Abstract
Context
The optimal measure of vitamin D status is unknown.
Objective
To directly measure circulating free 25-hydroxyvitamin D [25(OH)D] concentrations and relationships to total 25(OH)D in a clinically diverse sample of humans.
Design
Cross-sectional analysis.
Setting
Seven academic sites.
Patients
A total of 1661 adults: healthy (n = 279), prediabetic (n = 479), outpatients (n = 714), cirrhotic (n = 90), pregnant (n = 20), nursing home resident (n = 79).
Interventions
Merge research data on circulating free 25(OH)D (directly-measured immunoassay), total 25(OH)D (liquid chromatography/tandem mass spectrometry), D-binding protein [DBP; by radial (polyclonal) immunodiffusion assay], albumin, creatinine, intact parathyroid hormone, and DBP haplotype.
Main outcome measures
Distribution of free 25(OH)D (ANOVA with Bonferroni correction for post hoc comparisons) and relationships between free and total 25(OH)D (mixed-effects modeling incorporating clinical condition, DBP haplotype with sex, race, estimated glomerular filtration rate (eGFR), body mass index (BMI), and other covariates).
Results
Free 25(OH)D was 4.7 ± 1.8 pg/mL (mean ± SD) in healthy persons and 4.3 ± 1.9 pg/mL in outpatients, with levels of 0.5 to 8.1 pg/mL and 0.9 to 8.1 pg/mL encompassing 95% of healthy persons and outpatients, respectively. Free 25(OH)D was higher in patients with cirrhosis (7.1 ± 3.0 pg/mL; P < 0.0033) and nursing home residents (7.9 ± 2.1 pg/mL; P < 0.0033) than in other groups and differed between whites and blacks (P < 0.0033) and between DBP haplotypes (P < 0.0001). Mixed-effects modeling of relationships between free and total 25(OH)D identified clinical conditions (patients with cirrhosis > nursing home residents > patients with prediabetes > outpatients > pregnant women) and BMI (lesser effect) as covariates affecting relationships but not eGFR, sex, race, or DBP haplotype.
Conclusions
Total 25(OH)D, health condition, race, and DBP haplotype affected free 25(OH)D, but only health conditions and BMI affected relationships between total and free 25(OH)D. Clinical importance of free 25(OH)D needs to be established in studies assessing outcomes.
Free 25(OH)D levels were affected by clinical conditions as well as by race, BMI, and DBP haplotype. Relationships between free and total 25(OH)D were affected only by clinical conditions and BMI.
The adequacy of vitamin D status is usually assessed by measurement of total circulating 25-hydroxyvitamin D [25(OH)D] levels. Total circulating 25(OH)D includes 25(OH)D bound to vitamin D–binding protein (DBP), estimated to be about 85% of total with about 10% to 15% bound to albumin and a very small fraction as free or unbound 25(OH)D. Because DBP is the main carrier for 25(OH)D and other vitamin D metabolites, its concentration and affinity are the main drivers of the free concentration of 25(OH)D and other D metabolites. If the free hormone hypothesis applies to vitamin D biology, only free 25(OH)D is available for conversion to active 1α,25(OH)2 D, which interacts with the vitamin D receptor regulating hundreds of genes in most cells. It has been shown that health conditions, such as cirrhosis, that is associated with protein synthetic dysfunction (resulting in decreased DBP), as well as albumin; and pregnancy, that is associated with increased protein synthesis and DBP in the second and third trimesters, alter levels of free 25(OH)D inversely to the changes in DBP (1–3). There is uncertainty regarding DBP genetic variant effects on free 25(OH)D levels, but in vitro DBP affinity constants for 25(OH)D that differ between DBP haplotypes would predict altered 25(OH)D binding and differing free 25(OH)D levels (4–7). Altered albumin concentrations, such as the lower levels reported in the frail elderly or nursing home residents (8), could also alter free 25(OH)D concentrations, albeit to a smaller extent than changes in DBP. Thus, total 25(OH)D may not accurately reflect levels available for cellular uptake, with the exception of cells in the kidney or parathyroid capable of megalin/cubilin-mediated internalization of DBP-bound 25(OH)D (9).
The goal of this work was to combine data from human investigations involving direct measurement of free 25(OH)D to address two objectives. The first is to describe the distribution of circulating free 25(OH)D concentrations in adult humans with and without various conditions or disease states known to alter DBP, in people with differing DBP haplotypes that might alter DBP levels or binding, in people with a wide range of body weights because higher body mass indexes (BMIs) seen with obesity, metabolic syndrome, or prediabetes alter total 25(OH)D and calculated free 25(OH)D (10) and in groups for which no data on free 25(OH)D are currently available such as the very elderly, nursing home residents, and women with osteoporosis who are likely to receive D supplementation or receive exogenous female sex hormones. The second objective is to determine relationships between free and total 25(OH)D in these clinical conditions and disease states and among different DBP haplotypes. Our findings provide a measure of the normal range of free 25(OH)D concentrations as well as observations on factors that do and do not alter relationships between free and total 25(OH)D in clinical populations.
Participants and Methods
Participants
Investigators who directly measured free 25(OH)D in clinical investigations contributed de-identified data. Adult groups sampled included healthy persons, medically stable community-dwelling outpatients enrolled in longitudinal or D dosing studies, persons with prediabetes, medically stable nursing home residents over age 65 years, stable patients with cirrhosis, and pregnant women (second or third trimester) (2, 10–26). Participants provided informed consent for the research, which was approved by the institutional review board of the respective organizations. For investigators, sites, and participant descriptions see the Supplemental Material.
Laboratory measurements
Free 25(OH)D levels
Direct measurement of free 25(OH)D concentrations was by immunoassay (Future Diagnostics B.V., Wijchen, Netherlands) as described (23). In brief, an antibody to 25(OH)D is precoated onto a microtiterplate and serum samples and calibrators added. Free 25(OH)D is captured during this first incubation step, and after washing, a second incubation with biotin-labeled 25(OH)D analog reacts with nonoccupied antibody binding sites (competitive immunoassay). Finally, after washing and incubating with a streptavidin-peroxidase conjugate, absorbance (A450 nm) is measured by using a plate spectrophotometer, where concentration of free 25(OH)D in the sample is inversely proportional to absorbance in each sample well. Assay calibration was against a symmetric dialysis method. Limit of detection for blank serum is 0.7 pg/mL; at 5.02 pg/mL, between-run coefficient of variation (CV) was 6.2% and between-day CV was 4.5%, with a total imprecision CV of 15.7%. Biotin at 4 mg/dL of free 25(OH)D was tested for assay interference and mean percentage interference was 1% at 6.5 pg/mL, 4% at 10.6 pg/mL, and 1% at 15.7 pg/mL. Assays were performed at Future Diagnostics B.V. except for measurements in patients with prediabetes, which were performed in Tromsø using the Future Diagnostics B.V. kit (Wijchen, Netherlands) with the same technique calibrated over the range of 0.1 to 35 pg/mL with a limit of detection of 2.8 pg/mL; inter- and intra-assay CVs were < 10% (24).
Total 25(OH)D
Total 25(OH)D was determined by liquid chromatography/tandem mass spectrometry using National Institute of Standards and Technology reference standard. US sites participated in the National Institutes of Health Office of Dietary Supplements–funded quality assurance program for analysis of D metabolites in human serum. European sites participated in the National Institute of Standards and Technology or Vitamin D External Quality Assessment Scheme, with the exception that two thirds of samples from patients with cirrhosis were by immunoassay (Liaison; DiaSorin, Stillwater, MN). The results converted to liquid chromatography/tandem mass spectrometry equivalent by the manufacturer-provided calibration factor).
DBP
We measured DBP by using radial immunodiffusion (polyclonal) assay (KU Leuven, Leuven, Belgium) for all groups except pregnant patients (monoclonal ELISA; R&D Systems, Minneapolis, MN).
Albumin, creatinine, and calcium
Albumin, creatinine, calcium, were measured with autoanalyzers in clinical laboratories. Intact parathyroid hormone (iPTH) was measured by multiple immunoassays: two-site sandwich immunoassay using direct chemiluminometric technology (for University of California, San Francisco, samples: ADVIA Centaur; Siemens, Malvern, PA), DiaSorin immunoradiometric assay (for Creighton University samples, Stillwater, MN), automated clinical chemistry analyzer (for samples from Tromsø, Norway, and from the United Kingdom: Immulite 2000, Siemens Healthcare Diagnostics, Los Angeles, CA), and Scantibodies immunoradiometric assay (Santee, CA) for osteoporotic fractures in men samples. The assay method was coded.
DBP haplotyping
DBP haplotyping was performed for 959 participants. In 471 patients with prediabetes from the University of Tromso, haplotyping was done by KBioscience (Hoddenson, United Kingdom) by using the KBioscience Competitive Allele-specific PCR genotyping system; in 205 young and older men and women from Sheffield England at Sheffield Children’s Hospital, United Kingdom, a pyrosequencing assay was developed with PSQ software, version 1.0.6 (Qiagen, Manchester, United Kingdom) to detect rs4588 and rs7041 polymorphisms; in 254 older male community outpatients (multiple US osteoporotic fractures in men sites), two nonsynonymous GC single-nucleotide polymorphisms were used to define GC haplotypes [rs4588 (Thr436Lys) and rs7041 (Asp432Glu)], and in 29 young healthy participants (MRC/Gambia), samples were analyzed at Vesalius Research Center (Katholieke Universiteit, Leuven, Belgium) by iPLEX technology on a MassARRAY compact analyzer (Sequenom, San Diego, CA).
Data analysis
Demographic, clinical characteristics, and assay results are presented as mean ± SD. ANOVA for trends followed by post hoc analyses for between-group comparisons using Bonferroni correction for multiple comparisons was used to test for differences in total, free, or percentage free 25(OH)D between clinical groups, DBP haplotypes, or self-reported racial groups. Relationships between free and total 25(OH)D were examined by using a mixed-effects model incorporating clinical condition and DBP haplotypes with sex, race, estimated glomerular filtration rate (eGFR), BMI, and other biologically plausible covariates and interactions. Relationships between free or total 25(OH)D and iPTH were examined in the same manner, including iPTH assay method as a covariate. Analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria). The fixed-effect part of the model takes the following form: free = a + (b + c COV)total, where a is the intercept, b is the slope of the relationship free vs total, and c is a vector of parameters quantifying the relationship of the slope with covariates. Slopes are assumed to be normally distributed across individuals. Model selection was conducted by using standard procedures according to the Akaike criterion (27) and visual inspection of diagnostic plots. After model selection, comparisons to the reference group were computed according to a two-sided t test using the Satterthwaite approximation (R lmerTest). Exploratory analyses of effects of sex hormones in women were performed by using linear regression.
Results
Participant data
Data were from 1661 participants. Demographic characteristics by clinical group (healthy, prediabetic, community-dwelling outpatient, cirrhotic, pregnant, nursing home resident), eGFR, albumin, calcium, albumin-corrected calcium, DBP, total and free 25(OH)D, and iPTH are listed in Table 1. 25-Hydroxyvitamin D2 [25(OH)D2] was detected in no pregnant participants, 10% of nursing home residents, 25% of patients with cirrhosis, and 61% of healthy participants and outpatients). Average 25(OH)D2 was <7% of total in healthy persons and outpatients and 24% of total in patients with cirrhosis. No relationship was detected between free 25(OH)D and 25(OH)D2. Assays measuring C3 epimer of 25(OH)D were used in 498 samples, and C3 epimer was detected in 296 (59%). C3 epimer concentrations > 1 ng/mL were not detected until total 25(OH)D exceeded 20 ng/mL; C3 epimer was <2 ng/mL at a total 25(OH)D level up to 30 ng/mL.
Table 1.
Description of Populations Sampled and Serum Measurements by Clinical Subgroups
| Characteristic | Normal | Community-Dwelling Outpatients | Patients With Prediabetes | Patients With Cirrhosis | Nursing Home Residents | Pregnant Women |
|---|---|---|---|---|---|---|
| Participants, n (%) | 279 (16.8) | 714 (43) | 479 (28.8) | 90 (5.4) | 79 (4.8) | 20 (1.2) |
| Age, y | 36.6 ± 8.5 | 68.7 ± 8.5 | 62 ± 8.6 | 58.0 ± 8.8 | 87.4 ± 8.0 | 30.7 ± 6.9 |
| Sex, n (%) | ||||||
| Women, n (%) | 178 (63.8) | 324 (45.4) | 184 (38.4) | 36 (40) | 51 (64.6) | 20 (100) |
| Men | 90 (32.3) | 390 54.6) | 295 (61.6) | 54 (60) | 28 (35.4) | 0 (0) |
| Unknown | 11 (3.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Race/ethnicity, n (%) | ||||||
| White | 187 (67) | 518 (72.5) | 479 (100) | 69 (76.7) | 78 (98.7) | 15 (75) |
| Black | 65 (23.3) | 191 (26.8) | 0 (0) | 11 (12.2) | 0 (0) | 4 (20) |
| Asian | 12 (4.3) | 3 (0.4) | 0 (0) | 6 (6.7) | 1 (1.3) | 1 (5) |
| Other | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Native American | 2 (7.2) | 2 (0.3) | 0 (0) | 4 (4.4) | 0 (0) | 0 (0) |
| Unknown | 12 (4.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Weight, kg | 78.5 ± 18.6 | 83.7 ± 16.7 | 88.4 ± 16.6 | 85.5 ± 18.8 | 69.9 ± 16.4 | 81.1 ± 20.9 |
| BMI, kg/m2 | 28.0 ± 6.2 | 29.4 ± 6.0 | 29.9 ± 4.3 | 29.1 ± 5.8 | 27.3 ± 5.8 | 32.1 ± 7.4 |
| eGFR, mL/min/1.73 m2 | 107.1 ± 15.3 | 79.6 ± 18.1 | 93.4 ± 12.2 | NA | 63.8 ± 19.4 | 81.6 ± 25.6 |
| Creatinine, mg/dL | 0.8 ± 0.1 | 1.0 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.8 | 0.9 ± 0.3 | — |
| Albumin, mg/dL | 4.3 ± 0.4 | 4.3 ± 0.3 | 4.5 ± 0.2 | 3.2 ± 0.8 | 3.6 ± 0.4 | 3.6 ± 0.3 |
| Calcium, mg/dL | 9.3 ± 0.4 | 9.4 ± 0.4 | 9.2 ± 0.3 | 8.8 ± 0.7 | 9.0 ± 0.4 | 9.1 ± 0.6 |
| Corrected calcium, mg/dL | 9.1 ± 0.3 | 9.1 ± 0.4 | 8.8 ± 0.3 | 9.4 ± 0.6 | 9.4 ± 0.2 | NA |
| iPTH, pg/mLa | 42.2 ± 20.0 | 44.1 ± 24.7 | 52.8 ± 20.8 | 38.8 ± 35.3 | 48.1 ± 25.5 | 21.8 ± 18.0 |
| Free 25(OH)D, pg/mL)b,c,d,e | 4.3 ± 1.9d | 4.5 ± 1.8d | 5.5 ± 1.7d | 7.1 ± 3.0d | 9.5 ± 3.8d | 4.0 ± 1.1d |
| Total 25 (OH)D, ng/mLc,e,f,g | 21.9 ± 9.9g | 22.5 ± 9.1g | 24.4 ± 8.7g | 18.7 ± 10.6g | 34.9 ± 12.8g | 26.7 ± 10.0g |
| Percentage free 25(OH) Dc,h | 0.020 ± 0.006h | 0.021 ± 0.008h | 0.023 ± 0.006h | 0.040 ± 0.020h | 0.028 ± 0.006h | 0.016 ± 0.006h |
| DBP, μg/mLc,i,j | 293 ± 51.1i (n=159) | 294.1 ± 36.5i (n=495) | 299.2 ± 41.4i (n=476) | 175.5 ± 64.7i (n=58) | 264.2 ± 38i (n=78) | 529 ± 49.5i (n=20) |
Data expressed with a plus/minus sign are mean ± SD. NA, not available.
Measured in clinical laboratories by multiple methods.
Assays performed at Future Diagnostics BV, except for in patients with prediabetes; in those patients, assays were performed by using the same method at the investigator site.
Significant effect of clinical group (ANOVA, P < 0.0001).
Post hoc between-group comparisons were significant at P < 0.0033 for all but healthy persons vs pregnant women or outpatients and for pregnant women vs outpatients.
εMultiple samples of total and free 25(OH)D from some individuals from dose titrations studies.
Assays were by liquid chromatography/tandem mass spectrometry except in 69 (of 90) patients with cirrhosis; in those patients, the LIAISON assay (DiaSorin) was used, and values were corrected by a calibration factor provided by the manufacturer.
Post hoc between-group comparisons were significant at P < 0.0033 for all but healthy persons vs outpatients, pregnant women, or patients with cirrhosis and for pregnant women vs outpatients or patients with prediabetes.
Post hoc between-group comparisons were significant at P < 0.0033 for all but healthy persons compared with pregnant women or outpatients and for pregnant women vs outpatients.
iPost hoc between-group comparisons were significant at P < 0.0033 for all but healthy persons vs outpatients or patients with prediabetes and for patients with prediabetes vs outpatients.
DBP measurements by radial immunodiffusion assay (Leuven) except for pregnant women; in that group, DBP was determined by R&D assay (last column).
Free 25(OH)D Distribution
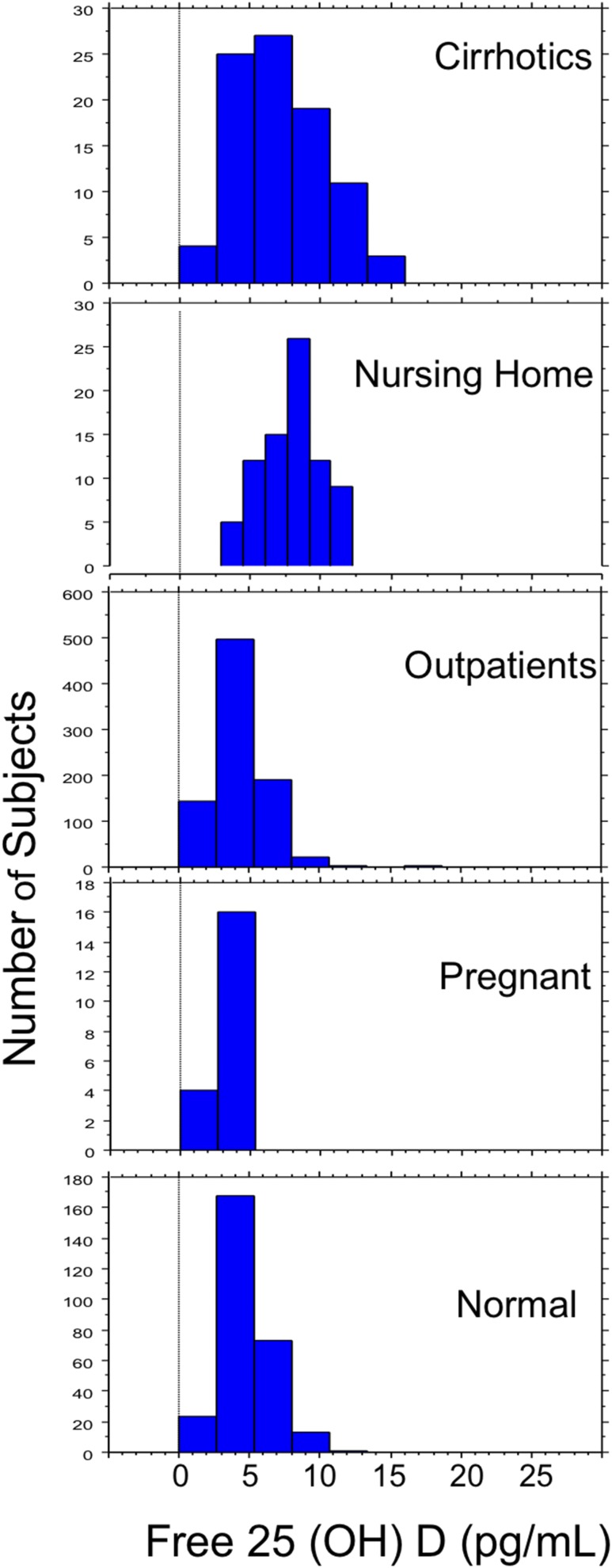
Distribution of free 25(OH)D concentrations by clinical group is shown in Fig. 1. Data reflect steady-state conditions with and without D supplementation as part of clinical care (but not during dose titration studies). Free 25(OH)D levels from 0.5 to 8.1 pg/mL include 95% of healthy participants and is similar to the 0.9 to 8.1 pg/mL range encompassing 95% of the almost three times larger group of stable outpatients. Significant effects of clinical condition on free 25(OH)D, DBP, total 25(OH)D, and percentage free 25(OH)D were detected (ANOVA P < 0.0001; Table 1). The highest mean free 25(OH)D was in nursing home residents, accompanied by higher total 25(OH)D and lower DBP than in healthy persons, outpatients, patients with prediabetes, and pregnant women, but higher DBP than in patients with cirrhosis (P < 0.0033). The next highest mean free 25(OH)D was in patients with cirrhosis (higher than in healthy persons, pregnant women, patients with prediabetes, and outpatients (post hocP < 0.0033 for all comparisons). Between-group differences were detected for all comparisons (post hocP < 0.0033) except for healthy persons vs pregnant women or outpatients, and for pregnant women vs outpatients. Both DBP and total 25(OH)D were lowest in patients with cirrhosis. Pregnant women had the second highest total 25(OH)D levels and the highest DBP (post hocP < 0.0033), despite measurement by a less sensitive assay. Albumin concentrations were not correlated with DBP (r2 = 0.0004; P = 0.83) in the absence of pregnancy or cirrhosis. Percentage free 25(OH)D was higher in patients with cirrhosis and nursing home residents than in other clinical groups (post hocP < 0.0033), and between-group comparisons were significant for all but healthy persons compared with pregnant or outpatients and for pregnant women vs outpatients.
Figure 1.
Distribution of free 25(OH)D concentrations are shown for healthy ("normal") persons, stable community-dwelling outpatients, pregnant women, elderly nursing home residents, and patients with cirrhosis. Free 25(OH)D concentrations are on the horizontal axis, and the number of participants is plotted on the vertical axis. Data are concentrations at study entry (baseline) for any participants enrolled in vitamin D supplementation or dose titration studies.
Effects of race and DBP haplotype
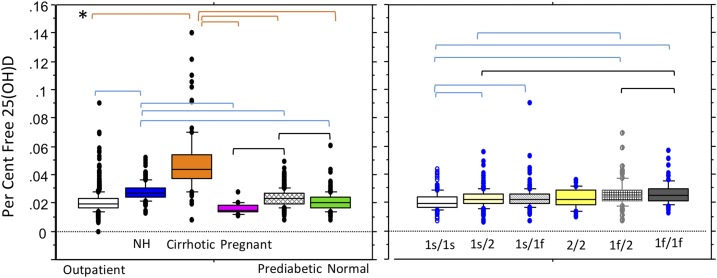
Genotype data were available for 959 participants (outpatients, patients with prediabetes, and healthy persons; Table 2). Ninety-eight were of self-reported black race, 860 were white, and 1 was of self-reported other race. Differences in free 25(OH)D between whites and blacks were detected (4.9 ± 1.9 vs 4.0 ±1.5 pg/mL, respectively; P < 0.0033). As expected, the 1f allele was more common in blacks and the 1s allele more common in whites. (Table 2). Gc 2/2 haplotype was present in 5.5% of whites and no blacks. DBP haplotype had significant effects on total 25(OH)D, free 25(OH)D, and DBP (ANOVA, P < 0.0001). The lowest total and free 25(OH)D were seen with the least frequent Gc 2/2 haplotype (4.2 ± 2.2 pg/mL). Total and free 25(OH)D were higher in the presence of 1s alleles. Post hoc analyses detected lower free 25(OH)D levels among participants with the 2/2 haplotype than among those with 1s/1s or 1s/1f haplotypes and among those with 1f/1f haplotypes compared to those with 1f/1s haplotypes (P < 0.0033). DBP haplotype also affected percentage free 25(OH)D (P < 0.0001) (Fig. 2). The percentage free level was lower with the 1s/1s haplotype than with the 1s/1f, 1f/2, 1f/1f, or 1s/2 haplotype (P < 0.0033). Percentage free 25(OH)D was higher with the 1f/1f haplotype than with 1s/2 and 1f/2 and was higher with 1f/2 than with 1s/2 (P < 0.0033). The magnitude of differences, however, was less than observed between some clinical conditions. DBP haplotypes differed in DBP concentrations: Participants with the 2/2 haplotype had the lowest DBP, total 25(OH)D, and free 25(OH)D levels (post hocP < 0.0033) but a percentage free 25(OH)D level that was in the middle of observed means. The highest DBP was seen among participants with the 1s/1s haplotype that had the highest total and free 25(OH)D but lowest percentage free 25(OH)D. DBP levels were higher for the 1s/1s haplotype compared with any haplotype with at least one Gc2 allele (P < 0.0033) but not when compared with haplotypes 1s/1f or1f/1f. DBP levels were significantly lower for haplotype 2/2 than for 1f/2,1f/1f, and 1s/1f (P < 0.0033). No differences were detected between haplotypes 1s/1s vs 1s/1f or 1f/1f; 1s/2 vs 1f/2, 1s/2 vs 1f/1f, or 1s/1f vs 1f/1f. Differences between haplotypes 1s/1f vs 1f/2 approached significance (P = 0.0045) but failed to reach the post hoc criteria for significance (P < 0.0033).
Table 2.
Free, Total, and Percentage Free 25(OH)D and DBP by DBP Haplotype
| DBP Haplotype |
Frequency (%)
a |
Free 25(OH)D (pg/mL) b | Total 25(OH)D (ng/mL) b | Percentage Free 25(OH)D b | DBP (RID) (μg/mL) b | ||
|---|---|---|---|---|---|---|---|
| White (n = 860) | Black (n = 98) | Other (n = 1) | |||||
| 1s/1s | 31.9 | 1 | 0 | 5.1 ± 1.8 | 25.6 ± 10.0 | 0.021 ± 0.006 | 308.6 ± 40; n = 209 |
| 1s/2 | 29 | 1 | 100 | 5.1 ± 2.1 | 23.1 ± 8.4 | 0.023 ± 0.007 | 287.9 ± 36.2; n = 182 |
| 1s/1f | 22.4 | 27 | 0 | 5.4 ± 2.0 | 24.2 ± 9.0 | 0.023 ± 0.007 | 304.5 ± 39.7; n = 189 |
| 2/2 | 5.5 | 0 | 0 | 4.1 ± 2.0 | 17.8 ± 7.3 | 0.023 ± 0.007 | 260.4 ± 25.1; n = 24 |
| 1f/2 | 8.3 | 18 | 0 | 4.7 ± 1.8 | 19.6 ± 7.7 | 0.026 ± 0.010 | 289.3 ± 34.1; n = 73 |
| 1f/1f | 3 | 51 | 0 | 4.4 ± 1.6 | 18.2 ± 8.2 | 0.026 ± 0.008 | 300.1 ± 43.5; n = 73 |
Significant differences in frequencies of haplotypes between the races for all haplotypes except for Gc1s/1f were detected.
Statistically significant effects of DBP haplotype were detected for total, free, and percentage free 25(OH)D concentrations and DBP (ANOVA, P < 0.0001; see Fig. 2 and text for individual between-haplotype post hoc comparisons).
Figure 2.
Percentage free 25(OH)D concentrations are presented by clinical subgroup (left) and by DBP haplotypes (right) (subset of n = 974). The box plot shows the 10th, 25th, median, 75th, and 90th percentile values. Individual points represent values above the 90th and below the 10th percentiles. Both clinical subgroup and DBP genotype had significant effects on percentage free 25(OH)D (ANOVA, P < 0.0001). *Horizontal brackets indicate statistically significant post hoc between-group comparisons (meeting Bonferroni criteria of P < 0.0033). Post hoc between-group comparisons were significant for all but healthy persons compared with pregnant women or outpatients, or for pregnant women compared with outpatients. For DBP haplotypes, smaller but significant differences were detected between the 1s/1s haplotype and the 1s/1f, 1f/2, 1f/1f, and 1s/2 haplotypes and between the 1s/2 and 1f/2 and 1f/1f haplotypes and between the 1s/1f and 1f/1f haplotypes. NH, nursing home.
Relationships between free and total 25(OH)D
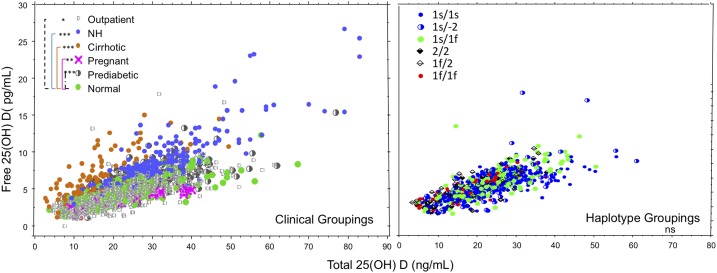
Individual data are plotted by clinical group and DBP haplotype in Fig. 3. Linear mixed-effects modeling identified significant contributors to the relationship as the clinical condition and BMI. (see Table 3). Rejected covariates included eGFR and race. Clinically healthy persons were associated with the baseline slope (b) of the model. The steepest slope (b + 0.1577) was in patients with cirrhosis with the lowest DBP, the second steepest slope was in nursing home residents with the second lowest DBP levels, and the least steep slope was in pregnant women with the highest DBP. After exclusion of cirrhosis and pregnancy from the model, sex was selected for inclusion (male sex with coefficient estimate of 0.03 ± 0.004). Effects of DBP haplotype on the relationship between free and total 25(OH)D were not detected in participants (n = 959) with these data.
Figure 3.
Relationships between free and total 25(OH)D by clinical subgroup and DBP haplotype. Total 25(OH)D concentration is plotted on the x-axis, and free 25(OH)D concentration is plotted on the y-axis. (Left) Open circles represent data from community-dwelling outpatients, closed blue circles represent data from older nursing home (NH) residents, closed brown circles represent data from patients with cirrhosis, pink x's represent data from pregnant women, half-filled circles represent data from patients with prediabetes, and closed green circles indicate data from healthy persons. Data include multiple measures in a subset of healthy persons and nursing home residents enrolled in vitamin D supplementation studies (n = 243 samples). (Right) Closed blue circles represent the 1s/1s DBP haplotype, half blue and half white circles represent 1s/2 haplotypes, solid green circles represent 1s/1f, solid diamonds represent 2/2, open cross-hatched diamonds represent 1f/2, and solid red circles represent 1f/1f. DBP haplotype data were from healthy persons, community-dwelling outpatients, and patients with prediabetes. Linear mixed modeling detected significant effects of clinical groupings on the relationship between free and total 25(OH) D. *P < 0.05, **P < 0.0001, ***P < 0.000001 for comparisons with healthy persons. Significant effects of DBP haplotype on the relationship were not detected. ns, not significant.
Table 3.
Linear Mixed Effects-Model Analysis of Relationship Between Free and Total 25(OH)D
| Model: Linear Mixed-Effects Regression | Coefficient | SEM | t Value | P Value |
|---|---|---|---|---|
| Model selected covariates | ||||
| a (intercept) | 1.291 | 0.0781 | 16.521 | <0.000001 |
| b (slope) | 0.186 | 0.0085 | 22.024 | <0.000001 |
| Selected covariates | ||||
| Clinical class | ||||
| Community-dwelling/outpatients | −0.0094 | 0.0046 | −2.026 | <0.05 |
| Patients with diabetes | 0.0245 | 0.0049 | 5.010 | <0.000001 |
| Patients with cirrhosis | 0.1577 | 0.0080 | 19.763 | <0.000001 |
| Nursing home residents | 0.0873 | 0.0064 | 13.585 | <0.000001 |
| Pregnant women | −0.0450 | 0.0126 | −0.357 | <0.0001 |
| BMI | −0.0013 | 0.0003 | −4.926 | <0.000001 |
The mixed-effect model takes the form free = a + (b +c COV)total, where a is the intercept, b is the slope of the relationship free vs total 25(OH)D, and c is a vector of parameters quantifying the relationship of the slope with covariates. Variables tested but not selected included eGFR and race. Sex was not tested in this model. The t and P values represent comparisons to the baseline slope of the model (healthy persons).
Relationships between free and total 25(OH)D and iPTH
Both total and free 25(OH)D concentrations were negatively related to iPTH levels, but the mixed-effects model fits favored total 25(OH)D (coefficient estimate of −0.96 ± 0.51). Covariates selected included BMI (continuous variable), with a small effect (0.02 ± 0.004), and iPTH assay method, which varied within the sites; this precluded further clinical group analyses.
Exploratory analyses: female sex hormones
Forty young nonpregnant and noncirrhotic women reported taking oral contraceptives. Total and free 25(OH)D levels were 21.0 ± 13.1 ng/mL and 3.4 ± 2.2 pg/mL, respectively; these did not differ from the total and free 25(OH)D levels of 20.1 ± 8.3 ng/mL and 3.6 ± 1.5 pg/mL in 21 young nonpregnant noncirrhotic women not taking oral contraceptives. Relationships between free and total 25(OH)D in oral contraceptive users had a slope of 0.150 (95% CI, 0.126 to 0.175) compared with a slope of 0.125 (95% CI, 0.066 to 0.185) in nonusers (P = not significant). Thirty-five postmenopausal women reported estrogen use for hormone replacement, and 82 age- and health-matched women reported no use. Total 25(OH)D concentrations were 24.8 ± 11ng/mL in estrogen users vs 26.1 ± 10.2 in nonusers. Free 25(OH)D was 4.4 ± 2 pg/mL in estrogen users and 4.6 ± 2.2 pg/mL in nonusers (P = not significant), and the slope of relationships between free and total 25(OH)D did not differ [users: 0.164 (95% CI, 0.136 to 0.195); nonusers: 0.158 (95% CI, 0.124 to 0.192)]. DBP data were not available.
Discussion
There is currently debate about the best serum measurement to determine vitamin D status (4). Circulating levels of 25(OH)D are the most commonly used marker for several reasons: (1) The concentration in blood is higher than that of other D metabolites, making it easier to measure; (2) its conversion from vitamin D is substrate dependent, with minimal regulation; and (3) it has a relatively long circulating half-life. However, the free hormone hypothesis postulates that only the nonbound or “free” fraction of hormones that circulates in blood can enter cells and exert biologic effects. This would suggest that the free fraction is key to the intracrine functions of vitamin D, except in cells such as those in the kidney or parathyroid gland that are capable of megalin/cubilin-mediated internalization of DBP-bound 25(OH)D (9).
Assays to directly measure free 25(OH)D are not currently applied in clinical care but have been used in research investigations. Directly measured free 25(OH)D concentrations differ from estimated (calculated) free 25(OH)D concentrations based on DBP assays using monoclonal or polyclonal antibodies and single or DBP haplotype estimated DBP dissociation constants (2, 3, 6, 19, 21–23, 28). Directly measured free 25(OH)D has been reported to correlate better than total 25(OH)D with some biologic measurements (2, 3, 6, 19, 21–23, 28), whereas other reports have not found a stronger relationship (4–7). Most investigations, however, have had small sample sizes or selected populations; thus, the distribution of free 25(OH)D concentrations in many clinical populations is unknown.
The current study compiled data from an international working group of vitamin D investigators in order to describe free 25(OH)D concentrations in a wide range of people with various clinical conditions. The data were from healthy young and older people, people with prediabetes, community-dwelling outpatients enrolled in longitudinal studies or vitamin D studies, pre- and postmenopausal women with low vitamin D status, pregnant women, patients with cirrhosis, and nursing home residents with multiple morbidities enrolled in observational or vitamin D studies. A major strength is that our international data represent the largest and most diverse sample of adults studied to date and included patients with conditions that alter both free 25(OH)D levels and the relationship between free and total 25(OH)D, groups for whom these data have not been previously available. Importantly, 98% of DBP measures were performed with one polyclonal method at one laboratory, and 95.8% of 25(OH)D measures were performed by laboratories participating in quality standardization programs (National Institute of Standards and Technology or Vitamin D External Quality Assessment Scheme) and 100% of free 25(OH)D measurements were performed using the same method.
A strict definition of healthy persons was used to identify people with normal laboratory chemistry test results, no known chronic medical diseases, and no long-term oral medications except for thyroid or hormone replacement therapy, oral contraceptives, or dietary supplements. In these individuals, the mean concentration of free 25(OH)D was 4.3 ± 1.9 pg/mL when mean total 25(OH)D concentration was 21.9 ±9.9 ng/mL. A range from 0.5 to 8.1 pg/mL included 95% of healthy participants and was similar to the 0.9-to-8.1 pg/mL range encompassing 95% of the stable outpatients (a group that was nearly three times larger). Mean free and total 25(OH)D concentrations as well as percentage free 25(OH)D were slightly higher in patients with prediabetes, yet the upper bound of the 95% CI was similar at 8.9 pg/mL. Free 25(OH)D in patients with prediabetes was measured by using the same technique but at a different site than all other assays. Some assay variation may explain the small differences; some patients with diabetes were included in the outpatient samples and did not show higher free or percentage free 25(OH)D (data not shown).
In our prior observations in pregnant women and a subset of patients with cirrhosis, DBP was measured by using a monoclonal antibody DBP assay (1–3, 23). In the current analyses, a polyclonal antibody was used in the radial immunodiffusion assay, which was performed at the same laboratory for all groups with the exception of the pregnant women. The data on the current larger group of patients with cirrhosis are consistent with early reports of lower DBP with higher directly measured mean free 25(OH)D despite lower total 25 (OH)D levels (10). The data from pregnant women mirror the almost twofold higher DBP initially reported in pregnant women in the second and third trimesters compared with nonpregnant women (29, 30) and with less variability in free 25(OH)D. Although the group of pregnant women was small, similar mean free 25(OH)D with lesser variability than in other groups has been reproduced by using the same method in a larger group of about 300 white women, despite somewhat higher DBP in the second and third trimesters when measured by ELISA with a polyclonal antibody (31).
We had limited data on women reporting oral contraceptive use or hormone replacement therapy with estrogen, but free 25(OH)D levels and relationships between total and free 25(OH)D did not appear to be significantly influenced by use of these agents at currently prescribed dosages and routes of administration.
An unexpected observation was that mean free 25(OH)D was higher in the nursing home residents, with distribution of values shifted toward higher concentrations. Likely contributors were both the lower DBP levels and the higher total 25(OH)D in the nursing home residents compared with the healthy persons, patients with prediabetes, community-dwelling outpatients, and pregnant women. Mean albumin concentrations were slightly lower in the nursing home residents than in the healthy persons, outpatients, or patients with prediabetes; however, because only 12% to 15% of 25(OH)D is bound to albumin, it is unlikely to have been a major factor. Inflammation and/or elevated cytokines that accompany very old age (32) or multiple morbidities could also alter affinity of 25(OH)D to DBP. Whatever the underlying mechanisms, both percentage free 25(OH)D concentrations and the relationship between free and total 25(OH)D differ in pregnant women, people with cirrhosis, and elderly people with multiple morbidities compared with healthy persons or community-dwelling outpatients; relationships are affected by BMI to a much smaller extent in all groups. It also appears that stable medical conditions, such as hypertension, prediabetes, diabetes, osteoporosis, or mild renal disease, do not appear to significantly alter relationships between free and total 25(OH)D.
Free 25(OH)D concentrations are related to total 25(OH)D concentrations as well as albumin and DBP and their binding affinities for 25(OH)D (29). DBP is a highly polymorphic protein (33). Our sample included whites and blacks and several Asians, and distribution of DBP haplotypes mirrored reported racial differences in that black (and Chinese) populations are more likely to carry the Gc1f allele and whites more likely to possess the Gc1s and the less frequent Gc2 allele (34). DBP haplotype affected DBP and both total and free 25(OH)D concentrations. The Gc2/2 haplotypes and presence of 1f alleles were associated with lower total 25(OH)D concentrations as previously reported (35). Gc1f has been reported to have the highest affinity and Gc2 the lowest affinity for vitamin D and its metabolites, but this has not been uniformly detected (7, 33, 36, 37). In our sample, the highest percentage free 25(OH)D was seen with the 1f/1f haplotype and 1f/2 haplotypes and the lowest was seen with 1s/1s despite similar DBP concentrations. Mean percentage free 25(OH)D in people with the 2/2 haplotype was in the midpoint of the range and did not differ significantly from the 1s/1f or 1s/2 haplotypes. These data do not support the earlier report of Gc1f having the highest and Gc2 having the lowest affinity for 25(OH)D. The maximum mean percentage differences between haplotypes was on the order of about 19% to 24%. DBP concentrations differed between some haplotypes, and free 25(OH)D concentrations were in the expected relationship [i.e., higher free 25(OH)D concentrations with lower DBP], but the percentage free 25(OH)D did not show the same relationship.
In contrast to differences in percentage free 25(OH)D by DBP haplotype, haplotype was not selected as a significant covariate in the linear mixed-effects model of relationships between free and total 25(OH)D in these individuals. This suggests that haplotype does not have a marked effect on the relationship. We did not have DBP haplotype data on patients with cirrhosis, nursing home residents, or pregnant women to allow comparisons of clinical condition effects to haplotype effects in the same model. Nevertheless, the magnitude of differences seen between the clinical groups was greater than that seen between DBP haplotypes.
This study has limitations. Data were not from random population-wide samples, and analyses of BMI, sex, race, or other subgroup effects might not be representative of all populations. Samples were from medically stable individuals and may not apply to acute medical conditions. The only potential biomarker for vitamin D status analyzed was iPTH, and differing methods in clinical laboratories limited our analyses. However, the parathyroid gland has the megalin/cubilin mechanism for cellular uptake of DBP, so parathyroid hormone levels are unlikely to discriminate between free and total 25(OH)D effects on biological function. Bone biomarkers were not assessed. Bone density has been reported to correlate better with measures of free than total 25(OH)D in the patients with prediabetes included in the current analyses (19), but others have found similar relations between markers of bone metabolism and free or total 25(OH)D (38). However, D and bone relationships are somewhat difficult to interpret because measures of vitamin D and its metabolites are often done only at a single timepoint, whereas bone density is the result of cumulative time effects. Because many participants sampled received D supplementation, we could not address seasonal effects.
Conclusions
Free 25(OH)D concentrations are affected by health conditions in addition to total 25(OH)D concentrations and DBP haplotype. Free 25(OH)D distributions were similar in healthy individuals and stable community-dwelling outpatients, with 95% within the range of 0.5 to 8.1 pg/mL and 0.9 to 8.1 pg/mL, respectively. Percentage free 25(OH)D was affected by clinical condition (patients with cirrhosis > nursing home residents > outpatients > healthy persons > pregnant women), self-reported race (black > white > Asian), and DBP haplotype (1f/1f + 1f/2 > 1f/1s, 2/2, 1s/2 > 1s/1s). Relationships between free and total 25(OH)D were influenced to a small extent by BMI and to a larger extent by health conditions; patients with cirrhosis and nursing home residents had the steepest slopes and pregnant women had the least steep slopes, without significant effects of DBP haplotype detected in mixed-effects models. Clinical outcomes data other than PTH levels are needed to determine the role of free 25(OH)D measurements in clinical decision-making, with the growing recognition of the role of vitamin D and its metabolites in promoting optimal health beyond bone and calcium absorption metabolism (39). Currently, most vitamin D intake recommendations are based on immunoassay-measured total 25(OH)D levels associated with lower risk for osteoporotic fractures in postmenopausal women (40).
As of May 30, 2018, ClinicalTrials.gov lists >600 completed phase 2, 3, and 4 trials of vitamin D relationships to various health conditions, 59 trials that are active and not recruiting, 149 trials that are currently recruiting, and 36 that are in the planning stages. Results from two very large randomized double-blind trials investigating vitamin D supplementation effects on cancer, cardiovascular disease, and mortality [VITAL (NCT01169259) and VIDAL (ISRCTN46328341)] will soon be available and will provide data on relationships with total 25(OH)D. However, to the extent that the free hormone hypothesis applies to cellular availability of vitamin D metabolites, total 25(OH)D measurements may be misleading in persons with altered total-to-free relationships, and analysis of free 25(OH)D could provide further insights.
Supplementary Material
Acknowledgments
Financial Support: The project received financial support in part by Future Diagnostics, BV, and Diasource Inc. (J.B.S.). Original research investigations were supported as follows: US support (National Institutes of Health unless otherwise stated): R21 AG 041660 (J.B.S., J.L.), P30 DK026743 (J.L.), R01 AR050023 (J.L.), R01 AG 15982 (J.B.S.), R56 AG15982 (J.B.S.), U01 AG027810 (E.O.), U01 AG042124 (E.O.), U01 AG042139 (E.O.), U01 AG042140 (E.O.), U01 AG042143 (E.O.), U01 AG042145 (E.O.), U01 AG042168 (E.O.), R01-AG28168 (E.O., J.C.G.), U01 AR066160 (E.O.), UL1 TR000128 (E.O.), P41GM103493, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) K01 AR062655, NIAMS R01 AR063910, T32 DK007674-20, an American College of Gastroenterology Clinical Research Award (J.L.), Department of Defense (W81XWH-07-1-201; J.C.G.), and Department of Energy (contract DE-AC05-76RL0 1830). United Kingdom support: Department of Health (policy research program 024/0052; R.E.) and by the Sheffield National Institute for Health Research Clinical Research Facility (R.E.), the Medical Research Council (MRC; R.E.), and the Department for International Development (DFID; (R.E.) [under the MRC/DFID Concordat; MRC unit programs U105960371 (R.E.) and U123261351; I.S., R.E.]. MC-A760-5QX00 (I.S.) and DFID (under MRC/DFID Concordat agreement), Research Foundation Flanders (G. 0858.11; I.S.) and KU Leuven (GOA 15/0/01; R.B.). In Norway: Novo Nordisk foundation (grant number R195-A16126; R.R.), North Norway Regional Health Authorities (grant number 6856/SFP1029-12; R.J.), UiT The Arctic University of Norway (R.J.), the Norwegian Diabetes Association (R.J.), and the Research Council of Norway (grant number 184766).
Author Contributions: J.B.S.: original data collection, combined study design, data analysis, and manuscript preparation; J.C.G.: original data collection, manuscript review; R.J.: original data collection, assay performance, manuscript review; V.B.: data collection, assay performance, manuscript review; J.W.: data collection and analysis, manuscript review; R.E.: data collection, manuscript review; A.L.E.: data collection and analysis; S.B.: data collection and analysis; K.E.N.: data collection and analysis and manuscript review; K.S.J.: data collection, analysis, and manuscript review; I.S.: data collection, analysis, and manuscript review; M.H.: data collection and analysis; E.O.: data collection and analysis; C.N.: data collation, study design, and manuscript review; M.K.: assay analysis and manuscript review; G.J.: assay analysis; R.B.: assay analysis and manuscript review; J.L.: data collection and analysis; D.V.: statistical analysis and manuscript preparation; D.B.: study design, manuscript preparation, and manuscript review.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D2
25-hydroxyvitamin D2
- BMI
body mass index
- CV
coefficient of variation
- DBP
vitamin D–binding protein
- eGFR
estimated glomerular filtration rate
- iPTH
intact parathyroid hormone
References
- 1. Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74(6):1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai JC, Bikle DD, Lizaola B, Hayssen H, Terrault NA, Schwartz JB. Total 25(OH) vitamin D, free 25(OH) vitamin D and markers of bone turnover in cirrhotics with and without synthetic dysfunction. Liver Int. 2015;35(10):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz J, Lai J, Lizaola G, Kane L, Weyland P, Terrault N, Stotland N, Bikle D.. Variability in free 25(OH)D levels in clinical populations. J Steroid Biochem Mol Biol. 2013;144 (Pt A):156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bikle D, Bouillon R, Thadhani R, Schoenmakers I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J Steroid Biochem Mol Biol. 2017;173:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880–881. [DOI] [PubMed] [Google Scholar]
- 6. Bouillon R, Jones K, Schoenmakers I. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879. [DOI] [PubMed] [Google Scholar]
- 7. Bouillon R, van Baelen H, de Moor P. Comparative study of the affinity of the serum vitamin D-binding protein. J Steroid Biochem. 1980;13(9):1029–1034. [DOI] [PubMed] [Google Scholar]
- 8. Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015;81(1):17–27. [DOI] [PubMed] [Google Scholar]
- 9. Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3(4):256–266. [DOI] [PubMed] [Google Scholar]
- 10. Walsh JS, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I, Jacques RM, Eastell R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016;103(6):1465–1471. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carbone LD, Rosenberg EW, Tolley EA, Holick MF, Hughes TA, Watsky MA, Barrow KD, Chen TC, Wilkin NK, Bhattacharya SK, Dowdy JC, Sayre RM, Weber KT. 25-Hydroxyvitamin D, cholesterol, and ultraviolet irradiation. Metabolism. 2008;57(6):741–748. [DOI] [PubMed] [Google Scholar]
- 13. Gallagher JC, Sai A, Templin T II, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156(6):425–437. [DOI] [PubMed] [Google Scholar]
- 14. Gallagher JC, Jindal PS, Smith LM. Vitamin D does not increase calcium absorption in young women: a randomized clinical trial. J Bone Miner Res. 2014;29(5):1081–1087. [DOI] [PubMed] [Google Scholar]
- 15. Gallagher JC, Jindal PS, Smith LM. Vitamin D supplementation in young White and African American women. J Bone Miner Res. 2014;29(1):173–181. [DOI] [PubMed] [Google Scholar]
- 16. Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98(3):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210. [DOI] [PubMed] [Google Scholar]
- 18. Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njølstad I. Cohort profile: the Tromso Study. Int J Epidemiol. 2012;41(4):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D [published correction appears in Scand J Clin Lab Invest. 2014;74(5):464]. Scand J Clin Lab Invest. 2014;74(3):177–183. [DOI] [PubMed] [Google Scholar]
- 20. Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane L, Moore K, Lütjohann D, Bikle D, Schwartz JB. Vitamin D3 effects on lipids differ in statin and non-statin-treated humans: superiority of free 25-OH D levels in detecting relationships. J Clin Endocrinol Metab. 2013;98(11):4400–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Schepmoes AA, Zmuda JM, Lapidus J, Cauley JA, Bouillon R, Schoenmakers I, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Group . Free 25-hydroxyvitamin d: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101(5):2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz JB, Kane L, Bikle D. Response of vitamin D concentration to vitamin D3 administration in older adults without sun exposure: a randomized double-blind trial. J Am Geriatr Soc. 2016;64(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sollid ST, Hutchinson MY, Berg V, Fuskevåg OM, Figenschau Y, Thorsby PM, Jorde R. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174(4):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 27. Akaike A. A new look at the statistical model identification problem. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- 28. Nielson CM, Jones KS, Bouillon R, Chun RF, Jacobs J, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Zmuda JM, Lapidus J, Cauley JA, Schoenmakers I, Orwoll ES. for the Osteoporotic Fractures in Men (MrOS) Research Group . Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations. N Engl J Med. 2016;374(17):1695–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 30. Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67(3):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsyprykov O, Buse C, Skoblo R, Haq A, Hocher B. Reference intervals for measured and calculated free 25-hydroxyvitamin D in normal pregnancy. J Steroid Biochem and Molec Metab. 2018;181:80–87. [DOI] [PubMed] [Google Scholar]
- 32. Srikanth P, Chun RF, Hewison M, Adams JS, Bouillon R, Vanderschueren D, Lane N, Cawthon PM, Dam T, Barrett-Connor E, Daniels LB, Shikany JM, Stefanick ML, Cauley JA, Orwoll ES, Nielson CM; Osteoporotic Fractures in Men (MrOS) Study Research Group . Associations of total and free 25OHD and 1,25(OH)2D with serum markers of inflammation in older men. Osteoporos Int. 2016;27(7):2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993;92(2):183–188. [DOI] [PubMed] [Google Scholar]
- 34. Speeckaert M, Huang G, Delanghe JR, Taes YEC. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372(1-2):33–42. [DOI] [PubMed] [Google Scholar]
- 35. Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 36. Kawakami M, Imawari M, Goodman DS. Quantitative studies of the interaction of cholecalciferol (vitamin D3) and its metabolites with different genetic variants of the serum binding protein for these sterols. Biochem J. 1979;179(2):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boutin B, Galbraith RM, Arnaud P. Comparative affinity of the major genetic variants of human group-specific component (vitamin D-binding protein) for 25-(OH) vitamin D. J Steroid Biochem. 1989;32(1, 1A):59–63. [DOI] [PubMed] [Google Scholar]
- 38. Aloia J, Dhaliwal R, Mikhail M, Shieh A, Stolberg A, Ragolia L, Fazzari M, Abrams SA. Free 25(OH)D and calcium absorption, PTH, and markers of bone turnover. J Clin Endocrinol Metab. 2015;100(11):4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.