV pathogenicity have also been identified, such as bile, sialic acid, and intestinal bacteria [2,5,6,8]. Additionally, host mucosal cytokine interferon lambda (IFN-λ) has recently been identified as a potent anti-NoV molecule, opening the possibility of its therapeutic use for HNoV in the future [9]. Multiple strains of MNoV allow for the study of both chronic (MNV.CR6, MNV-3) and acute (MNV-1, MNV-1.CW3) infection, further adding to the strengths and complexity of this model system [10].
Despite some differences in symptom presentation and species-specific receptors, HNoV and MNoV exhibit many similarities in cellular tropism, requirement of carbohydrate attachment factors, and potential for persistent viral shedding after symptom resolution [3,10]. However, some clinical symptoms do differ between MNoV and HNoV infections, most notably the lack of vomiting and inability to produce more than statistically significant mild diarrhea in mice [11].
Question 2: Do all strains of norovirus depend on the commensal microbiota for infection?
A large and diverse population of commensal microbes, consisting of bacteria, viruses, fungi, and parasites, reside within the intestinal lumen. NoV, being an enteric pathogen, encounters and interacts with members of this community, resulting in outcomes beneficial or detrimental to the host. HNoV has been found to interact directly with commensal (Enterobacter cloacae) and pathogenic (Clostridium difficile) bacterial species via the viral capsid and histo-blood group antigen (HGBA)-like carbohydrates expressed on bacterial surface membranes [12,13]. In addition, both HNoV and MNoV have been reported to bind sialic acid residues, which can be expressed on bacteria, suggesting that MNoV could also interact directly with the enteric microbiota [4,14].
Experimental alteration of the enteric microbiota with oral antibiotics drastically depletes the intestinal bacterial population. This in turn reduces the severity of acute MNoV infection (reduced MNV-1 titers) and also prevents or reduces persistent infections (drastically reduced MNV.CR6 and MNV-3 titers) in the ileum and colon [15]. Additionally, infection by MNV.CR6 can be rescued by fecal microbiota transplant (FMT) from nonantibiotic-treated to antibiotic-treated mice, highlighting the importance of commensal bacteria for MNoV infection [15]. While all murine NoV strains tested and reported thus far exhibit a dependence upon commensal bacteria for infection, further studies will be needed to determine whether this is a phenomenon affecting all HNoV and MNoV strains and to define strain-specific mechanisms.
One putative explanation by which bacteria can promote MNoV infection comes from the recent description of tuft cells as the physiologic target cell of persistent MNoV infection and propagation. Within the mouse intestine, tuft cells are regulated by commensal bacteria such that antibiotic treatment correlates with reduced numbers of tuft cells and leads to reduced viral titers [6]. In addition to commensal bacteria, parasitic worms (such as Trichinella spiralis) also exhibit a proviral effect in the context of MNoV infection [16,17] via induction of tuft cells by type 2 immune responses (IL-4, IL-25 cytokines) [6]. Thus, both bacteria and enteric metazoans can regulate NoV infection.
In addition to microbe–NoV and microbe–host interactions impacting viral pathogenesis, NoV infection itself can also alter the enteric microbial communities of the host. This virus-induced dysbiosis is characterized by an enhanced Firmicutes to Bacteroidetes ratio. This alteration is seen both in a subset of HNoV infections and early acute MNoV infections (MNV-1) [18,19]. However, this effect was not detected in longitudinal studies of both acute and persistent strains of MNoV (MNV-1, MNV.CR6, and MNV-4), suggesting potential temporal or facility-based effects [20].
Question 3: How do commensal bacteria regulate enteric virus infections via immune skewing?
The host intestinal immune system is highly regulated by a complex interplay of various lymphoid tissues, immune cells, cytokines, and their receptors [21–23] and possesses three distinct layers: mucus, epithelia, and lamina propria. In the small intestine, mucus-secreting goblet cells and antimicrobial peptide-secreting Paneth cells form the mucosal barrier that segregates commensal bacteria from the intestinal epithelia [24]. Intestinal epithelial cells directly interact with and survey the gut environment in coordination with innate lymphoid cells, which communicate with the immune system via secretion of cytokines and chemokines [24–26]. Dendritic cells ferry antigen from the lumen across the epithelial barrier to draining lymph nodes and mucosal lymphoid tissues in the lamina propria [27], and innate inflammatory signals and other luminal signals activate T- and B-cell responses [26]. These interacting layers play a large role in maintaining the microbiota and host immune system in homeostasis as well as regulating infection, inflammation, and autoimmunity.
While the interactions between commensal bacteria, enteric viruses, and the intestinal immune system are still poorly understood, several recent studies have suggested important interplay between these factors. Innate immune responses are primed via commensal bacterial recognition by the enteric epithelium, which activates antiviral intestinal responses after a secondary viral-induced signal within the gut [22]. In contrast, mouse mammary tumor virus (MMTV) has evolved to evade innate immune responses by binding bacterial lipopolysaccharide, inducing the immunosuppressive cytokine IL-10 via Toll-like receptor signaling pathways [21]. This effect is entirely dependent upon the microbiota; mice receiving parenteral administration of MMTV do not experience a suppressed immune response, and antibiotic-treated or germ-free mice receiving MMTV orally fail to pass MMTV to their offspring. Microbial modulation of innate immunity also offers a second explanation of the preventive effect of antibiotics on MNoV infection: it is the result of the bacterial microbiota hindering a yet-to-be-identified immune pathway, which limits the antiviral efficacy of IFN-λ during persistent MNoV infection [23]. Evidence for this comes from experiments demonstrating that mice lacking IFN-λ signaling no longer require commensal bacteria for successful MNoV infection [23]. This triangle of interactions—commensals, viral pathogen, and host—produce anti- or proviral environments through many disparate and yet-to-be-characterized mechanisms.
Question 4: Does a dependence on the commensal microbiota apply to other viruses?
Other enteric viruses, including rotavirus and poliovirus, have been found to depend on enteric bacteria to infect, similar to MNoV [28,29]. Commensal bacteria act as a proviral factor during poliovirus infection, as antibiotic treatment results in mice being less susceptible to infection and a reduced viral load in the intestine [28]. The mechanism underlying this involves viral particles binding to bacterial lipopolysaccharide, causing enhanced host cell receptor binding and virion stability [28,30]. Paradoxically, microbial depletion was found to increase antibody responses against rotavirus, which may contribute to enhanced viral clearance during antibiotic treatment [29].
In contrast, nonenteric viral infections are enhanced in mice depleted of commensal bacteria. For neurotropic flavivirus (West Nile, dengue, Zika) infections, depletion of the enteric microbiota significantly increased viral susceptibility, viral burden, disease severity, and lethal outcomes in mice [31]. Additionally, respiratory influenza A virus (IAV) and lymphocytic choriomeningitis virus (LCMV) infection have been found to be intensified (sustained, high viral titers in lung tissue and serum, respectively) due to impaired immune responses secondary to depletion of gram-positive bacteria in the gut [32,33].
In these cases, antibiotic treatment reduced virus-specific cluster of differentiation 8+ (CD8+) T-cell responses, though there is apparent variation in the manifestation of defects. Antibiotic treatment resulted in decreased numbers of dendritic cells (DCs) for antigen presentation in the case of flavivirus infection, whereas in the case of IAV and LCMV, there was a defect in DC migration to lymph nodes attributed to reduced inflammasome activation upon infection [31,32]. The bacterial metabolite desminotyrosine was also found to regulate type I IFN signaling in the lung to control IAV infection [32,34]. These findings suggest that bacteria interact with both innate and adaptive immune systems to control both local and systemic antiviral responses, leading to distinct outcomes for enteric and nonenteric viruses.
Question 5: Does microbiome modulation have therapeutic potential for infectious diseases in humans?
While it is clear that the microbiome plays a significant role in both infectious and noninfectious diseases alike, much remains unknown about the exact mechanisms of action. FMTs have proven to be an effective treatment for Clostridium difficile (C. diff) infection and treatment-resistant irritable bowel syndrome (IBS) and may have potential in inflammatory bowel diseases [35]. It is likely that different underlying mechanisms contribute to the efficacy of these treatments; for example, specific bile acids regulated by intestinal bacteria are critical for resistance to C. diff infection [36,37]. Targeted administration of efficacious microbes would be ideal to prevent disease, and we are just beginning to identify the specific bacterial species that may regulate diseases from multiple sclerosis to diabetes to norovirus.
While it may appear that treating HNoV patients with antibiotics would prove beneficial since antibiotic treatment reduces MNoV titers in mice, it may actually cause more harm than good due to the overall beneficial impact of the microbiome on human health and the potential for increased susceptibility to other viral, fungal, or bacterial infections. Probiotics may be a better therapeutic option, such as adding helpful microbes to a patient’s microbiome to fight infection or as a form of biological vaccine adjuvant. And in the case of NoV, development of drugs that temporarily mitigate the effect of commensal microbes and their metabolites without clearing bacterial populations could prove to be a viable treatment option in the future.
Acknowledgments
We apologize to all colleagues whose work could not be cited due to length restrictions. We also wish to thank Mudra Hegde, Dylan Lawrence, and Elizabeth Kennedy for their helpful critiques of this review.
Funding Statement
MTB was supported by NIH grant K22 AI127846-01, DDRCC grant P30 DK052574, and the Global Probiotics Council’s Young Investigator Grant for Probiotics Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jones MK, Grau KR, Costantini V, Kolawole AO, de Graaf M, Freiden P, et al. Human norovirus culture in B cells. Nat Protoc [Internet]. 2015. December [cited 2018 Apr 30];10(12):1939–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26513671 10.1038/nprot.2015.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science (80-) [Internet]. 2016. September 23 [cited 2018 Apr 30];353(6306):1387–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27562956 10.1126/science.aaf5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wobus CE, Thackray LB, Virgin HW. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol [Internet]. 2006. June 1 [cited 2018 Apr 30];80(11):5104–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16698991 10.1128/JVI.02346-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartnicki E, Cunha JB, Kolawole AO, Wobus CE. Recent advances in understanding noroviruses. F1000Research [Internet]. 2017. [cited 2018 May 1];6:79 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28163914 doi: 10.12688/f1000research.10081.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard RC, Wilen CB, Doench JG, Baldridge MT, McCune BT, Lee Y-CJ, et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science [Internet]. 2016. [cited 2018 Apr 30];353(6302):933–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27540007 10.1126/science.aaf1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL, et al. Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science [Internet]. 2018. April 13 [cited 2018 Apr 30];360(6385):204–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29650672 10.1126/science.aar3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grau KR, Roth AN, Zhu S, Hernandez A, Colliou N, DiVita BB, et al. The major targets of acute norovirus infection are immune cells in the gut-associated lymphoid tissue. Nat Microbiol [Internet]. 2017. December 6 [cited 2018 Jun 19];2(12):1586–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29109476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube S, Perry JW, Yetming K, Patel SP, Auble H, Shu L, et al. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol [Internet]. 2009. May [cited 2018 May 1];83(9):4092–101. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19244326 10.1128/JVI.02245-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Wilen CB, Orvedahl A, McCune BT, Kim K-W, Orchard RC, et al. Norovirus Cell Tropism Is Determined by Combinatorial Action of a Viral Non-structural Protein and Host Cytokine. Cell Host Microbe [Internet]. 2017. October 11 [cited 2018 May 9];22(4):449–459.e4. Available from: https://www.sciencedirect.com/science/article/pii/S1931312817303542?via%3Dihub 10.1016/j.chom.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldridge MT, Turula H, Wobus CE. Norovirus Regulation by Host and Microbe. Trends Mol Med [Internet]. 2016. [cited 2018 Apr 30];22(12):1047–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27887808 10.1016/j.molmed.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Kahan SM, Jia Y, Karst SM. Primary high-dose murine norovirus 1 infection fails to protect from secondary challenge with homologous virus. J Virol [Internet]. 2009. July [cited 2018 Jun 19];83(13):6963–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19403675 10.1128/JVI.00284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Breiman A, le Pendu J, Uyttendaele M. Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress. Front Microbiol [Internet]. 2015. July 1 [cited 2018 May 1];6:659 Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2015.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura T, Sano D, Suenaga A, Yoshimura T, Fuzawa M, Nakagomi T, et al. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol [Internet]. 2013. September 1 [cited 2018 May 1];87(17):9441–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23804639 10.1128/JVI.01060-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almagro-Moreno S, Boyd EF. Bacterial catabolism of nonulosonic (sialic) acid and fitness in the gut. Gut Microbes [Internet]. 2010. January [cited 2018 May 1];1(1):45–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21327116 10.4161/gmic.1.1.10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science [Internet]. 2015. January 16 [cited 2018 Apr 30];347(6219):266–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25431490 10.1126/science.1258025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK, et al. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science [Internet]. 2010. June 11 [cited 2018 Apr 30];328(5984):1391–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20538949 10.1126/science.1187703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science [Internet]. 2014. August 1 [cited 2018 May 9];345(6196):578–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25082704 10.1126/science.1256942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson AM, Walk ST, Taube S, Taniuchi M, Houpt ER, Wobus CE, et al. Disruption of the human gut microbiota following Norovirus infection. PLoS ONE [Internet]. 2012. [cited 2018 May 1];7(10):e48224 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23118957 10.1371/journal.pone.0048224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickman D, Jones MK, Zhu S, Kirkpatrick E, Ostrov DA, Wang X, et al. The effect of malnutrition on norovirus infection. MBio [Internet]. 2014. March 4 [cited 2018 May 1];5(2):e01032–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24595373 10.1128/mBio.01032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AM, Elftman MD, Pinto AK, Baldridge M, Hooper P, Kuczynski J, et al. Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome [Internet]. 2013. February 18 [cited 2018 Apr 30];1(1):7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24451302 10.1186/2049-2618-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky A V, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science [Internet]. 2011. October 14 [cited 2018 May 9];334(6053):245–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21998394 10.1126/science.1210718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sansone CL, Cohen J, Yasunaga A, Xu J, Osborn G, Subramanian H, et al. Microbiota-Dependent Priming of Antiviral Intestinal Immunity in Drosophila. Cell Host Microbe [Internet]. 2015. November 11 [cited 2018 May 9];18(5):571–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26567510 10.1016/j.chom.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science [Internet]. 2015. January 16 [cited 2018 May 9];347(6219):266–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25431490 10.1126/science.1258025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res [Internet]. 2017. [cited 2018 Apr 30];4:14 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28465831 10.1186/s40779-017-0122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe [Internet]. 2012. October 18 [cited 2018 May 9];12(4):496–508. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23084918 10.1016/j.chom.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature [Internet]. 2013. June 6 [cited 2018 May 9];498(7452):113–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23698371 10.1038/nature12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol [Internet]. 2016. April [cited 2018 May 9];14(4):197–204. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26853118 10.1038/nrmicro.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper L V, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science [Internet]. 2011. October 14 [cited 2018 May 9];334(6053):249–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21998395 10.1126/science.1211057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis [Internet]. 2014. July 15 [cited 2018 May 9];210(2):171–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24436449 10.1093/infdis/jiu037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe [Internet]. 2014. January 15 [cited 2018 Jun 22];15(1):36–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24439896 10.1016/j.chom.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thackray LB, Handley SA, Gorman MJ, Murphy KM, Virgin HW, Correspondence MSD, et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. CellReports [Internet]. 2018. [cited 2018 Apr 30];22:3440–3453.e6. Available from: 10.1016/j.celrep.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A [Internet]. 2011. March 29 [cited 2018 Apr 30];108(13):5354–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21402903 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity [Internet]. 2012. July 27 [cited 2018 Apr 30];37(1):158–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22705104 10.1016/j.immuni.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science [Internet]. 2017. [cited 2018 May 9];357(6350):498–502. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28774928 10.1126/science.aam5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holleran G, Scaldaferri F, Ianiro G, Lopetuso L, Mc Namara D, Mele MC, et al. Fecal microbiota transplantation for the treatment of patients with ulcerative colitis and other gastrointestinal conditions beyond Clostridium difficile infection: an update. Drugs Today (Barc) [Internet]. 2018. February [cited 2018 May 9];54(2):123–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29637938 [DOI] [PubMed] [Google Scholar]
- 36.Theriot CM, Bowman AA, Young VB. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere [Internet]. 2016. [cited 2018 May 9];1(1). Available from: http://www.ncbi.nlm.nih.gov/pubmed/27239562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature [Internet]. 2015. January 8 [cited 2018 May 9];517(7533):205–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25337874 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]