Abstract
Introduction
Regulatory T cells (Treg) characterized by expression of FOXP3 and strong immunosuppressive activity play a key role in regulating homeostasis in health and disease.
Areas covered
Human Treg are highly diverse phenotypically and functionally. In the tumor microenvironment (TME), Treg are reprogrammed by the tumor, acquiring an activated phenotype and enhanced suppressor functions. No unique phenotypic markers for Treg accumulating in human tumors exist. Treg are heterogeneous and use numerous mechanisms to mediate suppression, which either silences anti-tumor immune surveillance or prevents tissue damage by activated T cells. Treg plasticity in the TME endows them with dual functionality. Treg frequency in tumors associates either with poor or improved survival. Treg responses to immune checkpoint inhibition (ICI) differ from the restorative effects ICIs induce in other immune cells. Therapies used to silence Treg, including ICIs, are only partly successful. Treg persistence and resistance to depletion are critical for maintaining homeostasis.
Expert Opinion
Treg emerge as a heterogeneous subset of immunosuppressive T cells, which usually, but not always, favor tumor progression. Treg are also engaged in non-immune activities that benefit the host. Therapeutic silencing of Treg in cancer requires a deeper understanding of Treg activities in human health and disease.
Keywords: Regulatory T cells (Treg), tumor microenvironment (TME), cancer, Treg phenotype, Treg suppressor functions, Treg and checkpoint inhibition
1. Introduction
Regulatory T cells (Treg) have been occupying a central place in research of immune regulatory responses in health and disease for the last 20 years [1,2]. Treg have a checkered history. More than 25 years ago, a subset of T cells with immunosuppressive functions has been described by Gershon and colleagues [3] only to be abandoned for the lack of an adequate identity certification. In the 1990s, Sakaguchi et al re-introduced the idea that peripheral tolerance is mediated by a unique small subset of CD4+CD25+ T cells, which upon adoptive transfers were able to suppress the development of autoimmunity in mice [4]. The name “Treg” has been coined to describe these cells, and since then, Treg have continuously occupied interest of the scientific community.
In cancer, Treg are viewed as suppressors of anti-tumor immunity, and their role in down-regulating anti-tumor immune responses has become an accepted paradigm [5]. Rapid progress made in defining the Treg phenotype and functions in mice and in man allowed for studies of Treg present in the peripheral circulation and infiltrating tumor sites. As a result of these studies, the current view of Treg has enlarged. Newer data suggest that while Treg play a major role in suppression of anti-tumor responses, they are subject to environmental factors which may alter their functional repertoire.
The objective of this review is to consider human Treg in light of newly emerging data on their role in modulating responses to oncologic therapies, especially to therapies with immune checkpoint inhibitors (ICIs). Recent progress in phenotypic characterization of circulating and tumor-infiltrating Treg has facilitated studies of mechanisms responsible for their functions, revealing dual or multiple roles of Treg in regulating anti-tumor immunity and responses of cancers to oncological therapies. In this context, the paradoxical association of Treg with better survival in some patients with cancer becomes explainable as a manifestation of Treg functional diversity and their plasticity. Further, this functional diversity may be useful in considering Treg subsets as potential biomarkers of cancer progression and/or response to therapy and allow for Treg monitoring based on their multifaceted involvement in the tumor-host interactions. Finally, a measure of caution to therapeutic elimination of Treg seen only as “bad” players in cancer has to be re-considered, given emerging evidence for beneficial or restorative functions attributable to Treg subsets.
2. Nomenclature and classification of Treg
Attempts made to define the origin of Treg and classify them based on site of their differentiation and phenotypic characteristics culminated in a relatively simple division into natural/thymic-derived (tTreg) and peripherally-induced cells (pTreg) [6]. Studies in mice indicate that tTreg are derived from CD4+CD8- thymocytes, which upon stimulation by the MHC-self-reactive peptide complex acquire CD25 expression, respond to IL-2 and, signaling via STAT5, promote Foxp3 expression and differentiate into Treg [7]. The pTreg are converted from conventional CD4+ T cells through TGF-β stimulation into FoxP3+ suppressor cells in mice [8]. However, in man, a variety of stimulatory signals, including microbiota, give rise to induced (i)Treg [5,9]. Neither the process of conversion nor the stability or suppressor functions of (i)Treg are clearly defined. These cells may or may not express FOXP3, and their regulatory functions may be transient or absent, depending on the microenvironment. FOXP3 is generally viewed as a “master regulatory transcription factor” for Treg. In reality, however, FOXP3 expression in activated conventional CD4+ T cells and its absence in subsets of highly suppressive iTreg in the tumor microenvironment (TME) compromises this designation [10,11]. Recently, Sakaguchi and colleagues have introduced a classification of human CD4+FOXP3+ Treg based on co-expression levels of FOXP3 and CD45RA, a marker of naïve T cells [12]. Table 1 presents this classification, which defines two distinct subpopulations of Treg: CD4+CD45RA+FOXP3low naïve or nTreg with weak suppressor function and CD4+CD45RA-FOXP3high effector or eTreg that are strongly suppressive and represent bona fide suppressor Treg. The third subset, CD4+CD45RA-FOXP3low cells do not mediate suppression, produce pro-inflammatory cytokines, including INF-γ, and are non-Treg [12]. This classification suggests that Treg are a heterogeneous subset of CD4+ T cells and that nTreg upon stimulation via the TCR can proliferate and differentiate into highly suppressive eTreg [13].
Table 1.
Current classification of CD4+FOXP3+ human Trega
| Cell subset | Phenotype | Functional characteristics |
|---|---|---|
| Fraction 1: Naïve Treg (nTreg) | CD4+CD45RA+FOXP3low CD25hiCD127low CTLA4low | Weak suppressor activity; proliferate and differentiate into effector Treg upon TCR stimulation |
| Fraction 2: Effector Treg (eTreg) | CD4+CD45RA-FOXP3hi CD25hiCTLA-4hi PD1+TIM-3+GITR+ Fas+IL-10+TGF3+ | Strong suppressor activity; prone to apoptosis; low stability Increase with age |
| Fraction 3: Non-Treg | CD4+CD45RA-FOXP3low IL2+IFN3+IL17+ | No suppressor activity |
Human CD4+FOXP3+ T cells are phenotypically and functionally heterogeneous. Based on expression levels of FOXP3 and CD45RA, they can be divided into three subsets [12]
3. Treg in the tumor microenvironment
3.1. Are Treg functions “good” or “bad” in patients with cancer?
Treg are an important component of the tumor microenvironment. They accumulate at tumor sites and in the peripheral circulation of patients with cancer, and their frequency as well as suppressor functions are increased relative to those found in healthy donors [14,15]. Why do Treg accumulate in tumor tissues, tumor-associated lymph nodes (LN) and in the peripheral circulation of cancer patients? The observed increases of the Treg frequency do not appear to be due to preferential expansion of Treg at these sites, or to increased conversion of nTreg to iTreg, as reported in a recent study [16]. This latter result was based on measurements of the Treg-specific demethylation region (TSDR) in the FOXP3 gene locus and the unaltered FOXP3/TSDR ratio in Treg isolated form the tumor, non-tumor tissues, LN or PBMC of cancer patients [16]. Nor was the increased frequency of Treg in cancer related to patients’ age in the same study. The most likely explanation for Treg accumulations in tumors is their ability to effectively traffic to tissue sites dependent on expression of multiple chemokine receptors including, for example, CXCR5 in Treg from LN of patients with lung cancer [16]. Interactions of other chemokine receptors on Treg with chemokines in the tumor, e.g., CCR4 with CCL12, CCR4 with CCL17, CCR10 with CCL28 and CXCR4 with CXCL1 have been reported to participate in the Treg recruitment to tumors [1,5,16–19].
Treg infiltration into tumor tissues has been extensively examined in the context of recent “immune signature” studies [20–22]. These studies confirmed the prominent presence of Treg among tumor-infiltrating lymphocytes (TIL), especially in tumors that contain large immune cell infiltrates [23]. Based on the rationale that Treg interfere with anti-tumor immunity and thus promote tumor progression, their presence in the TME is expected to associate with unfavorable prognosis and to predict short OS. Indeed, Treg infiltration into the tumor has been negatively correlated to OS in a majority of human solid tumors [24,25]. However, this correlation is highly variable, depending on the tumor type [26]. In cancers that share a common feature of prominent chronic inflammation, such as colon, breast, bladder or head and neck cancers, intra-tumor accumulations of Treg appear to associate with favorable prognosis and improved OS [23,27,28] This association has been explained by the capability of Treg to suppress “tumor promoting inflammation” (TPI). In this context, Treg emerge as “good citizens” responsible for down-regulating activity of infiltrating immune cells in cancers such as colorectal carcinomas (CRC) that are characterized by the presence of chronic inflammatory infiltrates. Nevertheless, the general perception of Treg as “bad” T cells that suppress antitumor immune responses and negatively influence disease outcome is prevalent. Recently, Sakaguchi et al provided compelling evidence that CRCs abundantly infiltrated with the FOXP3high subset of suppression-competent effector Treg have poor prognosis, while the presence in tumor tissues of proinflammatory cytokine-secreting CD4+CD45RA-FOXP3low non-Treg, is associated with favorable outcome [12]. This report emphasizes the need for careful re-examination of the phenotypic and functional attributes of Treg in the TME and blood of patients with cancer to separate the bona fide suppressor cells from CD4+ T lymphocytes that might be FOXP3+ but function as anti-tumor effector cells, secrete IFN-γ and are not suppressor cells. A proper classification of Treg and recognition of their heterogeneity is essential for evaluating their prognostic impact in each tumor type.
3.2. Phenotypic properties of human Treg in cancer
Much of what is known about phenotypic properties of Treg is based on their studies in normal or tumor-bearing mice [13]. Studies of Treg in human cancers have been hampered by the fact that they are a minor subset of CD4+ T cells, representing about 5% of the total, and thus are only available in limited numbers for phenotyping. Also, Treg lack a unique surface marker that could confirm their identity and for their isolation from blood or tissues. While FOXP3 reliably marks murine Treg, it is less reliable in man, because it is frequently absent or is down-regulated in (i)Treg or, conversely, is present albeit transiently in activated human T effector (Teff) cells [29]. In addition, most of the markers expressed by Treg are also carried by other lymphoid or non-lymphoid cells, and thus selective distinction between Treg and other immune cells is difficult if not impossible. With these hurdles in mind, it has to be acknowledged that cells referred to in the literature as “Treg” might, in fact, be mixtures of Treg and Teff rather than true Treg.
Human Treg are usually phenotyped as CD4+FOXP3+CD25+ T cells. In mice, all CD4+Foxp3+CD25+ T cells are suppression-competent T cells. In man, conventional CD4+ T cells can transiently up-regulate FOXP3 upon activation, but are not immunosuppressive [10,11]. Also, not all CD4+FOXP3+ T cells found in human blood mediate suppression [10]. In the absence of a single surface marker that defines Treg, panels of variously combined surface markers have been used for Treg phenotyping. Substantial efforts have been made to come up with panels that include a rational selection of markers that are co-expressed on Treg and that could differentiate various human Treg subsets based on their origin, phenotype and suppressive functions. The most common phenotypic markers used for identification of human Treg are: CD25, CD39, GARP, CD45RA (or CD45RO), ICOS, HLA-DR and Helios [12]. As shown in Table 1, CD4+FOXP3+CD25+ Treg can be subdivided further into naïve/resting nTreg, effector/activated eTreg, and FOXP3low non-Treg which do not mediate suppression but may secrete inflammatory cytokines like IFN-γ [12]. Recently, Sialyl Lewis x (CD15s) was reported to reliably identify highly differentiated and most suppressive FOXP3high Treg in humans [30]. This marker decorates the surface of Treg and thus is potentially useful for phenotypic as well as functional analyses of human Treg [30].
A number of earlier reports suggested that the phenotype of Treg in cancer patients was distinct from that found in the circulation of healthy donors [2]. The terminology of “induced” or iTreg has been used to denote the activated phenotype these cells exhibit in cancer. There is confusion about the stable Treg phenotype, and misidentification of CD4+ cells as Treg has been attributed to the presence of FOXP3 in activated CD4+ T cells on the one hand, and its absence from some iTreg on the other [2,16]. The Treg accumulating at the tumor site were initially thought to have a distinct phenotype. However, as recently pointed out, this may be a misconception due to up-regulation of various Treg-associated cell surface antigens rather than expression of new ones [16]. The challenge of Treg isolation from tumors or peripheral circulation of cancer patients continues to be a significant barrier in the absence of a surface marker specific for Treg. Expression of FOXP3 confined to the nucleus is not useful for Treg isolation. The selection of CD25highCD4+ T cells as Treg is biased by a variable definition of “high” vs “low” CD25 expression in the hands of different investigators [31]. Another surface marker, CD39 was also used for Treg isolation from blood and tumor tissues. However, this marker identifies only the subset of CD4+FOXP3+CD25high Treg that produces an excess of adenosine [32]. The absence of CD127 on the surface of human Treg has been broadly used for Treg isolation, but because this is negative selection, the presence in the isolated population of CD127low Teff represents a potential contamination [33].
A very recent study by Hancock’s group reports that none of the 35 Treg-associated flow cytometry markers that are commonly used for studies of Treg were uniquely expressed on Treg obtained from blood and various human tissues in 262 samples obtained from 71 patients with lung cancer and 23 healthy blood donors [16]. Further, none of the markers discriminated tumor-associated Treg from circulating Treg in this study [16]. Previously, the use of CD4+FOXP3+ as a “conventional” marker of Treg was reported to consistently detect increased percentages of Treg in blood and tissues of cancer patients relative to those in healthy donors [14,24,34–36]. However, phenotypic comparisons of circulating CD4+FOXP3+ Treg in lung cancer patients and healthy donors in the Hancock study using several additional markers (i.e., CD127, CD25, CD39 or Ki67) did not identify any distinctive phenotype for Treg in cancer [16].
3.3. Enhanced suppressor function of tumor-infiltrating Treg
Few functional studies that were performed with Treg freshly isolated from human tumors report highly elevated suppression levels in these cells [16,37,38]. Suppression is generally measured by Treg-mediated inhibition of activation, proliferation, cytokine production and/or gene expression levels in responder lymphocytes co-incubated with Treg [39,40]. In these assays, purity of isolated CD4+FOXP3+Treg is a critical factor [16]. Suppression mediated by Treg in the TME involves one or several molecular pathways, including production of immunosuppressive cytokines TGFβ, IL-1, IL-35; lysis of Teff by the perforin/granzyme B-based mechanisms; various metabolic effects and other well studied pathways, as listed in Table 2. The availability of so many suppressive mechanisms in tumor-infiltrating Treg suggests redundancy consistent with the importance of Treg for maintaining immune suppression in the TME.
Table 2.
Mechanisms used by Treg for immune suppression in the TME
| Mechanism | Reference |
|---|---|
| Inhibitory cytokine production: | [41,96–98] |
| IL-10, TGF-β, IL-35 | |
| Soluble inhibitory factors: | |
| IDO, PGE2 | [50,99] |
| T effector cell cytolysis: | |
| Granzyme B, perforin | [100,101] |
| Metabolic interruption: | |
| IL-2 deprivation | [102] |
| Adenosine production | [50,51] |
| cAMP-mediated effects | [103] |
| Receptor/ligand signaling: | |
| Fas/FasL, TGF-β/TGFRII | [96,104] |
| Checkpoint Inhibition: | |
| CTLA4, PD-1, TIM-3, LAG-3, TIGIT | [105] |
| Exosomes | [106,107] |
In general, tumor-derived Treg are the more powerful suppressors relative to Treg derived from the patient’s autologous blood. It should be pointed out that previously administered oncologic therapies have been reported to significantly increase the frequency and suppressor functions of Treg present in the tumor [41–43]. Increased suppression mediated by Treg in human tumors is associated with significant up-regulation on the following proteins on the surface of CD4+FOXP3+ Treg: CD25, CD39, CTLA-4, GARP, Helios, LAP, TIM3, PD-1 and TIGIT [42–44]. Among these markers, CD39, CTLA-4, GARP and Tim3 appeared to best discriminate Treg from Teff as reported by Hancock [16]. Further, up-regulated expression of FOXP3 mRNA and protein together with significantly increased mRNA expression levels of EOS, IRF4, SATB1 and GATA1 transcription factors characterized the activated Treg and was associated with elevated suppressor function of intra-tumoral Treg [16]. This set of transcription factors was shown to positively regulate FOXP3 mRNA at the posttranscriptional level. Importantly, this signature of Treg is not stable but is regulated by tumor-derived signals and factors [16].
3.4. Treg signaling in the TME
The phenotype and functions of Treg in the TME are a subject of intense and pervasive pressure exercised by the growing tumor. As a result of tumor-driven signals in the form of soluble factors or vesicular transfer of membrane-tethered ligands and biologically-competent proteins, Treg are continuously being re-programmed to meet the demands imposed by the environment. In the TME, plasticity of Treg is dependent on the tumor, and since escape from the host immune cells is of primary importance, intra-tumoral T cells, including Treg, are recipients of a variety of suppressive signals originating from the tumor. Table 3 lists some of the inhibitory molecules and factors responsible for negative signaling in the TME. Figure 1 illustrates how Treg that are activated and expanded in the TME are induced to overexpress multiple inhibitory checkpoint receptors (ICRs), including PD-1, TIM-3, LAG-3, TGIT and adenosine receptors (A2ARs), as discussed above. Nevertheless, Treg are not suppressed but rather invigorated functionally. The implication of these findings is that in contrast to functional blockade delivered via the ICRs to all other immune cells, Treg respond differently to signals delivered via these receptors. It appears that Treg stability and their activities, including suppressor functions, are not reduced following ICR engagement [45]. Treg in murine cancer models and in patients with cancer are invigorated and proliferate in an antigen/IL-2-driven milieu [46]. iTreg have been reported to express increased levels of intracellular PTEN, and to signal via STAT3 and STAT5 pathways, bypassing the AKT/mTOR pathway [47,48], as indicated in Figure 2, gaining in stability and suppressive functions. We and others have shown that human iTreg up-regulate surface expression of CD39 and CD73, efficiently hydrolyze ATP to 5’-AMP and adenosine (ADO), actively secrete ADO and mediate effective suppression of Teff cells via the A2AR [49,50]. Unlike Teff, human Treg express low levels of adenosine deaminase (CD26), do not degrade ADO to inosine and, instead, re-utilize ADO via A2AR expressed on the cell surface [32] This autocrine signaling of ADO generated by Treg and accumulating in the pericellular space appears to promote Treg stability and functions. In Teff cells, which also express A2AR, ADO signaling leads to cAMP-mediated functional downregulation [51] Thus, Treg in the TME appear to process inhibitory signals differently than Teff. While activation and proliferation of Teff are restored by ICR signaling, and the stability and suppression mediated by Treg in the TME are promoted by the ICR signals [52]. Autocrine utilization of ADO by Treg in situ represents an example of the mechanisms adapted by iTreg to ensure their survival in the highly immunosuppressive TME. An alternative explanation suggested for the observed up-regulation of ICRs in tumor-infiltrating Treg is that strong suppression these cells mediate has to be mitigated to prevent an excessive blockade that could interfere with immunological homeostasis which is already partly compromised by the presence of malignancy [53]. At present, the biological significance of simultaneous overexpression of activation markers and numerous ICRs in tumor-infiltrating Treg remain unclear, and are being actively evaluated in the context of immunotherapy with ICIs.
Table 3.
Molecular pathways implicated in Treg-mediated signaling in the TME
| Pathway | References |
|---|---|
| TGF-β/TGF- βRI/II: | [96] |
| LAP, GARP, active TGF-β | |
| Adenosine/A2AR: | |
| A2aR | [85] |
| CD39, CD73 ectoenzymes | [51] |
| ADA | [32] |
| COX-2/PGE2 /EP2R | [49] |
| Immune checkpoints: | |
| CTLA-4/ B7, PD-1/PD-L1, TIM- 3/Galectin-9, PVR | [13,105] |
| LAG-3/ MHC II, TIGIT/CD155 (PVR) | [69] |
| Chemokine receptors: | |
| CCR-4, 5, 6, 10 | [1,5] |
| Fas/FasL | [104] |
| TNFR2/TNF | [108,109] |
| GITR/GITRL | [110] |
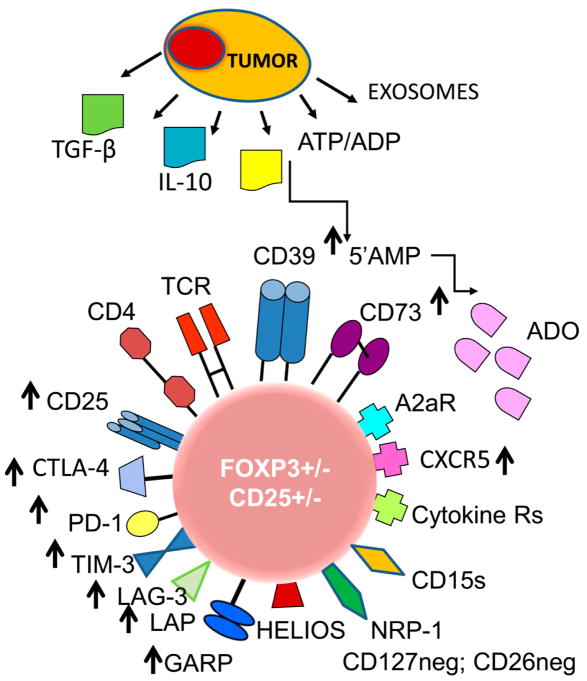
Figure 1.
The phenotypic profile of an iTreg in the tumor microenvironment (TME). The tumor produces cytokines, soluble factors or exosomes, all of which re-program functions of Treg in the TME. Highly activated iTreg up-regulate expression levels of checkpoint inhibitory receptors and other proteins involved in immunosuppressive activity of iTreg, such as TGF-β-associated LAP and GARP or CD39 and CD73 ectoenzymes (see arrows). iTreg in the TME may or may not be FOXP3+ and CD25+; they up-regulate expression levels of NRP1, which maintains contact with DCs via the semaphorin-4a and may play a role in Treg stability. Abbreviations: Treg, regulatory T cells, ATP, adenosine triphosphate, ADP, adenosine diphosphate; 5’AMP, adenosine-5’-monophosphate; ADO, adenosine; TCR, T-cell receptor; CTLA-4, cytotoxic lymphocyte antigen-4; PD-1, programmed death-1; TIM-3, T-cell immunoglobulin mucin-3; LAG-3, lymphocyte activation gene-3; TGF-β, transforming growth factor- beta; LAP, latency-associated protein; GARP, glycoprotein A repetition predominant; NRP-1, neuoropilin-1; CD15s, sialyl Lewis X.
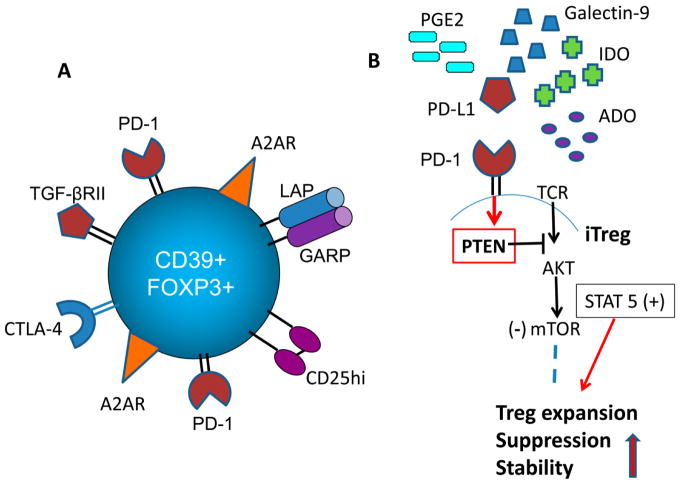
Figure 2.
Functions of iTreg in the TME. (A). An adenosine-producing CD4+CD39+ iTreg upregulates A2ARs expression levels thus facilitating autocrine signaling, which promotes iTreg suppressor functions. (B). In the TME, signals delivered by PD-L1 to PD-1 expressed on Treg upregulate PTEN, which blocks the AKT/mTOR pathway, so that Treg signal via STAT5. This increases Treg stability, promotes Treg proliferation and enhances Treg suppressor functions.
Another aspect of Treg signaling in the TME concerns a possibility that various Treg subsets might be selectively regulated to utilize specific inhibitory molecular pathways [54]). Table 3 lists several, but not all, of the inhibitory mechanisms known to operate in the TME. It seems unlikely that all Treg are able to utilize all these various mechanisms. In view of phenotypic and functional heterogeneity of Treg, it seems more realistic to propose that distinct subsets of Treg might acquire the preferential ability to mediate one type of suppression, depending on the inhibitory profile of the tumor. Thus, Treg in the TME might be instructed to adopt a suppressive pathway that best reflects the suppressive milieu of the tumor. For example, COX-2+ HNSCCs were shown to promote the in vitro development of iTreg that expressed COX-2 and produced PGE2, while COX-2(neg) HNSCCs induced Treg expressing few inhibitory receptors and producing low levels of immunosuppressive factors [55] . Similarly, in tumors producing TGF-β, such as HNSCCs, iTreg tend to up-regulate LAP and GARP on the cell surface, suggesting that the TGF-β pathway is involved in tumor-induced suppression mediated by Treg [54]. On the other hand, given the evidence that expression levels of multiple ICRs are up-regulated on Treg infiltrating human tumors, it could be hypothesized that all these iTreg are adequately equipped to respond to a wide variety of ligands present in the TME. This may be an example of redundancy often encountered with cellular processes regulating hematopoiesis.
3.5. Exosomes and Treg interactions
Tumor-derived exosomes, also dubbed “TEX”, are a major intercellular communication system in the TME. Tumors produce and release large quantities of exosomes that carry immunosuppressive and immunostimulatory receptors and ligands [56]. TEX partially mimic the molecular profiles of their parent tumor cells and deliver signals from tumor cell to other cells in the TME, including Treg [57]. The TEX cargo is generally enriched in immunoinhibitory proteins and factors known to suppress immune responses [56]. It has been noted TEX suppressed proliferation of CD8+ Teff but promoted expansion of CD4+ T cells [58]. More recently, we and other have reported that TEX promoted expansion and activity of Treg [59,60]. In vitro co-incubation experiments of TEX with T-cell subsets, including CD4+CD39+Treg isolated from peripheral blood of healthy donors, indicated that TEX simultaneously delivered a series of suppressive signals to T cells, up-regulated expression levels of multiple immunoregulatory genes in Treg and altered their functions [61]. For example, Treg co-incubated with TEX, which carried CD39 and CD73 ectonucleotidases [44] significantly up-regulated production of immunosuppressive adenosine by Treg. In aggregate, these data suggested that Treg were re-programmed differently by TEX than Teff subsets and that altered mRNA transcription in Treg upon co-incubation with TEX translated into enhanced production of immunosuppressive proteins [61]. Such re-programming of Treg by TEX might involve transfer of miRNAs from tumor to recipient cells, as commonly observed [62]. However, T cells do not readily internalize exosomes [63], and it is possible that Treg re-programming by TEX preferentially involves receptor-ligand signaling that leads to transcriptional activation in recipient cells. An interesting caveat of these in vitro experiments is that Treg appear to respond differently to TEX than CD4+ or CD8+ T cells, suggesting they might utilize distinct molecular mechanisms of activation than their lymphoid counterparts.
4. Treg and immunotherapy with immune check point inhibitors
While functions of Teff, NK and B cells, all of which express PD-1, are inhibited upon interactions with APC presenting PD-L1 (i.e., inhibitory ligand; Figure 3), Treg appear to respond to the ligand binding by increased proliferation, greater stability and increased suppressor functions as previously reported [52]. The PD-1/PD-L1 blockade with checkpoint inhibitors primarily blocks negative signaling in activated Teff and restores their anti-tumor functions. In the presence of pembrolizumab that blocks PD-1 signaling, immune cells are rejuvenated, except for Treg, which upon deprivation of PD-1 signaling in the presence of pembrolizumab are expected to be restrained in their suppressor functions but, in fact, do not seem to be (64). Targeting PD-1or PD-L1 by mAbs disrupts signaling necessary for FOXP3 expression, attenuates Treg functions but does not alter their frequency or phenotype. Elkord and colleagues who studied effects of pembrolizumab on various Treg subpopulations in healthy donors and in primary breast cancer patients treated with this blocking antibody, reported that despite decreased levels of PD-1 expression levels on Treg following pembrolizumab delivery, Treg phenotype or suppressor functions remained unchanged [45]. Similarly, Ribas et al also reported no change in Treg defined as CD45+CD4+CD25hiCD127lo cells in melanoma patients treated with pembrolizumab [64]. In both these studies, pembrolizumab therapy rejuvenated effector CD8+ T cell populations.
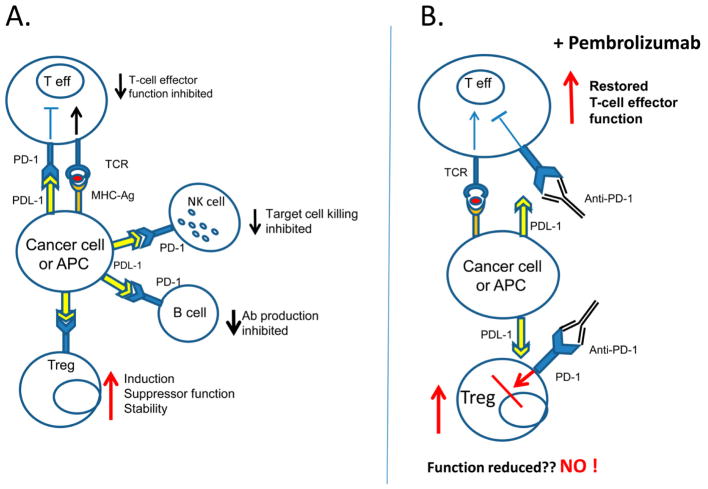
Figure 3.
Antigen-driven PD-1/PD-L1 signaling: (A) in patients with cancer, where tumor cells or antigen presenting cells express PD-L1, signaling via PD-1 leads to inhibition of effector functions in all immune cells except for Treg. PD-1/PD-L1 signaling in Treg leads to greater stability and more effective suppression. (B) in cancer patients receiving therapy with anti-PD-1 Abs such as pembrolizumab, immune cells are rejuvenated and regain anti-tumor functions. In contrast, PD-1/PD-L1 signaling does not seem to decrease the Treg frequency or block their functions. Instead, Treg stability and functions are not altered, suggesting that ICI restores Teff cell activity without depleting Treg or suppressing their functions.
It has been suggested on the basis of studies in mice that ipilimumab or other immune checkpoint blocking antibodies with the IgG1 isotype eliminate Treg by antibody-dependent cellular cytotoxicity (ADCC) mediated by the FcγR-expressing NK cells [65,66]. Anti-tumor efficacy of ipilimumab was shown to be dependent on the depletion of CTLA-4+ Treg by ADCC in mouse models [66]. Non-classical CD68+/CD163+/FcγR+ macrophages were reported to mediate ex vivo ADCC of Treg in patients with melanoma [67]. Preliminary in vitro studies from my laboratory suggest that pre-treatment of Treg isolated from PBMC of healthy donors with ipilimumab resulted in elimination by ADCC of about a third of Treg. The surviving Treg placed in culture proliferated rapidly, retained the Treg phenotype and mediated suppression in vitro (unpublished data). In aggregate, these data suggest that ICIs mainly restore immune competence of Teff without a major disturbance of Treg, which not only survive but may be encouraged to expand, replenishing the pool of Treg partially depleted by immunotherapy. However, the suggestion that Treg are not depleted or “muzzled” by therapies with ICIs, while Teff and other immune cells are restored in their anti-tumor effector functions should be considered with caution. The heterogeneity of human Treg, their plasticity in the TME and the lack of specific Treg markers represent a significant barrier in measuring Treg in tissues and blood of patients treated with ICIs. Therefore, conclusions on the Treg fate during and after immunotherapies remain unclear and warrant further investigation.
Resistance of Treg to checkpoint inhibition has been considered as a potential reason for unresponsiveness of some cancer patients to ICIs [68]. It could be surmised that Treg overexpressing ICRs in the TME, as previously reported [42,69,70], are not responsive to antibodies blocking receptor signaling and instead might benefit by greater proliferation or stability. In this context, elevated Treg-mediated immune suppression could contribute to resistance to therapy by interfering with rejuvenation of Teff in the presence of ICIs. Indeed, a recent study provides evidence that the recruitment of Treg into the TME may be a reason for a lack of response to ICI blockade, especially in tumor types that are heavily infiltrated with adaptive immune cells [71]. It has been suggested that in cancers with a lower number of non-self neoantigens, where shared antigen-specific CD8+T cells mainly control anti-tumor responses, ICI-driven rejuvenation of these CD8+ T cells may be inefficient, because of Treg which are especially potent inhibitors of self-antigen-reactive T cells [72]. This concept is based on the assumption that Treg mainly suppress autoimmune responses and only Treg corrupted by the tumor are engaged in restraining functions of tumor-specific T cells. This may or may not be the case, and a more convincing explanation for Treg resistance to ICIs could emerge by evaluating differential responses of Treg subsets to signals predominating in the TME as suggested above.
5. The role of ICRs on Treg in the TME
Figure 4 is a cartoon of iTreg expressing multiple ICRs. Each receptor interacts with a unique ligand or ligands expressed by immune or non-immune cells present in the TME. iTreg are thus seen as highly interactive cells. This scenario places Treg in a sensitive position of being modulated by many different signals generated in the TME. As PD-1, CTLA-4, TIM-3, LAG-3 and TIGIT are inhibitory receptors, the assumption is that they are meant to keep Treg from delivering excess suppression that could eliminate normal immune functions. In the TME, Treg are not only involved in controlling local cancer-promoting inflammation but also in down-regulating inflammation-induced tissue damage. Hence, Treg in the TME, and presumably in other inflammatory conditions, must play diverse regulatory functions and maintain a delicate balance between suppression and no suppression, depending on environmental cues. Treg appear to be well equipped with numerous regulatory receptors to effectively accomplish this task. They are a key part of the complex regulatory network driven by locally-produced cytokines and extracellular vesicles that operates smoothly in health and is disrupted and/or corrupted in the presence of disease.
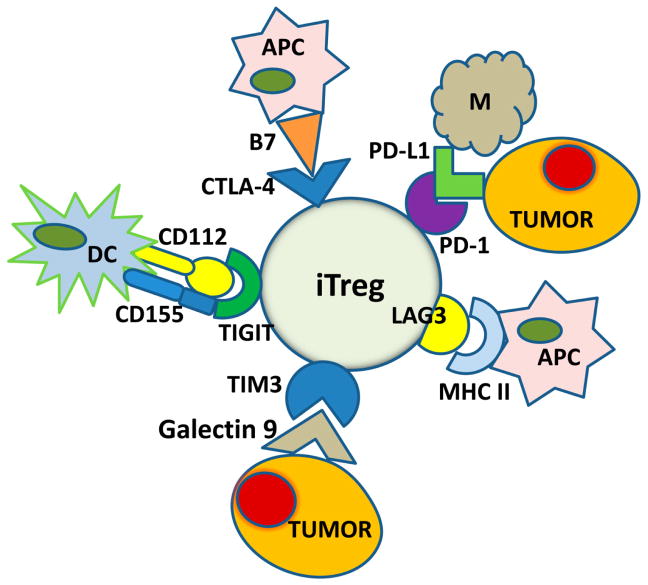
Figure 4.
iTreg in the TME are highly interactive and engage a variety of immune and non-immune cells. The immune checkpoint inhibitory receptors, CTL-4, PD-1, LAG-3, TIM-3 and TIGIT, are upregulated on the iTreg surface in the TME. The ligands for these receptors are present on a variety of tissue and immune cells so that Treg have an ample opportunity to interact with these various cells. It is possible that these receptors on iTreg control contextual responses of Treg to environmental signals. Engagements of Treg in immune and non-immune activities may be regulated by these receptors.
Recent literature provides intriguing evidence pointing to previously unsuspected functional diversity of Treg [73–79]. The engagement of Treg in a variety of non-immunosuppressive activities such as tissue repair, vascular re-programming, modulation of metabolic and secretory functions of immune or tissue cells, including tumor cells, and limiting TPI driven by gut microbiota (Table 4) suggests that this lymphocyte subset is capable of assuming a broad variety of functions and that this is a contextual process. Further, recent insight suggests that these constructive functions of Treg may be regulated by innate mechanisms such as cytokines (e.g., IL-18 or IL-33) and are independent of TCR signaling [75,76].
Table 4.
Functional diversity of Trega
|
Some of the non-immunoregulatory functions ascribed to Treg are listed. CRC, colorectal cancer; MMP2, metalloproteinase 2; NRP, neuropilin 1
6. Are Treg a barrier or not a barrier to the success of cancer immunotherapy??
Given the above discussed features of Treg and their functional diversity, the question of therapeutic attempts at their reduction/elimination in cancer deserves to be re-examined. Over last several years, many different strategies for Treg silencing or elimination have been experimented with both in pre-clinical and clinical settings (reviewed in [2]). Targeted Treg therapies often take advantage of expression on Treg of molecules such as CD25, CTLA-4, CCR4, OX40 or GITR. As neither of these proteins is selectively expressed on Treg, off-site effects may be anticipated and may interfere with the therapeutic goal. A good example is the use of antibodies targeting CD25 such as daclizumab, a humanized mAb with IgG isotype, that binds to the α-subunit of IL-2 R (CD25). Daclizumab reduced and re-programmed Treg but also diminished activated Teff and thus did not achieve the expected augmentation of T-cell responses [80]. Mogamulizumab is a humanized anti CCR4 mAb with ADCC activity which was initially approved in Japan for treatment of relapsed refractory adult T cell leukemia/lymphoma and later was used alone or in combination with nivolumab for therapy of solid tumors [81]. This therapy had a good safety profile, decreased FOXP3+ Treg, enhanced Teff activity and was associated with objective responses in some tumor types [81]. Based on the rationale that CCR4, a receptor for CCL17 and CLC22, is responsible for the Treg recruitment to tumors, therapy with megamulizumab alone or in combination with vaccines or other immune therapies promoting Teff responses may be a promising addition to the list of drugs targeting Treg [82]. Treg express CD39 and CD73, and blocking the adenosine pathway, which is utilized by Treg and promotes their growth and suppressor activity [51] via silencing these ectonucleotidases, is one strategy for targeting Treg. Preclinical studies indicate that the adenosine pathway can be targeted with antibodies, pharmacologic inhibitors or siRNAs to reduce or silence its pro-tumor activities [83–85]. The results of one clinical trial using the MED19447 MoAb which blocks CD73 activity were recently reported [86]. In the context of CD39+ Treg, antibodies inhibiting CD39 functions, are also undergoing preclinical in vivo testing [87]. Indoleamine 2, 3-dioxygenase (IDO) is expressed in many human cancers. Elevated expression of IDO in tumors is associated with advanced disease stage and metastasis. IDO inhibits activation of Teff by depletion of an essential amino acid, tryptophan, and promotes differentiation and activation of Treg and MDSC through kynurenine production [88]. Inhibitors of IDO such as epacadostat, alone or with the PD-1 blockade show promising results in recent clinical trials [89,90]. Treatment with checkpoint inhibitory Abs restore Teff functions but do not seem to alter phenotype or functions of Treg, as discussed above. As Treg commonly utilize the TGF-β pathway for suppression, antagonists of this pathway, including neutralizing anti-TGF-β, fresolimumab, as well as type I TGF-βR serine-threonine kinase inhibitor are in clinical trials with an objective of enhancing CD8+ T cell/Treg ratios and suppressing IDO expression by DC in the tumor-draining lymph nodes [91]. Recent data support the combination of fresolimumab with hypofractionated radiation for therapy of advanced solid tumors citing improved immune recovery achieved by the combination relative to Ab therapy alone [92]. Both the adenosine and COX2 pathways are targets of several recent clinical trials [86], and chemotherapies, including low and high doses of cyclophosphamide, are frequently in use to reduce Treg frequency [93]. Anti-VEGF therapies targeting VEGF-A or the VEGFR-2 are aimed at blockading blood vessel development also inhibit infiltration of immune suppressive cells, including Treg, into tumors and have been recently administered with ICIs in clinical trials of several solid malignancies [94]. Table 5 provides a listing of currently evaluated monotherapies and combination anti-tumor immunotherapies that target Treg aiming at their depletion or reprogramming.
Table 5.
Therapeutic depletion or re-programming of human Trega
|
Antibodies, inhibitors or drugs that have been clinically used for reducing numbers/functions of Treg in patients with cancer (reviewed in [2])
While multiple Treg-targeting therapies are available, the difficulty is to a priori discern which of the numerous inhibitory pathways play a key role in immune escape of a given tumor. Thus, a rational selection of therapeutic strategies for Treg depletion is a personal decision based on the prior knowledge of the TME in each cancer patient. The second difficulty is related to a concern about potential adverse systemic autoimmune effects of such therapies. So far, the reported adverse effects are few and relatively mild, but so is the efficacy of anti-Treg inhibitory strategies [95]. Although the Treg frequency is often rapidly reduced following anti-Treg therapy, it tends to reverse to the baseline level, making it difficult to judge clinical benefits of this therapy and to associate them to augmented anti-tumor immunity. It may be that higher or more frequent dosing of inhibitors might have greater effects on elimination of Treg, but an ever-present possibility for inducing autoimmune disease mitigates these approaches. Also, the question of whether it is prudent to silence Treg must be asked, given the new insights into their functional diversity and roles Treg assume in physiologically critical processes. Today, Treg must be viewed not only as immunoregulatory elements but as cells that can be programmed to mediate a broad range of “good” responses in different tissues and organs as indicated in Table 4. In this context, the concept of Treg silencing in cancer has to be re-examined, taking into account new insights into Treg functional heterogeneity and their phenotypic plasticity.
7. Expert Opinion
The question of whether FOXP3+ Treg serve or should serve as a therapeutic target for promoting anti-tumor immunity has no definite answer at this time. Current clinical studies have yet to demonstrate whether targeting Treg is beneficial for immunotherapy of cancer. Clearly, Treg play a critical role in tumor immunity and they are partly responsible for immune suppression in the TME. However, current literature also indicates that Treg might engage in various non-immune activities which are important for tissues and organs homeostasis. The contributions of Treg to current cancer immunotherapies are under investigation. This is proving to be exceptionally challenging because of the lack of selective phenotypic markers for human Treg and because expression of the FOXP3 transcription factor alone is not a stable characteristic of Treg in the TME. The lack of a reliable Treg biomarker that would allow for their isolation from tissues and more precise estimation of their frequency in situ is a barrier to further progress. Functional assessments of Treg in tumor tissues are complex and difficult to interpret because of re-programming mediated by cytokines, chemokines and exosomes that takes place in the TME. Recent data indicate that tumor-derived exosomes (TEX) carrying diverse molecular/genetic cargos reprogram Treg and are probably responsible for Treg plasticity in the TME. The reprogrammed Treg in the TME may operate differently than their normal counterparts, producing cytokines, soluble factors and their own exosomes that promote tumor progression. Imposing immune therapy on this complex network of cellular interactions without a better understanding of the multiple and diverse functions Treg can assume in the TME might lead to therapeutic disappointments.
The TME is uniquely shaped by the tumor to its own advantage. Treg differentiating in the TME acquire phenotypic and functional characteristics specified by the tumor. This means that each tumor can ”design” its own brand of Treg, depending on its need for protection from the host immune system and the repertoire of reprogramming factors it produces. Consequently, tumor-specific strategies for the Treg depletion might be needed to disrupt this cross-talk. Future Treg targeted therapies are likely to be “tumor and patient specific”. This, of course, will require a deeper understanding of the TME and the Treg in each tumor before strategies for Treg silencing can be effectively coupled with immune therapies to achieve tumor control.
Acknowledgments
Funding
This work was supported in part by NIH grants R0-1 CA168628 and R21 CA205644 to TL Whiteside.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 2•.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther. 2015;4:159–171. doi: 10.2147/ITT.S55415. This review summarizes an earlier (prior to 2015) view of Treg in the tumor microenvironment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu SK, Eardley DD, Cantor H, et al. Definition of two pathways for generation of suppressor T-cell activity. Proc Natl Acad Sci U S A. 1983;80:3779–3781. doi: 10.1073/pnas.80.12.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. This is the "must" paper which provides definitive evidence for suppressive activity of Treg. [PubMed] [Google Scholar]
- 5.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 7.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis GI, Reneer MC, Velez-Ortega AC, et al. Generation of induced regulatory T cells from primary human naive and memory T cells. J Vis Exp. 2012;62:3738. doi: 10.3791/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 11.Devaud C, Darcy PK, Kershaw MH. Foxp3 expression in T regulatory cells and other cell lineages. Cancer Immunol Immunother. 2014;63:869–876. doi: 10.1007/s00262-014-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. This paper points to the heterogeneity of Treg subsets in CRC and distinct roles of these subsets in prognosis. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 14•.Ondondo B, Jones E, Godkin A, et al. Home sweet home: the tumor microenvironment as a haven for regulatory T cells. Front Immunol. 2013;4:197. doi: 10.3389/fimmu.2013.00197. An explanation of why Treg accumulate and are activated in the TME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–334. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Akimova T, Zhang T, Negorev D, et al. Human lung tumor FOXP3+ Tregs upregulate four "Treg-locking" transcription factors. JCI Insight. 2017;2:e94075. doi: 10.1172/jci.insight.94075. A highly informative and high-impact study of human Treg in lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 18.Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 20.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 22••.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. This paper discusses the impact of the immune score on clinical outcome in solid cancers. [DOI] [PubMed] [Google Scholar]
- 23.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 25.Marshall EA, Ng KW, Kung SH, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. 2016;15:67. doi: 10.1186/s12943-016-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 27.Droeser R, Zlobec I, Kilic E, et al. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134. doi: 10.1186/1471-2407-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 29•.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. This paper provides an experimental basis for the role of FoxP3 as a "master regulatory transcription factor.". [DOI] [PubMed] [Google Scholar]
- 30•.Miyara M, Chader D, Sage E, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A. 2015;112:7225–7230. doi: 10.1073/pnas.1508224112. CD15s is presented as a potentially useful surface antigen for isolation of human Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 32.Mandapathil M, Szczepanski M, Harasymczuk M, et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology. 2012;1:659–669. doi: 10.4161/onci.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 35.Shigematsu Y, Hanagiri T, Shiota H, et al. Immunosuppressive effect of regulatory T lymphocytes in lung cancer, with special reference to their effects on the induction of autologous tumor-specific cytotoxic T lymphocytes. Oncol Lett. 2012;4:625–630. doi: 10.3892/ol.2012.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 37.Jie HB, Gildener-Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss L, Bergmann C, Szczepanski MJ, et al. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol. 2008;180:2967–2980. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside TL. Regulatory T Cell (Treg) Assays: Repertoire, Functions, and Clinical Importance of Human Treg. In: Detrick B, Schmitz J, Hamilton R, editors. Manual of Molecular and Clinical Laboratory Immunology. ASM Press; Washington, DC: 2016. pp. 296–299. [Google Scholar]
- 40•.Akimova T, Levine MH, Beier UH, et al. Standardization, Evaluation, and Area-Under-Curve Analysis of Human and Murine Treg Suppressive Function. Methods Mol Biol. 2016;1371:43–78. doi: 10.1007/978-1-4939-3139-2_4. This methods-oriented paper is required reading for researchers who want to study functions of Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 42.Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75:2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Schuler PJ, Harasymczuk M, Schilling B, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res. 2013;19:6585–6596. doi: 10.1158/1078-0432.CCR-13-0900. This paper provides evidence for changes that conventional cancer therapy may induce in Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuler PJ, Saze Z, Hong CS, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin Exp Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toor SM, Syed Khaja AS, Alkurd I, et al. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol. 2017 doi: 10.1111/cei.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma MD, Shinde R, McGaha TL, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha S, Yang K, Guy C, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteside TL, Jackson EK. Adenosine and prostaglandin E2 production by human inducible regulatory T cells in health and disease. Front Immunol. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. This study describes the role(s) PD-1 signaling play in the development, function and stability of Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park HJ, Park JS, Jeong YH, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 54.Arce-Sillas A, Alvarez-Luquin DD, Tamaya-Dominguez B, et al. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J Immunol Res. 2016;2016:1720827. doi: 10.1155/2016/1720827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergmann C, Strauss L, Zeidler R, et al. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 56.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017;189:259–267. doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. An excellent, comprehensive review of extracellular vesicles (EVs) and their role in intercellular communication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Szajnik M, Czystowska M, Szczepanski MJ, et al. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. The first report showing that tumor-derived exosomes promote expansion and functions of Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107:363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 61.Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017;13:2583–2592. doi: 10.2217/fon-2017-0343. The most recent summary of the importance of tumor-derived exosomes in cancer progression and therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller L, Simms P, Hong CS, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6:e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 65.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 67••.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. This paper shows that non-classical monocytes in the presence of ipilimumab can induce death of Treg in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. One of several papers by Dr. Elkord's group demonstrating accumulation of Treg decorated by ICI receptors in the TME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Syed Khaja AS, Toor SM, El Salhat H, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8:33159–33171. doi: 10.18632/oncotarget.16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong A, Pan X, Shi M. Expression of PD-1 by CD4(+)CD25(+)CD127(low) Treg cells in the peripheral blood of lung cancer patients. Onco Targets Ther. 2015;8:1831–1833. doi: 10.2147/OTT.S90538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor NA, Vick SC, Iglesia MD, et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J Clin Invest. 2017;127:3472–3483. doi: 10.1172/JCI90499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda Y, Nishikawa H, Sugiyama D, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 73.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasanthakumar A, Kallies A. The Regulatory T Cell: Jack-Of-All-Trades. Trends Immunol. 2015;36:756–758. doi: 10.1016/j.it.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 76•.Arpaia N, Green JA, Moltedo B, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. One of several reports by Rudensky's group on non-immune functions of Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Green JA, Arpaia N, Schizas M, et al. A nonimmune function of T cells in promoting lung tumor progression. J Exp Med. 2017;214:3565–3575. doi: 10.1084/jem.20170356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali N, Zirak B, Rodriguez RS, et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell. 2017;169:1119–1129e1111. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maryanovich M, Frenette PS. T-Regulating Hair Follicle Stem Cells. Immunity. 2017;46:979–981. doi: 10.1016/j.immuni.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs JF, Punt CJ, Lesterhuis WJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16:5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]
- 81.Kurose K, Ohue Y, Wada H, et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin Cancer Res. 2015;21:4327–4336. doi: 10.1158/1078-0432.CCR-15-0357. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto H, Muro K, Ishii H, et al. Anti-C-C chemokine receptor 4 (CCR4) antibody mogamulizumab (Moga) and nivolumab (Nivo) combination phase I study in patients with advanced or metasatic solid tumors. Annals of Oncology. 2017;28(suppl 5):v605–v649. [Google Scholar]
- 83.Young A, Mittal D, Stagg J, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 84.Allard B, Longhi MS, Robson SC, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther. 2017;17:527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5:e1208875. doi: 10.1080/2162402X.2016.1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonnefoy N, Bastid J, Alberici G, et al. CD39: A complementary target to immune checkpoints to counteract tumor-mediated immunosuppression. Oncoimmunology. 2015;4:e1003015. doi: 10.1080/2162402X.2014.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munn DH, Sharma MD, Johnson TS, et al. IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment. Cancer Immunol Immunother. 2017;66:1049–1058. doi: 10.1007/s00262-017-2010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beatty GL, O'Dwyer PJ, Clark J, et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2017;23:3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamid O, Bauer TM, Spira AI, et al. Safety of epacadostat 100 mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: Phase 2 data from ECHO-202/KEYNOTE-037. Journal of Clinical Oncology. 2017;25(suppl) abstr. #3012. [Google Scholar]
- 91.Hanks BA, Holtzhausen A, Evans K, et al. Combinatorial TGF-β signaling blockade and anti-CTLA-4 antibody immunotherapy in a murine BRAFV600E-PTEN−/− transgenic model of melanoma. Journal of Clinical Oncology. 2014;32:5s. suppl:abstr 3011. [Google Scholar]
- 92.Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-0037. [DOI] [PMC free article] [PubMed]
- 93.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baur AS, Lutz MB, Schierer S, et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122:2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 96.Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-beta pathway for cancer therapy. Clin Cancer Res. 2012;18:4514–4521. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 97.Dennis KL, Blatner NR, Gounari F, et al. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. 2013;25:637–645. doi: 10.1097/CCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sawant DV, Hamilton K, Vignali DA. Interleukin-35: Expanding Its Job Profile. J Interferon Cytokine Res. 2015;35:499–512. doi: 10.1089/jir.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mellor AL, Lemos H, Huang L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grossman WJ, Verbsky JW, Barchet W, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Thornton AM, Donovan EE, Piccirillo CA, et al. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 103.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182:1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agarwal A, Fanelli G, Letizia M, et al. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. doi: 10.3389/fimmu.2014.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okoye IS, Coomes SM, Pelly VS, et al. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity. 2014;41:503. doi: 10.1016/j.immuni.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, Baumel M, Mannel DN, et al. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 109.Nagar M, Jacob-Hirsch J, Vernitsky H, et al. TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol. 2010;184:3570–3581. doi: 10.4049/jimmunol.0902070. [DOI] [PubMed] [Google Scholar]
- 110.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10. doi: 10.1016/j.ejca.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 111.Russler-Germain EV, Rengarajan S, Hsieh CS. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017;10:1375–1386. doi: 10.1038/mi.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang BQ, Zhang CM, Gao W, et al. Cancer-derived matrix metalloproteinase-9 contributes to tumor tolerance. J Cancer Res Clin Oncol. 2011;137:1525–1533. doi: 10.1007/s00432-011-1010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piechnik A, Dmoszynska A, Omiotek M, et al. The VEGF receptor, neuropilin-1, represents a promising novel target for chronic lymphocytic leukemia patients. Int J Cancer. 2013;133:1489–1496. doi: 10.1002/ijc.28135. [DOI] [PubMed] [Google Scholar]