Abstract
Purpose:
We investigated the effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on the sneeze induced continence reflex and on bladder function in rats with cerebral infarction.
Materials and Methods:
Using urethane anesthesia the effect of duloxetine (1 mg/kg intravenously) on the amplitude of urethral responses during sneezing as well as urethral baseline pressure at the mid urethra was evaluated in normal female adult rats and cerebral infarction rats. Tilt leak point pressure was also measured. In normal and cerebral infarction rats continuous cystometry was evaluated before and after duloxetine injection.
Results:
Incerebralinfarctionratsurethralbaselinepressurewas43%lowerthan in normal rats but the amplitude of urethral responses during sneezing did not differ in the 2 groups. Duloxetine increased the amplitude of urethral responses during sneezing and urethral baseline pressure by 31% and 21%, respectively, in normal rats but did not affect either in cerebral infarction rats. Also, in cerebral infarction rats leak point pressure was 29% lower compared with normal rats. Duloxetine increased leak point pressure in normal rats but not in cerebral infarction rats. Cerebral infarction reduced intercontraction intervals without affecting the amplitude of bladder contractions compared with normal rats. Duloxetine prolonged intercontraction intervals in cerebral infarction rats but not in normal rats.
Conclusions:
These results suggest that cerebral infarction induces not only bladder overactivity but also stress urinary incontinence, which may account for mixed incontinence in patients with cerebral infarction. After cerebral infarction duloxetine reduced bladder overactivity but failed to enhance active urethral closure mechanisms during sneezing, suggesting that disorganization of the brain network after cerebral infarction might influence the effect of duloxetine on lower urinary tract function.
Keywords: urinary bladder, urethra, urinary incontinence, stress, cerebral infarction, duloxetine
STRESS urinary incontinence, the most common type of urinary incontinence in women after middle age, can be caused by 2 impaired closure mechanisms of the urethra (ie urethral hypermobility due to a loss of bladder neck/proximal urethra support and intrinsic sphincter deficiency).1 In CI survivors there is also a high prevalence of urinary incontinence varying from 12% to 79% depending on time after CI.2,3 Although urinary incontinence has an important negative impact on quality of life,4 the mechanisms underlying urinary incontinence after CI have not been studied in detail. In CI the neurological dysfunction is caused by focal brain damage due to ischemia and/or hemorrhage. When brain damage is located in a small area in the right frontal region of the cerebrum, which is involved in the control of micturition, it may result in bladder overactivity and urge urinary incontinence.5 In addition, it has been reported that CI patients can also show SUI or mixed incontinence.6 Thus, we hypothesized that CI might be a pathophysiological condition not only of urge incontinence but also of SUI.
Norepinephrine and serotonin reuptake in-hibitors such as duloxetine have demonstrated clinical efficacy in the treatment of SUI7 as well as overactive bladder.8 We have previously established a rat model that can be used to examine sneeze induced active urethral closure mechanisms that are mediated by somatic nerve induced reflex contractions of the external urethral sphincter and the pelvic floor striated muscles.9 We have also reported that duloxetine enhances these mechanisms via activation of spinal noradrenergic and serotonergic systems in normal rats.10,11 However, to our knowledge it has not been investigated whether these mechanisms are affected by CI.
Therefore, we examined the effect of duloxetine on the sneeze induced continence by using urethral microtransducer tipped catheter methods and LPP measurements in normal rats and rats with CI. To clarify the effect of CI on bladder function we also investigated the effect of duloxetine on continuous cystometrograms using normal and CI rats.
MATERIALS AND METHODS
Animals
A total of 44 adult female Sprague Dawley® rats weighing 238 to 270 gm were studied using experimental protocols approved by the University of Ryukyus and University of Pittsburgh institutional animal care and use committees. Experiments were performed in normal rats and rats with CI.
Cerebral Infarction
CI was produced according to methods described previously.12 Briefly, rats were anesthetized with 2% isoflurane and the right carotid bifurcation was exposed through a midline incision in the neck. The common carotid artery was occluded and the branches of the external carotid artery were dissected and divided. The pterygopalatine branch was identified and ligated close to its origin. A 4-zero monofilament nylon thread with the tip rounded by exposure to a flame was introduced into the internal carotid artery and advanced 17 mm from the carotid bifurcation to the origin of the middle cerebral artery, where it occluded blood flow and, thus, induced infarction on the right side of the brain. After suturing the neck incision the rats were placed in a restraining cage and allowed to recover from isoflurane anesthesia. All rats showed postoperative neurological deficits characterized by left hemiparesis and eyelid ptosis. Experiments were done 3 days after CI induction
Sneeze Reflex and Sneeze Induced Urethral Continence Reflex
The sneeze reflex, which is a highly coordinated reflex evoked by irritation of the nasal mucosa, removes irritations and cleans the airway. In this study the sneeze reflex induced by a rat whisker cut and inserted gently in the nostril while under urethane anesthesia was used to increase abdominal pressure.
Duloxetine Effect Experiments
1). Mid Urethral Pressure Responses and Baseline Pressure.
A total of 12 normal and 6 CI rats were examined. The bladder was emptied. A 3.5Fr SPR-524 nylon catheter (Millar Instruments, Houston, Texas) with a side mounted microtransducer located 1 mm from the catheter tip was inserted in the urethra from the urethral orifice according to previously described methods.9,10,13
In normal and CI rats the sneeze reflex was induced, and A-URS and UBP were measured. A-URS was determined as the maximal pressure change in cm H2O from baseline. Average UBP was obtained from a plateau section of pressure recordings with a 1 to 2-minute duration just before the sneeze response according to our previous reports.10,13 Sneeze induced responses were measured before and after intravenous injection of duloxetine (1 mg/kg, Kemprotec, Middlesbrough, United Kingdom). Sneeze reflexes were evoked repeatedly to obtain at least 10 measurable responses before and after duloxetine treatment.
To evaluate the intensity of the induced sneeze, which varied among sneeze events, Pabd increases during sneezing were also measured via an intra-abdominal balloon catheter inserted through the rectum because it was not possible to measure intravesical pressure in the emptied bladder. Sneeze induced increases in Pabd were determined as pressure values in cm H2O measured from baseline to peak of the pressure responses according to our previous reports.10,13
2). Tilt LPP.
In 8 normal and 6 CI rats urethral function was measured using the vertical tilt/intravesical pressure clamp method.14 Intravesical pressure was increased in 1 to 2 cm H2O increments via the suprapubic tube by raising a reservoir with saline solution containing Evans blue (100 μg/ml, Sigma®). The pressure at which visible leakage from the urethral meatus occurred was defined as LPP. Mean values of 3 or 4 estimates were analyzed. After control LPPs were obtained duloxetine (1 mg/kg) was injected intravenously and intravesical pressure was increased again to evaluate the effect of the drug on LPP.
3). Continuous Cystometry in Normal and CI Rats.
In 6 normal and 6 CI rats physiological saline at room temperature was infused in the bladder at a rate of 0.05 ml per minute via the intravesical catheter. Bladder activity was monitored with the rat under urethane anesthesia. Cystometry was continued for at least 60 minutes, and ICI, PT (bladder pressure immediately before micturition), BP and VC (maximum amplitude of voiding pressure) were measured during the final 30 minutes as controls. Thereafter, duloxetine (1 mg/kg) was injected intravenously and bladder activity was recorded to compare with predrug control values.
Surgical Procedures for Experiments
With the rat under isoflurane anesthesia a polyethylene catheter (PE-10, Clay Adams, Parsippany, New Jersey) was inserted in a jugular vein for drug injection. The bladder was exposed through an abdominal incision. The ureters were cut bilaterally and the distal ends were ligated. The visceral branches of the pelvic nerves were transected bilaterally near the internal iliac vessels in experiments 1 and 2 to exclude the effects of reflex bladder contractions on the urethral continence reflex. A PE-90 catheter (Clay Adams) connected to a pressure transducer was then inserted in the bladder through the dome for recording intravesical pressure to detect tilt LPPs in experiment 2 and bladder activity in experiment 3. A handmade small balloon catheter with a 1 cm diameter balloon connected to a pressure transducer was inserted through the rectum into the abdominal cavity to record Pabd during sneezing. The abdomen was then closed with sutures.
In experiment 1 after surgery isoflurane anesthesia was turned off and replaced with urethane anesthesia (Sigma) at an initial dose of 0.72 gm/kg intraperitoneally. This was followed by additional doses of urethane (0.1 gm/kg per injection) as required before starting sneeze reflex testing to obtain the sufficient level of anesthesia. This was confirmed by negative reflex responses to toe pinch while preserving the sneeze reflex. The final dose of urethane ranged from 1.0 to 1.2 gm/kg in 18 animals. In experiments 2 and 3 rats were anesthetized by subcutaneous injection of urethane (1.2 gm/kg). Rats were placed supine for the experiments.
Drugs
Duloxetine was dissolved in distilled water and administered at 1 mg/kg in a volume of 0.1 ml/100 gm body weight based on the results of a previous study.10,11
Statistical Analysis
Data are expressed as the mean ± SE. Excessively large sneezes that induced increases in Pabd greater than 2 SD above average and small responses that induced increases in Pabd less than 3 cm H2O were excluded from data analyses. Values of A-URS and UBP as well as increases in Pabd during sneezing were averaged in each rat. The mean ± SE in a group of animals was then calculated from the average value of each rat. The paired t-test was used to compare these data before and after duloxetine and unpaired t-test data were used between normal and CI rats with p <0.05 considered significant.
RESULTS
1). Mid Urethral Pressure Responses and Baseline Pressure
Figure 1 shows representative traces of urethral pressure responses measured by a microtransducer tipped catheter. UBP was significantly lower (43%) in CI rats than in normal rats (p <0.01, see table). However, A-URS did not differ in normal and CI rats. In normal rats duloxetine (1 mg/kg intravenously) significantly increased A-URS (31%, p <0.05) but it did not affect A-URS in CI rats (see table and fig. 1). Duloxetine treatment also significantly increased UBP (21%) in normal rats (p <0.05) but it did not affect UBP in CI rats (see table and fig. 1). The average value of sneeze induced increases in Pabd measured by intra-abdominal catheters was not significantly different in normal and CI rats, and was not changed by duloxetine in either group (see table).
Figure 1.
Representative traces of urethral pressure (a) and Pabd (b) changes before and after duloxetine 1 mg/kg intravenously (i.v.) in normal (A) and CI (B) rats. Urethral pressure responses were measured by microtransducer tipped catheter inserted to mid urethra from urethral orifice. Pabd was measured by balloon catheter inserted in abdominal cavity. Sneeze induced increases in urethral pressure and Pabd were simultaneously recorded. Duloxetine enhanced A-URS amplitude and increased UBP in normal rats but did not affect either parameter in CI rats.
Effects of duloxetine on sneeze induced pressure changes and bladder activity in normal and CI rats
| Mean ± SE Normal | Mean ± SE Cl | |||
|---|---|---|---|---|
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | |
| Sneeze induced pressure changes: | ||||
| No. rats | 12 | 6 | ||
| A-URS (cm H2O) | 36.9 ± 4.3 | 48.3 ± 4.8* | 39.5 ± 8.6 | 36.8 ± 6.2 |
| UBP (cm H2O) | 27.0 ± 2.4† | 32.7 ± 3.8* | 15.5 ± 1.6 | 15.2 ± 1.9 |
| Pabd increase (cm H2O) | 8.6 ± 0.5 | 8.1 ± 0.6 | 8.2 ± 0.6 | 8.1 ± 0.8 |
| Bladder activity: | ||||
| No. rats | 6 | 6 | ||
| ICI (mins) | 6.2 ± 0.7‡ | 5.5 ± 0.7 | 3.9 ± 0.5 | 8.8 ± 1.2§ |
| VC (cm H2O) | 38.5 ± 2.8 | 41.1 ± 3.2 | 42.4 ± 2.7 | 45.0 ± 4.4 |
| BP (cm H2O) | 5.7 ± 0.5 | 6.0 ± 0.4 | 6.6 ± 0.6 | 5.9 ± 0.3 |
| PT (cm H2O) | 7.4 ± 0.7 | 7.3 ± 0.4 | 9.7 ± 1.2 | 8.7 ± 0.4 |
p <0.05 vs normal pretreatment.
p <0.01 vs CI pretreatment.
p <0.05 vs CI pretreatment
p <0.01 vs CI pretreatment.
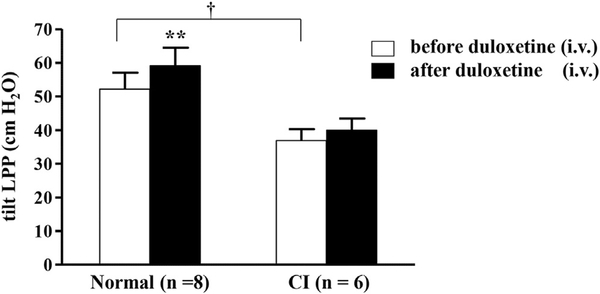
2). Tilt LPP
During passive intravesical pressure elevation fluid leakage from the urethral meatus was observed at a mean LPP of 36.9 ± 3.4 cm H2O in CI rats. This was significantly lower (29%, p <0.05) than in normal rats (52.2 ± 4.9 cm H2O) (fig. 2). Duloxetine (1 mg/kg intravenously) increased LPP in normal rats up to a mean of 59.1 ± 5.4 cm H2O but not in CI rats (40.0 ± 3.4 cm H2O, p <0.01).
Figure 2.
Tilt LPP values were significantly lower in CI than in normal rats (mean 36.9 ± 3.4 vs 52.2 ± 4.9 cm H2O, p <0.05). Duloxetine 1 mg/kg intravenously (i.v.) increased LPP in normal rats up to mean of 59.1 ± 5.4 cm H2O but not in CI rats, in which it was 40.0 ± 3.4 cm H2O. Asterisks indicate p <0.01 vs pretreatment in normal rats. Dagger indicates p <0.05 between normal and CI rats before duloxetine.
3). Bladder Activity
In normal rats ICI, VC, BP and PT became stable 1 hour after the start of continuous cystometry (see table and fig. 3, A). In CI rats ICI was significantly shorter (37%, p <0.05) than in normal rats (see table and fig. 3, B). In CI rats ICI was significantly prolonged (226%, p <0.01) after duloxetine, although VC, BP and PT were unchanged. In addition, duloxetine did not affect any cystometric parameter in normal rats (see table).
Figure 3.
Continuous cystometry in normal (A) and CI (B) rats under urethane anesthesia. Duloxetine 1 mg/kg intravenously (i.v.) did not affect intervals between bladder contractions or VC in normal rats. However, in CI rats intervals between bladder contractions were shorter than in normal rats but prolonged without affecting VC after duloxetine treatment.
DISCUSSION
Sneezing can induce active urethral closure, which is elicited by reflex contractions of the external urethral sphincter and pelvic floor muscles, while UBP reflects the activity of urethral smooth muscle innervated by sympathetic nerves carried through the hypogastric nerve in rats.9,13 This sneeze induced continence reflex is considered to be different from the bladder-to-urethral reflex, which is activated by afferent firing in the pelvic nerve in response to an increase in intravesical pressure.9,10,13 In the current study A-URS did not differ between normal and CI rats but UBP was 43% lower in CI rats than in normal rats. Duloxetine increased A-URS and UBP in normal rats but did not affect either in CI rats. These results suggest that in CI rats the sneeze induced urethral continence reflex is preserved but not enhanced by duloxetine. Thus, spinal noradrenergic and/or serotonergic systems controlling the pudendal nerve induced urethral continence reflex and sympathetic nerve dependent UBP might be impaired in CI rats.
Because microtransducer tipped catheters can only measure the local force per unit area exerted by the tissue on the inner surface on the transducer tip, recorded values do not necessarily reflect true urethral pressure. Thus, to assess overall urethral resistance we also measured changes in LPP, defined as the minimal intravesical pressure that induces fluid leakage from the urethral orifice. In accordance with the microtransducer tipped catheter experiments duloxetine increased LPP in normal rats but not significantly in CI rats. In CI rats UBP and tilt LPP were lower than in normal rats, suggesting that disruption of the serotonergic/ noradrenergic inputs from brain to lumbosacral spinal cord might be a pathophysiological basis for inducing SUI after CI. To our knowledge this is a novel finding showing the potential mechanism inducing SUI after CI.
In our previous study duloxetine enhanced A-URS and UBP via activation of the spinal noradrenergic and serotonergic system as well as the peripheral noradrenergic system in normal rats.10 However, in the current series duloxetine failed to enhance active urethral closure mechanisms during sneezing in CI rats. Therefore, normal brain activity might be needed to enhance active urethral closure mechanisms during sneezing.
Robinson and Bloom postulated that ischemic lesions may interrupt the biogenic amine containing axons ascending from the brainstem to the cerebral cortex, leading to decreased availability of biogenic amines in limbic structures of the frontal and temporal lobes as well as in the basal ganglia.15 The monoamine theory postulates that depression is associated with low levels of monoamines, particularly norepinephrine, serotonin and dopamine.16 In addition, descending pathways projecting through the dorsolateral spinal column are instrumental in the regulation of pain.17 Thus, ascending and descending pathways through the raphe nuclei and the locus ceruleus, which are the major sources of spinal serotonergic noradrenergic pathways, respectively,18 in the brainstem may be disrupted by the stroke lesion, which may result in SUI. To date there have been only a few reports of brain activity underlying lower urinary tract symptoms in patients with CI compared with other neurological diseases associated with bladder dysfunction such as Parkinson disease.19,20 Brain imaging, including functional magnetic resonance imaging, will help clarify the mechanisms inducing disorganization of the brain network affecting lower urinary tract function after CI.
In the current study ICI was reduced in CI rats compared with normal rats during continuous cystometry. Duloxetine prolonged ICI without affecting VC in CI rats but it did not affect ICI in normal rats. Thor and Katofiasc reported that duloxetine produced dose dependent increases in bladder capacity and increased periurethral striated muscle electromyographic activity under the condition of acetic acid infusion into the bladder.21 However, it did not significantly increase either parameter under the nonirritated conditions in α-chloralose anesthetized cats. These results are similar to ours showing that duloxetine works to improve bladder function only in CI rats. Also, it has been reported that duloxetine was effective over placebo for the treatment of symptoms of overactive bladder in women.8 Thus, it is assumed that the effects of duloxetine on bladder capacity are mediated by increases in the reduced levels of extracellular norepinephrine/serotonin under pathological conditions, including CI. In addition, because changes in ICI and VC seem to be related to the afferent and efferent limbs of the micturition reflex pathway, respectively,22 duloxetine might predominantly inhibit the afferent limb of the micturition reflex. Overall our results show that CI induces not only SUI but also bladder overactivity.
Tibaek et al reported that 42% of CI patients have urge incontinence, 8% have SUI and 50% have mixed incontinence.6 They also reported that pelvic floor muscle training had a significant effect in women with urinary incontinence after CI regardless of incontinence type. In the current series ICI in bladder activity and UBP in urethral activity were shortened and decreased, respectively, in CI rats compared with normal rats. Also, duloxetine failed to activate the spinal noradrenergic and/or serotonergic systems controlling the urethral continence reflex but it prolonged ICI during cystometry in CI rats. Thus, it can be speculated that CI induces not only bladder overactivity but also SUI. This may account for mixed incontinence in CI patients and the fact that the clinical efficacy of norepinephrine/ serotonin reuptake inhibitors for SUI could be decreased in SUI patients with CI while they are useful for overactive bladder. However, one should be cautious when applying findings in rat studies to humans because of the species difference. Further clinical studies are needed to clarify whether the findings of altered responses of urethral continence reflexes to duloxetine after CI in this rat study are similarly observed in humans.
In addition, there are some limitations to this study. 1) We used rats 3 days after CI because CI induced bladder overactivity is seen immediately after CI and lasts up to 4 months.12 It peaked 3 days after CI in our preliminary study (data not shown).However, it is possible that the effects of CI on urethral function are different on earlier or later days after CI. 2) All experiments were performed using urethane anesthesia, which might have affected the results of this series, including the altered responses of urethral continence reflexes to duloxetine after CI. 3) In the current study duloxetine failed to enhance active urethral closure mechanisms during sneezing while it ameliorated bladder overactivity in CI rats. One possible speculation to explain this discrepancy is that duloxetine effects on urethral continence reflexes may involve action in the brain while effects on bladder function may be more dependent on the spinal cord site, which would be less affected by CI. However, the site of action of duloxetine was not examined as we only tested the systemic application of duloxetine in this study. Thus, it is necessary to perform future studies to investigate the time course of alterations in urethral continence mechanisms after CI, the effects of anesthesia and the site as well as the dose dependence of duloxetine action using different routes, such as intrathecal or intracerebroventricular, with multiple doses of duloxetine.
CONCLUSIONS
CI rats showed SUI shown by reduced UBP and LPP, and bladder overactivity evidenced by reduced ICI, suggesting that brain dysfunction similar to that induced by CI might be a pathophysiological mechanism of inducing mixed urinary incontinence. Duloxetine intravenously enhanced A-URS and UBP by 31% and 21%, respectively, in normal rats but it did not affect either parameter in CI rats. It also increased ICI, which was shortened in CI rats, suggesting that disorganization of the brain network affecting lower urinary tract function after CI might influence the effect of duloxetine.
Acknowledgments
Supported by grants from the Institute for Food and Health Science, Yazuya and Mitsui Life Social Welfare Foundation, Japan, and National Institutes of Health Grant NIH DK067226.
Abbreviations and Acronyms
- A-URS
urethral pressure response amplitude during sneezing
- BP
baseline pressure
- CI
cerebral infarction
- ICI
intercontraction interval
- LPP
leak point pressure
- Pabd
abdominal pressure
- PT
pressure threshold
- SUI
stress urinary incontinence
- UBP
urethral baseline pressure
- VC
voiding bladder contraction pressure
Footnotes
Study received approval from the University of Ryukyus and University of Pittsburgh institutional animal care and use committees.
REFERENCES
- 1.Hampel C, Wienhold D, Benken N et al. : Definition of overactive bladder and epidemiology of urinary incontinence. Urology 1997; 50: 4. [DOI] [PubMed] [Google Scholar]
- 2.Brittain KR, Peet SM and Castleden CM: Strokeand incontinence. Stroke 1998; 29: 524. [DOI] [PubMed] [Google Scholar]
- 3.Kolominsky-Rabas PL, Hilz MJ, Neundoerfer Bet al: Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn 2003; 22: 322. [DOI] [PubMed] [Google Scholar]
- 4.Grimby A, Milsom I, Molander U et al. : The influence of urinary incontinence on the quality of life of elderly women. Age Ageing 1993; 22: 82. [DOI] [PubMed] [Google Scholar]
- 5.Fowler CJ, Griffiths D and de Groat WC: Theneural control of micturition. Nat Rev Neurosci 2008; 9: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tibaek S, Gard G and Jensen R: Pelvic floormuscle training is effective in women with urinary incontinence after stroke: a randomised, controlled and blinded study. Neurourol Urodyn 2005; 24: 348. [DOI] [PubMed] [Google Scholar]
- 7.Ghoniem GM, Van Leeuwen JS, Elser DM et al. : A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. Duloxetine/Pelvic Floor Muscle Training Clinical Trial Group. J Urol 2005; 173: 1647. [DOI] [PubMed] [Google Scholar]
- 8.Steers WD, Herschorn S, Kreder KJ et al. : Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int 2007; 100: 337. [DOI] [PubMed] [Google Scholar]
- 9.Kamo I, Torimoto K, Chancellor MB et al. : Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol 2003; 285: R356. [DOI] [PubMed] [Google Scholar]
- 10.Miyazato M, Kaiho Y, Kamo I et al. : Effect ofduloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 2008; 295: F264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitta T, Miyazato M, Chancellor MB et al. : Alpha2-adrenoceptor blockade potentiates the effect of duloxetine on sneeze induced urethral continence reflex in rats. J Urol 2010; 184: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama O, Komatsu K, Ishiura Y et al. : Changein bladder contractility associated with bladder overactivity in rats with cerebral infarction. J Urol 1998; 159: 577. [DOI] [PubMed] [Google Scholar]
- 13.Kaiho Y, Kamo I, Chancellor MB et al. : Role ofnoradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 2007; 292: F639. [DOI] [PubMed] [Google Scholar]
- 14.Conway DA, Kamo I, Yoshimura N et al. : Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2005; 16: 359. [DOI] [PubMed] [Google Scholar]
- 15.Robinson RG and Bloom FE: Pharmacological treatment following experimental cerebral infarction: implications for understanding psychological symptoms of human stroke. Biol Psychiatry 1977; 12: 669. [PubMed] [Google Scholar]
- 16.Krishnan V and Nestler EJ: The molecular neurobiology of depression. Nature 2008; 455: 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loubinoux I, Kronenberg G, Endres M et al. : Poststroke depression: mechanisms, translation and therapy. J Cell Mol Med 2012; 16: 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holstege JC and Kuypers HG: Brainstem projections to spinal motoneurons: an update. Neuroscience 1987; 23: 809. [DOI] [PubMed] [Google Scholar]
- 19.Kitta T, Kakizaki H, Furuno T et al. : Brain activation during detrusor overactivity in patients with Parkinson’s disease: a positron emission tomography study. J Urol 2006; 175: 99. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, Wu Z, Fu Y et al. : Reorganization offunctional brain networks during the recovery of stroke: a functional MRI study. Conf Proc IEEE Eng Med Biol Soc 2012; 2012: 4132. [DOI] [PubMed] [Google Scholar]
- 21.Thor KB and Katofiasc MA: Effects of duloxetine,a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloraloseanesthetized female cat. J Pharmacol Exp Ther 1995; 274: 1014. [PubMed] [Google Scholar]
- 22.de Groat WC and Yoshimura N: Mechanismsunderlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res 2006; 152: 59. [DOI] [PubMed] [Google Scholar]