Abstract
Objective
To date, there are no published guidelines to direct red blood cell (RBC) transfusion decision making specifically for critically ill children. We present the recommendations from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI).
Design
Consensus conference series of multidisciplinary, international experts in RBC transfusion management of critically ill children.
Setting
N/A.
Intervention
None.
Subjects
Children with, or children at risk for, critical illness who receive, or are at risk for receiving a RBC transfusion.
Methods
A panel of 38 content and 4 methodology experts met over the course of two years to develop evidence-based and when evidence lacking, expert consensus-based recommendations regarding decision making for RBC transfusion management and research priorities for transfusion in critically ill children. The experts focused on 9 specific populations of critically ill children: general, respiratory failure, non-hemorrhagic shock, non-life-threatening bleeding or hemorrhagic shock, acute brain injury, acquired/congenital heart disease, sickle cell/oncology/transplant, extracorporeal membrane oxygenation/ventricular assist/ renal replacement support, and alternative processing. Data to formulate evidence-based and expert consensus recommendations was selected based on searches of PubMed, EMBASE, and Cochrane Library from 1980 to May 2017. Agreement was obtained using the Research And Development/University of California, Los Angeles (RAND UCLA) appropriateness method. Results were summarized using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.
Measurements and Results
The TAXI consensus conference developed and reached consensus on a total of 102 recommendations (57 clinical [20 evidence-based, 37 expert consensus], 45 research recommendations). All final recommendations met agreement, defined a priori as >80%. A decision tree to aid clinicians was created based on the clinical recommendations.
Conclusions
The TAXI recommendations provide important clinical guidance and applicable tools to avoid unnecessary RBC transfusions. Research recommendations identify areas of focus for future investigation to improve outcomes and safety for RBC transfusion.
Keywords: blood, red blood cell, transfusion, hemoglobin, intensive care, child, pediatric critical care, evidence-based, consensus development conference
INTRODUCTION
Anemia is common in critically ill children, and is observed in 74% of patients with a length of stay in a pediatric intensive care unit (PICU) over 2 days (1). Anemia tolerance in this population has not been well studied. The transfusion of red blood cells (RBC) in the form of RBC units or whole blood units can be life-saving in hemorrhagic shock as well as in critically ill children with severe anemia (hemoglobin (Hb) levels <5.0 g/dL) (2–6). The immediate goal of RBC transfusion is to increase the Hb concentration of recipients, with the intent to improve oxygen delivery and oxygen consumption (7,8). However, over time, RBC storage may reduce oxygen delivery capacity to deliver oxygen, and RBC transfusion has been associated with morbidities and mortality, especially in the critically ill, raising important safety concerns (9). While infectious risks are low, non-infectious serious hazards of transfusion (NISHOT), such as transfusion-associated lung injury (TRALI) and transfusion-associated circulatory overload (TACO) are much more prevalent in critically ill children (10–14). Therefore, due to the risks of complications and the increased morbidity associated with transfusions, efforts are needed to ensure appropriate RBC transfusions decision-making.
Using a more restrictive, or lower Hb, RBC transfusion threshold for decision-making has been studied in critically ill children. In 2007, Lacroix and colleagues published the pivotal Transfusion strategies for Patients in Pediatric Intensive Care Units (TRIPICU) study, which compared a restrictive (Hb ≤7.0g/dL) to a liberal transfusion (Hb ≤9.5 g/dL) threshold in hemodynamically stable critically ill children (15). This multicenter international randomized controlled trial (RCT) enrolled 637 PICU patients and demonstrated that the restrictive transfusion strategy was as efficacious as liberal transfusion strategy based on similar new or progressive multiple organ dysfunction rates between study groups. Moreover, limiting RBC transfusion to children with Hb level ≤7.0 g/L reduced RBC transfusion incidence by half. An RCT published by Cholette et al that compared a restrictive vs. liberal RBC transfusion strategy (<9.0 vs. <13.0g/dL) in 60 children with cyanotic univentricular physiology, also showed that a restrictive strategy was non-inferior and reduced exposure to RBC transfusions (16). These seminal studies provide evidence that certain populations of critically ill children benefit from a restrictive approach towards RBC decision-making.
Despite evidence that a restrictive transfusion strategy in hemodynamically stable children is non-inferior and reduces exposure to blood products, multiple studies have shown that in practice, the Hb threshold is higher than the evidence indicates is needed, exposing additional children to the potential complications associated with RBC transfusion without any expectation of benefit (17–22). Multiple surveys and studies indicate that pediatric intensivists have only partially adopted a restrictive transfusion strategy (20–22). Furthermore, there remains a paucity of evidence to guide transfusion practice in critically ill children with hemodynamic instability. Guidelines for RBC transfusion practice in critically ill adults have been published (23), though the generalizability to critically ill children is uncertain. Pediatric RBC transfusion guidelines in 2004 have addressed RBC transfusion decision making in children (24), however despite additional data there have been no recent consensus statements or guidelines evaluating the practice of RBC transfusion specifically in critically ill children despite emerging data.
The need to update guidance for RBC transfusion decision making in critically ill children prompted the organization of the Pediatric Critical Care Transfusion and Anemia EXpertise Initiative (TAXI) through the Pediatric Critical Care Blood Research Network (BloodNet) and the Pediatric Acute Lung Injury and Investigators (PALISI) Network. The goals of the TAXI conference series were to bring together international, multidisciplinary experts to: 1) to develop evidence-based and when evidence is lacking, expert-based consensus statements to guide transfusion and blood management practices, with the first series focusing on RBC transfusion practices for those caring for critically ill children, 2) to create an implementation initiative in collaboration with implementation experts to develop specific strategies for adaptive dissemination and implementation into various clinical/research environments that would best ensure uptake, and 3) to develop future research priorities for study of RBC transfusion in critically ill children and foster international collaboration in pursuit of these goals.
METHODS
The methodology for TAXI was modeled after the Pediatric Acute Lung Injury Consensus Conference (PALICC) methodology (25) and followed the standards set by the Institute of Medicine for guideline development to create comprehensive evidence-based and when evidence was lacking, expert based recommendations for RBC decision-making in critically ill children. TAXI was proposed to and fully endorsed by the Pediatric Critical Care Blood Research Network (BloodNet). The focus on RBCs represents the first of multiple planned consensus series focused on developing guidelines for transfusion (e.g. RBC, plasma, platelets), and blood management decision-making in critically ill children. The TAXI Executive Committee, composed of two TAXI co-chairs, the BloodNet Executive Committee and evidence-based medicine experts from the Johns Hopkins Evidence-Based Practice Center provided oversight of the entire TAXI process. The details of the TAXI methodology and expert selection are fully described in this supplement of Pediatric Critical Care Medicine (26).
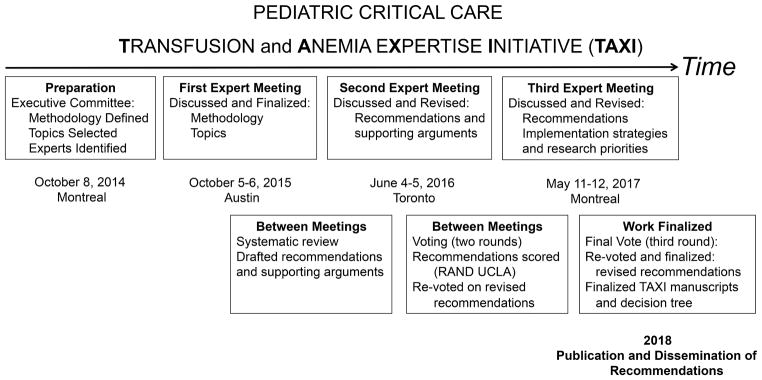
Briefly, the TAXI process included systematic reviews and three consensus meetings, with substantial work between meetings, conducted over the course of 2 years, with an overview provided in Figure 1. Thirty-eight content experts and 4 non-voting methodology and implementation experts, representing 8 countries, 29 academic institutions and 8 medical specialties, agreed and participated in all aspects of TAXI (Appendix 1).
Figure 1.
Timeline and overview of the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. RAND/UCLA= Research And Development/University of California, Los Angeles
During the first TAXI meeting, experts vetted and agreed upon the recommendation development methodology, common definitions, and the following 9 clinical subtopics: indications for RBC transfusion based on Hb and physiologic thresholds in critically ill children 1) in the general PICU population, with 2) respiratory failure, 3) non-hemorrhagic shock, 4) non-life threatening bleeding and hemorrhagic shock, 5) acute brain injury, 6) acquired and congenital heart disease, 7) sickle cell and oncologic disease, 8) support from extracorporeal membrane oxygenation (ECMO), ventricular assist devices (VAD), renal replacement therapy (RRT), and 9) the use of alternative processing of blood products.
The experts agreed upon common definitions to apply to all subgroups reviews and recommendations, as follows: a) RBC transfusion - any transfusion of RBC, whatever the volume or the type of blood product (RBC units or whole blood) transfused; b) critically ill children or those at risk for critical illness - pediatric patients within a an intensive care unit (ICU) which admits full term infants and any child up to at least 18 years of age; c) hemodynamically stable - mean arterial pressure is not less than 2 standard deviations below normal mean for age and cardiovascular support (vasopressors/inotropes and fluids) has not been increased in the last 2 hours, as defined in the TRIPICU study (15); d) severe pediatric acute respiratory distress syndrome (PARDS) - as defined by PALICC (27).
We conducted systematic review for the 9 subtopics and analyzed the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology and the GRADEPRO™ tool, and is described in detail, (with tables for search terms number of papers included, etc.). in the TAXI methodology of this supplement (26).
The recommendations and supporting arguments were drafted after completion of the systematic reviews, discussed in depth, and revised during the second expert meeting (Figure 1). The strength, “strong” (level 1) or “weak” (level 2), was based on weighing the balance between benefits, risks, burden and the costs and the level of evidence, “high quality” or level A, “moderate quality evidence” or level B and “low quality evidence” or level C, was based on the certainty of the evidence. Recommendations without pediatric evidence were presented with justification and rationale by the subgroups for expert consensus. Using the Research And Development/University of California, Los Angeles (RAND/UCLA) appropriateness method (28), the recommendations were scored anonymously using an online tool (Survey Monkey). Agreement was defined a priori as 80% of the experts rating the recommendation a 7, 8, or 9. Recommendations that did not achieve agreement were returned to the respective subgroup experts with the associated comments from the voting process for revision and subsequent re-scoring. All recommendations met >80% agreement after the 2nd round of scoring. During the third expert meeting, the recommendations were presented and any changes made to the recommendations were re-scored to confirm that the changes did not alter the intention of the recommendation. A total of 3 rounds of voting were performed.
Finally, TAXI was dedicated to formulating a TAXI decision tree, formalizing implementation goals and strategies to best support uptake into the pediatric critical care and transfusion medicine communities (29), as well as discussing TAXI research priorities. During the third meeting, a full day was dedicated to discussion/development of both implementation strategies (30) and knowledge gaps in RBC transfusion decision making to guide future research priorities.
RESULTS
The consensus recommendations of TAXI are presented below. All justifications, literature supporting the recommendations from the systematic review, as well as discussion of research priorities within the 9 subgroups are presented in separate subgroup manuscripts in a supplement of Pediatric Critical Care Medicine (31–39). The subgroups developed, scored and finalized 100 recommendations (55 specific clinical recommendations, and 45 research recommendations, which are presented separately, by subgroup) and 2 good practice statements, of which all met a priori > 80% agreement. Of the 119 recommendations initially developed, 95% (n=113) met agreement after the first round and the remaining 5% met agreement after the second round of voting. Nineteen recommendations were subsequently removed for redundancy. The level of evidence (GRADE) is provided for recommendations that are evidence-based.. Voting data (median and interquartile range [IQR]) are provided with each recommendation. Recommendations without direct pediatric evidence, but included based on strong expert opinion, are labeled as “consensus panel expertise”. The TAXI experts placed value on avoiding the rare, but potentially serious complications of RBC transfusion, therefore when evidence suggested no harm from transfusion, a restrictive decision-making strategy was recommended. The RBC transfusion Good Practice Statements, created by the TAXI experts, apply to all critically ill patients, when deciding to transfuse an individual patient. The TAXI consensus recommendations will not apply to all individual transfusion decisions and are not intended to be an absolute standard for transfusion decision making.
Good Practice Statements (31)
1. When deciding to transfuse an individual critically ill child, we recommend considering not only the Hb concentration, but also the overall clinical context (e.g. symptoms, signs, physiological markers, laboratory results) and the risks, benefits, and alternatives to transfusion. Consensus panel expertise, Voting Data (n=29): 97% Agreement (n=29), Median 9, IQR 9-9
2. In critically ill children or those at risk for critical illness, we recommend measuring a Hb concentration before prescribing each RBC transfusion; knowledge of Hb concentration is not required before RBC transfusion if the patient has life threatening bleeding. Consensus panel expertise, Voting Data (n=35): 100% Agreement, Median 9, IQR 8-9
Indications for RBC Transfusion for the General Critically Ill Child Based on Hemoglobin and Physiologic Thresholds (31)
The following recommendations are focused on transfusion decision-making in the general critically ill child and exclude the eight specific subpopulations of critically ill children discussed further in this text.
1.1 In critically Ill children or those at risk for critical illness we recommend a RBC transfusion if the Hb concentration is <5 g/dL. Strong recommendation, Low quality pediatric evidence (1C), 100% Agreement, (n=35), Median 9, IQR 8-9
1.2 In critically ill children or those at risk for critical illness, we cannot recommend a specific RBC transfusion decision-making strategy that is based upon physiologic metrics and biomarkers. Consensus panel expertise, 91% Agreement, n=35, Median 8, IQR 8-9
1.3 In critically ill children or those at risk for critical illness, who are hemodynamically stable and who have an Hb concentration ≥7 g/dL, we recommend not administering a RBC transfusion. Strong recommendation, Moderate quality pediatric evidence (1B), 97% Agreement, (n=29), Median 9, IQR 8-9
1.4 In critically ill children with acute post-operative non-hemorrhagic anemia (excluding cardiac surgery), who are hemodynamically stable, we recommend not administering a RBC transfusion if the Hb concentration is ≥7 g/dL. Weak recommendation, Low quality pediatric evidence (2C), 93% Agreement, (n=29), Median 8, IQR 8-9
1.5 There is insufficient evidence to make a recommendation regarding transfusion thresholds for critically ill children who have an Hb concentration between 5 and 7 g/dL. However, it is reasonable to consider transfusion based on clinical judgment in these children. Consensus panel expertise, 100% Agreement, (n=29), Median 9, IQR 9-9
1.6 In critically ill children or those at risk for critical illness who are hemodynamically stable, we recommend that the post-transfusion goal be to relieve the indication for transfusion and not necessarily achieve normal Hb for age. A reasonable Hb goal post-transfusion is a range between 7.0 g/dL and 9.5 g/dL. Weak recommendation, Low quality pediatric evidence (2C), 96% Agreement, (n=28), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Respiratory Failure (32)
2.1 We recommend RBC transfusion for critically ill children with respiratory failure who have an Hb concentration < 5g/dL. Strong recommendation, Low quality pediatric evidence (1C), 100% Agreement, (n=35), Median 9 IQR 8-9
2.2 In critically ill children with respiratory failure who do not have severe acute hypoxemia, a chronic cyanotic condition or hemolytic anemia, and whose hemodynamic status is stable we recommend not administering a RBC transfusion if the Hb concentration is ≥7 g/dL. Strong recommendation, Moderate quality pediatric evidence (1B), 100% Agreement, (n=29), Median 8.5, IQR 8-9
2.3 In critically ill children with respiratory failure who have severe hypoxemia, we cannot make a recommendation regarding optimal RBC transfusion strategy. Consensus panel expertise, 97% Agreement, (n=29), Median 8, IQR 8-9
2.4 There is insufficient evidence to make a recommendation regarding transfusion thresholds for critically ill children with respiratory failure who have an Hb concentration between 5–7 g/dL. However, it is reasonable to consider transfusion based on clinical judgment in these children. Consensus panel expertise, 97% Agreement, (n=35), Median 9 IQR 8-9
2.5 We cannot recommend a specific RBC transfusion decision-making strategy using physiologic based metrics and biomarkers in critically children with respiratory failure. Consensus panel expertise, 100% Agreement, (n=35), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child With Non-Hemorrhagic Shock (33)
3.1 In critically ill children with non-hemorrhagic shock, we recommend to consider all possible strategies to augment oxygen delivery and decrease oxygen demand and not RBC transfusion alone. Consensus panel expertise, 97% Agreement, (n=35), Median 9 IQR 8-9
3.2 We cannot recommend a specific RBC transfusion decision-making strategy using physiologic based metrics and biomarkers in critically ill children with non-hemorrhagic shock. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
3.3 We cannot make a recommendation regarding transfusion thresholds for critically ill children with unstable non-hemorrhagic shock. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
3.4 In hemodynamically stable critically ill children with a diagnosis of severe sepsis or septic shock, we recommend not administering a RBC transfusion if the Hb concentration is ≥ 7 g/dL. Weak recommendation, Low quality pediatric evidence (2C), 96% Agreement, (n=29), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child With Non-Life Threatening Bleeding or Hemorrhagic Shock (34)
4.1 In critically ill children with non-life threatening bleeding, we recommend that a RBC transfusion should be given for an Hb concentration <5 g/dL. Weak recommendation, Low quality pediatric evidence (2C), 100% Agreement, (n=35), Median 9, IQR 8-9
4.2 In critically ill children with non-life threatening bleeding, we recommend that a RBC transfusion should be considered for an Hb concentration between 5–7 g/dL. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
4.3 In critically ill children with hemorrhagic shock, we suggest that RBCs, plasma and platelets be transfused empirically in ratios between 2:1:1 to 1:1:1 for RBCs:plasma:platelets until the bleeding is no longer life-threatening. Consensus panel expertise, 94% Agreement, (n=35), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child With Acute Brain Injury (35)
5.1 In critically ill children with acute brain injury (e.g., trauma, stroke) a RBC transfusion could be considered if the Hb concentration falls between 7 – 10 g/dL. Consensus panel expertise, 90% Agreement, (n=30), Median 8, IQR 7-8
5.2 In critically ill children with acute brain injury (e.g., trauma, stroke), we cannot recommend the use of brain oxygen monitoring in determining when to administer a RBC transfusion. Consensus panel expertise, 91% Agreement, (n=35), Median 8 IQR 8-8
Indications for RBC Transfusion for the Critically Ill Child With Acquired and Congenital Heart Disease (36)
Good Practice Statements
6.1 In children with cardiac disease we recommend optimization of all the components contributing to oxygen delivery, including but not limited to achievement/maintenance of: normal sinus rhythm and/or heart rate control, optimal preload and contractility, optimal right ventricular and left ventricular afterload, adequate oxygenation and/or reduction of oxygen demand, as appropriate before initiation of RBC transfusion, except in the case of hemorrhagic shock. Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
6.2 For all children with congenital and acquired heart disease, the benefits and risks of transfusion must be considered before transfusion. Whenever possible, adoption of blood sparing and conservation procedures and guidelines should be implemented. Consensus panel expertise, 93% Agreement, (n=30), Median 8, IQR 8-9
6.3 In children undergoing cardiac surgery (repair or palliation) or heart transplants, when deciding to transfuse, we recommend considering not only the Hb concentration but also the overall clinical context (e.g. symptoms, signs, physiological markers, laboratory results) and the risk, benefits, and alternatives to transfusion. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
6.4 In infants and children with congenital heart disease we recommend investigating and treating pre-operative anemia in addition to implementing transfusion/blood management guidelines/blood conservation practices. Consensus panel expertise, 94% Agreement, (n=35), Median 9, IQR 8-9
6.5 In hemodynamically stable infants and children with CHD and adequate oxygenation (for their cardiac lesion) and normal end organ function who are awaiting cardiac surgery, we recommend that the risks, benefits, and alternatives of RBC transfusions must be carefully considered when deciding to give an RBC transfusion. Consensus panel expertise, 85% Agreement, (n=35), Median 8, IQR 7.25-9
Clinical recommendations
6.6 In children with documented right or left ventricular myocardial dysfunction (acquired or congenital), there is insufficient evidence to support transfusion to target a specific Hb concentration. Furthermore, there is no evidence that transfusion above an Hb level > 10 g/dL is beneficial. Consensus panel expertise, 83% Agreement, (n=30), Median 8 IQR 7.25-8.75
6.7 In children with structurally normal heart and idiopathic or acquired pulmonary hypertension, (defined as a mean pulmonary arterial pressure > 25 mmHg with normal pulmonary capillary wedge pressure) there is insufficient evidence to support transfusion to target a specific Hb concentration. Furthermore, there is no evidence that transfusion above an Hb level > 10 g/dL is beneficial. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
6.8 In hemodynamically stable critically ill infants and children with uncorrected CHD, we recommend RBC transfusion to maintain an Hb concentration of at least 7.0– 9.0 g/dL depending on the degree of cardiopulmonary reserve. Weak recommendation, Low quality pediatric evidence (2C), 81% Agreement, (n=35), Median 8, IQR 7-8
6.9 In infants and children undergoing cardiac surgery, we recommend development and adoption of intra- and postoperative blood-sparing and blood conservation procedures and guidelines with the goal of reducing the number and volume of RBCs transfused (pump prime, on cardio-pulmonary bypass (CPB), after separation from CPB, and post-op), and limiting donor exposures and other blood component transfusions. Strong recommendation, Low quality pediatric evidence (1C), 100% Agreement, (n=35), Median 9, IQR 8
6.10 In infants undergoing stage 1 palliation procedures (Norwood, Damus-Kaye-Stansel, Blalock-Taussig or central shunt, or pulmonary artery band) for single ventricle physiology who have stable hemodynamics, and adequate oxygenation (for their cardiac lesion) and normal end organ function we recommend avoiding reflexive (“solely Hb-based”) RBC transfusions if the Hb concentration is >9.0 g/dL. Weak recommendation, Low quality pediatric evidence (2C) 96% Agreement, (n=29), Median 8, IQR 7-9
6.11 In hemodynamically stable infants and children with single ventricle physiology undergoing stage 2 and 3 procedures with adequate oxygen delivery we recommend not administering a RBC transfusion if the Hb concentration is >9 g/dL. Weak recommendation, Low quality pediatric evidence (2C), 96% Agreement, (n=29), Median 8, IQR 8-9
6.12 In infants and children with CHD undergoing biventricular repair who are hemodynamically stable and have adequate oxygenation and normal end organ function, we recommend not administering a RBC transfusion if the Hb concentration is ≥7.0 g/dL. Strong recommendation, Moderate quality pediatric evidence (1B), 100% Agreement, (n=29), Median 8.5, IQR 7-9
6.13 Standard issue RBC transfusions should be utilized in children with acquired or congenital heart disease as there are insufficient data supporting the transfusion of RBCs of shortened storage age in this population. Weak recommendation, Low quality pediatric evidence (2C), 93% Agreement, (n=29), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child With Hematologic and Oncologic Diagnoses (37)
Sickle Cell Disease
7.1 In children with sickle cell disease who are critically ill or those at risk of critical illness, we recommend RBC transfusion to achieve a target Hb concentration of 10 g/dL (rather than a hemoglobin S (HbS) of <30%) prior to a surgical procedure requiring general anesthesia. Strong recommendation, Moderate quality pediatric evidence (1B), 96% Agreement, (n=29), Median 8, IQR 8-9
7.2 In children with sickle cell disease who are critically ill or at risk of critical illness, there is insufficient evidence to recommend an optimal Hb concentration threshold or percent HbS for RBC transfusion prior to minor surgical procedures. Consensus panel expertise, 91% Agreement, (n=35), Median 8, IQR 8-9
7.3 In children with sickle cell disease and acute chest syndrome (ACS) who are critically ill, we recommend an exchange transfusion over a simple (non-exchange) transfusion if the child’s condition is deteriorating (based on clinical judgment); otherwise a simple (non-exchange) RBC transfusion is recommended. Strong recommendation, Low quality pediatric evidence (1C), 97% Agreement, (n=35), Median 9, IQR 8-9
7.4 In children with sickle cell disease and pulmonary hypertension who are critically ill or at risk for critical illness, there is insufficient evidence to recommend the optimal Hb concentration threshold or percent HbS for RBC transfusion or the method of RBC transfusion. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
7.5 In children with sickle cell disease and acute stroke who are critically ill, there is insufficient evidence to recommend the optimal Hb concentration threshold or percent HbS for RBC transfusion; the preferred method of RBC transfusion is exchange transfusion if instituted quickly. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
Oncologic Disease
7.6 In children with oncologic diagnoses who are critically ill or at risk for critical illness, and hemodynamically stable, we suggest an Hb concentration of 7–8 g/dL be considered a threshold for RBC transfusion. Weak recommendation, Low quality pediatric evidence (2C) 88% Agreement, (n=35), Median 8, IQR 7-8
Bone Marrow Transplantation
7.7 In children undergoing hematopoietic stem cell transplant who are critically ill or at risk for critical illness, and are hemodynamically stable, we suggest a Hb concentration of 7–8 g/dL be considered a threshold for RBC transfusion. Weak recommendation, Low quality pediatric evidence (2C) 88% Agreement, (n=35), Median 8, IQR 7-8
Indications for RBC Transfusion for the Critically Ill Child Receiving Support from extracorporeal membrane oxygenation (ECMO), ventricular assist device (VAD) and renal replacement therapy (RRT) (38)
ECMO
8.1 In critically ill children on ECMO, we recommend reporting Hb concentration, rather than hematocrit, for RBC transfusion threshold algorithms. Consensus panel expertise 97% Agreement, (n=35), Median 8, IQR 8-9
8.2 In critically ill children on ECMO, we recommend measuring Hb concentration before all RBC transfusion, unless the patient experiences life-threatening bleeding. Consensus panel expertise. 97% Agreement, (n=35), Median 8, IQR 8-9
8.3 In critically ill children on ECMO, we recommend that adoption of blood sparing and conservation procedures and guidelines should be implemented. Consensus panel expertise. Voting Data (n=35): 94% Agreement, Median 8, IQR 8-9
8.4 In critically ill children on ECMO, we recommend taking measures to minimize the number of donor exposures. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
8.5 In critically ill children on ECMO, we recommend that all RBC exposure within circuit prime be reported in pediatric ECMO transfusion studies and quality improvement projects. Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
8.6 In critically ill children on ECMO, we recommend using physiologic metrics and biomarkers of oxygen delivery in addition to Hb concentration to guide RBC transfusion. Administration of a RBC transfusion should be based on evidence of inadequate cardiorespiratory support or decreased systemic and/or regional oxygen delivery. Weak recommendation, Low quality pediatric evidence (2C), 97% Agreement, (n=35), Median 8, IQR 8-9
8.7 In critically ill children on ECMO, there is insufficient evidence to recommend a specific RBC transfusion decision-making strategy using physiologic-based metrics and biomarkers. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
VAD
8.8 In critically ill children on VAD support, we recommend using physiologic metrics and biomarkers of oxygen delivery in addition to Hb concentration to guide RBC transfusion. Administration of a RBC transfusion should be based on evidence of inadequate cardiorespiratory support or decreased systemic and/or regional oxygen delivery. Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
RRT
8.9 In critically ill children on RRT support, we recommend using the smallest circuit size that will provide adequate RRT whilst minimizing a driver for RBC transfusion specific to RRT (i.e., loss of blood volume that arises with circuit dysfunction/replacement of the circuit). Consensus panel expertise, 100% Agreement, (n=35), Median 9 IQR 8-9
8.10 In critically ill children on RRT support who are hemodynamically stable with optimized intravascular volume status and no evidence of inadequate oxygen delivery or bleeding, we recommend not routinely administering a RBC transfusion if the Hb concentration is >7 g/dL. Consensus panel expertise, 100% Agreement (n=35), Median 8, IQR 8-9
Selection and Processing of RBC Components in Critically Ill Children (39)
9.1 We recommend the use of irradiated cellular blood components for all critically ill children at risk for transfusion-associated graft versus host disease (ta-GVHD) due to severe congenital or acquired causes of immune deficiency. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
9.2 We recommend the use of irradiated cellular blood components for all critically ill children when the blood donor is a blood relative of the child. Strong recommendation, Low quality pediatric evidence (1C), 100% Agreement, (n=35), Median 9, IQR 8-9
9.3 We recommend the use of the washed cellular blood components and avoidance of other plasma containing products (e.g. plasma, cryoprecipitate, etc.) for critically ill children with history of severe allergic reactions or anaphylaxis to blood transfusions, although patient factors appear to be critically important in the pathogenesis. Consensus panel expertise, 100% Agreement, (n=29), Median 9, IQR 8-9
9.4 For critically ill children with a history of severe allergic transfusion reaction(s), we recommend considering evaluation of allergic stigmata (anti-IgA antibodies in IgA deficient individuals, anti-haptoglobin antibodies—using a pre-transfusion specimen) prior to RBC transfusion. Consensus panel expertise, 96% Agreement, (n=29), Median 8, IQR 8-9
9.5 In critically ill children with suspected or documented severe IgA deficiency (undetectable), evidence of Anti-IgA antibodies, and/or a history of a severe transfusion reaction, we recommend using IgA deficient blood components obtained either from an IgA deficient donor and/or washed cellular components. Consensus panel expertise, 100% Agreement, (n=29), Median 8.5, IQR 8-9
TAXI Research Recommendations
Indications for RBC Transfusion for the General Critically Ill Child Based on Hemoglobin and Physiologic Thresholds (31)
R1.1 In critically ill children or those at risk for critical illness, we recommend creating clinical research programs specifically designed to determine the efficacy and safety of transfusion decision-making based upon physiologic metrics and biomarkers. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
R1.2 In children with critical illness or at risk for critical illness, we recommend investigation that identifies and evaluates biomarkers and/or physiologic measures that characterize anemia intolerance. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
R1.3 We recommend investigation to determine biomarkers or physiologic measures that identify anemia intolerance, defined as threat to O2 delivery and/or O2 consumption homeostasis, and manifested as an increase in global anaerobic metabolism. Consensus panel expertise 97% Agreement, (n=35), Median 8, IQR 8-9
R1.4 We recommend investigation that identifies and evaluates biomarkers and/or physiologic metrics of anemia intolerance specific to individual vital organs, which may be present and indicate patient-specific likelihood of benefit from transfusion, even in the absence of measures indicating systemic impairment of O2 delivery and/or O2 consumption homeostasis. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R1.5 We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in hemodynamically unstable critically ill children. Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8-9
R1.6 We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in subpopulations of hemodynamically stable critically ill children or those at risk for critical illness. Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8-9
R1.7 We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in hemodynamically stable critically ill children or those at risk for critical illness, when the Hb level is between 5 g/dL and 7 g/dL. Consensus panel expertise, 83% Agreement, (n=35), Median 8, IQR 7-8
R1.8 We recommend investigation that will inform priority (e.g. sequencing) of RBC transfusion relative to other interventions, which may either: (a) improve anemia tolerance or (b) improve O2 delivery homeostasis by supporting physiologic compensation for anemia. Consensus panel expertise, 91% Agreement, (n=35), Median 8, IQR 8-9
R1.9 In addition to investigation of physiologic metrics and biomarkers likely to indicate patient-specific likelihood of benefit of transfusion in patients with anemia, we recommend investigation that seeks evidence of patient-specific likelihood of harm from transfusion (both acute and long term). Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8-9
R1.10 We recommend investigations that seek evidence on thresholds or triggers that would tell practitioners that the risk/benefit ratio tolerating anemia is higher that the risk/benefit ratio of giving a RBC transfusion in critically ill children. Consensus panel expertise, 94% Agreement, (n=35) Median 9, IQR 8-9
R1.11 We recommend investigation that seeks evidence that, once the decision to transfuse has been made, will inform a titrated approach to administering RBCs, to maintain the risk of transfusion as low as reasonably achievable, while monitoring for resolution of the original indication for transfusion. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Respiratory Failure (32)
R2.1 We recommend future studies to evaluate the utility of physiologic markers of oxygen consumption and oxygen delivery that can guide RBC transfusion decisions for critically ill children with respiratory failure. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R2.2 We recommend further studies to determine the risk, benefits and alternatives of transfusion in unstable anemic children with respiratory failure, in particular if associated with severe hypoxemia or hemodynamic instability. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Non-Hemorrhagic Shock (33)
R3.1 We recommend future studies to evaluate the utility of physiologic markers of oxygen debt and oxygen delivery in conjunction with hemoglobin-based targets to guide RBC transfusion decisions for critically ill children with non-hemorrhagic shock. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R3.2 We recommend future studies to determine optimum transfusion thresholds for critically ill children with non-hemorrhagic shock undergoing acute resuscitation. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R3.3 The relative risks, benefits and alternatives of RBC transfusion to augment oxygen delivery remain unclear and should be the subject of future studies in critically ill children with non-hemorrhagic shock. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R3.4 We recommend future studies to determine long-term effects of anemia in children with non-hemorrhagic shock. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Non-Life Threatening and Hemorrhagic Shock (34)
R4.1 In children with non-life threatening bleeding, we recommend future studies to develop physiologic and laboratory measures to indicate the need for RBC transfusions. Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
R4.2 We recommend future studies to determine if goal directed hemostatic resuscitation improves survival compared to an empiric ratio approach in critically ill children with hemorrhagic shock. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
R4.3 We recommend future studies to determine if low titer group O whole blood is more efficacious and safe compared to reconstituted whole blood with components for critically ill children with hemorrhagic shock. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Acute Brain Injury (35)
R5.1 In critically ill children with acute brain injury (e.g., trauma, stroke), we recommend further clinical trials testing the transfusion threshold or Hb concentration that has the best long-term functional outcomes. In particular, specific populations need to be studied separately (e.g., trauma, stroke) since the physiology of oxygen delivery and extraction may differ. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R5.2 In critically ill children with acute brain injury (e.g., trauma, stroke), we recommend further clinical physiology studies to evaluate whether there is a role for brain oxygen monitoring in informing the decision whether to transfuse RBCs. Consensus panel expertise, 94% Agreement, (n=35), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child with Acquired and Congenital Heart Disease (36)
R6.1 We recommend further studies to determine the risks and benefits of RBC transfusion in critically ill children with documented right or left ventricular myocardial dysfunction (acquired or congenital). Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R6.2 We recommend further studies to determine the risks and benefits of transfusion in critically ill children with structurally normal hearts and idiopathic or acquired pulmonary hypertension (defined as a mean pulmonary arterial pressure > 25 mmHg with normal pulmonary capillary wedge pressure). Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R6.3 We recommend further studies in infants and children with CHD undergoing cardiac surgery to determine the impact of pre-operative anemia management on perioperative RBC transfusions and outcomes. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R6.4 In infants and children undergoing cardiac surgery with CPB, further research is needed to determine the benefits and risks associated with the administration of RBC to the CPB-prime, on-bypass and after separation of CPB. Consensus panel expertise, 97% Agreement, (n=35), Median 8, IQR 8-9
R6.5 In infants and children undergoing cardiac surgery further studies are needed to investigate the complex relationship between anemia, RBC transfusion, oxygen delivery and utilization and outcomes; with focus on which patient subgroups may benefit from, or be harmed by RBC transfusion. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
R6.6 We recommend that clinical trials on RBC transfusion in pediatric cardiac surgery report the volume of RBC transfused and number of donor exposures. Consensus panel expertise, 94% Agreement, (n=35), Median 9, IQR 8-9
R6.7 Further studies are needed in infants undergoing stage 1 surgical palliations for single ventricle physiology on Hb concentration and physiologic indications for RBC transfusion. Consensus panel expertise, 100% Agreement, (n=35), Median 9, IQR 8-9
R6.8 In children with acquired heart disease or CHD, further studies are warranted to determine if RBC storage time impacts clinical outcomes. Weak recommendation, Low quality pediatric evidence (2C), 90% Agreement, (n=30), Median 8, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child With Hematologic and Oncologic Diagnoses (37)
Thalassemia
R7.1 In critically ill children with thalassemia, we recommend undertaking well-designed registries or expanding current initiatives to determine measures and limits of anemia tolerance, examine current practice, and define clinical outcomes to inform future research investigating the risks, benefits and alternatives of RBC transfusion practice. Consensus panel expertise, 100% Agreement, (n=29), Median 9, IQR 8-9
Sickle Cell Disease
R7.2 In children with sickle cell disease who are critically ill or at risk for critical illness, we recommend a well-designed registry or enhancement of existing network databases to further clarify optimal transfusion management. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R7.3 In children with sickle cell disease who are critically ill or at risk for critical illness, we recommend future research studies to evaluate the optimal Hb concentration threshold and/or percent HbS to guide RBC transfusion decisions prior to minor surgical procedures. Consensus panel expertise, (n=35): 100% Agreement, (n=35), Median 9, IQR 8-9
Auto- or Allo-immune Hemolytic Anemia
R7.4 In children with auto- and/or allo-immune mediated hemolytic anemia who are critically ill or at risk for critical illness, we recommend undertaking well-designed registries to determine measures and limits of anemia tolerance, examine current practice, and define clinical outcomes to inform future research investigating the risks, benefits and alternatives of RBC transfusion practice. Consensus panel expertise, 100% Agreement, (n=29), Median 9, IQR 8-9
Oncologic Disease
R7.5 In children with oncologic disease who are critically ill or at risk of critical illness, we recommend undertaking well-designed registries or expanding current initiatives to inform future research investigating the risks, benefits and alternatives of transfusion practice. Consensus panel expertise, 97% Agreement, (n=29), Median 9, IQR 8-9
Radiation Therapy (XRT)
R7.6 In children receiving emergency radiation therapy (XRT) who are critically ill or at risk for critical illness, we recommend exploration of existing databases to investigate the impact of Hb concentration and RBC transfusion on disease response, survival and other toxicities to inform creation of contemporary registries to investigate these associations. Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
Bone Marrow Transplantation
R7.7 In children undergoing HSCT who are critically ill or at risk for critical illness, we recommend undertaking well-designed registries or expanding current initiatives to inform future research investigating the risks, benefits and alternatives of transfusion practice. Consensus panel expertise, 97% Agreement, (n=29), Median 9, IQR 8-9
Indications for RBC Transfusion for the Critically Ill Child Receiving Support from extracorporeal membrane oxygenation (ECMO), ventricular assist device (VAD) and renal replacement therapy (RRT) (38)
ECMO
R8.1 In critically ill children on ECMO, we recommend that Hb concentrations and correlations with physiologic indications for RBC transfusion be studied to determine minimum thresholds for safety and efficacy of RBC transfusion. Consensus panel expertise, 97% Agreement, (n=35), Median 9, IQR 8-9
R8.2 In critically ill children on ECMO, we recommend undertaking future studies of oxygen delivery/consumption markers (e.g., mixed venous saturation, cerebral oximetry, somatic oximetry, etc.) in patients maintained at different Hb thresholds. Such studies will aim to determine the optimal physiologic thresholds for RBC transfusion during pediatric ECMO. Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8-9
R8.3 In critically ill children who suffer from cardiac arrest pre-ECMO (i.e., extracorporeal cardiopulmonary resuscitation [ECPR]) and critically ill children with acute neurologic injury during ECMO (e.g., embolic stroke, intracranial hemorrhage, etc.), we recommend undertaking future studies for RBC transfusion strategies that optimize neuroprotection and recovery. Consensus panel expertise, 91% Agreement, (n=35), Median 8, IQR 8-9
R8.4 In critically ill children on ECMO, we recommend undertaking future studies of the types of RBC manipulations and attributes and their impact on outcomes (e.g., storage duration, irradiation, leukoreduction, filtration, matching for CMV/EBV serologic status, extended minor antigen matching, washing, etc.). Consensus panel expertise, 94% Agreement, (n=35), Median 8, IQR 8-9
VAD
R8.5 In critically ill children on VAD support, we recommend undertaking future studies of oxygen delivery/consumption markers (e.g., mixed venous saturation, cerebral oximetry, somatic oximetry, etc.). Such studies will aim to determine the optimal physiologic thresholds for RBC transfusion during pediatric VAD support. Consensus panel expertise, 100% Agreement, (n=35), Median 8.5, IQR 8-9
R8.6 In critically ill children on VAD/ECMO support, we recommend undertaking future studies to determine the impact of RBC transfusions on allo-sensitization, success of organ acquisition and/or risk of rejection. Consensus panel expertise, 100% Agreement, (n=35), Median 8, IQR 8-9
R8.7 In critically ill children on VAD support, we recommend undertaking future studies of the types of RBC manipulations and attributes and their impact on outcomes (e.g., storage duration, irradiation, leukoreduction, filtration, matching for CMV/EBV serologic status, extended minor antigen matching, washing, etc.). Consensus panel expertise, 100% Agreement, (n=35), Median 8, IQR 8-9
RRT
R8.8 In critically ill children on RRT support, we recommend undertaking future studies to determine how to optimize RRT length of use and hence minimize blood loss due to RRT circuit change/replacement. Consensus panel expertise, 100% Agreement, (n=35), Median 8, IQR 8-9
DISCUSSION
The breadth of recommendations presented in this manuscript aims to provide a comprehensive guide to RBC transfusion in a wide range of pediatric patients cared for in PICUs across the world. The goal of TAXI was to focus on the various subpopulations of children who have the highest risk of becoming anemic and receiving the most transfusions. TAXI used our best means of providing clear transfusion decision-making tools for PICU practitioners. The results of this effort have led to a combination of general guidance good practice statements, specific clinical recommendations backed by pediatric evidence, and a keen awareness of many areas still in need of evidence before any recommendation can be made.
The good practice statements are general principles that should apply to all clinical scenarios when a transfusion is being considered. Hb concentration can only be considered a surrogate marker of the capacity for oxygen delivery in critically ill children, so using it alone to determine RBC transfusion must be cautioned. The degree of compensation for anemia or anemia tolerance for critically ill patients through physiological metrics should factor into decision-making. The need for thoughtful consideration of the risks and benefits of RBC transfusion has become increasingly necessary, as the untoward effects of RBC transfusions, such as NISHOT, have emerged, particularly in the critically ill (10–14). The limitations of donor RBC’s to improve oxygen delivery deficits in the critically ill also have become more apparent (40), hence the recommendation to enhance all other means of improving oxygen delivery or decreasing oxygen demand prior to RBC transfusion. These good practice statements all seek to highlight a major tenet of patient blood management principles: avoid unnecessary RBC transfusions (41).
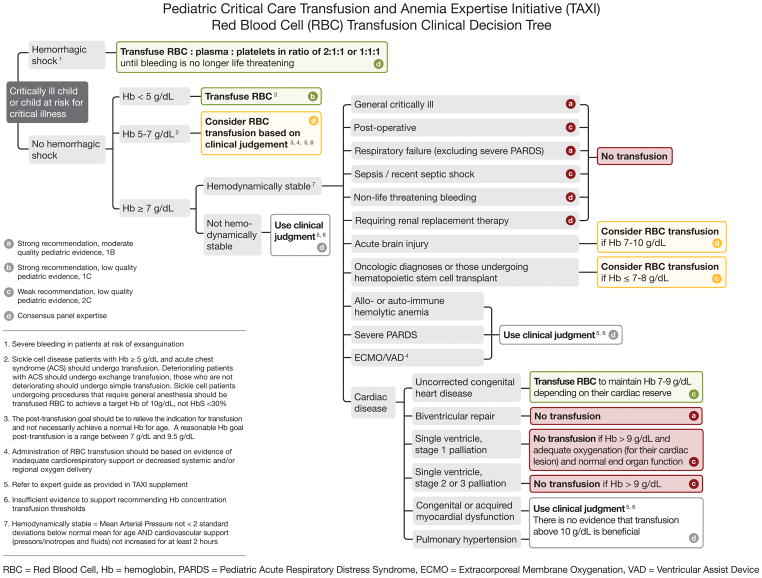
The clinical recommendations supported by pediatric evidence are presented across the various subgroups. The decision tree, displayed in Figure 2, summarizes these specific recommendations. It is important to highlight that only studies conducted in children were used to support our recommendations. That limited our data significantly, as much more adult data are available, but also strengthened our conclusions for children. Important data on RBC transfusions in critically ill children exist and provide high GRADE evidence that “restrictive” RBC transfusion practices in certain populations are safe and tolerated and decrease RBC transfusion events and volume.
Figure 2.
TAXI RBC Transfusion Decision Tree for Critically Ill Children
RBC: red blood cell, Hb: hemoglobin, PICU: pediatric intensive care unit, PARDS: pediatric acute respiratory distress syndrome, ECMO: extracorporeal membrane oxygenation, VAD: ventricular assist device.
Using Hb values to inform RBC transfusion decision-making remains the most common factor for pediatric intensivists (1) and has been the focus of most research on the topic. A Hb concentration <5 g/dL should always be seen as a threshold for RBC transfusion (except in the case of auto- or allo-immune hemolytic anemia) due to increased mortality noted in children with such a low Hb (2–6). When the hemoglobin level falls between 5.0 and 7.0 g/dL, it is unclear if the benefits outweigh the risks of RBC transfusion, necessitating further study. If the Hb concentration is equal to or above 7 g/dL and the patient is hemodynamically stable, then there are few situations where a transfusion is recommended (15,16, 42–45). In fact, our recommendations state to not transfuse children if the Hb is that high. Those few situations where a higher Hb may be preferred, such as single ventricle physiology, sickle cell disease with acute chest syndrome, oncology or HSCT patients, hemorrhagic and non-hemorrhagic shock, and acute brain injury, are highlighted above. These recommendations can be considered an adoption of a broad based restrictive RBC transfusion approach, also in line with the principles of patient blood management.
The TAXI recommendations have many similarities to those published in adults (23). Restrictive transfusion practices were first studied and found safe in critically ill adults (46) and has led to multiple large-scale adult studies solidifying the practice of lower Hb thresholds prior to RBC transfusion (47). Due to the inability to practically repeat many such studies in children, it is reassuring that the available pediatric data confirm and corroborate the adult findings. Subgroups incorporated adult data into their long text recommendation justification to provide a framework of available information. When stated, some adult data were used to inform expert consensus, if pediatric data were not available.
Our decision tree outlining the major recommendations of TAXI provides the first step in translating our recommendations into usable tools to improve uptake at the bedside. The TAXI implementation experts provided ongoing support, editing, and guidance on recommendation development (30, 48–50). We were thoughtful about dissemination of these recommendations, our target audience (primarily critical care practitioners, blood bankers), and publication strategy. The support of a broad range of organizations, such as BloodNet, PALISI, AABB, Society of Critical Care Medicine (SCCM), Society for the Advancement of Blood Management (SABM), National Institute of Child Health and Human Development (NICHD) and National Heart, Lung, and Blood Institute (NHLBI) ensure that our recommendations will be broadly accepted and adopted. We plan to continue to update the recommendations using our on-line repository of published literature as new data emerges. Important research continues to be conducted on this topic and will need to be integrated on an ongoing basis.
As can be noted from our recommendations, almost half are considered research. This was deliberate to 1) highlight what is not known in children, and 2) galvanize the research community to help answer these important RBC transfusion questions. A major theme of our research recommendations is an emphasis on anemia tolerance in children and finding other means of RBC transfusion indication besides Hb. Other physiological metrics easily obtainable from children need to be studied to help guide RBC transfusions decisions, as well as to allow following the amelioration of these indications after transfusion. We are aware of the difficulties of conducting clinical trials in critically ill children, but feel that we must encourage primary pediatric data to guide future recommendations. The funding priorities for research in RBC transfusions can hopefully be aligned with these recommendations. It is encouraging to see the research focus complement other efforts in pediatrics, such as the NHLBI state of the science initiative (51).
The strengths of TAXI are that it is the first consensus series to convene a group of international and multidisciplinary experts to use standardized guideline development principles to develop recommendations on RBC transfusion in critically ill children. It was a rigorous, large scale, formal systematic review with expert consensus achieved through multiple rounds of debate and refinement. Agreement of over 80% of our experts allows the recommendations to be highly acceptable to the pediatric critical care community. We engaged expertise from evidence-based and implementation science specialists to ensure that our systematic review of the literature and recommendation formation were performed according to published standards.
The TAXI recommendations are limited by the paucity of pediatric data in many subpopulations. There was heavy reliance on a few seminal articles that were applicable across multiple subpopulations. Other aspects of RBC transfusion, such as storage age of blood, volume of RBC to transfuse, whole blood factors, or RBC transfusion in active resuscitation could not be addressed. Expert consensus for clinical recommendations must always be appropriately scrutinized for legitimacy. Our systematic approach, standardized procedures, and careful objective guidance of TAXI participants provide reassurance and validity to the final product. Study design for answering some of the research recommendations could be a significant challenge. TAXI’s effort on RBC’s alone was also deliberate to allow for a focused approach to our recommendations. Similar efforts are needed in other blood products, such as platelets or plasma. TAXI’s RBC initiative is considered phase 1 of a comprehensive blood management program through BloodNet that will seek in the near future to engage experts in these other blood products to guide their use in children.
CONCLUSIONS
The TAXI Consensus Conference recommendations have the potential to impact global RBC transfusion practices for critically ill children. TAXI has developed pediatric specific recommendations regarding RBC transfusion management in the critically ill child across a variety of patient subpopulation, as well as recommendations to help guide future research priorities. Clinical recommendations emphasized relevant Hb thresholds and research recommendations emphasized a need for further understanding of anemia tolerance, physiological thresholds, alternatives to RBC transfusion, and Hb thresholds in populations with no pediatric literature. TAXI plans to continue to improve transfusion practices and ultimately outcomes in critically ill children receiving or at risk to receive an RBC transfusion by continuing to update its recommendations as new data emerges.
Acknowledgments
Sources of Funding: The Transfusion and Anemia Expertise Initiative was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Heart, Lung, and Blood Institute under award number 1 R13 HD088086-01, Society for the Advancement of Blood Management SABM-Haemonetics Research Starter Grant, CHU-Sainte-Justine Foundation, Washington University Children’s Discovery Institute (DCI-E1-2015-499) and the University of Massachusetts Medical School.
Dr. Bembea received support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number K23NS076674.
We thank all members of TAXI for their support and their comments. TAXI was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Heart, Lung, and Blood Institute (1 R13 HD088086-01), the Society for the Advancement of Blood Management SABM-Haemonetics Research Starter Grant, CHU-Sainte-Justine Foundation, Washington University Children’s Discovery Institute (DCI-E1-2015-499) and the University of Massachusetts Medical School. We also thank the World Federation of Pediatric Intensive and Critical Care Societies, Society for Critical Care Medicine and the AABB for their support of TAXI.
Appendix 1: Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Members
(* for Executive Committee) Co-chairs: Stacey L. Valentine MD MPH* and Scot T. Bateman MD*, University of Massachusetts, USA, Content Experts: Section 1. General pediatric critical care patient based on physiologic and hemoglobin thresholds: Andrew Argent MD MBBBCh, University of Cape Town, South Africa, Jeffrey L. Carson MD, Rutgers Robert Wood Johnson Medical School, USA, Jill M. Cholette MD*, University of Rochester, USA, Allan Doctor MD*, Washington University of St. Louis, USA, Jacques Lacroix MD*, Universite de Montréal, Canada, Kenneth Remy MD, Washington University of St. Louis, USA, Section 2. Respiratory failure: Pierre Demaret MD MSc, CHC Liege, Belgium, Guillaume Emeriaud MD PhD, Université de Montréal, Canada, Nabil E. Hassan MD, University of Illinois, USA, Martin C.J. Kneyber MD PhD, University of Groningen, Netherlands, Marisa Tucci MD*, Université de Montréal, Canada, Section 3. Shock, excluding hemorrhagic shock: Nina Guzzetta MD, Emory University, USA, Mark W. Hall MD, Ohio State University, USA, Jennifer A. Muszynski MD MPH, Ohio State University, USA, Philip C. Spinella MD, Washington University of St. Louis, USA, Duncan Macrae MB ChB, Imperial College London, UK, Section 4. Hemorrhagic shock and non-life-threatening bleeding, Oliver Karam MD PhD, Virginia Commonwealth University, Robert T. Russell MD MPH, University of Alabama, USA, Philip C. Spinella MD*, Washington University of St. Louis, USA, Paul Stricker MD, University of Pennsylvania, USA, Adam M. Vogel MD, Texas Children’s Hospital, USA, Section 5. Acute brain injury: Philip C. Spinella MD*, Washington University of St. Louis, USA, Robert C. Tasker MA MD MBBS, Harvard University, USA, Alexis F. Turgeon MD MSc, Université Laval, Canada, Section 6. Acquired or congenital heart disease, Jill M. Cholette MD*, University of Rochester, USA, Steven M. Schwartz MD, University of Toronto, Canada, Ariane Willems MD, University of Brussels, Belgium, Section 7. Sickle cell/ oncologic disease, Cassandra D. Josephson MD, Emory University, USA, Naomi LC Luban MD, George Washington University, USA, Leslie E. Lehmann MD, Harvard University, USA, Robert I. Parker MD*, Stony Brook University, USA, Simon J. Stanworth MD, NHS Blood and Transplant, Oxford, UK, Marie E. Steiner MD MS*, University of Minnesota, USA, Nicole D. Zantek MD PhD, University of Minnesota, USA, Section 8. Receiving support from extracorporeal, ventricular assist and renal replacement therapy devices: Melania M. Bembea MD PhD*, Johns Hopkins University, USA, Timothy Bunchman MD, Virginia Commonwealth University, USA, Ira M. Cheifetz MD, Duke University, USA, James Fortenberry MD, Emory University, USA, Marie E. Steiner MD MS*, University of Minnesota, USA, Section 9. Selection and processing of red blood cell components: Meghan Delaney DO, MPH, Children’s National Health System USA, Cassandra D. Josephson MD, Emory University, USA, Robert I. Parker MD*, Stony Brook University, USA, Leo van de Watering MD, Leiden University, Netherlands, Nicole D. Zantek MD PhD, University of Minnesota, USA, Evidenced-Based Medicine: Karen A. Robinson PhD, Johns Hopkins University, USA, Melania M Bembea MD PhD*, Johns Hopkins University, USA, Implementation Science: Sara Small MS, Washington University of St. Louis, USA, Katherine Steffen MD, Stanford University, USA
Footnotes
Conflicts of Interest: Dr. Zantek has the following disclosures not related to this project: Octapharma, Terumo BCT, Bayer HealthCare (research funding), Endo International PLC, Boston Scientific (financial interest), North American Specialized Coagulation Laboratory Association (executive board). Dr. Josephson has the following disclosures not related to this project: consultant for Biomet Zimmer, Immuncor and Octapharma. The other authors have no relevant disclosures.
Copyright form disclosure: Drs. Valentine, Bembea, Doctor, Steiner, Josephson, Luban, Zantek, Steffen, and Bateman received support for article research from the National Institutes of Health (NIH). Dr. Valentine also received support for article research from the Society for the Advancement of Blood Management (SABM) SABM-Haemonetics Research Starter Grant, CHU-Sainte-Justine Foundation, Washington University Children’s Discovery Institute (CDI), and the University of Massachusetts Medical School. Drs. Valentine, Bembea, and Steffen’s institutions received funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Heart, Lung, and Blood Institute (NHLBI) under award number 1 R13 HD088086-01; the SABM SABM-Haemonetics Research Starter Grant; and Washington University CDI (CDI-E1-2015-499). Dr. Bembea received other support from NIH/NINDS K23NS076674, and she disclosed off-label product use of ECMO (not FDA approved for use longer than 6 hours). Dr. Doctor’s institution received funding from the NIH, the Department of Defense, and KaloCyte. Dr. Steiner received funding from NIH R13. Dr. Argent received funding from N Kelly Attorneys (medicolegal report) and travel and accommodation to attend several national and international conferences as an invited speaker, as well as a consensus meeting to discuss this manuscript. Dr. Carson’s institution received funding from the NHLB, and he received funding from NICHD/NHLBI R13, Washington University CDI Grant (CDI-E1-2015-499) and the University of Massachusetts Medical School. Dr. Emeriaud’s institution received funding from Fonds de Recherche du Québec - Santé (research award) and Maquet Critical Care (supports the financial costs of a clinical study evaluating a neonatal ventilator that he is leading). Dr. Hall received funding from Bristol Myers-Squibb. Dr. Schwartz received funding from Novartis AG. Dr. Josephson received funding from consulting for Immucor LLC, Biomet Zimmer, and Octapharma. Dr. Luban’s institution received funding from the NICHD and NHLBI. Dr. Zantek’s institution received funding (all unrelated to the current study) from Octapharma, Bayer HealthCare, and Terumo BCT; she received funding from NICHD/NHLBI R13 (1 R13 HD088086-012) (funds from this grant were used to for travel accommodations for one of the study’s in-person group meetings); she disclosed her spouse is an employee of Boston Scientific and owns stock in Endo International PLC; and she disclosed other support from North American Specialized Coagulation Laboratory Association (member executive board), College of American Pathologists - American Society for Apheresis Inbound Liaison to CAP Transfusion Medicine Resource Committee (reimbursed for travel expenses), and the Thrombosis and Hemostasis Societies of North America (reimbursed for travel and meeting registration as a speaker at the 2018 meeting). Dr. Zantek disclosed that TAXI was supported, in part, by funding from the NIH NICHD/NHLBI, Washington University at St Louis CDI, SABM, the Canadian Critical Care Trials Group, the University of Massachusetts, as well as received support from the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the World Federation of Pediatric Intensive and Critical Care Society. Dr. Chiefetz received funding from Philips (medical adviser) and UptoDate (contributor). Dr. Fortenberry received funding from the American Board of Pediatrics Critical Care Subboard and Davis and Snyder LLC. Dr. Delaney received funding from Favros (expert witness case review, unrelated) and RedMed Ed, University of Cincinnati (speaking for CME course about specialized blood bank testing, unrelated). Dr. Van de Watering disclosed that he is employed by Sanquin Blood Supply, a national blood bank foundation. Dr. Robinson’s institution received funding from SABM/Haemonetics grant to fund the review work, and she received funding from Washington U CDI for travel to consensus meeting. Dr. Small’s institution received funding from Washington University CDI Grant (CDI-E1-2015-499). Dr. Steffen received support for article research from the CHU-Sainte-Justine Foundation and the University of Massachusetts Medical School. Dr. Bateman’s institution received funding from NICHD/NHLBI and SABM. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Noveck H, Berlin JA, et al. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.English M, Ahmed M, Ngando C, et al. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494–495. doi: 10.1016/S0140-6736(02)07666-3. [DOI] [PubMed] [Google Scholar]
- 5.Lackritz EM, Campbell CC, Ruebush TK, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 6.Lackritz EM, Hightower AW, Zucker JR, et al. Longitudinal evaluation of severely anemic children in Kenya: the effect of transfusion on mortality and hematologic recovery. AIDS. 1997;11:1487–1494. doi: 10.1097/00002030-199712000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–1442. doi: 10.1097/TA.0b013e3181ba7074. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano LM. Guideline compliance in trauma: evidence-based protocols to improve trauma outcomes? *. Crit Care Med. 2012;40:990–992. doi: 10.1097/CCM.0b013e3182411154. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 10.Reesink HW, Lee J, Keller A, et al. Measures to prevent transfusion-related acute lung injury (TRALI) Vox Sang. 2012;103:231–259. doi: 10.1111/j.1423-0410.2012.01596.x. [DOI] [PubMed] [Google Scholar]
- 11.Silliman CC, Fung YL, Ball JB, et al. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Rev. 2009;23:245–255. doi: 10.1016/j.blre.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung JP, Fraser JF, Nataatmadja M, et al. Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI) Crit Care. 2012;16:R19. doi: 10.1186/cc11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 14.Benson AB, Moss M, Silliman CC. Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. Br J Haematol. 2009;147:431–443. doi: 10.1111/j.1365-2141.2009.07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 16.Cholette JM, Powers KS, Alfieris GM, et al. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: results of a prospective, randomized, clinical trial. Pediatr Crit Care Med. 2013;14:137–147. doi: 10.1097/PCC.0b013e31826e741c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallman MD, Liu X, Harris AD, et al. Changes in transfusion practice over time in the PICU. Pediatr Crit Care Med. 2013;14:843–850. doi: 10.1097/PCC.0b013e31829b1bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy DJ, Needham DM, Netzer G, et al. RBC transfusion practices among critically ill patients: has evidence changed practice? Crit Care Med. 2013;41:2344–2353. doi: 10.1097/CCM.0b013e31828e9a49. [DOI] [PubMed] [Google Scholar]
- 19.Netzer G, Liu X, Harris AD, et al. Transfusion practice in the intensive care unit: a 10-year analysis. Transfusion. 2010;50:2125–2134. doi: 10.1111/j.1537-2995.2010.02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Pont-Thibodeau G, Tucci M, Ducruet T, et al. Survey on stated transfusion practices in PICUs*. Pediatr Crit Care Med. 2014;15:409–416. doi: 10.1097/PCC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 21.Demaret P, Tucci M, Ducruet T, et al. Red blood cell transfusion in critically ill children (CME) Transfusion. 2014;54:365–375. doi: 10.1111/trf.12261. [DOI] [PubMed] [Google Scholar]
- 22.Laverdière C, Gauvin F, Hébert PC, et al. Survey on transfusion practices of pediatric intensivists. Pediatr Crit Care Med. 2002;3(4):335–340. doi: 10.1097/00130478-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB. Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 24.Gibson BE, Todd A, Roberts I, et al. British Committee for Standards in Haematology Transfusion Task Force: Writing group. Transfusion guidelines for neonates and older children. Br J Haematol. 2004;124:433–453. doi: 10.1111/j.1365-2141.2004.04815.x. [DOI] [PubMed] [Google Scholar]
- 25.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bembea M, Valentine S, Bateman S, et al. The Pediatric Critical Care Transfusion and Anemia Expertise Initiative Consensus Conference Methodology. Pediatr Crit Care Med. 2018:19. doi: 10.1097/PCC.0000000000001593. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 28.Fitch K, Bernstein SJ, Aguilar MD, Kahan JP. The RAND/UCLA Appropriateness Method User’s Manual. RAND; Arlington, VA: 2001. [Google Scholar]
- 29.Feinstein AR. An analysis of diagnostic reasoning. III. The construction of clinical algorithms. Yale J Biol Med. 1974;1:5–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen KM, Bateman ST, Valentine SL, et al. Implementation of the recommendations for red blood cell transfusions for critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018:19. doi: 10.1097/PCC.0000000000001592. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doctor A, Cholette JM, Remy KE, et al. Recommendations on red blood cell transfusion in general critically ill children based on hemoglobin and/or physiologic thresholds from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001590. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demaret P, Emeriaud G, Hassan NE, et al. Recommendations on red blood cell transfusion in critically ill children with respiratory failure from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001619. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muszynski JA, Guzetta N, Hall MW, et al. Recommendations on red blood cell transfusions for critically ill children with non-hemorrhagic shock from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001620. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karam O, Russell RT, Stricker P, et al. Recommendations on red blood cell transfusion in critically ill children with non-life threatening or life-threatening bleeding from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001605. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tasker RC, Turgeon AF, Spinella PC, et al. Recommendations on red blood cell transfusion for critically ill children with acute brain injury from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001589. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cholette JM, Willems A, Schwartz SM, et al. Recommendations on red blood cell transfusion in infants and children with acquired and congenital heart disease from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. (19) 2018 doi: 10.1097/PCC.0000000000001603. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steiner ME, Zantek ND, Stanworth SJ, et al. Recommendations on red blood cell transfusion support in children with hematologic and oncologic diagnoses from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. (19) 2018 doi: 10.1097/PCC.0000000000001610. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bembea MM, Cheifetz IM, Fortenberry JD, et al. Recommendations on indications for red blood cell transfusions for critically ill children receiving support from extracorporeal membrane oxygenation, ventricular assist devices and renal replacement therapy devices from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19 doi: 10.1097/PCC.0000000000001600. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zantek ND, Parker RI, Van de Watering L, et al. Recommendations on selection and processing of red blood cell components for pediatric patients from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. (19) 2018 doi: 10.1097/PCC.0000000000001625. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spinella PC, Doctor A. Role of transfused red blood cells for shock and coagulopathy within remote damage control resuscitation. Shock. 2014;41:30–34. doi: 10.1097/SHK.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 41.National Blood Authority Australia. [Accessed June 14, 2017];Patient blood management guidelines. https://www.blood.gov.au/pbm-guidelines.
- 42.Rouette J, Trottier H, Ducruet T, et al. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg. 2010;251:421–427. doi: 10.1097/SLA.0b013e3181c5dc2e. [DOI] [PubMed] [Google Scholar]
- 43.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. 2010;38:649–656. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- 44.Karam O, Tucci M, Ducruet T, et al. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med. 2011;12:512–518. doi: 10.1097/PCC.0b013e3181fe344b. [DOI] [PubMed] [Google Scholar]
- 45.de Gast-Bakker DH, de Wilde RB, Hazekamp MG, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: a randomized controlled trial. Intensive Care Med. 2013;39:2011–2019. doi: 10.1007/s00134-013-3085-7. [DOI] [PubMed] [Google Scholar]
- 46.Hebert PC, Wells G, Blaichman M, et al. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 47.Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;12:10. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimshaw JM, Eccles MP, Lavis JN, et al. Knowledge translation of research findings. Implement Sci. 2012;7:50. doi: 10.1186/1748-5908-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 50.Berenholtz S, Pronovost PJ. Barriers to translating evidence into practice. Curr Opin Crit Care. 2003;9:321–325. doi: 10.1097/00075198-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Cure P, Bembea M, Chou S, et al. 2016 proceedings of the National Heart, Lung, and Blood Institute’s scientific priorities in pediatric transfusion medicine. Transfusion. 2017;57:1568–1581. doi: 10.1111/trf.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]