Abstract
Introduction:
Tumor-derived exosomes (TEX) and their role in tumor progression by accelerating angiogenesis are of great current interest. A better understanding of the mechanisms underlying TEX-blood vessels cross-talk may lead to improvements in current diagnosis, prognosis and treatment of cancer.
Areas covered:
For solid tumors, an adequate blood supply is of critical importance for their development, growth and metastasis. TEX, virus-size vesicles which circulate freely throughout body fluids and accumulate in the tumor microenvironment (TME), have been recognized as a new contributor to angiogenesis. TEX serve as a communication system between the tumor and various normal cells and are responsible for functional reprogramming of these cells. The molecular and genetic cargos that TEX deliver to the recipient cells involved in angiogenesis promote its induction and progression. The targeted inhibition of TEX pro-angiogenic functions might be a novel therapeutic approach for control of tumor progression.
Expert opinion:
TEX circulating in body fluids of cancer patients carry a complex molecular and genetic cargo and are responsible for phenotypic and functional reprogramming of endothelial cells and other normal cells residing in the TME.
Keywords: angiogenesis, endothelial cells, hypoxia, tumor-derived exosomes (TEX)
1.0. Introduction
The tumor microenvironment (TME) is organized by the developing tumor to promote its growth [1]. The TME consists of tumor stroma, which surrounds blood vessels and tumor cells and is crucial for tumor growth, drug resistance and metastasis [2,3]. A novel attempt to explain the molecular and genetic changes which tumor cells induce in the surrounding tissues and the host immune system has recently focused on extracellular vesicles (EVs). The subset of EVs with a diameter of 30–150 nm is known as “exosomes”. These virus-size membrane-bound vesicles serving as a universal communication system and are distributed like viruses in the interstitial fluid [4]. They circulate freely throughout body fluids, delivering “messages” to and from cells residing in the TME [5]. Exosomes are produced by all cells, but tumor cells are especially active exosome producers, potentially because the presence of stress or hypoxia stimulate tumor cells to increase exosome production [6]. Radiochemotherapy has also been reported to increase exosome production [7]. Exosomes carry a variety of signaling molecules, both receptors and ligands, on their surface membrane, and in their lumen are microRNAs (miRNAs), mRNA and DNA. Also, enzymes and various soluble factors are present in the vesicle lumen. These components of the exosome cargo partially mimic the content of the parent tumor cell [8]. They are biologically active and can trigger functional responses in targeted responder cells. Tumor cells secrete millions of exosomes and distribute them throughout the TME. Patients with cancer, especially those with advanced or metastatic disease, have greatly increased numbers of exosomes in the plasma compared to healthy blood donors [8]. The intercellular communication of the tumor exosomes usually runs bidirectionally between the tumor cells and the surrounding tissue. The cellular reprogramming driven by exosomes is dependent not only on the nature of the parent cell, but also on the recipient cell, which undergoes genetic and molecular alterations induced by an interaction with exosomes [9].
In the TME, exosomes mediate autocrine, juxtacrine and paracrine interactions [8,10]. The modulation of the microenvironment by tumors and the formation of a pre-metastatic niche is regarded as one of the major reprogramming events exosomes mediate [11]. Tumor-derived exosomes referred to as “TEX” play an important role in suppression of antitumor immune responses and thus in promoting tumor progression [9]. TEX are also involved in tumor angiogenesis and appear to modulate mechanisms responsible for the blood vessel development. In this review, we summarize current information available for the role TEX play in the process of angiogenesis.
2.0. Exosome Isolation for In vitro and In vivo Studies
Exosomes can be isolated from supernatants of tumor cell lines or body fluids using many different methodologies. These have been recently reviewed [12]. The most commonly used method for exosome isolation is based on differential ultracentrifugation [13]. However, the exosome quality and recovery may not be optimal, and the time of the procedure and equipment needed for ultracentrifugation are not practical for handling of large numbers of specimens. An alternative to ultracentrifugation is concentration of large volumes using ultrafiltration devices [14]. We prefer to use size exclusion chromatography for isolation of exosomes from plasma samples as well as cell culture supernatants, because the method allows for a removal of most, albeit not all, “contaminating” plasma proteins (albumin and immunoglobulins). Also, isolated exosomes, which elute in a void volume, are morphologically intact, non-aggregated and functional [15]. To date, no standardized, universally-accepted method for exosome isolation from supernatants of cells or human body fluids exists, and the quality of isolated exosomes remains an important and challenging variable in various studies. Compared to the isolation from cell culture supernatants, where exosomes originate exclusively from the cultured cells, the plasma-derived exosomes are mixtures of vesicles originating from many different tumor and non-tumor cells. The separation of TEX from non-tumor-derived exosomes in plasma of cancer patients using immunoaffinity capture has been recently reported by our group [16].
3.0. TEX and Neovascularization
The growth of new vessels, which begins at an early point of tumor development, has been related to the levels of exosomes the tumor produces. Glioblastomas, for example, are highly vascularized in comparison to other solid tumors [17]. They produce TEX, which influence the proliferation of endothelial cells (ECs). EVs secreted by glioblastomas contain angiogenic proteins and have pro-angiogenic properties both in vitro and in vivo [18,19]. Other solid tumors, such as pancreatic carcinoma or breast cancer, also produce exosomes that induce neovascularization [20,21]. In addition to these and other solid tumors, the exosomes produced by chronic myelogenous leukemia (CML) cells have been reported to influence the blood vessel formation by directly interacting with ECs [22,23]. In these studies, TEX were found to be potent inducers of angiogenesis in vitro and in vivo through functional reprogramming and phenotypic modulation of ECs. Specifically, they were able to stimulate proliferation, migration and tubulogenesis of ECs [19,24–27]. These angiogenic effects were observed using TEX derived from tumor cell lines and also with exosomes isolated from the urine of bladder cancer patients and exosomes from the blood of patients with various types of cancer [25,28–30]. In addition to modulating the formation of new blood vessels, TEX might also promote lymphangiogenesis [25], although little information is currently available on the TEX involvement in the formation of lymphatic vessels.
4.0. Uptake of Exosomes by ECs
In the TME, exosomes interact with ECs and other tissue or immune cells to accelerate tumor angiogenesis. The exosome-induced changes in these various cells are dependent on the exosome internalization. Whereas exosomes were observed to interact with T cells via surface-mediated receptor-ligand interactions, ECs internalize exosomes produced by cancer cells within 2–4 h [18,23,31–33]. Image analyses using confocal microscopy showed that ECs readily uptake exosomes labeled with the PKH26 dye within first 4 h of co-incubation [23,34]. Cells utilize a variety of mechanisms for the uptake of exosomes, e.g. phagocytosis, micropinocytosis or lipid raft-mediated internalization [35–37]. The main uptake mechanism used by ECs is presumably endocytosis [38]. Immediately after internalization, exosomes localize to the perinuclear region as demonstrated in Figure 1. During in vitro tubular formation, exosomes move to the cell periphery and enter into extending pseudopods [23]. At this point, exosomes are found predominantly in clusters. After complete remodeling, the neighboring ECs probably transport exosomes to other ECs and to other tissue cells in the TME through nanotubular structures visible by confocal microscopy. This appears to be the mechanism for communication via exosomal messages between ECs within the tubule network as well as between ECs and surrounding tissue cells [23,34]. Although the uptake of TEX by ECs is the most commonly described event in the literature, other mechanisms, which do not require TEX uptake, probably also occur, as indicated in Figure 2C and discussed below. Specifically, the receptor-ligand type interactions are also used by TEX to alter behavior of EC [39].
Figure 1:
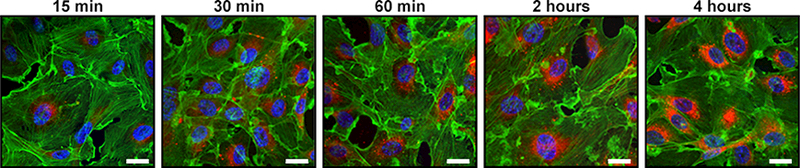
ECs internalize TEX within 4h and immediately after internalization, TEX localize to the perinuclear region. Shown are representative images of internalization of exosomes derived from plasma of a head and neck cancer patient by HUVECs after 15min, 30min, 60min, 2h and 4h of coincubation. Red fluorescence: exosomes, green fluorescence: F-actin, blue fluorescence: nuclei. Scale bar: 20μm (Figure was created in collaboration with Saigopalakrishna S. Yerneni).
Figure 2:
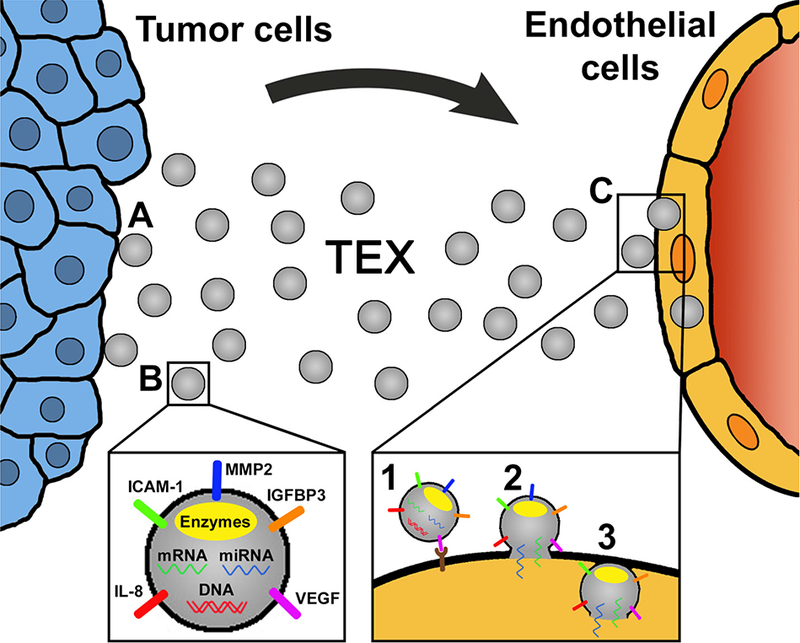
A schematic of EC reprogramming mediated by TEX. (A) Tumor cells produce and release exosomes. (B) TEX carry a variety of angiogenic proteins and in their lumen are enzymes, mRNA, miRNA and DNA. (C) TEX reach ECs carrying arrays or ‘bundles’ of several pro-angiogenic proteins. TEX reprogram ECs either by ligand/receptor signaling (1) and/or miRNA and mRNA transfers after fusion with the plasma membrane (2). ECs internalize TEX via endocytosis, phagocytosis, micropinocytosis or lipid raft-mediated internalization (3). All these pathways may result in an enhanced angiogenesis in vitro and in vivo. The molecular mechanisms engaged in EC reprogramming might include re-utilization of the delivered proteins and/or de-novo protein transcription from altered mRNA. The ECs reprogrammed by TEX express and secrete enhanced levels of angiogenic proteins. The proliferation, migration, and angiogenic activity of ECs are increased.
5.0. Molecular Mechanisms Used by TEX for EC Reprogramming
Reprogramming of recipient cells by exosomes through the transfer of mRNA, miRNAs and proteins leads to identifiable stable changes in their functional profile [18,19]. In addition, signals delivered by exosomes to receptors on recipient cells activate the relevant molecular pathways and also contribute to altered cellular responses [28]. Recent reports indicate that TEX take advantage of the molecular pathways operating in recipient cells and may rearrange the components of these pathways (e.g., by inducing protein aggregation), thus altering their activity [40].
Little is known about the mechanisms responsible for the induction by TEX of tumor angiogenesis. The increased angiogenic capability of recipient cells interacting with TEX was characterized in different types of cancer by studying pro-angiogenic proteins, which are carried by TEX (Figure 2B). The proteomic content of TEX has been shown to play an important part in cancer progression and, especially, in angiogenesis [41,42]. The TEX content of proteins with pro-angiogenic properties differs widely in different tumor types as indicated in Table 1. However, it is likely that the different protein rosters for TEX derived from various tumors might reflect the investigators’ bias in selecting the proteins as experimental targets. Glioblastomaderived exosomes carry Angiogenin, VEGF, TGFβ, IL-6, IL-8, TIMP-1 and TIMP-2. Moreover they carry proteolytic enzymes (MMP2 and MMP9) and the CXCR4 chemokine receptor [19,43]. Multiple myeloma-derived exosomes carry VEGF, bFGF, MMP-9, HGF and Serpin El among others [27]. In nasopharyngeal carcinoma, pro-angiogenic proteins including intercellular adhesion molecule-1 (ICAM-1), CD44 variant isoform 5 (CD44v5) and MMP13 are upregulated, whereas angio-suppressive protein, thrombospondin-1 (TSP-1) is down-regulated in TEX [24,44]. Studies of TEX obtained from colorectal carcinoma ascites carry proteins which might be involved in tumor progression, including Tetraspanin-8 and Plexin B2, which are known to be involved in angiogenesis [45]. Melanoma-derived exosomes carry the proangiogenic proteins IL-6, VEGF and MMP2 [46]. An important protein for exosome-facilitated angiogenesis in lung adenocarcinoma is sortilin [47]. TEX containing sortilin are able to upregulate expression levels of angiogenic proteins such as endothelin-1, IL-8, TSP-2, uPA and VEGF in ECs. Sortilin has been reported to promote the release of exosomes by tumor cells [47].
Table 1:
Proteins inducing tumor angiogenesis that are carried by TEX or exosomes produced by reprogrammed normal cells in the TME a
| Cellular origin of exosomes |
Angiogenic proteins | Reference |
|---|---|---|
| Tumor cells | ||
| Bladder cancer | EDIL-3 | [28] |
| Breast cancer | Annexin II, Heparanase, TGFß | [21,48,62] |
| Colorectal cancer | Plexin B2, Tetraspanin-8 | [45] |
| Glioblastoma | Angiogenin, CXCR4, FGFa, IL-6, IL-8, MMP2, | [19,43] |
| MMP9, TGFβ, TIMP-1, TIMP-2, VEGF | ||
| Glioma | IGFBP1, IGFBP3, IGFBP5, IL-8, LOXL2, VEGF | [25,64] |
| Leukemia | Heparanase | [48] |
| Lung cancer | Sortilin | [47] |
| Malignant melanoma | IL-6, MMP2, VEGF | [43] |
| Multiple myeloma | Angiogenin, Heparanase, HGF, MMP9, Serpin E1, | [27,48] |
| Serpin F1, VEGF | ||
| Nasopharyngeal carcinomi | CD44v5, HAX-1, ICAM-1, MMP13 | [24,44,51] |
| Reprogrammed stroma cells in the TME | ||
| Mesenchymal stem cells | Jaggedl, VEGF | [76,77] |
| ECs | MMP2, MMP9 | [78] |
TEX carry a variety of proteins known to be engaged in promotion of angiogenesis. The protein cargo is dependent on the cellular origin of exosomes and varies in different types of cancer. Exosomes can derive from both tumors and surrounding reprogrammed stroma cells in the TME. Microenvironmental factors and conditions may also contribute to tumor-mediated angiogenesis. Only limited information is available on pro-angiogenic cargos of exosomes produced by reprogrammed normal cells in the TME, including mesenchymal stem cells, cancer-associated fibroblasts, endothelial cells and immune cells.
In breast cancer, Annexin II is an important angiogenesis-promoting protein. Maji et al. [21] demonstrated, that Annexin II is present in exosomes and promotes tPA-dependent angiogenesis in vitro and in vivo. In exosomes derived from bladder cancer, EDIL-3 (EGF-like repeats and discoidin I-like domain-3) has a potential influence on the cell migration and angiogenesis and is overexpressed in urine-derived exosomes from bladder cancer patients compared to healthy donors’ exosomes [28]. In aggregate, angiogenesis appears to be driven, at least in part, by the variety of angiogenic proteins carried by TEX as well as exosomes produced by reprogrammed normal cells residing in the TME.
Another putative mechanism for tumor progression and enhanced angiogenesis via exosomes is heparanase. This enzyme is upregulated in tumors, and it enhances TEX secretion by tumor cells. Furthermore, it regulates the protein cargo of exosomes by upregulating levels of syndecan-1, VEGF and HGF. The recipient ECs showed an increased invasion and tube formation after interacting with heparanase-positive exosomes [48].
A crucial step for cancer progression is the activation of a myofibroblast-rich stroma. Prostate cancer exosomes triggered TGFβl-dependent fibroblast differentiation, to a distinctive myofibroblast phenotype, enhancing angiogenesis in vitro and promoting tumor growth in vivo [49].
TEX bearing tetraspanin (Tspan8) have been shown to efficiently induce angiogenesis upon internalization by ECs. Through regulating angiogenesis-related genes TEX enhanced EC migration, proliferation and vessel sprouting. Tspan8 also contributed to the maturation of endothelial progenitor cells (EPCs) [50]. In exosomes from nasopharyngeal carcinoma cells, HS1-associated protein X-l (HAX-1) was enriched in TEX and when transferred to ECs, it increased the proliferation, migration, and angiogenic activity of these cells [51].
6.0. Genetic Reprogramming of ECs by TEX
Based upon the results of several studies, transfer of mRNAs is one of the mechanisms used by TEX for reprogramming of recipient cells (Figure 2C and D). Exosomes derived from colorectal cancer cells are enriched in mRNAs such as CDK8, ERH and RAD21, which are mainly related to the cell cycle. This implies that TEX promote EC proliferation by mRNA transfer and, therefore, enhance tumor progression through stimulation of angiogenesis [19,52].
Another putative mechanism of EC reprogramming is described by Lang et al. [9]. Long noncoding RNAs (IncRNAs) were reported to promote angiogenesis. Specifically, long intergenic non-coding RNA CCAT2 (linc-CCAT2) was found to be overexpressed in glioma cells and was carried by glioma cell-derived exosomes. The transfer of linc-CCAT2 to ECs by exosomes enhanced in vitro and in vivo angiogenesis, upregulated VEGFA and TGFβ levels and conferred greater resistance to hypoxia-induced apoptosis to recipient cells [18,53].
A related mechanism implicated in reprogramming of ECs by exosomes involves transfer of miRNAs (Figure 2C and D). Numerous miRNAs are enriched in TEX and upon inter-cellular TEX transfer are reported to regulate biological properties of ECs [54,55]. Table 2 lists miRNAs with pro-angiogenic properties carried by TEX. Once delivered to recipient cells, these miRNAs proceed to induce transcriptional changes that eventually culminate in alteration of cellular proteins [55]. In lung cancer, miR-21 transferred in exosomes has been shown to play an important role in carcinogenesis. It is expressed by cancer cells and secreted to recipient cells mainly through TEX. In recipient cells, miR-21 increases the expression and secretion of VEGF and stimulates in vitro angiogenesis by ECs [56]. Moreover, miR-23a and miR-210 also participate in lung cancer progression. They are found in plasma of patients with lung cancer patients, suggesting that not only exosome-bound but also circulating miRNAs are involved in angiogenesis [29,57]. In addition, miRNAs enclosed in exosomes, especially miR-192, can also mediate anti-angiogenic effects [58]. The delivery of miRNAs by TEX represents a special advantage, because miRNAs carried in the vesicle lumen are protected from enzymatic degradation and thus are more stable compared to miRNAs freely circulating in plasma. Because of the key role the transfer of miRNAs plays in angiogenesis, therapeutic targeting of miRNAs may be one way of potentially inhibiting pro-angiogenic functions of TEX.
Table 2:
TEX carry miRNAs that are involved in inducing angiogenesisa
| Cellular origin of exosomes | miRNA | Reference |
|---|---|---|
| Tumor cells | ||
| Breast cancer | miR-126a | [79] |
| Lung cancer | miR-21, miR-23a, miR-210 | [29,56,57] |
| Ovarian cancer | miR-21 | [80] |
| Reprogrammed stroma cells in the TME | ||
| Mesenchymal stem cells miR-30b | miR-30b | [81] |
TEX carry a variety of miRNAs, which are transferred to ECs and result in an increased be carried by TEX and by angiogenesis. The miRNAs with pro-angiogenic properties can exosomes from reprogrammed stromal cells in the TME.
7.0. Environmental Factors Promoting Angiogenesis
Hypoxia is one of the main driving forces of tumor angiogenesis. Up to a size of 2–3 mm3, the supply of the tumor tissue occurs via diffusion through the surrounding tissue. In this tissue, cell proliferation and cell death are in equilibrium. When tumor tissue grows beyond the limit of oxygen diffusion, hypoxia triggers vessel growth by signaling through hypoxia-inducible transcription factors [59,60]. When cancer cells are exposed to hypoxia, the exosome production increases and leads to increased levels of signaling in the TME [6]. Exosomes derived from hypoxic cells show no differences in their size distribution and morphology compared to exosomes from normoxic cells. Normoxic and hypoxic exosomes are internalized by ECs in the same manner [29,61–63]. While the expression of exosome markers such as CD63, CD81 and HSP70 in exosomes remains constant in some studies, other studies describe higher expression of these markers on TEX from hypoxic cells [29,61,62]. While there exists some controversy regarding the similarity between exosomes derived from normoxic vs hypoxic cells, it appears that the protein cargo of exosomes is significantly influenced by hypoxia. For example glioma-derived exosomes from U373MG cells cultured under hypoxic conditions had higher levels of lysyl oxidase homolog 2 (LOXL2) and IGFBP3 and IGFBP5 than exosomes from the same cells cultured under normoxic conditions [64]. Also, Kucharzewska et al. [25] published a study, which demonstrates that hypoxia in glioblastomas leads to a distribution of exosomes that appeared to significantly alter the phenotypic characteristics of ECs in contrast to exosomes from normoxic cells. ECs treated with TEX from hypoxic cells significantly stimulated proliferation, migration and tube formation compared to TEX from normoxic cells. Similar results were shown for the proliferation of pericytes. Several components of TEX such as IGFBP3 and IL-8 were found to be hypoxia-regulated on the mRNA level and also at the protein level. The protein cargo and the molecular characteristics of TEX reflected the hypoxic status and aggressiveness of the tumor [25]. Similar results were reported for lung cancer cell- and leukemia cell-derived exosomes. Besides altered phenotypic characteristics, the exosomes of hypoxic lung cancer cells promoted angiogenesis and increased the permeability of ECs, leading to cancer cell intravasation/extravasation [29,61]. Cancer cells and their exosomes have altered miRNA profiles under hypoxic conditions which affects the behavior of ECs [61]. Table 3 lists the miRNA species upregulated under hypoxia, and while these data are mainly derived from one study, they highlight the potential for qualitative differences between TEX generated in normoxic vs hypoxic conditions [6,61,63,65]. Levels of miR-210 are upregulated soon after hypoxic exposure and increase gradually up to 72h [63]. miR-210 can be transferred to ECs and induce angiogenesis in vitro and in vivo [29]. Similar to miR-210, miR-135b has strong proangiogenic effects in multiple myeloma [63]. After being transferred to ECs, it enhances neovascularization in vivo and regulates HIF-1 signaling in ECs. In colorectal cancer the increased angiogenesis through hypoxia is inhibited by blocking Wnt4. The Wnt4 upregulation is hereby dependent on HIFI α [66]. Since hypoxic conditions prevail in the TME, it is important to conduct future studies of TEX and their cargo under hypoxic conditions to simulate the TME. Although the cell culture under hypoxia requires special technical equipment, it is a promising approach to searching for biomarkers which would best reflect the hypoxic status of the patients’ tumor.
Table 3:
Several pro-angiogenic components of TEX were found to be regulated by hypoxiaa
| Cellular origin of exosomes |
Pro-angiogenic factor | Reference |
|---|---|---|
| Proteins | ||
| Breast cancer | TGFβ | [62] |
| Glioma | IGFBP1, IGFBP3, IGFBP5, IL-8, LOXL2, VEGF | [25,64] |
| miRNAs | ||
| Leukemia | miR-18b, −20a, −24, −106b, −130b, −185, −210, −224, −379, −652 | [61] |
| Lung cancer | miR-23a | [29] |
| Multiple myeloma | miR-135b, −200c, −210, −223, −328, −335, −425 | [63] |
Cancer cells and exosomes they produce have altered protein cargo and miRNA profiles when cultured in hypoxic vs normoxic conditions. The qualitative differences between TEX generated in normoxic vs hypoxic conditions affect the behavior of targeted ECs.
8.0. Cancer Stem Cell-Derived Exosomes in Angiogenesis
A subpopulation of cancer cells with progenitor characteristics, also described as cancer stem cells (CSCs), might be involved in producing exosomes with a pro-angiogenic potential. Although there is no difference in morphology and size, the CSC-derived exosomes express CSC-specific markers, such as CD133, CD44, CD105 and CD90, compared to normal bulk cells [33,67,68]. CD90(+) liver cancer cell-derived exosomes affect ECs by promoting tube formation and cell-cell adhesion. After exosome treatment the production and secretion of VEGF as well as its receptor in ECs was increased. Effects were significantly increased compared to CD90(−) exosomes [33]. Similar results were found for CD105(+) exosomes derived from renal cancer [67]. In prostate cancer, the stem cell-derived exosomes have a different miRNA profile compared to the bulk cell derived exosomes [69]. Exosomes derived from a special subpopulation of cells with progenitor characteristics may, therefore, represent a potential target for personalized therapies.
Several studies have shown, that TEX also mediate the epithelial-mesenchymal transition (EMT) in various cancers. TEX are not only able to induce the EMT, but cells that undergo EMT use exosomes for communication with ECs and those exosomes contain VEGF-associated proteins and use Rac 1/PAK2 as an angiogenic promoter [70,71].
9.0. Therapeutic Targeting of TEX-promoting Angiogenesis
Investigations of TEX and the role they play in angiogenesis are essential for the future development of new approaches for cancer therapy. New therapeutic strategies might emerge based on the targeted elimination or silencing of angiogenesis-promoting TEX and thus blocking their reprogramming activities. Because most types of cancer are characterized by strong vascularization, it would be a significant advancement to identify the mechanisms used by exosomes to promote angiogenesis and to acquire an understanding of how to effectively inhibit TEX-driven angiogenesis and, therefore, improve the existing anti-angiogenic therapies. One reported therapeutic approach of targeting TEX-induced angiogenesis is Carboxyamidotriazoleorotate (CTO) for patients with CML [72]. While imatinib is very effective for chronic phase CML, an imatinib resistance due to point mutations can lead to decreased survival rates. CTO targets TEX-induced angiogenesis by exosome-stimulated increase of cell-to-cell adhesion molecules and IL-8 expression in ECs. Thus, CTO inhibits the adhesion of CML cells to ECs and blocks the exosome-stimulated migration of ECs. The tube formation of ECs and angiogenesis in a matrigel plug model were decreased after CTO treatment [72].
Another therapeutic approach for targeting exosome stimulated angiogenesis in CML is Curcumin [73]. The treatment of CML cells with Curcumin causes an increase of miR-21 in released exosomes. Compared to the non-treatment group, Curcumin-treated exosomes are depleted in pro-angiogenic proteins and enriched in proteins with anti-angiogenic effects. Although exosomes are internalized by ECs, they lose their pro-angiogenic phenotype and their pro-angiogenic effect after Curcumin treatment [73].
Hannafon et al. [74] reported that Docosahexaenoic acid (DHA), a preventative agent and an adjuvant to breast cancer therapy, can target exosomes and, therefore, inhibits exosome-driven angiogenesis. DHA treatments increased the number of exosomes internalized by ECs and inhibited tube formation by ECs without affecting VEGF levels. The study suggested, that mature miRNAs (miR-23b, miR-27b, and miR-320b), which are increased in number in cancer cells and exosomes after DHA treatment, are responsible for anti-angiogenic activity.
10.0. Conclusion
TEX appear to play an important role in tumor angiogenesis thus contributing to tumor growth and metastasis. TEX carry angiogenesis-related proteins, transfer RNA, miRNAs and proteins and induce phenotypic and functional changes in ECs and in other cells in the TME that may also contribute to the vessel formation or growth. TEX emerge as potential biomarkers of angiogenesis in cancer, and a putative target for anti-angiogenic therapies. However, it will be up to future studies to further characterize the biological role of TEX in tumor angiogenesis and to determine the clinical relevance of targeting TEX for inhibiting pro-angiogenic effects. Current investigations of the TEX role in angiogenesis are limited by a lack of a standardized method for exosome isolation from human body fluids. Another hurdle is the separation of TEX from nontumor-derived exosomes. The nomenclature of EVs has not been yet established, and the reasons for EV heterogeneity in size and cellular origins are not yet clear. Nevertheless, we are convinced that a deeper understanding of the TEX biology and their role in tumor angiogenesis will lead to improvements in current diagnosis, prognosis and treatment of cancer.
11.0. Expert Opinion
Cancer development, growth and metastasis are intimately linked to angiogenesis, which is regulated by a complex network of morphogenic and molecular pathways, soluble factors and exosomes. The effects of TEX on the process of angiogenesis have been only recently described. TEX were found to be potent inducers of angiogenesis in vitro and in vivo through phenotypic modulation and functional reprogramming of ECs and of collateral tissue as well as immune cells in the TME. The mechanisms underlying pro-angiogenic signaling of TEX appear to involve the delivery of angiogenesis-related proteins and the transfer by TEX of RNA and miRNAs from the tumor to recipient cells. The growth of new vessels, which begins at an early point of the tumor development, has been linked to the levels of exosomes the parent tumor produces. Cancer cells are active exosome producers, and plasma of patients with cancer is enriched in TEX. Total exosomes isolated from patients’ plasma contain variable TEX levels, and separation of TEX from “contaminating” non-tumor-derived exosomes would likely facilitate molecular “profiling” and correlations of the TEX cargo to vascular changes in the tumor and to clinical endpoints. As the TEX molecular and genetic cargos recapitulate contents of the parent cell, they emerge as potential non-invasive biomarkers of tumor-induced angiogenesis. This is encouraging for the development of a “liquid biopsy” paradigm, which would allow for assessment of the tumor angiogenic profile in real time and repeatedly. This liquid biopsy paradigm is an attractive but as yet not validated approach to TEX as cancer biomarkers. The isolation from body fluids of patients and profiling of TEX for their proangiogenic features is expected to significantly contribute to the efforts of establishing TEX as future biomarkers of cancer diagnosis, staging, response to therapy and prognosis. The use of TEX as biomarkers in serial monitoring of cancer progression or of responses to antiangiogenic therapies promises to greatly improve patient management and drug selection. Because TEX mimic the properties of their parent cells and are surrounded by lipid bilayers which protect their cargos from degradation en route, they have an advantage over soluble cancer biomarkers [75]. The potential for non-invasive isolation of exosomes in large quantities from all types of body fluids and their stability represent significant advantages compared to other forms of a liquid biopsy such as circulating tumor cells or circulating free nucleic acids. Should TEX prove to carry a marker or markers of angiogenesis that are specific for a given patients’ tumor, they could emerge as a tool for patient-specific diagnosis and fulfill the promise of personalized anti-angiogenic therapy. Moreover, future efforts should focus on eliminating or silencing TEX that selectively promote malignant, but not benign, angiogenesis, thereby adding new treatment options to existing anti-angiogenic therapies.
To implement many of the ideas advanced above, further studies of extracellular vesicles (EVs) from human body fluids are required. An important immediate goal in the field of EV research is to establish a uniform nomenclature, which recognizes the heterogeneity of EVs in body fluids. Exosome isolation from other EVs in body fluids and reliable separation of TEX from nontumor-derived vesicles are major hurdles that need to be resolved. For clinical applications, high throughput isolation/separation techniques will be obligatory. While the development of new exosome isolation techniques is proceeding, no standardized method currently exists. Although it is acknowledged that subsets of exosomes exist, separation of biologically-active TEX fractions from plasma is in infancy. The TEX profiles of angiogenic molecular and genetic markers that convincingly and reliably detect malignant vessels will have to be established. Next, validation of this profile in “liquid biopsy” specimens from large patient cohorts will be necessary to begin efforts aimed at translating TEX profiling to the daily clinical practice.
Since the first studies of exosome role in angiogenesis were reported in 2006, the field of exosome research has rapidly grown. An improved understanding of exosome-driven communication networks between the tumor and surrounding cells, including ECs, lends support to the concept of future anti-angiogenic therapy targeting TEX. Because nearly all malignancies are characterized by strong vascularization, and they all produce TEX, It would be a significant advancement to identify mechanisms used by these exosomes to promote angiogenesis. Then, it would only be another step to acquire an understanding of how to effectively inhibit exosome-driven angiogenesis, thereby adding new options for improving existing anti-angiogenic therapies.
Article highlights box.
TEX, virus-size vesicles, which circulate freely through system between throughout body fluids and accumulate in the TME, serve as a communication system between the tumor and various normal cells.
TEX appear to play an important role in tumor angiogenesis thus contributing to tumor growth and metastasis.
TEX carry angiogenesis-related proteins, transfer RNA, miRNA and proteins and are responsible for phenotypic and functional reprogamming of ECs and other normal cells residing in the TME.
TEX emerge as potential biomarkers of angiogenesis in cancer, and a putative target for anti-angiogenic therapies.
The characterization of TEX in body fluids of cancer patients and better understanding of their pro-angiogenic activities are required for the development of novel anti-angiogenic therapies.
Acknowledgments
Funding
Supported in part by NIH grants R0–1 CA168628 and R-21 CA205644 to TLW. Dr. Ludwig was supported by the Leopoldina Fellowship LPDS 2017–12 from German National Academy of Sciences Leopoldina.
Abbreviations
- CD44v5
CD44 variant isoform 5
- CML
chronic myelogenous leukemia
- CSC
cancer stem cell
- CTO
Carboxyamidotriazole-orotate
- DHA
Docosahexaenoic acid
- EC
endothelial cell
- EDIL-3
EGF-like repeats and discoidin I-like domain-3
- EMT
epithelial-mesenchymal transition
- EPC
endothelial progenitor cell
- EV
extracellular vesicle
- HAX-1
HS1-associated protein X-1
- ICAM-1
intercellular adhesion molecule-1
- linc-CCAT2
long intergenic non-coding RNA CCAT2
- lncRNA
Long non-coding RNA
- LOXL2
lysyl oxidase homolog 2
- miRNA
microRNA
- TEX
tumor-derived exosomes
- TME
tumor microenvironment–
- TSP-1
thrombospondin-1
- Tspan8
tetraspanin
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Whiteside T The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res. 2016;30:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6 [DOI] [PubMed] [Google Scholar]
- 4.Whiteside T The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Futur Oncol. 2017;13:2593–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79 [DOI] [PubMed] [Google Scholar]
- 6.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahlert C Exosomes in Tumor Microenvironment Influence Cancer Progression and Metastasis. J Mol Med. 2014;91:431–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016;74:103–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung T, Castellana D, Klingbeil P, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boriachek K, Islam MN, Möller A, et al. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2018; 14:1–21 [DOI] [PubMed] [Google Scholar]
- 13.Thierry, Théry C, Amigorena S, et al. Isolation and Characterization of Exosomes from Cell Culture Supernatants. Curr Protoc cell Biol. 2006;Chapter 3:1–29 [DOI] [PubMed] [Google Scholar]
- 14.Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong C-S, Funk S, Muller L, et al. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell vesicles. 2016;5:29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Ludwig S, Muller L, et al. Immunoaffinity-based isolation of melanoma cellderived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7:1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francescone R, Scully S, Bentley B, et al. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J Biol Chem. 2012;287:24821–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang H-L, Hu G-W, Chen Y, et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21:959–72 [PubMed] [Google Scholar]
- ••19.Skog J, Wurdinger T, Rijn S Van, et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol. 2012;10:1470–6 This is one of the first manuscripts highlighting the potential effects of TEX on angiogenesis. It is a high impact paper demonstrating that TEX contain mRNA, miRNA and angiogenic proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gesierich S, Berezovskiy I, Ryschich E, et al. Systemic Induction of the Angiogenesis Switch by the Tetraspanin D6.1A/CO-029. Cancer Res. 2006;7083–95 [DOI] [PubMed] [Google Scholar]
- 21.Maji S, Chaudhary P, Akopova I, et al. Exosomal Annexin A2 Promotes Angiogenesis and Breast Cancer Metastasis. Mol Cancer Res. 2016;93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taverna S, Flugy A, Saieva L, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2013;130:2033–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mineo M, Garfield SH, Taverna S, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a src-dependent fashion. Angiogenesis. 2012;15:33–45 • Numerous miRNAsThe authors describe important uptake mechanisms of TEX by ECs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YK, Zhang H, Liu P, et al. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int J Cancer. 2015;137:1830–41 [DOI] [PubMed] [Google Scholar]
- •• 25.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7 This is the most comprehensively manuscript on the role of hypoxia as a regulator for TEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greening DW, Ji H, Chen M, et al. Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci Rep. 2016; 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, De Veirman K, Faict S, et al. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239:162–73 [DOI] [PubMed] [Google Scholar]
- 28.Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192:583–92 [DOI] [PubMed] [Google Scholar]
- 29.Hsu Y-L, Hung J-Y, Chang W-A, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;4929–42 [DOI] [PubMed] [Google Scholar]
- 30.O’Brien K, Rani S, Corcoran C, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Cancer. 2013;49:1845–59 [DOI] [PubMed] [Google Scholar]
- 31.Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon YJ, Kim DK, Yoon CM, et al. Egr-1 activation by cancer-derived extracellular vesicles promotes endothelial cell migration via ERK1/2 and JNK signaling pathways. PLoS One. 2014;9:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conigliaro A, Costa V, Lo Dico A, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hood J, Pan H, Lanza G, et al. Paracrine Induction of Endothelium by Tumor Exosomes. Lab Invest. 2009;89:1317–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–87 [DOI] [PubMed] [Google Scholar]
- 36.Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58 [DOI] [PubMed] [Google Scholar]
- 37.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell vesicles. 2014;3:1–14 • A comprehensive review of the uptake mechanisms used by exosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang A, Dong J, Li S, et al. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 2015;11:961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheldon H, Heikamp E, Turley H, et al. New mechanism for Notch signaling to endothelium at a distance by delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–94 [DOI] [PubMed] [Google Scholar]
- 40.Manček-Keber M, Lainšček D, Benčina M, et al. Extracellular vesicle-mediated transfer of constitutively active MyD88 L265P engages MyD88 wt and activates signaling. Blood. 2018;blood-2017-09–805499 [DOI] [PubMed] [Google Scholar]
- 41.Henderson MC, Azorsa DO. The Genomic and Proteomic Content of Cancer Cell-Derived Exosomes. Front Oncol. 2012;2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JE, Sen Tan H, A Datta, et al. Hypoxic Tumor Cell Modulates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Exosomes. Mol Cell Proteomics. 2010;9:1085–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giusti I, Delle Monache S, Di Francesco M, et al. From glioblastoma to endothelial cells through extracellular vesicles: messages for angiogenesis. Tumor Biol. 2016;37:12743–53 [DOI] [PubMed] [Google Scholar]
- 44.You Y, Shan Y, Chen J, et al. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106:1669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi D-S, Park JO, Jang SC, et al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics. 2011;11:2745–51 [DOI] [PubMed] [Google Scholar]
- 46.Ekström EJ, Bergenfelz C, von Bülow V, et al. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer. 2014; 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson CM, Naves T, Vincent F, et al. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci. 2014;127:3983–97 [DOI] [PubMed] [Google Scholar]
- 48.Thompson CA, Purushothaman A, Ramani VC, et al. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webber JP, Spary LK, Sanders AJ, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302 [DOI] [PubMed] [Google Scholar]
- 50.Nazarenko I, Rana S, Baumann A, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–78 [DOI] [PubMed] [Google Scholar]
- 51.You B, Cao X, Shao X, et al. Clinical and biological significance of HAX-1 overexpression in nasopharyngeal carcinoma. Oncotarget. 2016;7:12505–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong B, Cho J-H, Kim H, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lang HL, Hu GW, Zhang B, et al. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38:785–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghayad SE, Rammal G, Ghamloush F, et al. Exosomes derived from embryonal and alveolar rhabdomyosarcoma carry differential miRNA cargo and promote invasion of recipient fibroblasts. Sci Rep. 2016;6:37088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umezu T, Ohyashiki K, Kuroda M, et al. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747-55 • This paper nicely illustrates the interactions of TEX and ECs via miRNAs. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Luo F, Wang B, et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–35 [DOI] [PubMed] [Google Scholar]
- 57.Cui H, Seubert B, Stahl E, et al. Tissue inhibitor of metalloproteinases-1 induces a protumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. 2015;34:3640–50 [DOI] [PubMed] [Google Scholar]
- 58.Valencia K, Luis-Ravelo D, Bovy N, et al. MiRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol. 2014;8:689–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folkman J Angiogenesis: initiation and control. Ann N Y Acad Sci. 1982;401:212–27 [DOI] [PubMed] [Google Scholar]
- 60.Carmeliet P Angiogenesis in health and disease. Nat Med. 2003;9:653–60 [DOI] [PubMed] [Google Scholar]
- 61.Tadokoro H, Umezu T, Ohyashiki K, et al. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rong L, Li R, Li S, et al. Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncol Lett. 2016;11:500–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon JH, Kim J, Kim KL, et al. Proteomic analysis of hypoxia-induced U373MG glioma secretome reveals novel hypoxia-dependent migration factors. Proteomics. 2014;14:1494–502 [DOI] [PubMed] [Google Scholar]
- 65.Jung KO, Youn H, Lee C-H, et al. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2016;8:9899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Z, Feng Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced β-Catenin Signaling in Endothelial Cells. Oncol Res Featur Preclin Clin Cancer Ther. 2017;25:651–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–56 [DOI] [PubMed] [Google Scholar]
- 68.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68 [DOI] [PubMed] [Google Scholar]
- 69.Sánchez C a., Andahur EI, Valenzuela R, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2015;7:3993–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gopal SK, Greening DW, Hanssen EG, et al. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget. 2016;7:19709–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng A-L, Yan W, Liu Y-W, et al. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene. 2017;5369–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrado C, Flugy AM, Taverna S, et al. Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis. PLoS One. 2012;7:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Taverna S, Fontana S, Monteleone F, et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget. 2016;7:30420–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hannafon BN, Carpenter KJ, Berry WL, et al. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer. 2015;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui S, Cheng Z, Qin W, et al. Exosomes as a liquid biopsy for lung cancer. Lung Cancer. Elsevier; 2018;116:46–54 [DOI] [PubMed] [Google Scholar]
- 76.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37 [DOI] [PubMed] [Google Scholar]
- 77.Gonzales-King H, Garcia N, Ontoria-Oviedo I, et al. Hypoxia Inducible Factor-1a Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells. 2017;35:1747–59 [DOI] [PubMed] [Google Scholar]
- 78.Taraboletti G, D’Ascenzo S, Borsotti P, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sondike SB, Pisetsky EM, Luzier JL. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;21:133–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cappellesso R, Tinazzi A, Giurici T, et al. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122:685–93 [DOI] [PubMed] [Google Scholar]
- 81.Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–12 [DOI] [PMC free article] [PubMed] [Google Scholar]


