Abstract
Background
Sex hormones in serum have been hypothesized to influence the risk of prostate cancer. We performed a collaborative analysis of the existing worldwide epidemiologic data to examine these associations in a uniform manner and to provide more precise estimates of risks.
Methods
Data on serum concentrations of sex hormones from 18 prospective studies that included 3886 men with incident prostate cancer and 6438 control subjects were pooled by the Endogenous Hormones and Prostate Cancer Collaborative Group. Relative risks (RRs) of prostate cancer by fifths of serum hormone concentration were estimated by use of conditional logistic regression with stratification by study, age at recruitment, and year of recruitment. All statistical tests were two-sided.
Results
No associations were found between the risk of prostate cancer and serum concentrations of testosterone, calculated free testosterone, dihydrotestosterone, dehydroepiandrosterone sulfate, androstenedione, androstanediol glucuronide, estradiol, or calculated free estradiol. The serum concentration of sex hormone–binding globulin was modestly inversely associated with prostate cancer risk (RR in the highest vs lowest fifth = 0.86, 95% confidence interval = 0.75 to 0.98; Ptrend = .01). There was no statistical evidence of heterogeneity among studies, and adjustment for potential confounders made little difference to the risk estimates.
Conclusions
In this collaborative analysis of the worldwide data on endogenous hormones and prostate cancer risk, serum concentrations of sex hormones were not associated with the risk of prostate cancer.
Prostate cancer is the second most common cancer in men worldwide (1), and the only established risk factors for prostate cancer are age, race, and a family history of the disease (2). Androgens are required for the normal growth and development of the prostate gland, and high levels of androgens have long been hypothesized to be possible risk factors for prostate cancer (3,4). Evidence that the development of prostate cancer has a hormonal component comes from a wide range of sources, including the historical observation that most prostate tumors respond to androgen deprivation therapy until they establish an androgen-independent growth mechanism. More recently, results from the Prostate Cancer Prevention Trial (5,6) indicated that inhibition of the conversion of testosterone to the more potent dihydrotestosterone (DHT) by finasteride, a 5α-reductase inhibitor, reduced the occurrence of prostate cancer by approximately 25% during a 7-year follow-up, although the risk of high-grade tumors was higher in the treated group than in the untreated group.
To date, 18 prospective studies have investigated whether differences in circulating levels of sex hormones are related to the risk of prostate cancer (7–27). The results have been somewhat inconsistent, with a number of studies finding small associations for some hormones. However, many of the studies individually had limited power.
The Endogenous Hormones and Prostate Cancer Collaborative Group was established to conduct pooled analyses of the original data from studies on the relationships among endogenous sex hormones, insulin-like growth factors (IGFs), and prostate cancer risk. The specific aims of the group were 1) to use uniform statistical methods to provide precise estimates of the associations of endogenous hormones and IGFs with prostate cancer risk; 2) to investigate whether the association of risk differs with time between blood collection and diagnosis; 3) to examine the relative risks (RRs) in different tumor subgroups; 4) to identify whether there are interactions among hormone concentrations, IGFs, and risk of prostate cancer; and 5) to examine the cross-sectional relationships between lifestyle factors and concentrations of sex hormones and IGFs. In this analysis, we addressed the first four of these objectives in relation to endogenous sex hormones; analyses of prostate cancer risk in relation to IGFs will be reported elsewhere.
Participants and Methods
Identification of Prospective Studies of Endogenous Sex Hormones and Prostate Cancer Risk
Principal investigators were invited to join this collaborative group if they had published studies on prostate cancer risk and endogenous sex hormone concentrations that had been determined from blood samples collected before diagnosis. Studies were identified by literature searches of computerized bibliographic systems, including PubMed, Web of Science, Cochrane Library, and CancerLit, and through discussions with colleagues. A total of 18 prospective studies (7–27) were identified. Investigators from one study with two relevant publications (12,15) were able to supply data from only one of these publications (12). Investigators from a study (28) of IGF concentration and prostate cancer risk also provided previously unpublished data on endogenous sex hormone concentrations. Investigators from one study (19) with 70 case patients with prostate cancer declined to participate in the collaboration. In summary, 18 prospective studies that included 3886 men with incident prostate cancer and 6438 control subjects contributed data to the collaborative group. This data represented more than 95% of the worldwide data.
Study Designs
Fourteen of the 18 studies used a matched case–control design nested within a prospective cohort collection (7,9,10,12–16, 18,20,22,23,25,27) or a randomized trial (17,21). Blood samples were collected from apparently healthy men who were then followed to identify those who developed prostate cancer. The laboratory analyses were performed on blood samples from the case patients with incident prostate cancer and from the control subjects who were matched to these case patients on criteria such as age and date of blood collection (for a full description of the matching criteria used in these studies, see Supplementary Table 1, available online). One study (28) was nested within a randomized trial that recruited healthy, apparently disease-free men, and those with an increased prostate-specific antigen (PSA) level at recruitment underwent a prostate biopsy examination, so that all cancers were detected shortly after recruitment. The same blood sample that was used for PSA testing was also used to determine serum hormone concentrations. Three studies (8,11,24,26) were carried out as full cohort or case–cohort analyses, in which hormone assays were performed on stored serum from some or all cohort participants. For this collaborative analysis, matched case–control sets were created for these three studies by randomly matching, when possible, up to three control subjects to each case patient by age at recruitment, date of recruitment, time of blood collection, and race. Converting cohort or case–cohort studies to nested case–control studies results in some loss of power for each individual study. However, because this loss happened for only three of the 18 studies, there was little impact on the overall power of the collaborative analysis.
Collection of Data
Principal investigators were asked to provide data on the following factors for each participant in their original study: case–control status and matched-set identifier (if a matched design was used); date of birth and date of recruitment or age at recruitment; date (or age) and time of blood collection with details of any overnight fasting or concurrent drug use; date of (or age at) diagnosis; stage and grade of the tumor; method of case-patient ascertainment (self-report, record linkage, or unknown); height; weight; waist and hip circumferences; history of prostate disease; family history of prostate cancer; smoking habits; alcohol intake; educational status; marital status; race; serum concentrations of androstenedione, androstanediol glucuronide, dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), total testosterone, DHT, total estradiol, estrone, sex hormone–binding globulin (SHBG), IGF-I, IGF-II, IGF-binding proteins 1, 2, and 3, and PSA at blood collection. Not all of the hormone and SHBG measurements were performed by each study, and investigators were asked to provide whatever measurements were available (for details of the assay methods used by individual studies, see Supplementary Table 2, available online).
Free testosterone and free estradiol were calculated from the reported measured serum concentrations of testosterone or estradiol and SHBG by use of the law of mass action (29) and by assuming a constant serum albumin concentration of 43g/L. Such calculated results have been found to correlate highly with the free fractions of these hormones measured by equilibrium dialysis (30,31).
For most studies, data supplied to the collaborative group were identical to those analyzed and published by the original research group. However, in the Northern Sweden Health and Disease Cohort (20) and the Prostate Testing for Cancer and Treatment (ProtecT) study (28), data on additional hormones were available that were not included in their published reports. The Health Professionals Follow-up Study (25) provided data on additional case–control sets that had not yet been reported. Finally, the Rancho Bernardo Study (8) had additional unpublished follow-up data that were contributed to this collaborative analysis.
Data Processing
Some studies (7,9–11,14,16,22,24,25) had published more than one investigation on the association between endogenous sex hormones and prostate cancer risk, sometimes with different matched case–control sets, different laboratory measurements, and different amounts of follow-up. For each study, a dataset was created in which each participant appeared only once. Thus, we treated any participant who appeared as both a control subject and a case patient in the original study datasets as a case patient in this analysis.
For this analysis, matched-set identifiers were removed and a series of strata (equivalent to matched sets) were generated in which participants in each study were grouped according to age at recruitment (2-year age bands) and date of recruitment (by year) because these matching criteria were common to most studies. The number of strata used in the collaborative analysis was slightly less than the number of matched sets used in the original analyses.
Each study provided data on prostate cancer stage and grade, if available. To provide a common definition across studies, if tumor stage information was available, we defined a cancer as being advanced if it was tumor–node–metastasis stage T3+/N1+/M1+ or the equivalent (a tumor extending beyond the prostate capsule with or without lymph node involvement and/or distant metastases) and otherwise we defined it as being localized. If tumor grade information was available, we defined a cancer as high grade if it had a Gleason sum of at least 7 or the equivalent (ie, moderately to poorly differentiated) and otherwise we defined it as low grade.
Statistical Analysis
Partial correlation coefficients were calculated between log-transformed serum hormone concentrations (to normalize the distribution) among control subjects, adjusted for age at blood collection (<50, 50–59, 60–69, or ≥ 70 years) and study. For each hormone, men were categorized into fifths of its serum concentrations, with cut points defined by the study-specific fifths of the distribution within control subjects. If a study measured serum hormone concentrations at more than one time point (and possibly with different assay methods), cut points were determined separately for each time point. To assess whether there was evidence of an association between sex hormone concentration and risk of prostate cancer at the upper or lower tails of the distribution, men were categorized into deciles in a similar way.
The main method of analysis was conditional logistic regression that was stratified by study, age at recruitment (2-year age bands), and date of recruitment (single year). To provide a summary measure of risk, a linear trend was calculated by replacing the categorical variable representing the fifths of the serum hormone concentration with a continuous variable that was scored as 0, 0.25, 0.5, 0.75, and 1. A unit change in this continuous trend variable is equivalent to the relative risk comparing the highest with the lowest fifth.
For each hormone, heterogeneity in linear trends among studies was assessed with a chi-square test. The chi-square statistic was calculated as the difference between the sum of the model chi-square values for each individual study and the model chi-square value from the all-studies analysis. This method is equivalent to an overall test of whether the study-specific relative risks are statistically significantly different from the overall relative risk. Heterogeneity among studies was also quantified by calculating the H and I2 statistics (32). The H statistic is a measure of the amount of heterogeneity between studies, with H = 1.0 indicating homogeneity and with heterogeneity increasing as H becomes greater than 1.0. The I2 statistic can be interpreted as the proportion of variation in study-specific estimates due to heterogeneity. Assay methods that include a purification or extraction step (ie, assays for testosterone, DHT, androstanediol glucuronide, and estradiol) may be more accurate than those that do not. Therefore, a chi-square test for heterogeneity in risk estimates between these methods was calculated.
The possible influences of participant characteristics on the associations between the concentration of sex hormones and prostate cancer risk were examined by adjusting the relevant conditional logistic regression models for body mass index (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, or ≥ 30 kg/m2 or not known), marital status (married or cohabiting, not married or cohabiting, or not known), educational status (did not attend college or university, attended college or university, or not known), smoking (never, previous, current, or not known), and alcohol consumption (<10 or ≥ 10 g/day or not known).
To test whether the linear-trend relative risk estimates for each sex hormone varied according to case patient characteristics, a series of subsets for each characteristic were estimated: stage at diagnosis (localized or advanced), grade at diagnosis (low or high), year of diagnosis (pre-1990, 1990–1994, or 1995 onward; these year cutoffs were chosen in an attempt to reflect differences in the use of the PSA test for cancer detection), age at diagnosis (<60, 60–69, or ≥ 70 years), and time between blood collection and diagnosis (<3, 3–6, or ≥ 7 years). Case patients were excluded from the analyses of stage and grade at diagnosis if the relevant information on stage or grade was not available. For each of these case patient characteristics, the heterogeneity test was calculated as the difference between the sum of the model chi-square values from each of the subset models and the overall model chi-square value (by use of data from the same case patients that were used in the subsets). This method is equivalent to performing a contrast test of whether, for each of the case patient characteristics, the estimated regression coefficients from the subsets were statistically significantly different from each other. To assess whether the relative risk estimate of the linear trend for each sex hormone varied according to PSA level at recruitment (<2 ng/mL or ≥ 2 ng/mL; grouping participants according to their probability of having a latent prostate cancer at recruitment), an interaction term was entered into the conditional logistic regression model for each hormone and the statistical significance of the interaction term was tested with a likelihood ratio test. The same method was used to examine whether there was an interaction among linear trends for serum concentrations of two (or more) sex hormones or among linear trends for serum concentrations of sex hormones and IGF-I concentration and prostate cancer risk.
Statistical significance was set at the 5% level. All statistical tests were two-sided. All statistical analyses were performed with Stata version 9.0 (Stata Corp., College Station, TX).
Results
Data on serum testosterone concentration were available for 3886 case patients with prostate cancer and 6438 control subjects from 18 prospective studies. A similar amount of data was available for SHBG, enabling the concentration of free testosterone to be calculated for 3550 case patients and 5815 control subjects. Data on estradiol were available for 2186 case patients and 3039 control subjects. Data for androstanediol glucuronide were available for 2453 case patients and 3035 control subjects. Individual-level data for DHT, DHEA-S, and androstenedione were available from at least 1000 case patients.
Among the control subjects, the mean age at recruitment ranged from 46 to 72 years and the date of study recruitment ranged from 1961 through 2001 (Table 1). Mean body mass index ranged from 22.4 to 28.2 kg/m2. Between 4% and 37% of control subjects smoked [except in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (17) and the Carotene and Retinol Efficacy Trial (21), which recruited either all smokers or an enriched sample of smokers, respectively], and the mean consumption of alcohol ranged from 11.5 through 21.7 g/day. There was considerable variation in the time between blood collection and diagnosis: in the ProtecT study (28), all case patients were diagnosed within 3 years of recruitment, but in studies from the Janus Serum Bank in Norway (22), more than 90% of case patients were diagnosed 7 or more years after recruitment (Table 2). Most case patients were diagnosed when older than 60 years, and many studies consisted of case patients who were older than 70 years at diagnosis. Among case patients with available data, 60%–70% had localized disease and a similar proportion had low-grade disease.
Table 1.
Participant characteristics by study and case–control status*
| Study (reference) | Case–control status | No. of participants | Mean age at recruitment, y | Date of blood collection | Mean BMI, kg/m2 | Married or cohabiting, % | With higher education, % | Current smoker, % | Mean ethanol consumption, g/day |
|---|---|---|---|---|---|---|---|---|---|
| ATBC (17) | Case | 116 | 60.5 | 1985–1988 | 26.5 | 77.6 | 9.5 | 100.0 | 17.1 |
| Control | 231 | 60.5 | 1985–1988 | 25.9 | 82.3 | 6.9 | 100.0 | 16.6 | |
| BLSA (11,24) | Case | 176 | 58.9 | 1961–1995 | 25.3 | 92.6 | 92.6 | 6.9 | N/A |
| Control | 220 | 60.5 | 1963–1994 | 26.1 | 88.2 | 93.2 | 14.5 | N/A | |
| CARET (21) | Case | 300 | 63.2 | 1986–1996 | 28.2 | 82.9 | 55.2 | 47.3 | 17.5 |
| Control | 300 | 62.8 | 1986–1996 | 27.7 | 85.9 | 55.3 | 50.0 | 16.1 | |
| CLUE (9,10) | Case | 124 | 62.7 | 1974 | N/A | 92.7 | 21.0 | 23.6 | N/A |
| Control | 181 | 62.3 | 1974 | N/A | 85.0 | 24.3 | 20.4 | N/A | |
| EPIC (27) | Case | 643 | 61.0 | 1992–1999 | 26.7 | 87.7 | 25.6 | 23.0 | 22.1 |
| Control | 636 | 60.9 | 1992–1999 | 27.0 | 89.1 | 23.1 | 27.9 | 21.7 | |
| FMC (18) | Case | 166 | 58.1 | 1968–1972 | 25.8 | 89.7 | N/A | 28.8 | N/A |
| Control | 300 | 57.6 | 1968–1972 | 26.0 | 84.7 | N/A | 33.9 | N/A | |
| HHS (NBSBWG) (22) | Case | 84 | 51.3 | 1980–1982 | 26.5 | N/A | N/A | N/A | N/A |
| Control | 295 | 51.0 | 1980–1982 | 26.4 | N/A | N/A | N/A | N/A | |
| HPFS (25) | Case | 682 | 65.3 | 1993–1995 | 25.9 | 93.4 | 100.0 | 4.8 | 11.9 |
| Control | 670 | 65.1 | 1993–1995 | 26.1 | 92.8 | 100.0 | 4.0 | 11.5 | |
| JACC (23) | Case | 40 | 68.7 | 1988–1991 | 22.4 | 100.0 | 12.9 | 54.5 | 17.8 |
| Control | 101 | 67.9 | 1988–1992 | 22.4 | 92.3 | 16.9 | 36.8 | 14.3 | |
| Janus (16) | Case | 60 | 58.3 | 1973–1989 | N/A | N/A | N/A | N/A | N/A |
| Control | 180 | 58.3 | 1973–1989 | N/A | N/A | N/A | N/A | N/A | |
| Janus (NBSBWG) (22) | Case | 530 | 45.9 | 1972–1990 | N/A | N/A | N/A | N/A | N/A |
| Control | 1538 | 46.3 | 1972–1990 | N/A | N/A | N/A | N/A | N/A | |
| JHCS (7,14) | Case | 188 | 62.1 | 1971–1975 | 24.1 | 92.5 | 19.1 | 27.8 | 12.4 |
| Control | 212 | 62.2 | 1971–1975 | 23.3 | 93.4 | 12.3 | 33.5 | 20.8 | |
| KPMCP (12) | Case | 45 | 71.5 | 1964–1969 | 25.7 | 86.8 | 25.0 | 20.0 | 18.7 |
| Control | 218 | 71.9 | 1964–1970 | 25.8 | 82.8 | 17.6 | 17.8 | 14.9 | |
| MCCS (26) | Case | 524 | 61.8 | 1990–1994 | 27.2 | N/A | 35.1 | 9.7 | 19.7 |
| Control | 932 | 59.1 | 1990–1994 | 27.2 | N/A | 36.7 | 12.0 | 20.9 | |
| NSHDC (22) | Case | 280 | 58.0 | 1987–2000 | 26.1 | N/A | N/A | 18.9 | N/A |
| Control | 555 | 58.0 | 1987–2000 | 26.6 | N/A | N/A | 20.4 | N/A | |
| PHS (13) | Case | 546 | 59.8 | 1982–1983 | 24.8 | N/A | N/A | 8.1 | N/A |
| Control | 701 | 60.0 | 1982–1984 | 24.7 | N/A | N/A | 8.0 | N/A | |
| ProtecT (28) | Case | 176 | 61.8 | 1999–2001 | 27.3 | 88.3 | N/A | 12.5 | N/A |
| Control | 324 | 61.7 | 1999–2001 | 27.0 | 88.8 | N/A | 10.8 | N/A | |
| RBS (8) | Case | 110 | 70.3 | 1984–1987 | 25.7 | 93.6 | 69.2 | 8.2 | 16.1 |
| Control | 322 | 70.2 | 1984–1986 | 26.1 | 90.1 | 80.5 | 7.1 | 14.6 |
The numbers of case patients and control subjects are the maximum number for whom hormone measurements were available, and numbers varied by hormone. BMI = body mass index; ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = Baltimore Longitudinal Study of Aging; CARET = Carotene and Retinol Efficacy Trial; CLUE = CLUE Study, Washington County, MD; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; NBSBWG = Nordic Biological Specimen Biobank Working Group; HPFS = Health Professionals Follow-up Study; JACC = Japan Collaborative Cohort Study; Janus = Janus Serum Bank; JHCS = Japan–Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; NSHDC = Northern Sweden Health and Disease Cohort; PHS = Physicians’ Health Study; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Rancho Bernardo Study; N/A = data not available for this study.
Table 2.
Characteristics of patients with prostate cancer by study*
| Study (reference) | Time from blood collection to diagnosis | Age at diagnosis | Date of diagnosis | Stage of disease | Grade of disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||
| <3 y | 3–6 y | ≥7 y | <60 y | 60–69 y | ≥70 y | Pre-1990 | 1990–1994 | 1995 onward | Localized | Advanced | Low | High | |
| ATBC (17) | 37.1 | 61.2 | 1.7 | 22.4 | 57.8 | 19.8 | 37.9 | 62.1 | 0.0 | 61.2 | 38.8 | 53.6 | 46.4 |
| BLSA (11,24) | 4.6 | 14.8 | 80.7 | 4.0 | 30.7 | 65.3 | 29.6 | 44.3 | 26.1 | 77.3 | 22.7 | 64.7 | 35.3 |
| CARET (21) | 55.3 | 39.7 | 5.0 | 13.7 | 54.0 | 32.3 | 5.3 | 39.7 | 55.0 | 68.5 | 31.5 | 59.8 | 40.2 |
| CLUE (9,10) | 16.1 | 25.0 | 58.9 | 8.9 | 39.5 | 51.6 | 100.0 | 0.0 | 0.0 | 75.3 | 24.7 | 71.4 | 28.6 |
| EPIC (27) | 42.5 | 52.9 | 4.7 | 19.1 | 63.1 | 17.7 | 0.0 | 0.9 | 99.1 | 68.7 | 31.3 | 67.7 | 32.3 |
| FMC (18) | 6.0 | 16.9 | 77.1 | 10.2 | 33.7 | 56.0 | 87.4 | 12.7 | 0.0 | N/A | N/A | N/A | N/A |
| HHS (NBSBWG) (22) | 2.4 | 15.5 | 82.1 | 32.1 | 67.9 | 0.0 | 21.4 | 54.8 | 23.8 | 62.1 | 37.9 | N/A | N/A |
| HPFS (25) | 45.3 | 54.4 | 0.3 | 13.6 | 38.3 | 48.1 | 0.0 | 11.3 | 88.7 | 82.9 | 17.1 | 60.4 | 39.6 |
| JACC (23) | 17.5 | 52.5 | 30.0 | 0.0 | 35.0 | 65.0 | 5.0 | 50.0 | 45.0 | N/A | N/A | N/A | N/A |
| Janus (16) | 13.3 | 16.7 | 70.0 | 8.3 | 53.3 | 38.3 | 53.3 | 46.7 | 0.0 | N/A | N/A | N/A | N/A |
| Janus (NBSBWG) (22) | 1.1 | 4.2 | 94.7 | 20.9 | 69.8 | 9.2 | 22.3 | 54.7 | 23.0 | 74.9 | 25.1 | 76.0 | 24.0 |
| JHCS (7,14) | 6.4 | 18.6 | 75.0 | 1.6 | 30.3 | 68.1 | 90.4 | 9.6 | 0.0 | 65.4 | 34.6 | N/A | N/A |
| KPMCP (12) | 17.8 | 17.8 | 64.4 | 0.0 | 6.7 | 93.3 | 100.0 | 0.0 | 0.0 | 61.9 | 38.1 | 90.0 | 10.0 |
| MCCS (26) | 29.8 | 41.0 | 29.2 | 11.3 | 55.9 | 32.8 | 0.0 | 17.4 | 82.6 | 91.3 | 8.7 | 62.6 | 37.4 |
| NSHDC (22) | 27.1 | 49.6 | 23.2 | 17.1 | 77.5 | 5.4 | 0.0 | 10.4 | 89.6 | 80.9 | 19.1 | 84.9 | 15.1 |
| PHS (13) | 9.2 | 21.4 | 69.4 | 11.7 | 48.4 | 39.9 | 31.5 | 64.8 | 3.7 | 69.1 | 30.9 | 65.7 | 34.3 |
| ProtecT (28) | 100.0 | 0.0 | 0.0 | 29.6 | 69.9 | 0.6 | 0.0 | 0.0 | 100.0 | 73.9 | 26.1 | 70.5 | 29.5 |
| RBS (8) | 15.5 | 35.5 | 49.1 | 1.8 | 17.3 | 80.9 | 30.9 | 37.3 | 31.8 | N/A | N/A | N/A | N/A |
Data are percentages of case patients among those with a known value of the characteristic. Percentages may not add to 100 due to rounding. Stage and grade of disease were unknown for some case patients, and the percentages displayed are among those with known information. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = Baltimore Longitudinal Study of Aging; CARET = Carotene and Retinol Efficacy Trial; CLUE = CLUE Study, Washington County, Maryland; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; NBSBWG = Nordic Biological Specimen Biobank Working Group; HPFS = Health Professionals Follow-up Study; JACC = Japan Collaborative Cohort Study; Janus = Janus Serum Bank; JHCS = Japan–Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; NSHDC = Northern Sweden Health and Disease Cohort; PHS = Physicians’ Health Study; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Rancho Bernardo Study; N/A = data not available for this study.
We next determined the median serum concentration and its interquartile range for each hormone by study and case–control status (Table 3). Although the absolute serum concentrations of sex hormones varied among studies, the concentrations for each hormone between case patients and control subjects were very similar within most studies. Within each study, the extent of between-subject variation in hormone levels was similar, with the interquartile range representing increased hormone concentrations of between 50% and 100%.
Table 3.
Sex hormone concentrations by study and case–control status*
| Study (reference) |
Case–control status |
Testosterone, nmol/L |
Calculated free testosterone, pmol/L |
DHT, nmol/L | Androstanediol glucuronide, nmol/L |
DHEA-S, nmol/L |
Androstenedione, nmol/L |
Estradiol, pmol/L |
Calculated free estradiol, pmol/L |
SHBG, nmol/L |
|---|---|---|---|---|---|---|---|---|---|---|
| ATBC (17) | Case | 20.8 (15.8–24.3) | 219 (184–272) | 1.85 (1.48–2.17) | 6.5 (3.9–8.8) | 3279 (2340–4952) | 4.78 (3.77–5.87) | 103 (84–125) | 1.06 (0.84–1.39) | 85.5 (65.0–109.5) |
| Control | 21.6 (16.7–24.5) | 219 (186–264) | 1.89 (1.48–2.27) | 5.7 (3.9–8.5) | 3075 (2259–4680) | 4.75 (3.92–5.68) | 103 (81–125) | 1.04 (0.86–1.31) | 85.0 (69.0–107.0) | |
| BLSA (11,24) | Case | 15.3 (12.5–17.9) | 183 (146–234) | N/A | N/A | 1330 (805–1790) | N/A | N/A | N/A | 72.9 (52.7–91.7) |
| Control | 15.2 (12.9–18.7) | 181 (134–233) | N/A | N/A | 1235 (841–1950) | N/A | N/A | N/A | 72.4 (53.0–97.3) | |
| CARET (21) | Case | 14.0 (10.8–17.5) | 308 (254–364) | N/A | 13.7 (9.2–18.8) | 2115 (1345–3407) | 2.72 (2.13–3.47) | 191 (165–215) | 3.27 (2.83–3.67) | 28.5 (20.5–36.9) |
| Control | 14.2 (11.3–17.8) | 326 (260–384) | N/A | 12.3 (8.7–18.1) | 1956 (1253–3091) | 2.63 (2.07–3.36) | 195 (168–219) | 3.36 (2.89–3.86) | 27.4 (20.8–36.2) | |
| CLUE (9,10) | Case | 14.3 (12.3–18.5) | N/A | 1.60 (1.23–2.27) | N/A | 2725 (1505–4025) | N/A | 233 (178–301) | 4.40 (3.46–5.65) | 38.1 (25.5–59.6) |
| Control | 13.7 (10.0–20.3) | N/A | 1.64 (1.23–2.27) | N/A | 2860 (1320–4590) | N/A | 221 (173–296) | 3.29 (2.64–4.28) | 42.7 (31.6–54.1) | |
| EPIC (27) | Case | 16.2 (11.8–21.2) | 284 (211–378) | N/A | 12.7 (8.6–19.2) | N/A | 4.59 (3.62–5.75) | N/A | N/A | 42.2 (31.8–53.9) |
| Control | 16.1 (12.2–20.7) | 277 (213–354) | N/A | 13.1 (9.0–19.3) | N/A | 4.75 (3.75–5.82) | N/A | N/A | 44.3 (33.3–55.2) | |
| FMC (18) | Case | 23.9 (17.9–31.0) | 404 (314–487) | N/A | N/A | N/A | 4.95 (3.80–7.55) | N/A | N/A | 53.0 (39.2–68.0) |
| Control | 23.5 (18.5–29.9) | 376 (306–470) | N/A | N/A | N/A | 4.90 (3.60–7.80) | N/A | N/A | 52.1 (38.5–68.9) | |
| HHS (NBSBWG) (22) | Case | 20.4 (16.6–24.0) | 325 (260–383) | N/A | N/A | N/A | N/A | N/A | N/A | 50.7 (34.9–73.2) |
| Control | 20.2 (16.2–24.5) | 327 (256–386) | N/A | N/A | N/A | N/A | N/A | N/A | 50.6 (36.5–71.3) | |
| HPFS (25) | Case | 16.1 (12.4–20.5) | 192 (141–332) | 1.34 (0.81–1.90) | 10.8 (7.2–16.5) | N/A | N/A | 114 (93–138) | 1.45 (0.87–2.23) | 63.5 (29.3–106.8) |
| Control | 16.0 (12.5–20.2) | 188 (144–316) | 1.34 (0.91–1.81) | 10.6 (7.0–15.7) | N/A | N/A | 115 (93–138) | 1.45 (0.91–2.20) | 63.2 (29.5–104.1) | |
| JACC (23) | Case | 16.4 (13.4–19.5) | 286 (237–356) | N/A | N/A | N/A | N/A | N/A | N/A | 39.3 (30.4–53.9) |
| Control | 15.5 (12.7–19.5) | 273 (226–327) | N/A | N/A | N/A | N/A | N/A | N/A | 41.7 (34.6–58.8) | |
| Janus (16) | Case | 18.2 (14.5–24.2) | N/A | 1.60 (1.19–2.08) | 14.0 (10.6–18.0) | N/A | N/A | N/A | N/A | N/A |
| Control | 18.1 (15.3–24.0) | N/A | 1.58 (1.21–2.04) | 12.5 (9.6–18.3) | N/A | N/A | N/A | N/A | N/A | |
| Janus (NBSBWG) (22) | Case | 22.1 (17.6–27.1) | 381 (298–476) | N/A | N/A | N/A | N/A | N/A | N/A | 46.9 (34.7–63.5) |
| Control | 23.1 (18.1–29.1) | 385 (300–487) | N/A | N/A | N/A | N/A | N/A | N/A | 49.2 (36.5–67.1) | |
| JHCS (7,14) | Case | 17.4 (12.9–23.6) | 346 (281–447) | 1.80 (1.29–2.52) | 10.0 (6.8–14.1) | N/A | 5.17 (4.33–6.25) | 66 (55–77) | 1.00 (0.83–1.17) | 39.7 (31.8–46.2) |
| Control | 17.6 (13.4–23.0) | 397 (329–516) | 1.86 (1.35–2.52) | 9.0 (6.4–12.6) | N/A | 5.10 (4.09–6.22) | 66 (51–84) | 0.97 (0.81–1.29) | 41.1 (30.3–47.8) | |
| KPMCP (12) | Case | N/A | N/A | N/A | N/A | 2844 (2027–4122) | N/A | N/A | N/A | N/A |
| Control | N/A | N/A | N/A | N/A | 2599 (1578–3864) | N/A | N/A | N/A | N/A | |
| MCCS (26) | Case | 15.5 (12.9–18.9) | 295 (251–342) | N/A | 13.4 (9.4–18.9) | 2500 (1600–4000) | 3.63 (2.61–4.51) | 104 (87–126) | 1.62 (1.35–1.94) | 38.5 (30.7–46.6) |
| Control | 15.8 (12.4–19.6) | 296 (248–360) | N/A | 14.3 (9.8–19.8) | 2900 (1800–4300) | 3.60 (2.76–4.58) | 107 (90–126) | 1.68 (1.36–2.01) | 37.7 (30.0–47.6) | |
| NSHDC (22) | Case | 21.6 (17.1–27.5) | 375 (299–472) | N/A | N/A | N/A | N/A | 74 (59–92) | 1.05 (0.81–1.31) | 46.3 (34.7–60.8) |
| Control | 21.7 (16.4–28.3) | 360 (293–462) | N/A | N/A | N/A | N/A | 75 (60–93) | 1.06 (0.83–1.36) | 48.3 (35.4–62.9) | |
| PHS (13) | Case | 16.7 (13.2–20.4) | 429 (334–521) | 1.16 (0.79–1.76) | 14.5 (11.1–19.2) | N/A | N/A | 121 (92–158) | 2.26 (1.66–3.02) | 20.2 (14.3–27.4) |
| Control | 16.2 (12.6–20.3) | 401 (325–504) | 1.24 (0.79–1.79) | 13.9 (10.0–18.6) | N/A | N/A | 125 (99–154) | 2.31 (1.81–2.95) | 21.8 (14.8–29.5) | |
| ProtecT (28) | Case | 14.3 (11.5–18.7) | 273 (199–338) | N/A | N/A | N/A | N/A | N/A | N/A | 37.6 (28.5–47.1) |
| Control | 13.8 (10.6–17.5) | 258 (200–312) | N/A | N/A | N/A | N/A | N/A | N/A | 37.7 (27.6–50.4) | |
| RBS (8) | Case | 10.9 (7.7–13.6) | N/A | 1.53 (0.98–1.98) | N/A | 2239 (1561–3474) | N/A | 77 (59–92) | N/A | N/A |
| Control | 10.4 (8.7–13.3) | N/A | 1.43 (1.10–1.87) | N/A | 2063 (1276–2904) | N/A | 73 (59–95) | N/A | N/A |
Data are median serum concentration (interquartile range). The numbers of case patients and control subjects for whom hormone measurements were available for a particular study varied by hormone. DHT = dihydrotestosterone; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin; ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = Baltimore Longitudinal Study of Aging; CARET = Carotene and Retinol Efficacy Trial; CLUE = CLUE Study, Washington County, MD; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; NBSBWG = Nordic Biological Specimen Biobank Working Group; HPFS = Health Professionals Follow-up Study; JACC = Japan Collaborative Cohort Study; Janus = Janus Serum Bank; JHCS = Japan–Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; NSHDC = Northern Sweden Health and Disease Cohort; PHS = Physicians’ Health Study; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Rancho Bernardo Study; N/A = data not available for this study.
Serum concentrations of testosterone, free testosterone, and DHT were positively correlated with each other (all r > 0.29) but were not correlated with the serum concentration of DHEA-S (all r < 0.12; Table 4). The serum concentration of androstanediol glucuronide was weakly correlated with that of the other androgens (r of between 0.1 and 0.3). Serum concentrations of the androgens (except for DHEA-S) were moderately correlated with that of estradiol (r of between 0.2 and 0.3), but the serum concentration of free estradiol was correlated only with that of free testosterone (r = 0.31). The serum concentration of SHBG was positively correlated with those of testosterone (r = 0.51) and DHT (r = 0.33) but was negatively correlated with that of free testosterone (r = –0.15) and, to a greater extent, with that of free estradiol (r = –0.42). PSA levels at recruitment were not correlated with concentrations of the androgens and estradiol, except for a weak positive correlation with the concentration of free testosterone (r = 0.11). The concentration of IGF-I was weakly negatively correlated with the concentration of SHBG (r = −0.12) but not correlated with concentrations of the sex hormones.
Table 4.
Partial correlation coefficients among control subjects between concentrations of selected serum hormones, sex hormone–binding globulin, insulin-like growth factor I, and prostate-specific antigen*
| Compound | Testosterone (n = 7143) | Free testosterone (n = 6479) | DHT (n = 1715) | Androstanediol glucuronide (n = 3377) | DHEA-S (n = 2147) | Androstenedione (n = 2525) | Estradiol (n = 3399) | Free estradiol (n = 3062) | SHBG (n = 6626) | PSA (n = 3937) |
|---|---|---|---|---|---|---|---|---|---|---|
| Testosterone | 1 | |||||||||
| Free testosterone | 0.76 | 1 | ||||||||
| DHT | 0.60 | 0.29 | 1 | |||||||
| Androstanediol glucuronide | 0.17 | 0.24 | 0.14 | 1 | ||||||
| DHEA-S | 0.04 | 0.12 | 0.01 | 0.15 | 1 | |||||
| Androstenedione | 0.24 | 0.25 | 0.26 | 0.19 | 0.47 | 1 | ||||
| Estradiol | 0.29 | 0.29 | 0.22 | 0.18 | 0.05 | 0.17 | 1 | |||
| Free estradiol | −0.02 | 0.31 | −0.07 | 0.18 | 0.09 | 0.11 | 0.87 | 1 | ||
| SHBG | 0.51 | −0.15 | 0.33 | −0.03 | −0.07 | 0.08 | 0.05 | −0.41 | 1 | |
| PSA | 0.08 | 0.11 | −0.01 | 0.02 | 0.05 | 0.05 | −0.04 | −0.01 | −0.03 | 1 |
| IGF-I | −0.02 | 0.07 | 0.01 | −0.04 | 0.05 | 0.02 | −0.03 | 0.02 | −0.12 | 0.07 |
Data are partial correlation coefficients of log-transformed hormone concentrations that were adjusted for age at blood collection (in four groups) and study. n = the number of control subjects with a measurement for that hormone; DHT = dihydrotestosterone; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin; PSA = prostate-specific antigen; IGF-I = insulin-like growth factor I.
Associations Between Serum Sex Hormone Concentrations and Prostate Cancer Risk
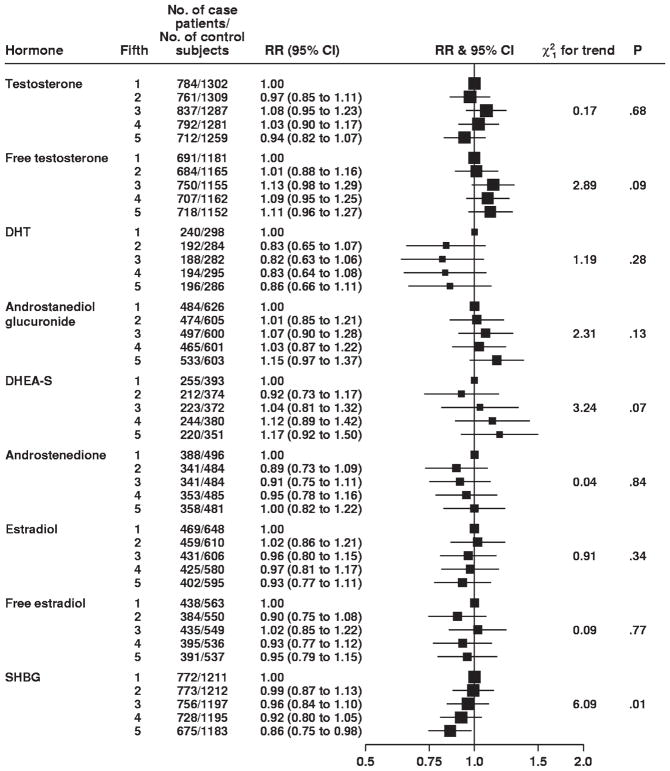
There were no statistically significant associations between serum concentrations of any of the androgens or estrogen and risk of prostate cancer, comparing the highest fifth with the lowest fifth (eg, for testosterone, RR = 0.94, 95% confidence interval [CI] = 0.82 to 1.07 and, for free testosterone, RR = 1.11, 95% CI = 0.96 to 1.27) (Fig. 1). Although comparing the highest with the lowest fifth of DHT concentrations and risk of prostate cancer indicated a possible inverse association (RR = 0.86, 95% CI = 0.66 to 1.11), there was no evidence of a dose–response relationship. There was also no evidence of an association of risk of prostate cancer with concentrations of androstanediol glucuronide, androstenedione, DHEA-S, estradiol, or free estradiol (Fig. 1). SHBG was statistically significantly and inversely related to prostate cancer risk (RR = 0.86, 95% CI = 0.75 to 0.98, comparing the highest fifth with the lowest fifth), and evidence of a statistically significant dose–response relationship was also found (Ptrend = .01). When we categorized the concentrations of each hormone into deciles, we found no evidence of an association between prostate cancer risk and either very high or very low serum concentrations of sex hormones (results not shown).
Fig. 1.
Associations between risk of prostate cancer and increasing fifths of hormone concentrations. The position of each square indicates the magnitude of the relative risk, and the area of the square is proportional to the amount of statistical information available (inverse of the variance of the logarithm of the relative risk). The length of the horizontal line through the square indicates the 95% confidence interval. The chi-square 1 degree of freedom statistic for linear trend is calculated by replacing the categorical variables with a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. The P value was two-sided for statistical significance of the chi-square linear trend statistic. RR = relative risk; CI = confidence interval; DHT = dihydrotestosterone; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin.
There was no evidence of heterogeneity between studies for the estimates of the linear trend for any serum hormone concentration and prostate cancer risk (all P>.10 and only two of the nine I2 estimates were >5%, indicating that there was very little evidence of heterogeneity in linear trends between studies; Supplementary Figs. 1–9, available online). There was no statistically significant heterogeneity between the estimates of linear trend for concentrations of testosterone, DHT, androstanediol glucuronide, and estradiol and prostate cancer risk according to whether or not the assays included an extraction or purification step (all P>.10).
Adjustment for Potential Confounders
The associations between concentrations of serum sex hormones and risk of prostate cancer were examined before and after adjustment for the following characteristics: body mass index, marital status, educational status, smoking, and alcohol consumption. Age was dealt with through stratification. Adjustment for these variables, either individually or mutually, made no appreciable difference to the associations between serum hormone concentrations and risk of prostate cancer (results not shown).
Subgroup Analyses by Patient Characteristics
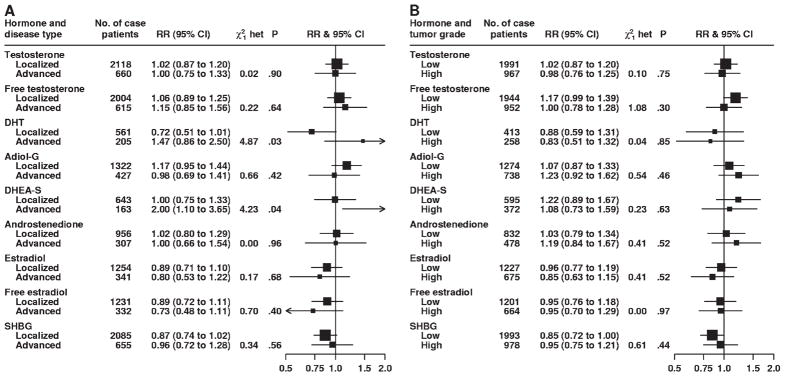
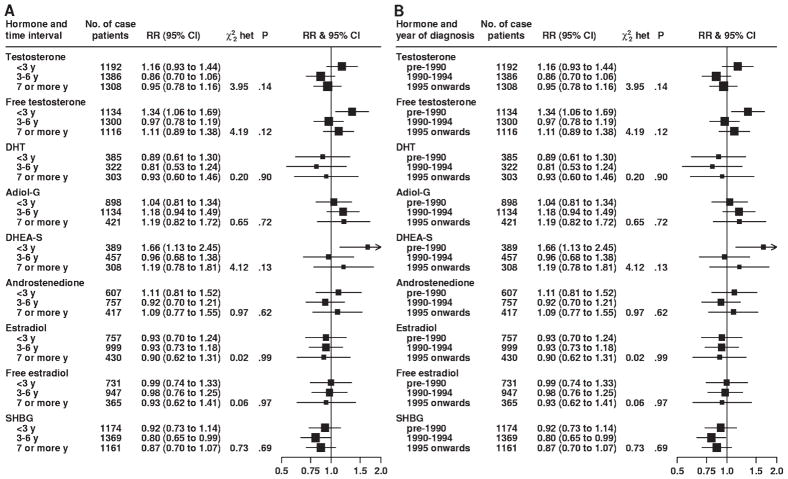
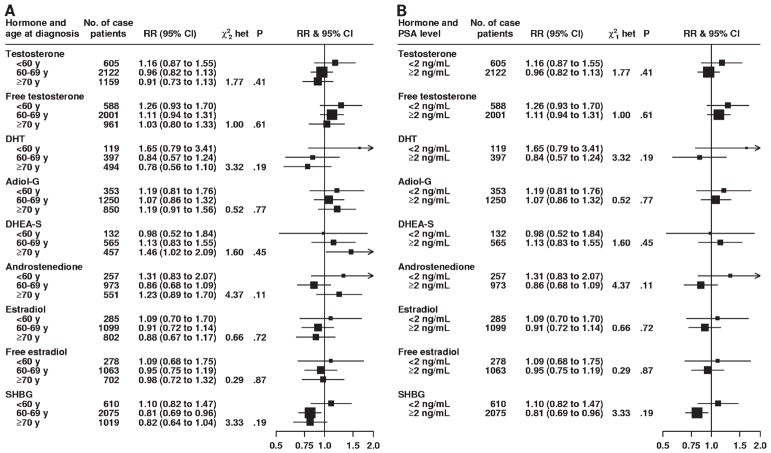
There was no statistically significant heterogeneity for either stage or grade of prostate cancer at diagnosis in associations between prostate cancer risk and serum concentrations of testosterone, free testosterone, androstanediol glucuronide, androstenedione, estradiol, free estradiol, and SHBG (Fig. 2). However, although DHT concentration was not associated with the risk of localized (RR = 0.72, 95% CI = 0.51 to 1.01) or advanced (RR = 1.47, 95% CI = 0.86 to 2.50) prostate cancer in linear trend analyses, these associations were statistically significantly different from each other (P for heterogeneity = .03) (Fig. 2, A). Risks of localized vs advanced prostate cancer associated with serum concentration of DHEA-S were also statistically significantly different from each other (P for heterogeneity = .04); the linear trend estimate for DHEA-S serum concentration was not associated with the risk of localized disease (RR = 1.00, 95% CI = 0.75 to 1.33) but was statistically significantly associated with an increased risk of advanced disease (RR = 2.00, 95% CI = 1.10 to 3.65) (Fig. 2, A). There was no variation in the associations between serum concentrations of any of the sex hormones and prostate cancer risk for time between blood collection and diagnosis, year of diagnosis, age at diagnosis, or PSA level at recruitment (Figs. 3 and 4). Subgroup results remained unchanged after adjustment for potential confounding variables, including body mass index (results not shown). Analyses that jointly classified tumors by both stage and grade did not detect evidence of additional heterogeneity in risk for any subgroup compared with the differences observed in the analyses of stage and grade (results not shown).
Fig. 2.
Association between risk of prostate cancer and sex hormone concentrations according to stage of disease (A) and grade of disease (B). The relative risk is the estimate of the linear trend for each sex hormone obtained by replacing the categorical variables representing the fifths with a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. The position of each square indicates the magnitude of the relative risk, and the area of the square is proportional to the amount of statistical information available (inverse of the variance of the logarithm of the relative risk). The length of the horizontal line through the square indicates the 95% confidence interval. The chi-square statistic for heterogeneity ( het) is to assess whether the relative risk estimates for each characteristic are different from each other. The P value was two-sided for statistical significance of the chi-square heterogeneity statistic. RR = relative risk; CI = confidence interval; DHT = dihydrotestosterone; Adiol-G = androstanediol glucuronide; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin.
Fig. 3.
Association between risk of prostate cancer and sex hormone concentrations according to time interval between blood collection and diagnosis (A) and year of diagnosis (B). The relative risk is the estimate of the linear trend for each sex hormone obtained by replacing the categorical variables representing the fifths with a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. The position of each square indicates the magnitude of the relative risk, and the area of the square is proportional to the amount of statistical information available (inverse of the variance of the logarithm of the relative risk). The length of the horizontal line through the square indicates the 95% confidence interval. The chi-square statistic for heterogeneity ( het) is to assess whether the relative risk estimates for each characteristic are different from each other. The P value was two-sided for statistical significance of the chi-square heterogeneity statistic. RR = relative risk; CI = confidence interval; DHT = dihydrotestosterone; Adiol-G = androstanediol glucuronide; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin.
Fig. 4.
Association between risk of prostate cancer and sex hormone concentrations according to age at diagnosis (A) and prostate-specific antigen level at recruitment (B). The relative risk is the estimate of the linear trend for each sex hormone obtained by replacing the categorical variables representing the fifths with a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. The position of each square indicates the magnitude of the relative risk, and the area of the square is proportional to the amount of statistical information available (inverse of the variance of the logarithm of the relative risk). The length of the horizontal line through the square indicates the 95% confidence interval. The chi-square statistic for heterogeneity ( and het) is to assess whether the relative risk estimates for each characteristic are different from each other. The P value was two-sided for statistical significance of the chi-square heterogeneity statistic. RR = relative risk; CI = confidence interval; DHT = dihydrotestosterone; Adiol-G = androstanediol glucuronide; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin.
Relationship Between Serum Concentrations of Multiple Sex Hormone and Prostate Cancer Risk
The joint relationships between concentrations of two or more hormones and the risk of prostate cancer were examined. For the combination of testosterone and estradiol and the combination of free testosterone and free estradiol (hence simultaneously adjusting for SHBG), the estimated linear trend from the univariate model was virtually unchanged when serum concentrations of either combination of hormones were included in a joint model (both Pinteraction >.10) (Table 5). Simultaneous adjustment for serum concentrations of free testosterone, free estradiol, androstanediol glucuronide, and DHT or that of androstenedione or of DHEA-S did not alter the results obtained from univariate analyses of these sex hormones (Table 5).
Table 5.
Prostate cancer risk associated with sex hormone concentrations with and without mutual adjustment for other hormones*
| No. of case patients/No. of control subjects | Unadjusted RR (95% CI)† | Mutually adjusted RR (95% CI)‡ | |
|---|---|---|---|
| Mutual adjustment set 1 | |||
| Testosterone | 2107/2937 | 1.00 (0.85 to 1.18) | 1.03 (0.87 to 1.22) |
| Estradiol | 0.92 (0.78 to 1.09) | 0.92 (0.77 to 1.10) | |
| Mutual adjustment set 2 | |||
| Free testosterone | 1977/2653 | 1.11 (0.93 to 1.32) | 1.12 (0.94 to 1.35) |
| Free estradiol | 0.99 (0.83 to 1.18) | 0.95 (0.80 to 1.14) | |
| Mutual adjustment set 3 | |||
| Free testosterone | 701/823 | 1.09 (0.80 to 1.48) | 1.15 (0.82 to 1.62) |
| Free estradiol | 1.10 (0.81 to 1.50) | 0.98 (0.70 to 1.38) | |
| Androstanediol glucuronide | 1.17 (0.87 to 1.56) | 1.19 (0.88 to 1.60) | |
| DHT | 0.84 (0.62 to 1.13) | 0.79 (0.57 to 1.09) | |
| Mutual adjustment set 4 | |||
| Free testosterone | 834/1203 | 1.09 (0.83 to 1.42) | 1.09 (0.82 to 1.44) |
| Free estradiol | 0.89 (0.69 to 1.16) | 0.87 (0.66 to 1.13) | |
| Androstanediol glucuronide | 1.09 (0.85 to 1.41) | 1.08 (0.84 to 1.41) | |
| Androstenedione | 1.11 (0.86 to 1.43) | 1.08 (0.83 to 1.41) | |
| Mutual adjustment set 5 | |||
| Free testosterone | 818/1187 | 1.11 (0.85 to 1.46) | 1.15 (0.87 to 1.52) |
| Free estradiol | 0.86 (0.66 to 1.12) | 0.82 (0.63 to 1.08) | |
| Androstanediol glucuronide | 1.07 (0.83 to 1.40) | 1.05 (0.81 to 1.37) | |
| DHEA-S | 1.09 (0.84 to 1.42) | 1.09 (0.84 to 1.43) | |
| Mutual adjustment set 6 | |||
| SHBG | 2339/3138 | 0.83 (0.71 to 0.97) | 0.88 (0.75 to 1.04) |
| IGF-I | 1.55 (1.32 to 1.82) | 1.51 (1.29 to 1.78) | |
RR = relative risk; CI = confidence interval; DHT = dihydrotestosterone; DHEA-S = dehydroepiandrosterone sulfate; SHBG = sex hormone–binding globulin; IGF-I = insulin-like growth factor I.
Relative risks were from separate univariate analyses.
Relative risks were from model with hormones adjusted for each other.
We next considered mutual adjustment for SHBG and IGF-I on the basis of their biological relationship (Table 5). When the serum concentration of IGF-I was included in the statistical model as an additional variable, associations between the risks for prostate cancer and serum concentration of SHBG or IGF-I were essentially unchanged, but the association between the serum concentration of SHBG and prostate cancer risk became non–statistically significant after adjustment for IGF-I (before, RR = 0.83, 95% CI = 0.71 to 0.97, P = .02; and after, RR = 0.88, 95% CI = 0.75 to 1.04, P = .12). Mutual adjustment of concentrations of testosterone, estradiol, free testosterone, and free estradiol for IGF-I concentration did not change any of the results appreciably (results not shown).
Discussion
The main finding of this pooled analysis of approximately 3900 patients with prostate cancer and 6500 control subjects was that prediagnostic serum concentrations of testosterone, free testosterone, DHT, androstanediol glucuronide, DHEA-S, androstenedione, estradiol, or free estradiol were not associated with the risk of subsequent prostate cancer. There was no heterogeneity in the estimated trends among the studies for any of the hormones, and adjustment for potential confounders made little difference to the risk estimates.
This collaboration was successful in collecting more than 95% of the identified prospective worldwide data on endogenous sex hormones and prostate cancer risk. Through this collaborative approach, we were also able to obtain additional, as yet unpublished, data, although it is possible that we were unaware of other unpublished studies.
There have been many reviews and commentaries on the role of sex hormones in the development of prostate cancer, which hypothesize that high circulating levels of androgens are associated with an increased risk (3,4,33–37). The findings from this pooled analysis of the worldwide data, however, showed no association between prostate cancer risk and serum concentrations of testosterone, free testosterone, DHT, androstenedione, or DHEA-S. Testosterone is converted to the more androgenic DHT (by the action of 5α-reductase) within the prostate, but the proportion of prostate-produced DHT that reaches the serum has not been determined. Thus, the relationship between the concentration of DHT in serum and in the prostate is unclear and could explain the lack of an association between serum levels and prostate cancer risk. However, we did observe that higher concentrations of DHT were associated with a non–statistically significant increase in the risk of advanced disease and a non–statistically significant decrease in the risk of localized disease. Furthermore, we cannot rule out chance as an explanation for the heterogeneity observed because of the large numbers of statistical tests performed.
The serum DHT concentration reflects not only the activity of 5α-reductase type 2 within the prostate but also that of 5α-reductase type 1 in the skin and, to a lesser extent, the liver (38). An alternative serum marker of 5α-reductase activity is the concentration of androstanediol glucuronide, a terminal metabolite of DHT. However, like the serum concentration of DHT, the concentration of serum androstanediol glucuronide also reflects the activities of both 5α-reductase types 1 and 2. However, administration of a selective 5α-reductase type 2 inhibitor leads to an approximate 85% reduction in the concentration of serum androstanediol glucuronide and a 70% reduction in the concentration of serum DHT. These results indicate that intraprostatic androgen activity may be more closely related to the serum concentration of androstanediol glucuronide than to serum DHT concentration (39,40). Assays of serum androstanediol glucuronide usually measure only androstanediol 17-glucuronide, one of the two isomers of this metabolite. Because this isomer is the predominant isomer in the circulation, representing more than 80% of the total circulating concentration of androstanediol glucuronide (41–43), androstanediol 17-glucuronide may be a useful proxy for total serum concentration of androstanediol glucuronide. If the concentration of serum androstanediol glucuronide is a valid marker of intraprostatic androgen activity, the findings of this collaborative analysis do not support the hypothesis that such activity is strongly related to the risk of prostate cancer. However, without data directly comparing serum concentrations of these hormones with intraprostatic concentrations, any biological interpretation must be viewed with caution.
The findings from this collaborative study indicate that endogenous estrogen concentrations are not related to prostate cancer risk. It has been proposed (13) that a combination of estrogens and androgens may be more strongly associated with the risk of prostate cancer than either estrogen or androgen alone. However, the results from this pooled analysis show that mutual adjustment for serum concentrations of free testosterone, free estradiol, and androstanediol glucuronide did not change the estimated trends compared with analyses of the individual hormones. Further, there was no evidence of interactions between concentrations of any of the hormones considered and risk of prostate cancer.
This study found a modest inverse association between serum SHBG concentration and prostate cancer risk, with a relative risk reduction of 14% (95% CI = 2% to 25%) when the highest fifth was compared with the lowest fifth. Further adjustment for body mass index, which was correlated with SHBG level (r = −0.27 in this study), did not appreciably alter this risk estimate. SHBG is a major determinant of the serum concentrations of free testosterone and estradiol, which regulate steroid hormone production through a negative feedback loop (44). Insulin and, to a lesser extent, IGF-I inhibit SHBG production (33). Most studies did not measure the concentration of insulin, but we observed a negative correlation between IGF-I and SHBG serum concentrations (r = −0.12). After adjustment of SHBG for IGF-I serum concentrations, the estimated trend was only slightly attenuated toward the null but was no longer statistically significant. Although this slight attenuation could be a consequence of the mutual adjustment and errors in the measurement of IGF-I, it is also possible that the inverse association observed between SHBG serum concentration and prostate cancer risk is a consequence of the negative relationship between concentrations of SHBG and IGF-I, which itself is positively associated with risk (The Endogenous Hormones and Prostate Cancer Collaborative Group, unpublished results).
One of the aims of this analysis was to examine whether the associations of serum concentrations of sex hormones with risk of prostate cancer varied according to the clinical characteristics of the disease. Such questions are important because there have been substantial changes in prostate cancer detection since the late 1980s, when the introduction of PSA testing led to a sharp increase in incidence rates, with many more localized cancers being detected than advanced cancers (45). The combination of an increasing proportion of early, often asymptomatic, disease and a decreasing proportion of advanced cancers at diagnosis can introduce difficulties in the interpretation of results from individual studies (46). The introduction of PSA testing has further complicated the situation by increasing the lead time (ie, the number of years earlier the tumor is detected as a result of testing) by a period that was estimated to be as long as 12 years in men aged 55 years (47,48). Moreover, approximately 30% of early-stage PSA-detected prostate cancers may be the result of overdetection; that is, if left undetected, these cancers would never progress to clinical disease (47). It has been shown that up to 80% of men older than 80 years at autopsy have small foci of incidental prostate cancer (49). Unfortunately, we did not have detailed information on each participant’s PSA screening history or on which of the cancers were detected by PSA screening. Therefore, we attempted to address these concerns in various subgroup analyses. First, we examined the consistency of the associations of hormone concentrations with risk of prostate cancer by stage and grade of the disease at diagnosis. Although there were some small differences in the estimated risks, these differences could be due to chance because of repeated statistical testing. In addition, there are no clear biological mechanisms underlying such associations. Second, we found no statistically significant heterogeneity in the risks of prostate cancer according to the age at diagnosis, time between recruitment and diagnosis, year of diagnosis, or the PSA level at recruitment, which suggests that the introduction of PSA testing and differences in its use in various populations may not have unduly influenced the associations.
Our study had several limitations in common with most prospective studies of prostate cancer risk. There are possible biases that can be introduced due to the long latency associated with the disease, which would result in some control subjects having occult disease. However, in a large study in which the relationship between the exposure and disease is at least moderate, it is unlikely that associations would be missed as a result of some control subjects having occult disease (46). In this pooled analysis with many case patients and with no variation in the estimated risks according to time between blood collection and diagnosis or age at diagnosis, it seems unlikely that such misclassification would have had a major effect.
A further limitation is that many different laboratory methods were used in the different studies to measure serum concentrations of sex hormones, which may be responsible for much of the variation in hormone concentrations among studies (for detailed descriptions of laboratory methods, see Supplementary Table 2, available online). However, our primary method of analysis allowed for this problem by defining within-study fifths of hormone concentration and by pooling study-specific estimates of relative risk. This method assumes that the fifths are comparable among studies; if this assumption was not valid, estimated relative risks could be biased. However, because we found no evidence of heterogeneity among studies for the association of sex hormone concentration and prostate cancer risk and have no reason to expect that the distributions of hormone concentrations would be very different among the men in the different studies, this assumption appears reasonable.
Another possible limitation is that this pooled analysis relied on the measurement of serum hormone levels in only one sample at only one time. These single measurements provide an imperfect estimate of a man’s usual hormonal status and can be influenced both by within-person errors and analytic errors. Both types of error are likely to lead to attenuation of the relationship between hormone concentration and risk (50). Therefore, such attenuation could have masked a moderate relative risk in our analysis, although because most hormones showed no statistical evidence of a dose–response relationship, the combined effect of both within-person and analytic error would have to have been substantial. Although a single measurement of hormone concentration can reliably reflect average exposure over a short time interval (25,51), it is not clear whether one measurement also adequately reflects a lifetime exposure. Little is known about whether hormonal status early in life, such as during adolescence (52) or in utero (53), is important for the subsequent development of prostate cancer.
In summary, the results of this collaborative analysis of the existing worldwide data on the associations between endogenous hormone concentrations and prostate cancer risk indicate that circulating concentrations of androgens or estradiol do not appear to be associated with the risk of prostate cancer.
Supplementary Material
CONTEXT AND CAVEATS.
Prior knowledge
Sex hormones in serum could, hypothetically, influence the risk of prostate cancer.
Study design
Pooled analysis of 18 prospective studies on the association between sex hormone concentrations in serum and the risk of prostate cancer. A total of 3886 men with incident prostate cancer and 6438 control subjects were included in this analysis.
Contribution
No associations were found between the risk of prostate cancer and serum concentrations of testosterone, calculated free testosterone, dihydrotestosterone, dehydroepiandrosterone sulfate, androstenedione, androstanediol glucuronide, estradiol, or calculated free estradiol. A modest inverse association was observed between the risk of prostate cancer and the serum concentration of sex hormone–binding globulin.
Implications
Sex hormones apparently do not influence the risk of prostate cancer.
Limitations
Possible biases could have been introduced because of the long latency associated with prostate cancer, which could result in some control subjects having occult disease. Different laboratory methods were used in different studies to measure sex hormone concentrations in serum. Hormone concentrations were measured in only one sample for each participant.
Acknowledgments
The central pooling and analysis of these data were supported by Cancer Research UK. Cancer Research UK had no role in the design, conduct, data management and analysis; in the manuscript preparation or review; or in the authorization for submission.
Appendix
Notes
Endogenous Hormones and Prostate Cancer Collaborative Group.
Authors/writing committee: Andrew W. Roddam, Naomi E. Allen, Paul Appleby, and Timothy J. Key (Cancer Research UK Epidemiology Unit, University of Oxford, Oxford, UK).
Authors/members of the collaboration:
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: Joanne F. Dorgan (Fox Chase Cancer Center, Philadelphia, PA), Demetrius Albanes and Philip R. Taylor (Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MA). (The ATBC study would like to acknowledge the support of J. Virtamo, O. Heinonen (who is deceased now), D. W. Chandler, M. Galmarini, L. M. McShane, M. J. Barrett, and J. Tangrea.)
Baltimore Longitudinal Study of Aging: Luigi Ferrucci, Director (Clinical Research Branch, National Institute on Aging, Baltimore, MD); H. Ballentine Carter (The James Buchanan Brady Urological Institute, The Johns Hopkins Medical Institutes, Baltimore, MD); E. Jeffrey Metter (Clinical Research Branch, National Institute on Aging, Baltimore, MD). (The Baltimore Longitudinal Study of Aging is supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging).
Carotene and Retinol Efficacy Trial (CARET): Chu Chen, Noel S Weiss (University of Washington and Fred Hutchinson Cancer Research Center, Seattle, WA); Gary Goodman (Fred Hutchinson Cancer Research Center and Swedish Cancer Institute, Seattle, WA). (CARET would like to acknowledge the support of F. Z. Stanczyk, S. K. Lewis, R. Etzioni, M. Barnett, D. DiTommaso, and the CARET study participants.)
CLUE Study, Washington County, MD: Ann W. Hsing (Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD); George Comstock (who is deceased now; Johns Hopkins Bloomberg School of Public Health, Baltimore, MD); Kathy Helzlsouer (Prevention and Research Center, The Weinberg Center for Women’s Health and Medicine, Mercy Medical Center, Baltimore, MD and Johns Hopkins Bloomberg School of Public Health, Baltimore, MD).
European Prospective Investigation into Cancer and Nutrition: Ruth Travis (Cancer Research UK Epidemiology Unit, University of Oxford, Oxford, UK); Elio Riboli (Department of Epidemiology and Public Health, Imperial College, London, UK); Rudolf Kaaks (Division of Cancer Epidemiology, Deutsches Krebsforschungszentrum, Heidelberg, Germany).
Finnish Mobile Clinic Health Examination Survey: Paul Knekt, Markku Heliövaara (National Public Health Institute, Helsinki, Finland).
Health Professionals Follow-up Study (HPFS): Elizabeth A. Platz (Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, the Brady Urological Institute and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD); Walter C. Willett, Edward Giovannucci (Departments of Nutrition and Epidemiology, Harvard School of Public Health and the Channing Laboratory, Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA). (The HPFS investigators would like to acknowledge Dr Nader Rifai, whose laboratory performed the hormone assays.)
Janus Serum Bank: Lars Vatten (Department of Community Medicine and General Practice, University of Trondheim, Trondheim, Norway); Giske Ursin (Department of Preventive Medicine, Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA, and Department of Nutrition, University of Oslo, Norway). (The Janus Serum Bank Study would like to acknowledge the help of the late R. Ross, F. Stanczyk, R. Lobo, S. Harvei, and E. Jellum.)
Japan Collaborative Cohort Study: Akiko Tamakoshi (National Center for Geriatrics and Gerontology, Obu, Japan); Kotaro Ozasa (Kyoto Prefectural University of Medicine, Kyoto, Japan).
Japan-Hawaii Cancer Study (JHCS): Abraham M. Y. Nomura (Japan-Hawaii Cancer Study, Kuakini Medical Center, Honolulu, HI); Grant N. Stemmermann (Department of Pathology, University of Cincinnati Medical Center, Cincinnati, OH). (JHCS would like to acknowledge the support of F. Z. Stanczyk and H. L. Judd.)
Kaiser Permanente Medical Care Program: Catherine Schaefer, Charles P. Quesenberry Jr (Division of Research, Kaiser Permanente Northern California, Oakland, CA); Joseph H. Vogelman (Orentreich Foundation for the Advancement of Science, Inc., New York).
Melbourne Collaborative Cohort Study: Gianluca Severi, Dallas R. English, Graham G. Giles (Cancer Epidemiology Centre, The Cancer Council Victoria and Centre for Molecular, Environmental, Genetic, and Analytic Epidemiology, The University of Melbourne, Melbourne, Victoria, Australia).
Nordic Biological Specimen Biobank Working Group—Finland: Tapio Luostarinen (Finnish Cancer Registry, Helsinki, Finland); Ulf-Håkan Stenman (Department of Clinical Chemistry, Helsinki University Central Hospital, Helsinki, Finland); Leena Tenkanen (Helsinki Heart Study, Helsinki, Finland, and University of Tampere, Tampere, Finland).
Nordic Biological Specimen Biobank Working Group—Norway: Randi Gislefoss (The Norwegian Cancer Registry, Oslo, Norway). (The Janus Serum Bank, owned by The Norwegian Cancer Society, provided serum samples.)
Northern Sweden Health and Disease Cohort: Pär Stattin (Department of Surgical and Perioperative Sciences, Urology and Andrology, Umeå University Hospital, Umeå, Sweden); Göran Hallmans (Department of Public Health and Clinical Medicine, Nutritional Research, Umeå University Hospital, Umeå, Sweden); Tanja Stocks (Department of Surgical and Perioperative Sciences, Urology and Andrology, Umeå University Hospital, Umeå, Sweden).
Physicians’ Health Study: June M. Chan (Departments of Epidemiology and Biostatistics and Urology, University of California, San Francisco, CA); Meir Stampfer (Departments of Nutrition and Epidemiology, Harvard School of Public Health and the Channing Laboratory, Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA); Peter Gann (Department of Pathology, University of Illinois at Chicago, Chicago, IL).
Prostate Testing for Cancer and Treatment: Steven E. Oliver (Department of Health Sciences, University of York and the Hull York Medical School, York, UK); Jeff M. Holly (Division of Surgery, Bristol Royal Infirmary, Bristol, UK); Jenny Donovan (Department of Social Medicine, University of Bristol, Bristol, UK). (ProtecT would like to acknowledge the support of D. Gunnell, T. J. Peters, R. Persad, D. Gillatt, A. Pearce, D. E. Neal, F. C. Hamdy, and the ProtecT research team.)
Rancho Bernardo Study: Elizabeth Barrett-Connor, Cedric Garland (University of California, San Diego, CA).
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. Version 2.0. Lyon, France: IARC Press; 2004. [Google Scholar]
- 2.Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101:2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 3.Hsing AW. Hormones and prostate cancer: what’s next? Epidemiol Rev. 2001;23:42–58. doi: 10.1093/oxfordjournals.epirev.a000795. [DOI] [PubMed] [Google Scholar]
- 4.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–253. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 6.Canby-Hagino E, Hernandez J, Brand TC, Thompson I. Looking back at PCPT: looking forward to new paradigms in prostate cancer screening and prevention. Eur Urol. 2007;51:27–33. doi: 10.1016/j.eururo.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Nomura AMY, Heilbrun LK, Stemmermann GN, Judd HL. Prediagnostic serum hormones and the risk of prostate-cancer. Cancer Res. 1988;48:3515–3517. [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Garland C, McPhillips JB, Khaw KT, Wingard DL. A prospective, population-based study of androstenedione, estrogens, and prostatic-cancer. Cancer Res. 1990;50:169–173. [PubMed] [Google Scholar]
- 9.Hsing AW, Comstock GW. Serological precursors of cancer—serum hormones and risk of subsequent prostate-cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:27–32. [PubMed] [Google Scholar]
- 10.Comstock GW, Gordon GB, Hsing AW. The relationship of serum dehydroepiandrosterone and its sulfate to subsequent cancer of the prostate. Cancer Epidemiol Biomarkers Prev. 1993;2:219–221. [PubMed] [Google Scholar]
- 11.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of serum androgen levels in men with and without prostate-cancer. Prostate. 1995;27:25–31. doi: 10.1002/pros.2990270106. [DOI] [PubMed] [Google Scholar]
- 12.Corder EH, Friedman GD, Vogelman JH, Orentreich N. Seasonal-variation in vitamin-D, vitamin-D-binding protein, and dehydroepiandrosterone—risk of prostate-cancer in black and white men. Cancer Epidemiol Biomarkers Prev. 1995;4:655–659. [PubMed] [Google Scholar]
- 13.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 14.Nomura AMY, Stemmermann GN, Chyou PH, Henderson BE, Stanczyk FZ. Serum androgens and prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:621–625. [PubMed] [Google Scholar]
- 15.Guess HA, Friedman GD, Sadler MC, et al. 5 alpha-reductase activity and prostate cancer: a case-control study using stored sera. Cancer Epidemiol Biomarkers Prev. 1997;6:21–24. [PubMed] [Google Scholar]
- 16.Vatten LJ, Ursin G, Ross RK, et al. Androgens in serum and the risk of prostate cancer: a nested case-control study from the Janus serum bank in Norway. Cancer Epidemiol Biomarkers Prev. 1997;6:967–969. [PubMed] [Google Scholar]
- 17.Dorgan JP, Albanes D, Virtamo J, et al. Relationships of serum androgens and estrogens to prostate cancer risk: results from a prospective study in Finland. Cancer Epidemiol Biomarkers Prev. 1998;7:1069–1074. [PubMed] [Google Scholar]
- 18.Heikkila R, Aho K, Heliovaara M, et al. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma—a longitudinal study. Cancer. 1999;86:312–315. [PubMed] [Google Scholar]
- 19.Mohr BA, Feldman HA, Kalish LA, Longcope C, McKinlay JB. Are serum hormones associated with the risk of prostate cancer? Prospective results from the Massachusetts Male Aging Study. Urology. 2001;57:930–935. doi: 10.1016/s0090-4295(00)01116-x. [DOI] [PubMed] [Google Scholar]
- 20.Stattin P, Rinaldi S, Stenman UH, et al. Plasma prolactin and prostate cancer risk: a prospective study. Int J Cancer. 2001;92:463–465. doi: 10.1002/ijc.1191. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Weiss NS, Stanczyk FZ, et al. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003;12:1410–1416. [PubMed] [Google Scholar]
- 22.Stattin P, Lumme S, Tenkanen L, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108:418–424. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 23.Ozasa K, Nakao M, Watanabe Y, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95:65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, Metter EJ. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–2260. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 25.Platz EA, Leitzmann MF, Rifai N, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–1269. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 26.Severi G, Morris HA, MacInnis RJ, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 27.Travis RC, Key TJ, Allen NE, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:1331–1338. doi: 10.1002/ijc.22814. [DOI] [PubMed] [Google Scholar]
- 28.Oliver SE, Gunnell D, Donovan J, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer. 2004;108:887–892. doi: 10.1002/ijc.11631. [DOI] [PubMed] [Google Scholar]
- 29.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis. 2000;3:157–172. doi: 10.1038/sj.pcan.4500421. [DOI] [PubMed] [Google Scholar]
- 34.Eaton NE, Reeves GK, Appleby PN, Key TJ. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999;80:930–934. doi: 10.1038/sj.bjc.6690445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaneyfelt T, Husein R, Bubley G, Mantzoros CS. Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol. 2000;18:847–853. doi: 10.1200/JCO.2000.18.4.847. [DOI] [PubMed] [Google Scholar]
- 36.Bosland MC. Chapter 2: the role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;(27):39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 37.Hsing AW. Hormones and prostate cancer: where do we go from here? J Natl Cancer Inst. 1996;88:1093–1095. doi: 10.1093/jnci/88.16.1093. [DOI] [PubMed] [Google Scholar]
- 38.Russell DW, Wilson JD. Steroid 5-α-reductase—2 genes 2 enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 39.Norman RW, Coakes KE, Wright AS, Rittmaster RS. Androgen metabolism in men receiving finasteride before prostatectomy. J Urol. 1993;150:1736–1739. doi: 10.1016/s0022-5347(17)35882-2. [DOI] [PubMed] [Google Scholar]
- 40.Stanczyk FZ, Skinner EC, Mertes S, Spahn MF, Lobo RA, Ross RK. Alterations in circulating levels of androgens and PSA during treatment with finasteride in men at high risk of prostate cancer. In: Li JJ, Li SA, Gustafsson J, Nandi S, Sekely LI, editors. Hormonal Carcinogenesis II. New York: Springer; 1996. pp. 404–407. [Google Scholar]
- 41.Rittmaster RS, Thompson DL, Listwak S, Loriaux DL. Androstanediol glucuronide isomers in normal men and women and in men infused with labeled dihydrotestosterone. J Clin Endocrinol Metab. 1988;66:212–216. doi: 10.1210/jcem-66-1-212. [DOI] [PubMed] [Google Scholar]
- 42.Thompson DL, Rittmaster RS, Rodriguez AM, Moore PHJ, Rao PN. Synthesis of new steroid haptens for radioimmunoassay—VIII. Development and validation of a specific radioimmunoassay for serum 5 alpha-androstane-3 alpha, 17 beta-diol 17-glucuronide. J Steroid Biochem. 1990;36:345–349. doi: 10.1016/0022-4731(90)90227-j. [DOI] [PubMed] [Google Scholar]
- 43.Rao PN, Burdett JEJ, Moore PHJ, Horton R. Isolation and identification of androstanediol glucuronide from human plasma. J Steroid Biochem. 1987;28:565–569. doi: 10.1016/0022-4731(87)90516-4. [DOI] [PubMed] [Google Scholar]
- 44.Tiitinen A, Simberg N, Stenman UH, Ylikorkala O. Estrogen replacement does not potentiate gonadotropin-releaseing hormone agonist-induced androgen suppression in treatment of hirsutism. J Clin Endocrinol Metab. 1994;79:447–451. doi: 10.1210/jcem.79.2.8045961. [DOI] [PubMed] [Google Scholar]
- 45.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 46.Platz EA, De Marzo AM, Giovannucci E. Prostate cancer association studies: pitfalls and solutions to cancer misclassification in the PSA era. J Cell Biochem. 2004;91:553–571. doi: 10.1002/jcb.10700. [DOI] [PubMed] [Google Scholar]
- 47.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 48.Stenman UH, Leinonen J, Hakama M, Knekt P, Aromaa A, Teppo L. Serum concentrations of prostate specific antigen and its complex with [α]1-antichymotrypsin before diagnosis of prostate cancer. Lancet. 1994;344:1594–1598. doi: 10.1016/s0140-6736(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 49.Breslow N, Chan CW, Dhom G, et al. Latent carcinoma of prostate at autopsy in 7 areas—collaborative study organized by International Agency For Research On Cancer, Lyons, France. Int J Cancer. 1977;20:680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 50.McShane LM, Midthune DN, Dorgan JF, Freedman LS, Carroll RJ. Covariate measurement error adjustment for matched case-control studies. Biometrics. 2001;57:62–73. doi: 10.1111/j.0006-341x.2001.00062.x. [DOI] [PubMed] [Google Scholar]
- 51.Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long-term hormonal milieu in men. J Clin Endocrinol Metab. 1992;74:939–942. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]
- 52.Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson BE. Serum testosterone levels in healthy-young black-and-white men. J Natl Cancer Inst. 1986;76:45–48. [PubMed] [Google Scholar]
- 53.Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero estrogen and testosterone environment of blacks and whites—potential effects on male offspring. Br J Cancer. 1988;57:216–218. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.