Abstract
Objectives:
To evaluate the role of caveolin-1 as a predictor of disease reclassification in men with early prostate cancer undergoing active surveillance.
Patients and Methods:
We analyzed archived plasma samples prospectively collected from men with early prostate cancer in a single-institution active surveillance study. Of 825 patients enrolled, 542 had 1 or more years of follow-up. Baseline and longitudinal plasma caveolin-1 levels were measured using an enzyme-linked immunosorbent assay. Tumor volume or Gleason grade increases were criteria for disease reclassification. Logistic regression analyses assessed associations between clinicopathologic characteristics and reclassification risk.
Results:
In 542 patients, 480 (88.6%) had stage cT1c disease, 542 (100.0%) had a median prostate-specific antigen level of 4.1 ng/mL, and 531 (98.0%) had a median Cancer of the Prostate Risk Assessment score of 1. In all, 473 (87.3%) had a Gleason score of 3+3. After a median of 3.1 years’ follow-up, disease was reclassified in 163 (30.1%). Baseline caveolin-1 levels were 2.2±8.5 ng/mL (mean) and 0.2 ng/mL (range, 0–85.5 ng/mL) (median). In univariate analysis, baseline caveolin-1 was a significant predictor for risk of disease reclassification (OR, 1.82, 95% CI 1.24–2.65, p=0.002); in multivariate analysis, with adjustments for age, tumor length, group risk stratification, and number of positive cores, reclassification risk associated with caveolin-1 remained significant (OR 1.91, 95% CI 1.28–2.84, p=0.001).
Conclusion:
Baseline plasma caveolin-1 level was an independent predictor of disease classification. New methods for refining active surveillance and intervention may result.
INTRODUCTION
The last decade witnessed a paradigm shift to managing low-risk prostate cancer with active surveillance (AS) not only in academic centers but also in community-based practices [1]. In part because prostate cancer’s biologic behavior varies widely and early disease progression is not clearly defined, no consensus exists about surveillance for early prostate cancer, including selection criteria for eligibility or clinicopathologic changes constituting disease progression [2].
AS is based on the tenet that prostate cancer’s natural history is predictable. Although the molecular basis of disease progression in early prostate cancer is not well delineated, we know from next-generation sequencing studies in advanced prostate cancer that molecular changes accompanying histologic changes in early prostate cancer progression may not be linear, suggesting tumor heterogeneity [3]. Additionally, prostate biopsy, the primary method of characterizing disease, is vulnerable to inherent sampling bias (undersampling), which threatens identification of true disease progression. In radical prostatectomy series of patients considered eligible for AS, approximately 30% are found to have upstaging and upgrading at prostatectomy [4]. Based on the results of AS cohorts with at least 5 years of follow up, 24–40% of AS patients will pursue active treatment and 0.1–2.8% will develop metastatic disease [5].
To improve risk stratification, individualize monitoring strategy, and reduce active treatment during AS, we need to refine patient selection at diagnosis with biomarkers. Given the morbidities [6] of and limited tumor tissue from prostate biopsies, identifying circulating biomarkers able to distinguish indolent from aggressive disease and able to predict early disease progression could transform management.
Caveolin-1 (Cav-1), a major structural component of caveolae, is secreted by prostate cancer cells and associated with malignant progression through multiple mechanisms and signaling pathways [7]. We have shown that Cav-1 is implicated in the transition from high-grade prostatic epithelial neoplasia to prostate cancer through c-Myc regulation and Akt signaling induction [8]. Other studies show that Cav-1 levels rise during prostate cancer progression and mediate resistance to hormone therapy [9, 10] by inducing glycolytic activities in prostate cancer cells and promoting hormone resistance under androgen deprivation through upregulation of acetyl-CoA carboxylase 1 and fatty acid synthase [10, 11]. Finally, investigations show serum Cav-1 associated with high-risk prostate cancer [12]; biochemical recurrence after prostatectomy [13]; and, when levels are high, with castration-resistant prostate cancer rather than with hormone-naive disease [14].
Hypothesizing that Cav-1 participates in disease progression in early prostate cancer, we evaluated Cav-1 as a biomarker for upgrading and disease reclassification (DR). We used archived plasma samples from men undergoing AS, which we also used to assess longitudinal measurements of Cav-1 in early disease progression.
METHODS
We used archived plasma samples prospectively collected from patients with early prostate cancer who participated in a single-institution cohort study of AS at The University of Texas MD Anderson Cancer Center as reported previously [15]. The study (clinicaltrials.gov NCT00490763) was initiated in February 2006, and 825 patients had enrolled by February 2014. The Institutional Review Board approved the protocol, and all enrollees signed an informed consent. Patients with clinically organ-confined prostate cancer diagnosed within 6 months of study enrollment were stratified to one of three groups. Enrollment in group I included those with favorable risk and required no more than one core of 3+3 Gleason score (GS) (tumor focus, <3.0 mm) or 3+4 GS (tumor focus, <2.0 mm). Study-entry prostate-specific antigen (PSA) level had to be <4 ng/mL or adjusted for prostate volume [16]. Group II included patients with a 3+3 GS or 3+4/4+3 GS who did not meet group I criteria and chose AS. Group III included patients with comorbidities that precluded local therapy, as determined by the physician treating the patient. Twice-annual clinical examination, laboratory study, and biopsy protocols along with protocol amendments are reported elsewhere [15].
At twice-annual clinical examinations, a digital rectal examination and laboratory studies (levels of testosterone and serum PSA), recording of concomitant medications, including 5α-reductase inhibitors and statins, and body mass index were performed. In 2014, scheduling of post baseline measures of testosterone were amended to an annual schedule. Biopsy of the prostate was performed every 1 to 2 years; the following year’s biopsy was omitted if the biopsy was negative, unless requested by the patient. Biopsies were performed via a standard approach using transrectal ultrasonography, and samples were taken according to the 11-core multisite-directed scheme that included sextant locations, one posterior midline, and left and right anterior horns [17].
At repeat biopsy, having an increase in tumor volume (increase in the number of positive cores and/or total tumor length) or any Gleason grade 4 or 5 component meant DR. For 3 patients in group II who had a primary Gleason grade 4 component in Gleason score 7 tumors, any primary Gleason grade 5 meant DR. Investigators did not reclassify disease based on the location of disease, including when it was found in the contralateral lobe, if the repeat biopsy otherwise met criteria for stable disease. A PSA change of a greater than 30% increase from baseline was considered clinically significant, and the level was retested a month later or three months after biopsy. Given that an elevated PSA level triggers additional tests, no patient came off study solely based on the elevated PSA level. No multiparametric endorectal MRI was required at baseline, and during surveillance it was left to the managing physician whether MRI was indicated for cause (e.g., mismatching PSA level and pathologic findings on biopsy). Patients remained on protocol until they requested withdrawal, chose active treatment after DR, or were diagnosed with a second malignancy. With the managing physician’s agreement, patients who declined repeat biopsy could stay on protocol.
Additionally, we calculated for each case the University of California, San Francisco, Cancer of the Prostate Risk Assessment (CAPRA) score [18, 19]. To calculate PSA velocity, we fit a linear regression model on ln(PSA) values over time. A reliable measure of PSA velocity requires, according to the American Urological Association, at least three measures of PSA after diagnosis over 18 months [20]. To calculate PSA doubling time, we regressed In(PSA) over time, obtaining the slope m and defining it as ln(2)/m.
Venous blood was collected into one 10-mL EDTA-coated tube (Becton, Dickinson, Franklin Lakes, NJ, USA) at baseline and every 6 months during surveillance as an optional procedure. The Vacutainer was inverted gently to allow proper additive mixture. Samples were centrifuged at 4ºC for 20 minutes at 1000–1200 rcf. The supernatant was removed, and vial contents were aliquoted into multiple cryovials (minimum of three). Blood samples were processed and frozen within 2 hours of collection. Cav-1 was measured by a direct sandwich enzyme-linked immunosorbent assay (ELISA), as previously described [13, 21]. To the best of our knowledge there is no available commercial assay to measure Cav-1 levels. We have described characterization of the ELISA parameters, including the inter-assay and intra-assay coefficient of variability in our previous publication [21].
Patient characteristics were summarized using median and range for continuous variables and frequency with percentage for categorical variables. The skewed distribution prompted application of logarithmic transformation after shifting Cav-1 values by 1. The log-transformed Cav-1 at each time point was summarized using descriptive statistics. A BLiP plot was used to show the distribution of log-transformed Cav-1 over time. Patient characteristics of those with and without DR were compared using the Wilcoxon rank sum test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables.
Univariate logistic regression analyses were performed to assess the association between each patient characteristic and risk of DR. The optimal cutoff value for Cav-1 was selected based on the Youden index [22]. A multiple logistic regression model was fit by including all covariates that were statistically significant in the univariate analysis, and a scoring system was developed based on the multivariate logistic regression model for predicting risk of DR. The assigned score for each covariate was determined based on the estimated odds ratio (OR) obtained from the regression model. A bootstrapping method was used to validate the scoring system. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Cohort
Of 825 patients enrolled between February 2006 and February 2014, 616 had at least 1 year of follow-up. Of these, 542 patients in groups I and II had baseline blood specimens. Two patients with GS5 tumor in groups I and II, and fourteen patients in group III were excluded. Baseline patient characteristics by DR status show that 163 patients (30.1%) were reclassified, and of these, 86 met the DR cutoff because of tumor upgrading (Table 1). They were more likely to be older (p=0.04) and have longer total tumor length at baseline (p=0.01) than those whose disease was not reclassified.
Table 1.
Baseline characteristics
| All patients (n = 542) | No disease reclassification | Disease reclassification | |||||
|---|---|---|---|---|---|---|---|
| n/Value | %/Range | n/Value | %/Range | n/Value | %/Range | P | |
| No. pts | 542 | 100 | 379 | 100 | 163 | 100 | |
| Median age (yr) | 64 | 64–87 | 63 | 36–87 | 65 | 45–83 | 0.04 |
| Race/Ethnicity (n = 540) |
0.92 | ||||||
| White | 442 | 81.9 | 307 | 81.2 | 135 | 83.3 | |
| Black | 43 | 8.0 | 30 | 7.9 | 13 | 8.0 | |
| Hispanic | 45 | 8.3 | 33 | 8.7 | 12 | 7.4 | |
| Asian | 10 | 1.9 | 8 | 2.1 | 2 | 1.2 | |
| Median body mass index (n = 531) |
28.44 | 17.59–62.67 | 28.43 | 17.59–62.67 | 28.51 | 18.84–42.49 | 0.93 |
| Median PSA values (ng/mL) |
|||||||
| PSA (n = 542) | 4.1 | 0.2–34 | 4.1 | 0.2–34 | 4.1 | 0.2–12 | 0.85 |
| PSA density (n = 523) | 0.09 | 0.01–1.05 | 0.09 | 0.01–1.05 | 0.1 | 0.01–0.39 | 0.30 |
| PSA velocity (n = 378) |
0.22 | -3.09–10.92 | 0.21 | -3.09–10.92 | 0.38 | −0.78–2.7 | 0.10 |
| PSA doubling time (n = 377) |
4.12 | -206.72–163,111.6 | 4.03 | -206.72–163,111.6 | 4.42 | -87.3–220.07 | 0.44 |
| Median testosterone (ng/dL) (n =541) |
379 | 25–1118 | 382 | 25–1118 | 378 | 78–798 | 0.61 |
| Median total prostate volume by TRUS (mL) (n = 523) |
40.1 | 13.5–196 | 41.7 | 13.5–196 | 38.5 | 17.3–105 | 0.16 |
| Median caveolin-1 (n = 542) |
0.19 | 0–85.49 | 0.18 | 0–79.17 | 0.33 | 0–85.49 | 0.27 |
| Median CAPRA score (n = 531) |
1 | 0–6 | 1 | 0–6 | 1 | 0–5 | 0.48 |
| Gleason score (n = 542) |
0.27 | ||||||
| 5* | 2 | 0.4 | 2 | 0.5 | 0 | 0 | |
| 6 | 473 | 87.3 | 335 | 88.4 | 138 | 84.7 | |
| 7 | 67 | 12.4 | 42 | 11.1 | 25 | 15.3 | |
| Clinical stage (n = 542) | 0.86 | ||||||
| cT1a/cT1b | 8 | 1.5 | 6 | 1.6 | 2 | 1.2 | |
| cT1c | 480 | 88.6 | 337 | 88.9 | 143 | 87.7 | |
| cT2 | 54 | 10 | 36 | 9.5 | 18 | 11 | |
| Median number of surveillance biopsies (n = 542) |
3 | 1–9 | 3 | 1–8 | 4 | 2–9 | <0.001 |
| Tumor length (mm) (n = 531) |
0.01 | ||||||
| < 3 | 376 | 70.8 | 275 | 74.1 | 101 | 63.1 | |
| ≥ 3 | 155 | 29.2 | 96 | 25.9 | 59 | 36.9 | |
| Median number of positive biopsy cores (n=531) |
1 | 1–6 | 1 | 1–6 | 1 | 1–5 | <0.001 |
| 5-α-reductase inhibitor: current or history of treatment (n = 541) |
0.11 |
||||||
| Yes | 61 | 11.3 | 48 | 12.7 | 13 | 8.0 | |
| No | 480 | 88.7 | 330 | 87.3 | 150 | 92.0 | |
| Family history, first degree (n = 538) |
0.79 | ||||||
| Yes | 121 | 22.5 | 86 | 22.8 | 35 | 21.7 | |
| No | 417 | 77.5 | 291 | 77.2 | 126 | 78.3 | |
| Family history, second degree (n = 539) |
0.25 | ||||||
| Yes | 70 | 13.0 | 45 | 11.9 | 25 | 15.5 | |
| No | 469 | 87.0 | 333 | 88.1 | 136 | 84.5 | |
| Statin use (n = 542) | 0.27 | ||||||
| Yes | 250 | 46.1 | 169 | 44.6 | 81 | 49.7 | |
| No | 292 | 53.9 | 210 | 55.4 | 82 | 50.3 | |
CAPRA, Cancer of the Prostate Risk Assessment; PSA, prostate-specific antigen; TRUS = transurethral ultrasonography.
Gleason score 5 tumors were detected in the transurethral resection of the prostate specimens in 2 patients in Group I.
Associations between Cav-1 Levels and Reclassification
As seen in the distribution of log-transformed Cav-1 levels by reclassification status at 12, 24, and 36 months (fig. 1), Cav-1 levels tended to be higher in patients with DR than those without. The mean log-transformed Cav-1 levels and their 95% confidence intervals (CIs) by reclassification status and distribution across time indicate that between patients with DR and those without, we observed statistically significant differences at 6, 12, 18, 24, and 36 months (fig. 2).
Figure. 1.
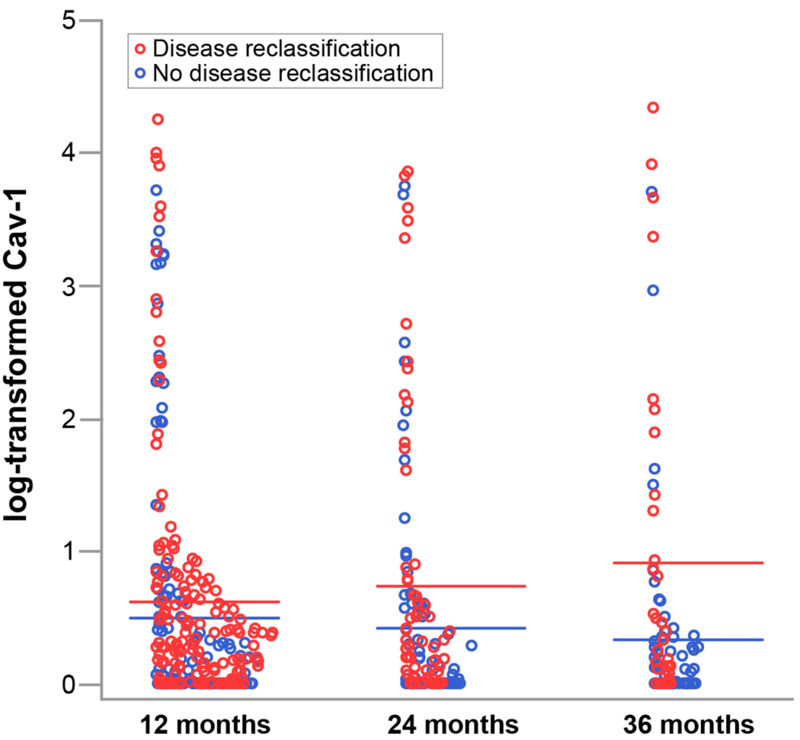
Distribution of log-transformed caveolin-1 (Cav-1) values of 542 patients by disease reclassification status at specific time points (12, 24, and 36 months). Mean log-transformed Cav-1 values for patients with disease reclassification (red bars) tended to be higher than those for patients who had no disease reclassification (blue bars).
Figure 2.
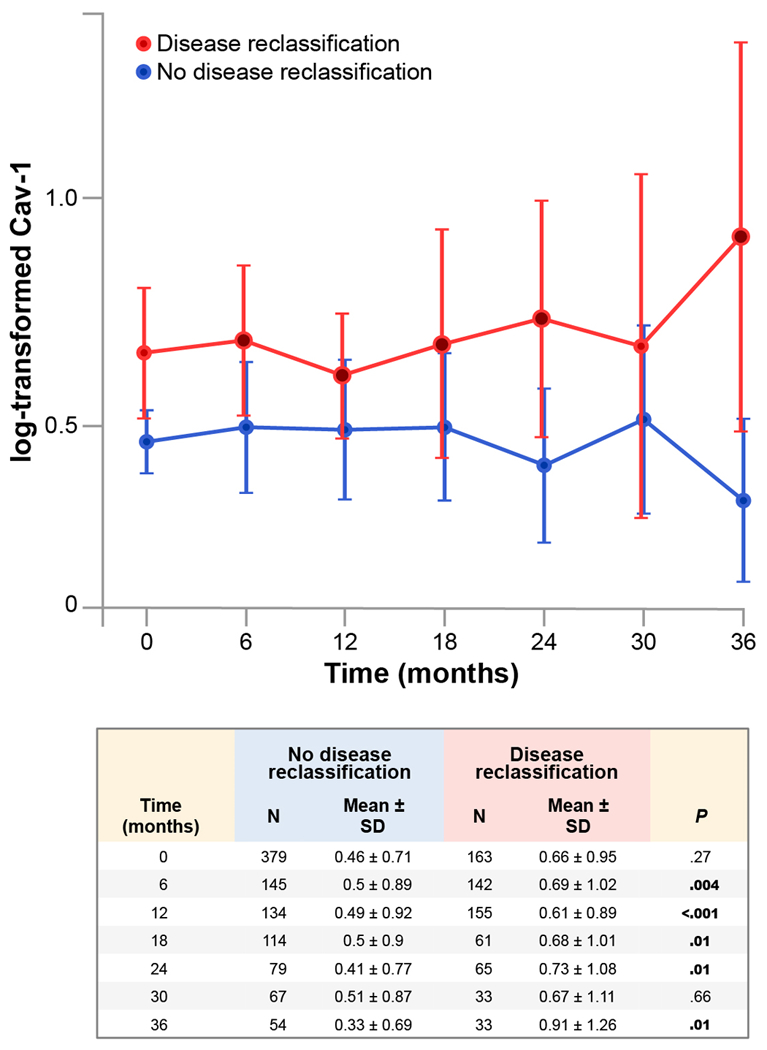
Mean log-transformed caveolin-1 (Cav-1) values and their 95% confidence intervals by reclassification status over time among 542 patients. Differences in log-transformed Cav-1 levels were significant between the group with reclassified disease (red) and the group without reclassified disease (blue) at 6, 12, 18, 24, and 36 months (closed red circles).
Table 2 shows the results of univariate and multivariate logistic regression analyses. As determined by the Youden index, the optimal cutoff point of Cav-1 was 0.624 ng/mL. In univariate analysis, the risk of DR was significantly associated with having a higher baseline Cav-1 level (OR 1.82, 95% CI 1.24–2.65, p=0.002), being older (OR 1.45, 95% CI 1.00–2.10, p=0.05), belonging to a subgroup of AS patients who chose AS (OR 2.31, 95% CI 1.52–3.51, p=0.001), having a longer total tumor length at baseline (OR 1.67, 95% CI 1.13–2.49, p=0.01) or having more than one positive biopsy core (OR 2.31, 95% CI 1.56–3.42, p<0.001). In the multivariate regression, baseline Cav-1 level (p=0.001), subgroup assignment (p=0.001), and number of positive biopsy cores (p=0.02) were significantly associated with DR. Patients who had a higher level of baseline Cav-1, belonged in a subgroup of patients who chose AS (group II), had more than one positive biopsy core, and had a higher risk of DR. We also performed a subgroup analysis for the 473 patients with 3+3 GS and observed similar multivariate analysis results (data not shown).
Table 2.
Univariate and multivariate logistic regression models for reclassification among all patients
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Odds ratio |
95% Confidence interval |
p | Odds ratio |
95% Confidence interval |
P | |
| Caveolin-1 | ≥0.624 vs. <0.624 |
1.82 | 1.24 – 2.65 | 0.002 | 1.91 | 1.28 – 2.84 | 0.001 |
| Age (years) | ≥64 vs. <64 | 1.45 | 1.00 – 2.10 | 0.05 | 1.34 | 0.90 – 1.99 | 0.14 |
| Risk group | 2 vs. 1 | 2.31 | 1.52 – 3.51 | < 0.001 | 1.97 | 1.19 – 3.26 | < 0.001 |
| Gleason score | 7 vs. 5/6 | 1.45 | 0.85 – 2.48 | 0.17 | |||
| Prostate-specific antigen level (ng/mL) |
≥4 vs. <4 | 1.15 | 0.80 – 1.66 | 0.45 | |||
| Tumor length (mm) | ≥3 vs. <3 mm | 1.67 | 1.13 – 2.49 | 0.01 | 0.80 | 0.48 – 1.34 | 0.39 |
| Family history, first degree | Yes vs. No | 0.94 | 0.60 – 1.47 | 0.79 | |||
| Clinical stage* | cT2a/c vs. cT1c | 1.18 | 0.65 – 2.14 | 0.59 | |||
| Statin use | Yes vs. No | 1.23 | 0.85 – 1.77 | 0.27 | |||
| Number of positive biopsy cores |
≥1 vs. 1 | 2.31 | 1.56 – 3.42 | <0.001 | 1.85 | 1.12 – 3.07 | 0.02 |
Patients with cT1a and cT1b were excluded.
Based on the fitted multiple logistic regression model and the estimated OR for each covariate, a score of 0, 1, or 2 was assigned to each level of a prognostic factor, resulting in a total score for each patient, ranging from 0 to 8 (Table 3). The Hosmer-Lemeshow test suggested a goodness of fit of the model (p=0.13). The area under the receiver operating characteristic curve (AUC) of 0.66 confirmed adding Cav-1 to clinical features improved on relying on clinical features alone and indicated a good performance by the scoring system (Figure S1). Table 3 also includes the sensitivity and specificity associated with each total score when dichotomizing patients into low-risk vs. high-risk groups, using total score as a cutoff value. The odds of DR for patients with a total score at least 4 were 125% higher than the odds for those with a total score less than 4 (OR 2.25, 95% CI 1.46–3.46, p<0.001). We also assessed the internal validity of the scoring system using 1000 bootstrap samples, and 96.4% of the time the scoring system was shown to be significantly associated with the risk of DR. Finally, by multivariate logistic regression analysis of DR among all 542 patients, adjusting for CAPRA score, baseline Cav-1 was significantly associated with DR (p=0.002).
Table 3.
Factors identified as prognostic of disease reclassification and the derived scoring system
| Factors Prognostic of Disease Reclassification | |||
|---|---|---|---|
| Comparison values | Odds ratio | Score | |
| Caveolin-1 | <0.624 | 1.00 | 0 |
| ≥0.624 | 1.91 | 2 | |
| Age | <64 | 1.00 | 0 |
| ≥64 | 1.35 | 1 | |
| Risk group | 1 | 1.00 | 0 |
| 2 | 2.34 | 2 | |
| Tumor length | <3 | 1.00 | 0 |
| ≥3 | 1.05 | 1 | |
| Number of positive | 1 | 1.00 | 0 |
| biopsy cores | ≥1 | 1.85 | 2 |
| Scoring system | |||
| Total score | DR cases/Total (%) | Sensitivity* | Specificity* |
| 0 | 1/3 (33%) | 100% | 0% |
| 1 | 0/2 (0%) | 99% | 1% |
| 2 | 16/87 (18%) | 99% | 1% |
| 3 | 17/83 (20%) | 90% | 20% |
| 4 | 50/155 (32%) | 79% | 37% |
| 5 | 41/112 (37%) | 48% | 65% |
| 6 | 26/64 (41%) | 23% | 84% |
| 7 | 8/24 (33%) | 7% | 94% |
| 8 | 4/12 (33%) | 2% | 98% |
For example: Grouping patients using a total score of 4 (i.e., <4 vs. ≥4) results in a sensitivity of 79% and a specificity of 37%.
Other Associations
There was no significant association between log-transformed Cav-1 levels and GS and testosterone levels or log-transformed Cav-1 levels and statin use. However, GS was significantly associated with baseline Cav-1, and statin use was significantly associated with testosterone level. The multiple logistic regression analysis indicated that there was a significant association between the log-transformed baseline Cav-1 level and reclassification related to upgrading of GS (OR 1.4, p=0.01) or reclassified related to other reasons (OR 1.32, p=0.05) (data not shown).
DISCUSSION
In this study of 542 patients enrolled in AS, baseline plasma Cav-1 levels were significantly associated with DR (OR 1.82, p=0.002). Furthermore, a multivariate prognostication model, adjusted for age, baseline total tumor length, risk group, and number of positive biopsy cores indicated that a high baseline Cav-1 level was significantly associated with the risk of DR (OR 1.91, 95% CI 1.28–2.84, p=0.001). CAPRA was not predictive of early DR, and, in fact, after adjusting for CAPRA in a multivariate regression analysis, we found Cav-1 was still predictive of DR, further supporting the utility of Cav-1 as prognostic biomarker. As a secondary analysis, we also fitted a multivariable Cox regression model for the time to DR. The results are similar in that Caveolin-1 remained a significant predictor for the risk of DR (p=0.003) after adjusting for the effects of age, risk group, baseline total tumor length, and number of positive biopsy cores. Validation of these results could lead to adding new biologic criteria that will refine AS patient selection by improving performance of the biopsy, individualize monitoring strategy, and improve timing of active intervention.
Currently, any triggers for early intervention in AS are mainly pulled by changes in disease on surveillance biopsies, including histologic grade, number of tumor-containing cores, and tumor length. This explains why a close relationship ties increases in tumor volume to dedifferentiation [23, 24]. However, prostate biopsies are vulnerable to inherent sampling bias, which may threaten identification of true dedifferentiation (progression). Sampling bias vulnerability was a major focus in two AS studies characterized by a short interval between biopsies [25, 26].
The strengths of our investigation include a large sample size, strict criteria for patient inclusion, and use of high-sensitivity assays. Moreover, to the best of our knowledge this is the first longitudinal investigation of a potential blood-based biological biomarker in AS. Limitations include the narrow patient sample: most patients were white and had clinical stage cT1c disease with a GS of 6 and also there was lack of MRI data to analyze. This study focused on only one plasma biomarker, whereas a panel of biologically related biomarkers has long been sought to improve early progression prediction accuracy. We decided to longitudinally study Cav-1 levels in an attempt to find if biomarker fluctuation occurred close to the time progression occurred, but no association was found. However, our results showed consistently elevated Cav-1 levels for those with DR from the beginning of the surveillance period. These results could mean that patients with high Cav-1 levels harbored cryptic, unbiopsied prostate cancer cells of greater clinical potential, and possibly higher GS, at the time of initial biopsy.
Aside from Cav-1, other plasma biomarkers have been suggested as predictive of DR. Tosoian et al. [27] associated [−2]proPSA with biopsy-detected DR in patients with prostate cancer undergoing AS, and after studying 167 men for a median of 4.3 years, these investigators found that measures based on [−2]proPSA, such as phi, could predict reclassification by biopsy. The 4Kscore was also found to be a significant predictor of reclassification as defined by a Gleason score ≥7 [28].
Lin et al [29] analyzed scores of PCA3, a prostate-specific noncoding RNA, and TMPRSS2-ERG generated from urine samples from nearly 400 patients undergoing AS and showed that both stratify risk of having aggressive cancer as defined by higher tumor volume or higher GS. When authors evaluated the ROC analysis of these biomarkers for predicting high Gleason grade (≥7), they found that each one of them independently had an AUC near 0.66; nevertheless, the addition of the markers from the AUC analysis was not more significant than PSA prediction alone (p=0.08).
The threshold for triggering clinical intervention is often left to the treating physician and to patients, and three commercial biomarker tests have been validated for prostate cancer: Oncotype Dx (Genomic Health, Redwood City, CA, USA), Prolaris (Myriad Genetics, Salt Lake City, UT, USA), and Decipher (GenomeDx Biosciences, Vancouver, BC, Canada). Thus far, Decipher, designed for use after prostatectomy, is the only one that has not been prospectively evaluated in patients undergoing AS. Additionally, these tissue-based gene tests are limited by the usual vagaries of biopsy in men with early stage prostate cancer (i.e., invasiveness, small tumor size, and negative biopsy).
Although MRI has not been incorporated in any AS guidelines yet, several studies support that MRI can predict an increased risk of DR [5]. However, in this era of cost containment, unfortunately, a large proportion of insurance companies decline covering the cost of MRIs in the setting of AS in the United States. Schoots et al. [30] reviewed MRI use in men undergoing AS and, despite the small number of patients, it became evident that MRI-targeted biopsies led to discovery of DR in 1:3 to 1:2 men. Based on these results, it seems that MRI could become part of the ongoing surveillance of AS cohorts. Although data are insufficient at this point to support replacing standard repeat biopsy with MRI-targeted biopsy [30], integrating MRI imaging with detection by blood based-biomarkers may be the best way to improve patient selection, stratification, and monitoring (e.g., decrease in the frequency of surveillance biopsies) in AS.
Given the complexities of the molecular basis and the multistep processes involved in disease progression of early prostate cancer, blood-based biomarkers prognostic of true disease progression possess transformative promise. Cav-1 is the first biomarker detectable in blood that has been shown not only to participate in prostate cancer progression but also to be predictive of early disease progression, and the discovery of this duality expands the library of known clinicopathologic parameters in patients undergoing AS. Though these findings have had no impact on clinical practice so far, if validated, monitoring plasma Cav-1 levels may improve risk stratification and with it increase predictive capacity for identifying AS patients at higher risk of early disease progression. This will lead to a personalized monitoring strategy of hypervigilance for patients at higher risk of disease progression and a less intensive schedule for those who could be spared biopsies and/or early active intervention.
CONCLUSIONS
Cav-1 not only has been shown to participate in prostate cancer progression but also to predict DR. The discovery of this duality expands the library of known clinicopathologic parameters in patients undergoing AS and may increase predictive capacity for identifying AS patients at higher risk of DR. This capability will lead to a personalized monitoring strategy of hypervigilance for patients at higher risk of DR and a less intensive schedule for those who could be spared biopsies and/or early active intervention.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge support from The University of Texas MD Anderson Cancer Center Moon Shots Program and from the Prostate Cancer Foundation—Young Investigator Award (S.P.B.).
Grant: P30 CA016672, and from the Prostate Cancer SPORE P50 CA140388.
Abbreviations and Acronyms
- AS
active surveillance
- AUC
area under the receiver operating characteristic curve
- CAPRA
Cancer of the Prostate Risk Assessment
- Cav-1
Caveolin-1
- CI
confidence interval
- DR
disease reclassification
- GS
Gleason score
- MRI
magnetic resonance imaging
- OR
odds ratio
- PSA
prostate-specific antigen
Footnotes
Presented in part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, June 3–7, 2016.
AUTHORS' DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Contributor Information
Spyridon P. Basourakos, Department of Genitourinary Medical Oncology The University of Texas MD Anderson Cancer Center, Houston, Texas.
John W. Davis, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Brian F. Chapin, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
John F. Ward, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Curtis A. Pettaway, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Louis L. Pisters, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Neema Navai, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Mary F. Achim, Department of Urology The University of Texas MD Anderson Cancer Center, Houston, Texas
Xuemei Wang, Department of Biostatistics The University of Texas MD Anderson Cancer Center, Houston, Texas.
Hsiang-Chun Chen, Department of Biostatistics The University of Texas MD Anderson Cancer Center, Houston, Texas.
Seungtaek Choi, Department of Radiation Oncology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Deborah Kuban, Department of Radiation Oncology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Patricia Troncoso, Department of Pathology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Sam Hanash, Department of Clinical Cancer Prevention The University of Texas MD Anderson Cancer Center, Houston, Texas.
Timothy C. Thompson, Department of Genitourinary Medical Oncology The University of Texas MD Anderson Cancer Center, Houston, Texas.
Jeri Kim, Department of Genitourinary Medical Oncology The University of Texas MD Anderson Cancer Center, Houston, Texas.
REFERENCES
- [1].Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 2015. July 7: 314:80–2 [DOI] [PubMed] [Google Scholar]
- [2].Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol 2016. March: 13:151–67 [DOI] [PubMed] [Google Scholar]
- [3].Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015. May 21: 161:1215–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Davis JW, Kim J, Ward JF, et al. Radical prostatectomy findings in patients predicted to have low-volume/low-grade prostate cancer diagnosed by extended-core biopsies: an analysis of volume and zonal distribution of tumour foci. BJU Int 2010. May: 105:1386–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tosoian JJ, Carter HB, Lepor A, Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol 2016. April: 13:205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol 2013. November: 190:1742–9 [DOI] [PubMed] [Google Scholar]
- [7].Thompson TC, Tahir SA, Li L, et al. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis 2010. March: 13:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang G, Goltsov AA, Ren C, et al. Caveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Mol Cancer Res 2012. February: 10:218–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nasu Y, Timme TL, Yang G, et al. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med 1998. September: 4:1062–4 [DOI] [PubMed] [Google Scholar]
- [10].Karantanos T, Karanika S, Wang J, et al. Caveolin-1 regulates hormone resistance through lipid synthesis, creating novel therapeutic opportunities for castration-resistant prostate cancer. Oncotarget 2016. June 16: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tahir SA, Yang G, Goltsov A, et al. Caveolin-1-LRP6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Res 2013. March 15: 73:1900–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gumulec J, Sochor J, Hlavna M, et al. Caveolin-1 as a potential high-risk prostate cancer biomarker. Oncol Rep 2012. March: 27:831–41 [DOI] [PubMed] [Google Scholar]
- [13].Tahir SA, Frolov A, Hayes TG, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res 2006. August 15: 12:4872–5 [DOI] [PubMed] [Google Scholar]
- [14].Sugie S, Mukai S, Tsukino H, et al. Increased plasma caveolin-1 levels are associated with progression of prostate cancer among Japanese men. Anticancer Res 2013. May: 33:1893–7 [PubMed] [Google Scholar]
- [15].Davis JW, Ward JF 3rd, Pettaway CA, et al. Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int 2016. July: 118:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Babaian RJ, Miyashita H, Evans RB, Ramirez EI. The distribution of prostate specific antigen in men without clinical or pathological evidence of prostate cancer: relationship to gland volume and age. J Urol 1992. March: 147:837–40 [DOI] [PubMed] [Google Scholar]
- [17].Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol 2000. January: 163:152–7 [PubMed] [Google Scholar]
- [18].Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 2005. June: 173:1938–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst 2009. June 16: 101:878–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].American Urological Association. PSA Testing for the Pretreatment Staging and Posttreatment Management of Prostate Cancer: 2013 Revision of 2009 Best Practice Statement Linthicum, MD: American Urological Association, 2013 [Google Scholar]
- [21].Tahir SA, Ren C, Timme TL, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res 2003. September 1: 9:3653–9 [PubMed] [Google Scholar]
- [22].Perkins NJ, Schisterman EF. The Youden Index and the optimal cut-point corrected for measurement error. Biom J 2005. August: 47:428–41 [DOI] [PubMed] [Google Scholar]
- [23].McNeal JE, Bostwick DG, Kindrachuk RA, Redwine EA, Freiha FS, Stamey TA. Patterns of progression in prostate cancer. Lancet 1986. January 11: 1:60–3 [DOI] [PubMed] [Google Scholar]
- [24].McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer 1990. September 15: 66:1225–33 [DOI] [PubMed] [Google Scholar]
- [25].Choo R, Danjoux C, Morton G, et al. How much does Gleason grade of follow-up biopsy differ from that of initial biopsy in untreated, Gleason score 4–7, clinically localized prostate cancer? Prostate 2007. November 1: 67:1614–20 [DOI] [PubMed] [Google Scholar]
- [26].Sheridan TB, Carter HB, Wang W, Landis PB, Epstein JI. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J Urol 2008. March: 179:901–4; discussion 4–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tosoian JJ, Loeb S, Feng Z, et al. Association of [−2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol 2012. October: 188:1131–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin DW, Newcomb LF, Brown MD, et al. Evaluating the four kallikrein panel of the 4K score for prediction of high-grade prostate cancer in men in the Canary Prostate Active Surveillance Study. European urology 2016. November 23: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin DW, Newcomb LF, Brown EC, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res 2013. May 01: 19:2442–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. European urology 2015. April: 67:627–36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


