Abstract
Necrotizing enterocolitis (NEC) is a devastating disease that typically affects formula-fed premature infants, suggesting that dietary components may influence disease pathogenesis. Triglycerides are the major fat component of infant formula, and their digestion requires pancreatic lipases, which may be naturally deficient in premature neonates. We hypothesize that NEC develops in part from the accumulation of incompletely digested long chain triglyceride-containing unsaturated fatty acids within the intestinal epithelial cells, leading to oxidative stress and enterocyte damage. We further hypothesize that the administration of a formula that contains reduced triglycerides (“pre-digested fats”) that don’t require lipase action may reduce NEC severity. To test these hypotheses, we induced NEC in neonatal mice using three different fat formulations, namely “standard fat”, “pre-digested fat”, or “very low fat”, and determined that mice fed “standard fat” developed severe NEC, which was significantly reduced in mice fed “pre-digested fat” or “very low fat”. The expression level of the critical fat digesting enzyme carboxyl-ester lipase was significantly lower in the newborn compared to older pups, leading to impaired fat digestion. The accumulation of mal-digested fat resulted in the dramatic accumulation of fat droplets within the intestinal epithelium of the distal ileum, resulting in the generation of reactive oxygen species and intestinal inflammation. Strikingly, these changes were prevented in pups fed “predigested fat” or “very low fat” formulas. These findings suggest that nutritional formula containing a predigested fat system may overcome the natural lipase deficiency of the premature gut, and serve as a novel approach to prevent NEC.
INTRODUCTION
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants(1; 2). While the pathogenesis of NEC remains incompletely understood, its development is nearly always seen after the administration of enteral feeds, suggesting that specific components of infant formula such as dietary fat may contribute to NEC pathogenesis (2). In a rat model of experimental NEC, Caplan and colleagues showed that the administration of polyunsaturated fatty acids arachidonic acid (AHA) and docosahexaenoic acid (DHA) reduced the incidence of NEC(3), while Lu and colleagues showed that polyunsaturated fatty acids reduced NEC severity in part through the reduction in expression of the lipopolysaccharide receptor, toll like receptor 4 (TLR4) (4), supporting findings from our lab and others that TLR4 signaling plays a critical role in NEC pathogenesis(5; 6; 7; 8). These findings place the spotlight on how fat is handled in the premature intestine, and how failure of appropriate fat digestion may lead to NEC.
In the presence of the principal fat digesting enzyme namely carboxyl ester lipase, ingested triglycerides are normally digested in the lumen of the duodenum and jejunum into free fatty acids (FFAs) and 2-monoglycerides, which are then emulsified into soluble micelles that are taken up by intestinal epithelial cells. In the intestinal epithelial cells, FFAs and 2-monoglycerides are then converted back to triglycerides. The triglycerides then combine with cholesterol, protein and phospholipids to form chylomicrons that are transported out via lymph to the rest of the body(9). Importantly, premature infants display a relative inability to digest and absorb triglyceride fat(10; 11) and may have underdeveloped capacities for chylomicron synthesis(12). This potential inability of the immature intestine to adequately digest ingested fat could lead to the accumulation of triglyceride-containing unsaturated fatty acids in the ileum, where they would be at risk of becoming oxidized. The ensuing production of lipid peroxides could then induce oxidative stress and inflammation and contribute to the development of NEC.
We now seek to explore the role of fat digestion in the premature gut in greater detail in the pathogenesis of NEC. As described above, the critical enzyme required for fat digestion in the lumen of the intestine is carboxylic ester lipase (CEL) - also called bile salt dependent lipase (BSDL) – which is secreted into the intestinal lumen by the exocrine pancreas(13). Exocrine pancreatic function is not fully developed at birth, and as a result CEL release is insufficient to adequately support fat absorption in the newborn (14; 15). In a remarkable demonstration of the nutritional synergy that exists between infant and mother, CEL is also secreted by the lactating mammary gland into the breast milk, and upon ingestion of breast milk, the infant has an immediately available source of CEL which can at least partially compensate for its otherwise low endogenous production (16). The lack of CEL in infant formula explains in part why premature infants may encounter incomplete fat digestion when administered standard infant fat formula (17; 18). One of the main components of the resultant incompletely digested fat in the intestine of the premature infant is free fatty acid (FFA), whose persistence in the lumen of the premature gastrointestinal tract may accumulate within cells and disrupt the cell membrane(19), leading to significant toxicity to the enterocyte (20). Supplementation of preterm formula with polyunsaturated fatty acids (PUFA) has been shown to reduce the incidence of necrotizing enterocolitis (NEC) in animal models, which has been linked to the ability of PUFA to suppress Toll-like receptor (TLR) 4 and platelet-activating factor receptor (PAFR) gene expression, molecules that are important in the pathogenesis of NEC in epithelial cells(4). However, while these studies have focused on the potential role of unsaturated fatty acids in the pathogenesis of NEC, the potential roles – if any – of using hydrolyzed or pre-digested fats for NEC prevention to essentially bypass the lack of CEL in the gastrointestinal tract of the premature infant remain incompletely understood.
Based upon these findings, we hypothesize that incomplete fat digestion in the premature intestine leads to intestinal inflammation and the development of NEC, in part due to the accumulation of undigested triglyceride-containing unsaturated fatty acids within the epithelium of the distal ileum, leading to the accumulation of reactive oxygen intermediates which induces an inflammatory response. We sought to test this hypothesis by studying the extent of NEC development, using a) standard infant formula, b) pre-digested fat (PDF), and c) a very low fat formula, the nutritional components of which are described in Table 1.
Table 1.
Nutritional composition of study formulations
| Standard Formula (per L) | PDF Formula (per L) | Very Low Fat Formula (per L) | |
|---|---|---|---|
| Protein, g | 56.5 | 57.1 | 58.1 |
| Fat, g | 71 | 71 | 3.7 |
| High Oleic Safflower Oil, g | 27.66 | 24.75 | 0 |
| Soy oil, g | 20.7 | 0 | 0 |
| Coconut oil, g | 19.8 | 10.5 | 0 |
| Mono-acyl glycerol palmitate, g | 0 | 14.25 | 0 |
| Soybean oil free fatty acids, g | 0 | 12.4 | 0 |
| Lecithin, g | 0 | 7.3 | 0 |
| Distilled monoglycerides, g | 1.5 | 0 | 0 |
| DHA, mg | 146 | 135 | 161 |
| ARA, mg | 312 | 322 | 355 |
| Carotenoids, mg | 59 | 59 | 61 |
| Remaining fat from proteins, carrier oils from vitamins, carotenoids, DHA, and ARA, g | 0.82 | 1.28 | 3.12 |
| Carbohydrate, g | 62 | 63 | 138 |
| Vitamins | |||
| Vitamin A Palm, IU | 6146 | 6677 | 5584 |
| Vitamin E, mg | 18 | 18 | 19 |
| Vitamin C, mg | 375 | 375 | 375 |
| B1, mg | 2.45 | 2.25 | 2.45 |
| B2, mg | 6.1 | 6.02 | 6.7 |
| B6, mcg | 783 | 802 | 832 |
| B12, mcg | 10.2 | 10.1 | 11.4 |
| Pantothenic acid, mg | 12 | 12.38 | 12.7 |
| Folic acid, mcg | 334 | 297.4 | 328.2 |
| Niacin, mg | 16.4 | 16.22 | 17 |
| Biotin, mcg | 118 | 115.4 | 125.9 |
| Total Choline, mg | 317 | 474 | 341 |
| Minerals | |||
| Sodium, mg | 762 | 755 | 802 |
| Potassium, mg | 2228 | 2319 | 2539 |
| Chloride, mg | 1175 | 1175 | 1184 |
| Calcium, mg | 2402 | 2371 | 2432 |
| Phosphorus, mg | 1269 | 1362 | 1280 |
| Magnesium, mg | 154 | 158.1 | 158.2 |
| Iron, mg | 20 | 20.2 | 21.2 |
| Zinc, mg | 13.1 | 12.69 | 13.77 |
| Copper, mg | 1.13 | 1.09 | 1.18 |
In all cases, formula was supplemented with stool bacteria cultured from the intestine of an infant with surgical NEC (12.5μl original stool slurry in 1ml formula), and the fat content as described above.
METHODS
Chemical reagents
Reagents were obtained as follows: Oil Red ‘O’ kit (Cat # ab150678, Abcam, Cambridge, MA), RNeasy® kit (Cat # 74106, Qiagen, Germantown, MD) and QuantiTect® Reverse Transcription (Cat # 205313, Qiagen, Germantown, MD), Dihydroethidium (DHE, Cat # D7008, Sigma, St. Louis, MO), 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Cat # D9542, Sigma, St. Louis, MO), CEL (BSDL) ELISA kit (Cat # MBS098208, MyBiosource, Inc., San Diego, CA) (forward and reverse primers (custom designed using NCBI Primer-BLAST online program and ordered from Integrated DNA Technologies, Coralville, Iowa).
Animal study approval
All mice experiments described in this study were carried out following in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and are approved by Johns Hopkins University protocol, according to the ‘NC3R’s The ARRIVE Guidelines(21). C57BL/6J mice were purchased from the Jackson laboratory and bred in the pathogen free facility at Johns Hopkins University for multiple generations to stabilize intestinal microbiota. All mice were given ad libitum access to water, food, and housed in a temperature controlled room (22°C) with 12 hours’ light and dark cycles. All mice used in the study were euthanized humanely using isoflurane anesthesia by inhalation (~3–4% isoflurane) followed by cervical dislocation.
Induction of NEC in neonatal mice
Experimental NEC was induced in 7–8 day old (~3.0g body weight) neonatal mouse pups as previously described(22), (23; 24). Neonatal pups were randomly divided into control and treatment groups and experimental models were repeated at least 3 times with 8 or more mice per treatment group. Neonatal pups were gavage fed (40μL/g) five times/day (7am to 7pm) with formula containing one of three types of fat (described below and illustrated in Figure 1), that was supplemented with bacterial stock that had been cultured from the stool of an infant with severe NEC (12.5μl stool slurry in 1ml formula). The stool mixed formula (50μl per gram of mouse body weight) (25) was administered using a 24-French angiocatheter placed into the mouse esophagus. Mice were exposed to hypoxia (5% O2, 95% N2) for 10 minutes in a chamber (Billups-Rothenberg INC. Del Mar, CA), twice daily (7am and 1pm immediately after feeding) for the 4-days, and the bacterial slurry was added to the formula on each day. Additional breast-fed control groups - exposed to hypoxia only, bacteria only and hypoxia + bacteria, and formula fed groups - exposed to formula only, formula + bacteria (no hypoxia), and formula +hypoxia (no bacteria) were also performed to evaluate the role of the individual components of the model of the development of intestinal inflammation. For mice subjected to breast fed/hypoxia alone, mice were given hypoxia (5% O2, 95% N2) for 10 minutes, twice daily for 4 days (at 7am and 1pm) and immediately returned with dams similar to NEC treated groups. For the breast fed/bacteria control group, mice were gavage fed bacteria (equal amount of bacteria given to NEC treated mice i.e., 10μL NEC bacterial stock slurry, diluted in 100μL saline/pup) once daily for 4 days. We and others have previously demonstrated that under administration of the standard formula, this experimental model induces significant intestinal inflammation expression of pro-inflammatory cytokines, Interleukin-6 (IL-6), Interleukin 1-beta (IL-1β), and Tumor Necrosis factor-alpha (TNF-α) that closely mimics human NEC (26), (27). To determine the effect of antioxidant on ROS generation, NEC formula was supplemented with N-acetylcysteine (NAC) (100mg/kg) and admistered to mice in the NEC model. Control mice were gavage fed once daily with similar dose of 100mg/kg) of NAC.
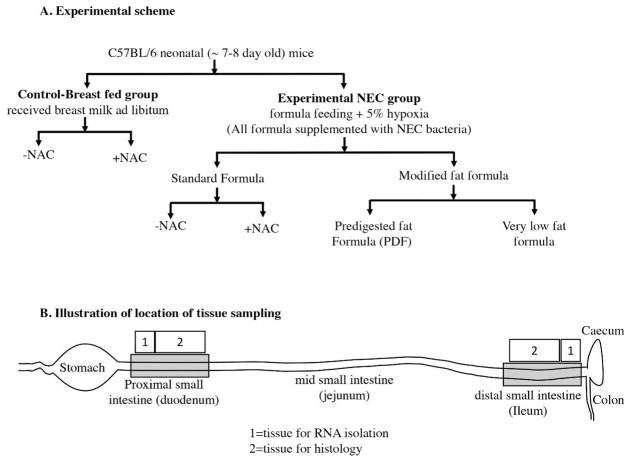
Figure 1.
Experimental scheme and location of tissue sampling used in the current studies.
Nutritive formulas
Three different study formulas were used, each with varying types and amounts of fats (manufactured by Abbott Laboratories, Columbus, OH) and which contained identical ingredients except for the fat composition. Standard formula was composed of 100% triglyceride (TG) rich oils (mixture of 39 % high oleic safflower oil (HOSO), 29 % soy oil, and 27.9% coconut oil), and these are described in detail in Table 1. Pre-digested fat formula (PDF-formula) contained approximately 50% triglycerides composed of a mixture of soybean FFAs (17.5%), 2-monoacylglycerol palmitate (20%), and phospholipid lecithin (10.3%) along with HOSO (34.8%) and coconut oil (14.8%). Soybean FFA were added into the formula in the calcium salt form where the FFAs were first mixed with calcium hydroxide during manufacturing. Very low fat formula was formulated with no added triglycerides except DHA and ARA and lipid soluble vitamins to match the standard and PDF formulas. In an attempt to close the gap in caloric content of the low fat formula as compared with the other formulas, lactose was used to replace the fat energy, and caloric values were similar between the three formulas. All three study formulas were manufactured using the same homogenization and thermal treatment conditions which are representative of commercial RTF (Ready to Feed) formulas. The nutrition profiles of the formulas are shown in Table 1 and the experimental scheme for the NEC model is shown Figure 1. The breast fed (control) animals remained with their mothers and received breast milk ad libitum.
NEC severity assessment
NEC severity was determined based upon a validated scoring system applied to de-identified, paraformaldehyde (PFA) fixed/paraffin embedded/H&E-stained intestinal sections from both proximal and distal intestine in consultation with a pediatric pathologist who was blinded to the group allocation. Histological NEC severity score were assigned as described previously(28; 29), 0 (no injury), 1 (minor-submucosal, lamina propria separation), 2 (moderate separation of submucosa(28), lamina propria, and edema in submucosal and muscular layers), and 3 (severe separation of submucosa, lamina propria, severe edema, and villous sloughing or loss of villi).
Ontogeny of transcripts levels of carboxyl ester lipase (CEL) in pancreas by qRT-PCR
To measure the transcripts levels of CEL also called bile-salt dependent lipase (BSDL) within the pancreas of breast fed neonatal (postnatal day, p2) to weanling (postnatal day, p15) mice by qRT-PCR, whole pancreas was carefully dissected out under dissection stereoscope (Nikon, Nikon Instruments Inc., Tokyo, Japan) and snap frozen until processing for total RNA isolation and qRT-PCR assay as described below.
Ontogeny of CEL protein levels by Enzyme linked immunosorbent assay (ELISA) assay
To assess the ontogeny of CEL by ELISA, the stomach content of neonatal mice (postnatal days 2, 5, 10, and 15) was collected immediately after euthanasia and snap frozen in liquid nitrogen. CEL in stomach contents was measured using mouse lipase, CEL (i.e. BSDL) ELISA kit as per manufacturer instructions.
Quantitative Real-time Polymerase Reaction (qRT-PCR)
Total RNA was isolated from snap frozen whole intestine (~1 cm length) and pancreas using RNeasy® kit, checked for RNA purity, and concentration on SpectraMax® microplate reader (Molecular Devices, San Jose, CA). 0.5μg of total RNA was reverse transcribed for cDNA synthesis using the QuantiTect® Reverse Transcription kit. qRT-PCR was then performed on a Bio-Rad CFX96 Real-Time System (Bio-Rad labs, Hercules, CA) using Sybr green mix (Bio-Rad labs, Hercules, CA), forward and reverse primers (Table 2). The mRNA expression relative to the housekeeping gene ribosomal protein large P0 (Rplp0) was calculated using the 2-ΔΔCT method as described(30).
Table 2.
Primer sequences
| Gene | Forward sequence | Reverse sequence | Amplicon Size (bp) |
|---|---|---|---|
| Akr1b7 | GGTGGTGATCCCCAAGTCTG | GCCCTCCAGTTCCTGTTGAA | 120 |
| CEL (BSDL) | ACGATAACCAGCGCTTCCAT | TCATCCTCAGGGGGAGTGAG | 125 |
| Gpx2 | TCAATGGGCAGAACGAGCAT | CGCACGGGACTCCATATGAT | 118 |
| IL-1β | AGTGTGGATCCCAAGCAATACCCA | TGTCCTGACCACTGTTGTTTCCCA | 175 |
| Keap1 | CTCAACCGCTTGCTGTATGC | TTCAACTGGTCCTGCCCATC | 194 |
| MPO | GACAGTGTCAGAGATGAAGCTACT | TTGATGCTTTCTCTCCGCTCC | 189 |
| Nox2 | GACACGCATGCCTTTGAGTG | TGCACAGCAAAGTGATTGGC | 143 |
| Nqo1 | GGTAGCGGCTCCATGTACTC | CCAGACGGTTTCCAGACGTT | 198 |
| Nrf2 | AGCACTCCGTGGAGTCTTCCATTT | TGTGCTTTAGGGCCGTTCTGTTTG | 115 |
| TNF-α | TTCCGAATTCACTGGAGCCTCGAA | TGCACCTCAGGGAAGAATCTGGAA | 144 |
| Rplp0 | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 143 |
Oil Red O′ staining for lipid droplets
To assess the presence of intracellular lipid uptake, we performed ‘Oil Red O’ staining on proximal and distal intestinal tissues harvested 45 min after formula or breast milk feeding. To do so, fresh intestinal tissues were fixed with 4% PFA for 4 hours, immersed in 30% sucrose solution for 2 days, and frozen blocks were prepared in Tissue-Tek media and cryomolds. 10μm cryo-sections were then cut using a Cryostat (Thermofisher, Waltham, MA), dehydrated in PBS, and stained for lipid droplets using ‘Oil Red O’ kit according to the manufacturer instructions. Sections were briefly counter-stained with Hematoxylin (Modified Mayer’s), mounted using Gelvetol mount media, and imaged using the EVOS imaging system (Invitrogen, Carlsbad, CA). Cryo-tissue sections were also prepared without PFA fixation for Oil-red O′ staining, no difference in staining was observed, but tissue integrity was far superior in PFA fixed tissues, therefore all data was collected from PFA fixed cryo-sections.
Immunohistochemistry and Hematoxylin and Eosin (H&E) staining
Specific parts of proximal and distal intestinal tissues as illustrated in Figure 1 were obtained for histological examination. For detection of ROS accumulation in mouse intestinal tissue, we used the dihydroethidium (DHE) oxidation staining method, based upon its oxidation to ethidium in the presence of ROS, which then intercalates into DNA producing bright red nuclear fluorescence (Sigma, St. Louis, MO). In brief, 10μm cryo-sections were hydrated in PBS and stained with 5μm dihydroethidium for 30min in the dark, counterstained nuclei with DAPI, mounted using Gelvetol media and immediately imaged using Nikon A1 confocal microscope (Nikon, Nikon Instruments Inc., Tokyo, Japan). H&E staining was performed on 4% PFA fixed/paraffin embedded sections (5μm thickness). Malondialdehyde (MDA) immunofluorescence staining was performed as described (31).
Statistical analysis
Statistical analysis was performed using PRISM version 7.0 (Graph Pad). The pups were randomized to treatment group, and blinded analyses were performed using either Fisher’s exact test or ANOVA with multiple comparisons, and post-hoc analyses were performed whenever statistical differences were determined in the multiple group analyses. Statistical significance was accepted at p<0.05. Based upon the predicted effects of both the pre-digested fat system and the very low fat formula, with an alpha error of 0.05 and a beta error of 0.10, in order to assess a 50% reduction in cytokine expression by RT-PCR, ROS generation, ELISA expression of CEL, we calculate a sample size of 8 pups in each group, which is also the expected survival rate based upon technical variability, between litters.
RESULTS
Establishment of a model of necrotizing enterocolitis in newborn mice and determination of appropriate controls for assessment of infant formula
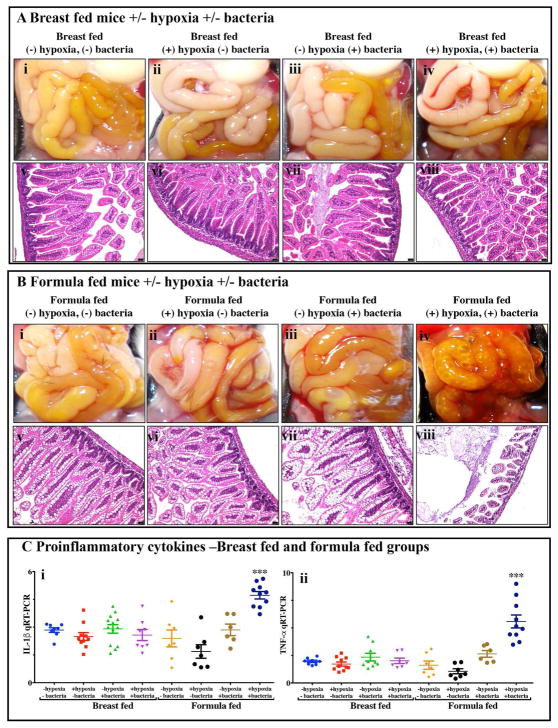
We first sought to develop a platform for the assessment of different fat compositions in experimental NEC in newborn mice, and thus evaluated the relative contribution of each of the individual components - namely the administration of infant formula, the induction of hypoxia and the administration of the cultured bacterial slurry from an infant with severe NEC – to the development of intestinal inflammation. As shown in Figure 2, newborn mice that were exposed to breast milk and either hypoxia or bacterial slurry did not develop NEC, as revealed by gross examination of the intestine, intestinal histology, and qRT-PCR analysis of pro-inflammatory gene expression in the intestinal mucosa (Figure 2A and 2C). Similarly, mice that were administered “standard fat”-containing formula feeds along with either hypoxia or bacteria had no evidence of NEC (Figure 2B and 2C). By contrast, mice who were administered the combination of “standard fat”-containing formula feeds, hypoxia and bacteria developed severe NEC as revealed by the presence of air within the wall of the bowel (Figure 2Biv), histologic evidence of mucosal disruption (Figure 2Bviii) and the induction of pro-inflammatory genes in the distal ileum (Figure 2C). These findings reveal that the induction of NEC requires a combination of formula feeding, bacterial supplementation and exposure to hypoxia together. Subsequent studies were thus designed in which NEC was induced using these three variables, and in which only the fat component of the formula was varied, in comparison with control mice who were left with their mothers and were breast fed. This approach allowed us to determine the effect of fat type on the development of NEC, as examined below.
Figure 2. Establishment of a model of necrotizing enterocolitis in newborn mice and determination of appropriate controls for assessment of infant formula.
A–B: Shown are results from representative distal ileal samples of mice subjected to breast fed and formula feedings. A: Gross morphology (Ai–iv), H&E stained histology (Av–viii) of breast fed groups; B: Gross morphology (Bi–iv), H&E stained histology (Bv–viii) of formula fed groups; C: Levels of Pro-inflammatory cytokines, IL-1β (Cii) and TNF-α (Cii);
The administration of formula containing “pre-digested” fat attenuates the severity of NEC in mice
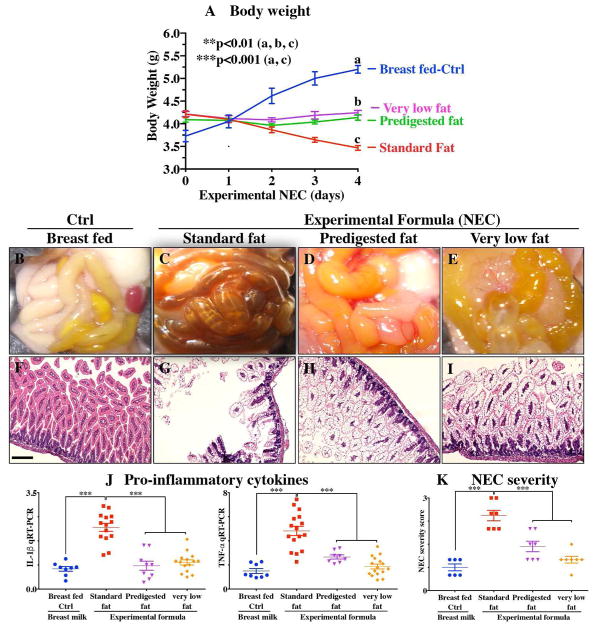
We next administered formulas containing varying fat compositions to newborn mice and assessed the effects on NEC severity. All formulas contained approximately 71g of the various types of fat except the “very low fat” formula which contained 3.7g of fat; formulas contained approximately 56.5 to 58.1g of protein per liter (Table 1), which approximates that seen in rodent milk (32). The “standard” formula contained 100% triglycerides in the form of high oleic safflower, soybean and coconut oils as shown in Table 1. As shown in Figure 3, and using an evaluation scheme that was consistent with our prior studies(26),(5), (33), the administration of this “standard fat formula” to newborn mouse pups resulted in progressive weight loss (red curve in Figure 3A) in comparison to breast fed mice (blue curve in Figure 3A), and the development of severe mucosal injury and marked morphological change to the small intestine, characterized by edema, air within the bowel wall (pneumatosis intestinalis), and patchy intestinal necrosis (Figure 3C and G) consistent with the findings in Figure 2. These morphologic changes were accompanied by an increase in pro-inflammatory cytokines IL-1β and TNF-α (Figure 3L), and an increase in the NEC severity score (Figure 3K). Breast milk fed control mice showed normal gross morphology (Figure 3B), intact architecture of intestinal epithelium (Figure 3F), low expression of pro-inflammatory cytokines (Figure 3G). Based upon these findings, we next hypothesized that the removal of long chain triglycerides from the formula would reduce NEC severity. To test this specifically, we administered a formula that was deficient in long chain triglyceride oil, which we termed “very low fat” formula, which resulted in less weight loss (pink curve, Figure 3A), less mucosal and gross morphological injury (Figure 3E, I), reduced pro-inflammatory cytokine expression (Figure 3J) and reduced NEC severity scores (Figure 3K), as compared to mice in the “standard fat” formula group. To further assess the role of fat in NEC development, we next administered formula with reduced triglycerides containing fat in a “pre-digested” composition, that was not dependent upon the action of lipases within the lumen of the intestine for digestion. Specifically, “pre-digested fat” containing formula (i.e. “PDF formula”) contains approximately 50% long chain triglycerides and a mixture of soybean oil free fatty acids, monoacylglycerol palmitate, and soy lecithin compared to 100% long chain triglyceride fats that is present in the “standard fat” formula. As shown in Figure 3, the administration of “predigested fat” (which contains DHA and ARA at concentrations similar to that of standard formula) resulted in marked preservation of infant weight (green curve, Figure 3A), and significantly reduced gross morphological injury (Figure 3D), histological injury (Figure 3H), pro-inflammatory cytokine expression (Figure 3J) and NEC severity (Figure 3K). Importantly there was no difference observed in body weight loss, gross morphology of the intestine, histological examination of the terminal ileum, expression of pro-inflammatory cytokines, and NEC severity scores between the “pre-digested fat” and “very low fat” formula groups. Taken together, the above findings suggest that the type of fat used in formula may contribute to NEC pathogenesis, leading us to next determine the potential mechanisms involved.
Figure 3. Administration of “pre-digested fat” containing formula to neonatal mice reduces the incidence and severity of experimental NEC.
A: Body weights; B–E: Photomicrographs of gross images; F–I: H&E stained images; J: qRT-PCR of pro-inflammatory cytokines; K: NEC severity scores, in neonatal mice subjected no treatment (Ctrl, Control-Breast fed) or experimental NEC treatments. PDF, pre-digested fat; IL-1b, interleukin-1 beta; TNF-a, Tumor necrosis factor-alpha. **p<0.01, ***p<0.001, each dot in dot-graphs represents data from individual mouse, scale bar =10μm.
Pancreatic carboxylic ester lipase is reduced at the time of NEC development in mice
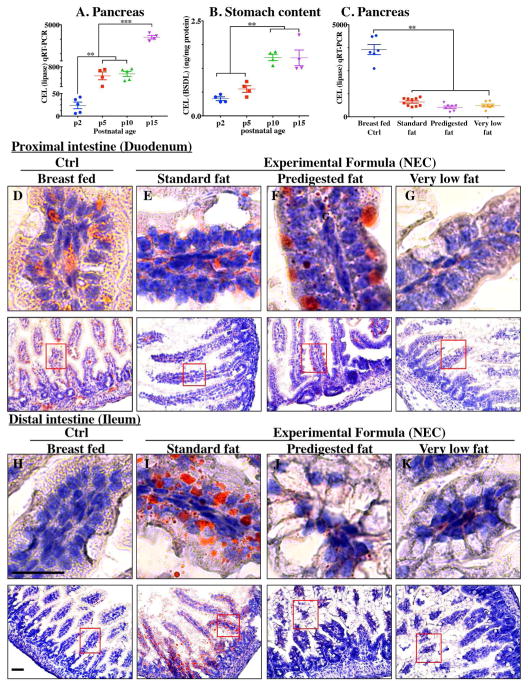
The content of lipases in the lumen of the intestine can be assessed by measurement of both pancreatic and gastric lipases. To determine whether lipases are reduced in the intestine of newborn mice at the time of NEC development, we assessed the expression of carboxyl ester lipase within the pancreas of neonatal to weaned breast fed mice by qRT-PCR, and also measured the amount of CEL (also called BSD-lipase) in the gastric contents of mice at varying post-natal ages from days 2 through 15 by ELISA(15). As shown in Figure 4A, the pancreatic expression of CEL was naturally low immediately after birth and increased significantly over time. These findings were supported by the observation shown in Figure 4B that the concentration of CEL is also significantly greater in the gastric contents of mice as they mature toward post-natal day 15 as compared with early newborn mice. Not surprisingly, the composition of fat within the administered formula had no effect on the expression of individual lipases (measured at the end of the experimental NEC model), as mice that were fed “standard fat” formulas were found to have similar expression of CEL as compared with mice that were fed either “pre-digested fat” or “very low fat” formula (Figure 4C). We therefore next sought to investigate the potential effects of impaired lipase content of the newborn mouse intestine on the accumulation of fat within the proximal and distal intestinal epithelium.
Figure 4. Low lipase activity in neonatal mice impairs normal fat absorption in the proximal intestine and triggers the accumulation of fat droplets in ileal enterocytes.
A: Ontogeny of carboxyl ester lipase (also called bile salt dependent lipase, BSDL) in pancreas by qRT-PCR; B: CEL/BSDL ELISA; C: qRT-PCR of carboxyl ester lipase in pancreas of control and NEC mice; D–K: ‘Oil Red O’ staining (red droplets) counter stained blue nuclei with Hematoxylin stain (D–G proximal intestine and H–K distal intestine, 10 μM cryo-sections) (Ctrl, Control-Breast fed or experimental NEC treatments with hypoxia and formula feeding). PDF, pre-digested fat. **p<0.01, ***p<0.001, each dot represents data from individual mouse, scale bar =10μm.
Administration of formula containing “pre-digested fat” results in reduced accumulation of lipid and ROS generation in the intestinal mucosa as compared with standard infant formula
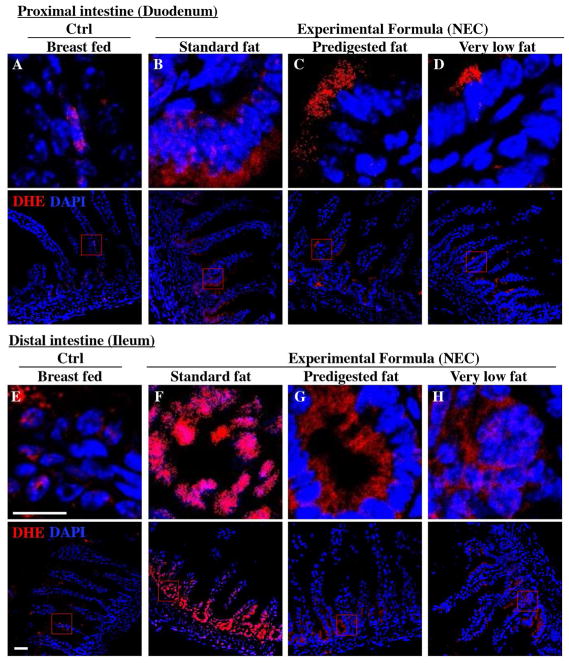
Having shown reduced expression of lipases within the pancreas and stomach of the developing mice, and given the potential cytotoxic effects that non-digested fats may exert on the ileal mucosa (15), we next sought to evaluate the effects – if any – on the accumulation of fat within the intestinal epithelium of mice subjected to models of NEC using formulas containing various amounts of fat as in Table 1. As shown in Figure 4, there was a significant accumulation of lipid droplets in the duodenal epithelium of mice that received breast milk (Figure 4D) as well as the three infant formulas tested, as determined by Oil-Red O′ staining (Figure 4E–G). By contrast, examination of the distal ileum - where NEC-induced injury most commonly develops - revealed that the administration of “standard fat” containing formula (Figure 4I) to newborn mice resulted in significantly increased lipid accumulation in the intestinal epithelium, as compared to breast fed control mice (Figure 4H) and to mice who were administered a “pre-digested fat” (PDF) formula (Figure 4J) or a “very low fat” formula (Figure 4K). To assess the potential link between intracellular fat accumulation and ROS-induced mucosal injury, we next sought to measure ROS accumulation in both the proximal and distal intestine by staining the intestinal tissue with the ROS-sensitive fluorescent dye DHE(27). Examination of the duodenum of mice who were either breast fed, or who were fed “standard fat” or “pre-digested fat” containing formula revealed the accumulation of intracellular Oil Red O′ stained fat droplets (Figure 4D–G) –yet very little nuclear DHE staining (Figure 5A, D). By contrast, the administration of “standard fat” containing formula resulted in the marked accumulation of ROS as manifested by increased DHE staining (Figure 5F) corresponding to the accumulation of intracellular Oil Red O′ stained fat droplets (Figure 4I) in the distal intestine (ileum), suggesting a link between lipid accumulation and ROS generation in the distal intestine, where NEC disease normally develops. Interestingly, in the distal intestine (ileum), the administration of either a “pre-digested fat” or a “very low fat” containing formula resulted in significantly less accumulation of intracellular Oil Red O′ stained fat droplets (Figure 4J, K) and ROS accumulation (Figure 5G, H) as compared to the “standard formula” group (Figure 4I and 5F). Based upon these findings, we next sought to evaluate the degree of lipid peroxidation of polyunsaturated lipids by ROS, and then sought to determine whether a ROS inhibitor could reduce the incidence of experimental NEC in mice.
Figure 5. The effect of fat composition on the accumulation of reactive oxygen species in ileal enterocytes of mice induced to develop NEC.
A–H: Immunofluorescence images of DHE (red fluorescence) and DAPI (nuclei, blue) staining from control and NEC mice. A–D proximal (duodenum) and E–H distal (ileum), 10μm: Cryo-sections (Ctrl, Control-Breast fed or experimental NEC treatments with hypoxia and formula feeding). PDF, pre-digested fat, scale bar =10μm.
The administration of formula containing “pre-digested fat” (PDF) leads to a reduction in the accumulation of ROS-degraded polyunsaturated lipids in the distal small intestine of mice with NEC
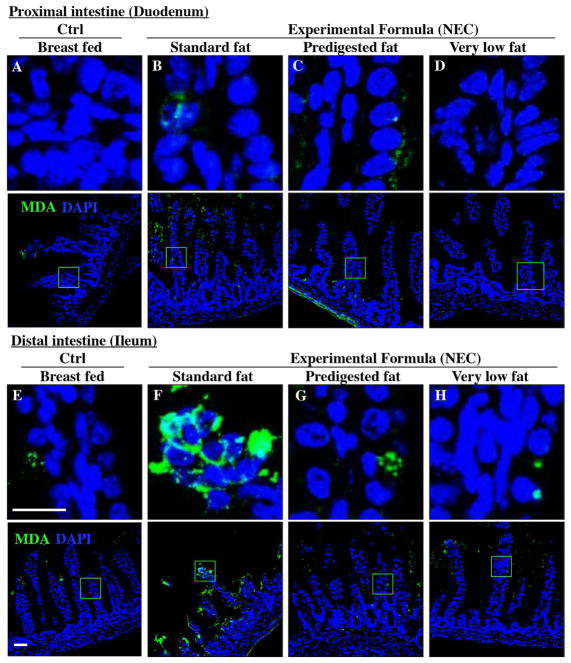
Given that ROS accumulation can lead to the degradation of polyunsaturated lipids to form reactive electrophile species including malondialdehyde (MDA)(34) which can cause tissue injury(35), we next measured the extent of MDA accumulation within the intestinal epithelium of newborn mice after exposure to either breast milk, or infant formulas containing different types of fat as described in Methods and Table 1. As shown in Figure 6A and 6E, breast-fed mice showed low levels of MDA in the duodenum and ileum, consistent with the fact that this diet induces minimal ROS accumulation inside the cells as described above in Figure 5A, E. By contrast, examination of the distal small intestine (ileum) of mice who were administered “standard fat” formula revealed marked accumulation of MDA in the ileum (Figure 6F), consistent with the accumulation of ROS that was detected by DHE staining (Figure 5F), and the histological development of NEC (Figure 3C, G, J–K). Strikingly, the administration of formula containing “pre-digested fat” showed a markedly reduced degree of MDA accumulation in the distal bowel (Figure 6G), similar to the observation found in either breast fed control (Figure 6E) or “very low fat” formula fed mice (Figure 6H). Taken together, these findings illustrate that the degree of ROS generation and lipid peroxidation can be influenced in part by the composition of fat (i.e. intact triglycerides vs. hydrolyzed triglycerides) provided to the newborn gut.
Figure 6. The effect of fat composition on the accumulation of oxidized lipids in ileal enterocytes of mice induced to develop NEC.
A–H: Immunofluorescence images of Malondialdehyde (MDA-green) and DAPI stained (nuclei, blue) from control and NEC mice proximal (duodenum, A–D) and distal (ileum, E–H) small intestine 10μm, cryo-sections) (Ctrl, Control-Breast fed or experimental NEC treatments with hypoxia and formula feeding). PDF, pre-digested fat, scale bar =10μm.
The administration of N-acetylcysteine (NAC) with standard infant formula prevents the accumulation of ROS and the oxidation of lipids, and reduces the severity of NEC in newborn mice
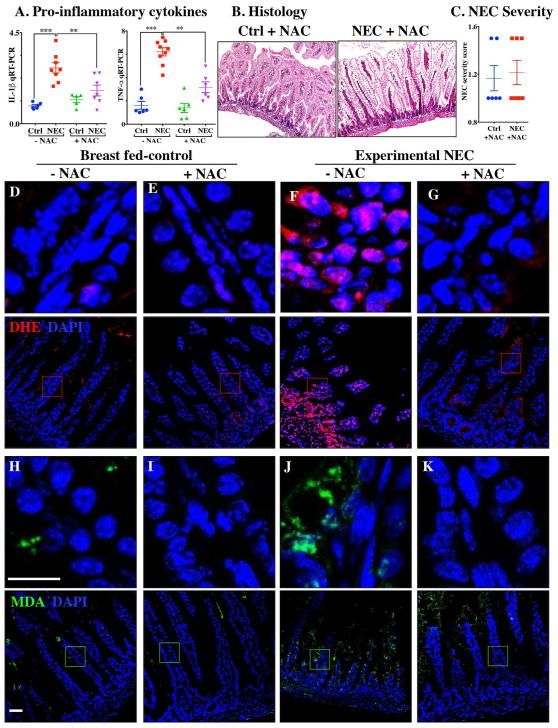
Having shown that the fat composition of various infant formulas can influence the degree of accumulation of ROS generation and severity of NEC, we next sought to investigate whether the use of a ROS quencher could reverse these effects. To do so, we administered NAC to mice which were then induced to develop NEC in the presence of formulas containing various fat compositions. As shown in Figure 7, the combined oral administration of NAC to the standard fat containing NEC formula significantly reduced NEC severity as demonstrated by reduced pro-inflammatory cytokine expression (Figure 7A), as well as preservation of histology of the terminal ileum and NEC severity scores (Figure 7B, C). The levels of ROS generation as determined by DHE staining were significantly reduced in the terminal ileum of mice exposed to NEC using NEC formula that was supplemented with NAC (Figure 7D–G). Furthermore, the addition of NAC led to a marked reduction in ROS-mediated lipid peroxidation in the terminal ileum, as revealed by reduced MDA staining (Figure 7H–K). We therefore sought to evaluate the mechanisms by which lipid oxidation occurred in the distal intestine, and to determine whether the expression of components of thethus anti-ROS machinery could play a role in NEC development.
Figure 7. Administration of the ROS scavenger N-acetylcysteine (NAC) prevents ROS generation and NEC development.
A: qRT-PCR of pro-inflammatory cytokines; B: H&E stained images; C: NEC severity score; D–G: Immunofluorescence images of Dihydroxyethidium (DHE-red) and DAPI stained (nuclei, blue); H–K: Immunofluorescence images of malondialdehyde (MDA-green) and DAPI stained (nuclei, blue) from distal intestine (ileum) of control and NEC mice (Ctrl, Control-Breast fed or experimental NEC treatments with and without N-acetylcysteine supplementation). **p<0.01, ***p<0.001 by a Student t test when comparisons of two groups were made, and by ANOVA for multiple comparisons, each dot represents data from individual mouse, scale bar =10μm.
The expression of the anti-ROS machinery in the newborn intestine contributes to the pattern of NEC development in the distal bowel after fat administration
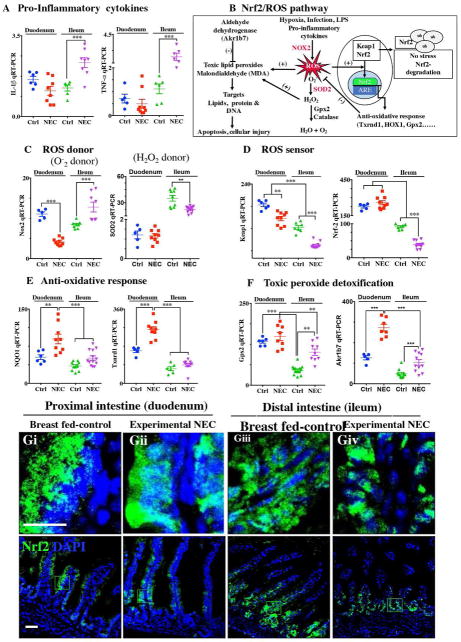
In the final series of studies, we sought to explore the potential mechanisms by which the proximal intestine of mice is protected from NEC induced injury despite the accumulation of fat in duodenal enterocytes (Figure 4E–G). As shown in Figure 8A, the expression of the pro-inflammatory cytokines IL-1β and TNF-α were significantly elevated in the ileum but not in the duodenum, consistent with the development of NEC in the more distal regions of the bowel. We thus next studied the expression of genes within the Nrf2/ROS pathway as illustrated in Figure 8B. As shown in Figure 8C–F, the expression of the anti-ROS enzymatic machinery in the proximal (duodenum) versus distal (ileum) bowel correlated with the observed distribution of NEC after the accumulation of fat. Specifically, the expression of the ROS donor NADPH oxidase 2 (NOX2) and the H2O2 donor superoxide dismutase 2 (SOD2) were significantly increased in the ileum of NEC mice and decreased in the duodenum (Figure 8C), while the ROS sensors and cytoprotective transcription factors Kelch-like ECH-associated protein 1 (Keap1) and Nuclear factor erythroid 2-related factor (Nrf2) were expressed at very low levels in the ileum as compared to the duodenum, suggesting the presence of an anti-oxidant environment under physiological conditions of the duodenum. The gene expression data of Nrf2 was verified by immunostaining for Nrf2 in duodenum (Figure 8Gi–ii) and ileum (Figure 8Giii–iv), revealing low levels in the ileum. Furthermore, the mRNA levels of Nfr2 dependent anti-oxidative enzymes NAD(P)H Quinone Dehydrogenase 1(NQO1), Thioredoxin reductase 1 (Txnr1) and toxic peroxide detoxification enzymes intestinal Glutathione peroxidase 2 (Gpx2), aldo-keto reductase family 1, member B7 (Akr1b7) were significantly lower in ileum compared to duodenum and further reduced in the ileum of NEC mice (Figure 8E, F). These data suggest that the proximal intestine exists in an anti-oxidant environment which contributes to reduced inflammation after the administration of lipid, as compared with the pro-inflammatory, pro-oxidant environment in the distal intestine which predispose to intestinal injury and NEC.
Figure 8. The anti-oxidative environment of the proximal small intestine versus the distal small intestine correlates with the location of NEC.
A: qRT-PCR of pro-inflammatory cytokines IL-1b and TNF-a; B: Schematic of Nrf2/ROS oxidative injury pathway in intestinal epithelium; C: qRT-PCR of ROS donor enzymes Nox2 and SOD2; D: qRT-PR of ROS sensor Keap1 and Nrf2; E: qRT-PCR of antioxidants NQO1and Txnrd1; F: qRT-PCR of peroxide detoxifier Gpx2 and Akr1b7; G: Immunofluorescence images of Nrf2 showing cytoplasmic and nuclear translocation (Nrf2-green) and DAPI (nuclei, blue) in control and NEC mice (Ctrl, Control-Breast fed or experimental NEC. **p<0.01, ***p<0.001 by a Student t test when comparisons of two groups were made, and by ANOVA for multiple comparisons, each dot represents data from individual mouse. ROS (reactive oxygen species), Nox2 (NADPH oxidase), SOD2 (Superoxide dismutase, mitochondrial), Keap1 (Kelch Like ECH Associated Protein 1), Nrf2 (NF-E2 p45-related factor), NQO1 (NADPH Quinone Dehydrogenase 1), Txnrd1 (Thioredoxin Reductase 1), Gpx2 (Glutathione Peroxidase 2, intestinal), Akr1b7 (Aldo-keto reductase family 1, member B7), scale bar =10μm.
DISCUSSION
The current study provides evidence that the composition of fat in infant formula has a significant effect on the severity of NEC, and further, that the administration of a novel formula containing “pre-digested fat”, composed of soybean oil free fatty acids, monoacyl glycerol palmitate, and soy lecithin can reduce NEC severity. In seeking to understand the mechanisms involved, we focused on the observation that in the premature gastrointestinal tract, the expression of lipases is relatively low, resulting in the delivery of un-digested lipid constituents to the distal ileum, where their intracellular accumulation can lead to the generation of ROS, oxidative stress and the inflammation that characterizes NEC. The oral administration of the broad acting anti-oxidant NAC reversed the accumulation of ROS and limited the degree of NEC and NEC-induced injury in mice, providing support for the role of ROS in NEC. We further determined that the proximal bowel has a greater expression of anti-oxidant genes as compared with the distal bowel, which may explain in part the greater susceptibility of the distal bowel to NEC as compared to the proximal bowel, while also explaining how the intracellular accumulation of lipid may have variable effects in different parts of the bowel. Taken together, these findings shed light on how the rational development of infant formulas through the provision of a “pre-digested fat” substrate may offer preventative strategy for infants at risk for the development of NEC.
The modified formula containing a novel “pre-digested fat” system that was used in the current study merits additional discussion. Free fatty acids included in the “pre-digested fat” are normal metabolic breakdown products of triglycerides after lipase action in the gastrointestinal tract. These free fatty acids are packaged into micelles that are absorbed readily by diffusion in proximal intestine into epithelial cells where they are then converted back into triglyceride with monoglyceride and subsequently transported out to the rest of the body as chylomicrons via the lymphatics. The calcium salt of FFAs present in the “pre-digested fat” formula could mimic metabolites of the normal digestive process and be readily absorbed in the proximal intestine. The “pre-digested fat” formula is different from a typical medium chain triglyceride (MCT)-rich infant formula, as the fat content utilized is a mixture of soybean free fatty acids (17.5%), monoacyl glycerol palmitate (20%), and phospholipid lecithin (10.3%) to bring the fatty acid profile closer to that of human milk. Soybean free fatty acids are produced from naturally occurring soybean oil (fatty acid in triglyceride form), which is highly processed by hydrolysis and distillation in order to separate glycerol from free fatty acids to achieve their final composition. In contrast, “standard fat” containing formula contains non-hydrolyzed oils (soybean oil, high oleic safflower oil and coconut oil) in 100% triglyceride form. This oil composition thus requires endogenous lipase activity for successful digestion and absorption, without which the accumulation of undigested triglycerides containing especially unsaturated fatty acids e.g. linoleic and alpha linolenic leads to the accumulation of ROS and cellular injury. It is noteworthy that in the current model of experimental NEC, mice displayed significantly reduced pancreatic lipase activity as compared with breast fed controls, perhaps as a result of hypoxia during the highly vulnerable window of the first week or so of life. This lack of lipase explains in part the reduced ability of these mice to undergo fat digestion, leading to NEC. Importantly, these cytotoxic effects could be reversed by the addition of the pre-digested formula, thus linking the presence of undigested fat with NEC development.
With respect to understanding further how breast milk protects against NEC, it should be mentioned that the fat digestive enzymes carboxyl ester lipase (CEL, also called bile salt dependent lipase, BSDL) is not only released by the pancreas, but is also present at very high levels in the breast milk of several species including mouse and human (17; 18; 36; 37), where it plays a critical key role in digestion and absorption of milk fat, which itself exists as triglyceride form. Several investigators have demonstrated that in preterm infants, the pancreas is unable to secrete sufficient lipases for triglyceride fat digestion due to enzyme insufficiency(13; 38; 39), and indeed the data in Figure 4A indicating the ontogeny of CEL secretion are consistent with this concept. Although mice which were induced to develop NEC were unable to digest the triglyceride fat completely when fed “standard formula”, the breast-fed pups were able to digest the milk fat (100% triglyceride fat), likely due to the presence of the fat digestive enzymes that are present in the breast milk, accounting in part for why undigested fat did not accumulate in the lumen of breast-fed mice. Casper et al (16) have demonstrated that by supplementing recombinant human CEL in formula, they facilitated fat digestion and absorption in preterm infants. The presence of lipases (36) in the breast milk supports the rationale for the pre-digested fat approach, which bypasses the requirement for CEL for fat digestion and absorption.
The current study adds to a growing body of work regarding the role of fat composition of infant formula on the maintenance of infant health. Caplan and colleagues have shown in experimental systems that the administration of a combination of long chain polyunsaturated fatty acids (LC-PUFA), specifically ARA and DHA can reduce intestinal inflammation and attenuate the degree of experimental NEC in a neonatal rat model, while DHA alone was unable to show any beneficial effect in terms of reducing NEC or TLR4 expression(40). In these studies, the authors used formula supplemented with 34mg/100 ml ARA and 23mg/100 ml DHA. Although in the present study, the formulas (Standard, PDF) contain approximately same amount of both ARA (~32 mg/100 ml) and DHA (~15 mg/100 ml) (Table 1), these two formulas showed different outcomes indicating that fat digestion and absorption in the current study may play an important role in NEC development. There are additional studies over the past several decades that have confirmed the various health benefits of polyunsaturated fatty acids, whose effects include improved absorption, enhanced membrane integrity, anti-inflammatory effects, and salutary effects on cell function(3; 41; 42).
We readily acknowledge that the current study has several limitations which may prevent its translation to the human population. First, we have shown data from a mouse model, which although shares many similarities with the human NEC, is limited by the short life span of the mouse compared to the human, and the fact that the mouse model cannot be subjected to surgical intervention or ostomy creation for further translatability of the current findings. Further, we acknowledge that there may be additional subtle differences in the various formulas that we have tested here, although the major differences lie in the type of fat components. Finally, we recognize that one of the key and perhaps underappreciated effects of the fat component in the diet on the premature infant will be on the microbiome, which itself can have major effects on the propensity for NEC development. Additional studies will be required to determine in greater detail the degree to which the predigested fat formula can impact the microbiome, and the effects to which changes in the microbiome can lead to NEC.
Acknowledgments
Financial support: DJH is supported by R01GM078238 and R01DK083752 from the National Institutes of Health and this research was funded in part by a Sponsored Research Grant from Abbott Nutrition. MG is supported by K08DK101608 and R03DK111473 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17-79, and the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital.
Abbreviations
- AHA
arachidonic acid
- BSDL
bile salt dependent lipase
- DHA
docosahexaenoic acid
- CEL
carboxyl ester lipase
- FFA
free fatty acid
- MDA
malondialdehyde
- NAC
N-acetylcysteine
- NEC
necrotizing enterocolitis
- PFA
paraformaldehyde
predigested fat
- ROS
reactive oxygen species
Footnotes
The amount of all nutrients used in animal studies could reasonably be expected to be achieved in the human population as they are derived from ready to feed infant formulas.
There are no in vitro studies, so the statement as requested by the British Journal of Nutrition that the “molecular form of the nutrient or nutrients used in in vitro studies is the same as that which the cell type in which the test was performed would encounter in vivo” does not apply.
This work does not involve any probiotics.
Disclosure: This work was supported in part by a sponsored research grant from Abbott Nutrition to DJH. No author has any financial relationship or conflict of interest with the current work.
References
- 1.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016;13:590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan MS, Russell T, Xiao Y, et al. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatric research. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Jilling T, Li D, et al. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2007;61:427–432. doi: 10.1203/pdr.0b013e3180332ca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan CE, Sodhi CP, Good M, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126:495–508. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soliman A, Michelsen KS, Karahashi H, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PloS one. 2010;5:e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y, Liu F, Li Y, et al. mRNA expression of TLR4, TLR9 and NF-kappaB in a neonatal murine model of necrotizing enterocolitis. Molecular medicine reports. 2016;14:1953–1956. doi: 10.3892/mmr.2016.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Li Y, Zhou B, et al. Inflammation and Apoptosis: Dual Mediator Role for Toll-like Receptor 4 in the Development of Necrotizing Enterocolitis. Inflamm Bowel Dis. 2017;23:44–56. doi: 10.1097/MIB.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 9.Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr. 2011;2:275–283. doi: 10.3945/an.111.000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armand M, Hamosh M, Mehta NR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–437. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Martin CR, Cheesman A, Brown J, et al. Factors Determining Optimal Fatty Acid Absorption in Preterm Infants. Journal of pediatric gastroenterology and nutrition. 2016;62:130–136. doi: 10.1097/MPG.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black DD. Effect of intestinal chylomicron secretory blockade on apolipoprotein synthesis in the newborn piglet. Biochem J. 1992;283(Pt 1):81–85. doi: 10.1042/bj2830081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care. 2010;13:314–320. doi: 10.1097/MCO.0b013e328337bbf0. [DOI] [PubMed] [Google Scholar]
- 14.Lebenthal E, Lee PC. Development of functional responses in human exocrine pancreas. Pediatrics. 1980;66:556–560. [PubMed] [Google Scholar]
- 15.Howles PN, Stemmerman GN, Fenoglio-Preiser CM, et al. Carboxyl ester lipase activity in milk prevents fat-derived intestinal injury in neonatal mice. Am J Physiol. 1999;277:G653–661. doi: 10.1152/ajpgi.1999.277.3.G653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casper C, Carnielli VP, Hascoet JM, et al. rhBSSL improves growth and LCPUFA absorption in preterm infants fed formula or pasteurized breast milk. J Pediatr Gastroenterol Nutr. 2014;59:61–69. doi: 10.1097/MPG.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis LA, Hamosh M. Bile salt stimulated lipase: comparative studies in ferret milk and lactating mammary gland. Lipids. 1992;27:917–922. doi: 10.1007/BF02535873. [DOI] [PubMed] [Google Scholar]
- 18.Freed LM, York CM, Hamosh M, et al. Bile salt-stimulated lipase in non-primate milk: longitudinal variation and lipase characteristics in cat and dog milk. Biochim Biophys Acta. 1986;878:209–215. doi: 10.1016/0005-2760(86)90148-7. [DOI] [PubMed] [Google Scholar]
- 19.Badwey JA, Curnutte JT, Robinson JM, et al. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984;259:7870–7877. [PubMed] [Google Scholar]
- 20.Penn AH, Altshuler AE, Small JW, et al. Effect of digestion and storage of human milk on free fatty acid concentration and cytotoxicity. J Pediatr Gastroenterol Nutr. 2014;59:365–373. doi: 10.1097/MPG.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good M, Sodhi CP, Egan CE, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8:1166–1179. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Good M, Sodhi CP, Hackam DJ. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert review of clinical immunology. 2014;10:875–884. doi: 10.1586/1744666X.2014.913481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodhi CP, Jia H, Yamaguchi Y, et al. Intestinal Epithelial TLR-4 Activation Is Required for the Development of Acute Lung Injury after Trauma/Hemorrhagic Shock via the Release of HMGB1 from the Gut. J Immunol. 2015;194:4931–4939. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshiba J. Method for hand-feeding mouse pups with nursing bottles. Contemporary topics in laboratory animal science. 2004;43:50–53. [PubMed] [Google Scholar]
- 26.Good M, Sodhi CP, Yamaguchi Y, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. The British journal of nutrition. 2016;116:1175–1187. doi: 10.1017/S0007114516002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afrazi A, Branca MF, Sodhi CP, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289:9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern MD, Weitkamp JH, Mount Patrick SK, et al. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. American journal of physiology Gastrointestinal and liver physiology. 2010;299:G623–631. doi: 10.1152/ajpgi.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yazji I, Sodhi CP, Lee EK, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A. 2013;110:9451–9456. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Chen SY, Hsu T, et al. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis. 2002;23:207–211. doi: 10.1093/carcin/23.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Yajima M, Kanno T, Yajima T. A chemically derived milk substitute that is compatible with mouse milk for artificial rearing of mouse pups. Experimental animals. 2006;55:391–397. doi: 10.1538/expanim.55.391. [DOI] [PubMed] [Google Scholar]
- 33.Sodhi C, Richardson W, Gribar S, et al. The development of animal models for the study of necrotizing enterocolitis. Disease models & mechanisms. 2008;1:94–98. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. The Journal of organic chemistry. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 35.Yin G, Wang Y, Cen XM, et al. Lipid peroxidation-mediated inflammation promotes cell apoptosis through activation of NF-kappaB pathway in rheumatoid arthritis synovial cells. Mediators of inflammation. 2015;2015:460310. doi: 10.1155/2015/460310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivecrona T, Hernell O. Human milk lipases and their possible role in fat digestion. Padiatrie und Padologie. 1976;11:600–604. [PubMed] [Google Scholar]
- 37.Miller R, Lowe ME. Carboxyl ester lipase from either mother’s milk or the pancreas is required for efficient dietary triglyceride digestion in suckling mice. The Journal of nutrition. 2008;138:927–930. doi: 10.1093/jn/138.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brady MS, Rickard KA, Fitzgerald JF, et al. Specialized formulas and feedings for infants with malabsorption or formula intolerance. Journal of the American Dietetic Association. 1986;86:191–200. [PubMed] [Google Scholar]
- 39.Nevin-Folino NL, Loughead JL, Loughead MK. Enhanced-calorie formulas: considerations and options. Neonatal Netw. 2001;20:7–15. doi: 10.1891/0730-0832.20.1.11. [DOI] [PubMed] [Google Scholar]
- 40.Diehl-Jones WL, Askin DF, Friel JK. Microlipid-induced oxidative stress in human breastmilk: in vitro effects on intestinal epithelial cells. Breastfeeding medicine: the official journal of the Academy of Breastfeeding Medicine. 2007;2:209–218. doi: 10.1089/bfm.2007.0017. [DOI] [PubMed] [Google Scholar]
- 41.Kapoor V, Glover R, Malviya MN. Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. The Cochrane database of systematic reviews. 2015:Cd009172. doi: 10.1002/14651858.CD009172.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vegge A, Thymann T, Lauritzen L, et al. Parenteral lipids and partial enteral nutrition affect hepatic lipid composition but have limited short term effects on formula-induced necrotizing enterocolitis in preterm piglets. Clinical nutrition (Edinburgh, Scotland) 2015;34:219–228. doi: 10.1016/j.clnu.2014.03.004. [DOI] [PubMed] [Google Scholar]