Abstract
Opioid use and abuse has reached epidemic levels in the United States. As these drugs are frequently used by women of reproductive age, there has been a significant increase in the number of infants born to opioid dependent women. Few preclinical studies have examined voluntary opioid intake during pregnancy, and none have used intravenous self-administration. Thus, the purpose of the current set of studies was to utilize a translational model of oxycodone self-administration in rats to determine the effects of oxycodone intake during pregnancy on early postnatal outcomes. Females were trained to intravenously self-administer oxycodone several weeks prior to mating and then continuously throughout pregnancy followed by withdrawal around the time of parturition. Offspring were monitored for weight gain and separation-induced ultrasonic vocalizations (i.e. number of calls) while dams were examined for motivated maternal responding. Neural expression of the mu opioid receptor gene OPRM1 was examined in offspring on postnatal day 1 (PND1). Results indicate that females self-administer oxycodone during pregnancy at levels similar to those observed in cycling females. Postpartum, oxycodone withdrawn females demonstrate impaired maternal responding. In offspring, while no significant group effects were observed on body weight or call number, age-dependent alterations in weight gain and call number correlated with the dams cumulative oxycodone dose during pregnancy. In addition, offspring demonstrated region specific effects of oxycodone exposure on OPRM1 on PND1. Overall, these findings demonstrate that pregnant females will voluntarily self-administer oxycodone at levels similar to cycling females when using a short access model. Further, maternal oxycodone self-administration alters the maternal-offspring dyad in a manner that is dose-dependent and results in sex-and region-specific effects on early measures of neurodevelopment.
1. Introduction
Recent data from the Centers for Disease Control (CDC) reveal that women of reproductive age are frequently prescribed opioid pain medications, with 39% of Medicaid and 28% of privately insured female patients between the ages of 15–44 filling at least one prescription each year (http://www.cdc.gov/media/releases/2015/p0122-pregnancy-opioids.html 2015). These findings led the CDC to recommend that physicians carefully consider the appropriateness of treating women who are pregnant, or may become pregnant, with opioids due to the risk of potential birth defects, including the risk of neonatal abstinence syndrome (NAS). Data regarding birth defects associated with gestational opioids are extremely limited, particularly regarding commonly prescribed opioids, like codeine or oxycodone. It is clear, however, that the rates of prenatal opioid exposure are rising with recent reports suggesting a 5 fold increase in maternal opioid use from 2000–2009 and a 3 fold increase in infants born to women dependent on, or addicted to, prescription opioids (Patrick, Schumacher et al. 2012). The extent to which rising rates of opioid prescriptions in women are related to these outcomes is open for debate; however, it should be noted that states with the highest number of opioid prescriptions are also those with the highest number of infants born with NAS (Patrick, Davis et al. 2015). Moreover, rates of prescription opioid misuse and opioid use disorder remain stubbornly high despite efforts to improve prescribing habits. Indeed, the number of women misusing prescription opioids is substantially higher than the number addicted to heroin (Han, Compton et al. 2017). Thus, prenatal exposure to prescription opioids, the growing population of NAS infants, and the potential long-term neurodevelopment effects of both exposure and withdrawal remain significant areas of concern.
Most animal models of prenatal opioid exposure have examined the effects of morphine. Many rely on either continuous release pumps/pellets or experimenter administered drug, neither of which is representative of human use. There are a few studies that have used oral gavage or delivery via the animal’s water-bottle to replicate oral ingestion of pain medication (Nasiraei-Moghadam, Sahraei et al. 2005, Schrott, Franklin et al. 2008, Davis, Franklin et al. 2010), however, those studies must consider potential issues related to first-pass metabolism (Iwamoto and Klaassen 1977) which may be further complicated by the increased circulatory demands of pregnancy. Finally, the vast majority of studies begin their opioid administration on or around the first day of pregnancy, a model which does not represent the likely pattern of use in women.
Drug self-administration represents an animal model with high face, construct, and predictive validity with regard to the abuse liability of compounds across drug class. By training the animal to perform a specific operant response to attain an intravenous drug infusion, this paradigm allows the animal to control the timing and amount of drug intake. Studies have demonstrated significant differences between this type of voluntary drug intake, when compared to both experimenter administered and non-contingent drug infusion across a number of different drugs (Jacobs, Smit et al. 2003). These differential effects are observed on measures of withdrawal, gene expression and neurochemical effects in a number of critical brain regions (Mutschler and Miczek 1998, Miguens, Crespo et al. 2008, Orejarena, Berrendero et al. 2009, Metaxas, Bailey et al. 2010). Of particular relevance to modeling drug use during pregnancy, significant alterations in both endocrine and immune function are observed following opioid self-administration that are not similarly observed following with the same amounts of opioids administered using non-contingent infusions (Weber, Gomez-Flores et al. 2009). These differences could impact how prenatal opioid exposure affects both the mother and her offspring.
The purpose of the current set of studies was to utilize a model of prenatal oxycodone self-administration in rats to examine the impact of voluntary intake on both the dam and her offspring during the early postnatal period. As there are currently no published data on opioid self-administration during pregnancy, we first determined whether the rate of responding for a moderate dose of oxycodone was altered by the state of pregnancy. We also examined the effects of oxycodone prior to mating on fertility and fecundity. We then examined the effects of prenatal oxycodone self-administration on response rates following postpartum drug withdrawal as well as on maternal responding and developmental parameters in offspring, including effects on body weight and number of separation-induced ultrasonic vocalizations. Finally, we examined mu opioid receptor gene (OPRM1) expression in three regions of the developing brain (postnatal day 1; brainstem, hypothalamus, and forebrain) as modifications in the regulation of this gene are correlated with increased risk of neonatal abstinence syndrome (Wachman, Hayes et al. 2013, Wachman, Hayes et al. 2014). Indeed, OPRM 1 expression is of interest because it’s role in withdrawal and correlation with the severity of neonatal abstinence syndrome. In addition, stimulation of this receptor in utero may have a profound impact on subsequent gene expression which could alter neurodevelopmental trajectories for the offspring.
2. Materials and Methods:
2.1. Animals:
Nulliparous female Sprague-Dawley rats (approximately 70 days of age) were purchased from Charles River Breeding Laboratories (Kingston, NY, USA) and housed in a light (0700–1900 hours) and temperature (21–24°C) controlled room. Food and water were provided ad libitum throughout the experiment. All procedures were conducted in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Tufts University.
2.2. Oxycodone Self-Administration
2.2.1. Catheterization Surgery:
Animals were anesthetized using a ketamine/ dexmedetomidine cocktail (80 mg/kg and 1 mg/kg, respectively). The catheter (CamCaths, Cambridge, UK) was composed of silastic tubing that was fed into the right external jugular vein and routed to a mesh backmount platform secured subcutaneously between the shoulder blades. Following surgery, animals were housed individually. Catheters were flushed daily with an antibiotic (Cefazolin, 0.02 mg/ml) dissolved in heparinized saline and sealed with plastic obturators when not in use.
2.2.2. Apparatus:
Self-administration was conducted in operant chambers housed within sound-attenuating cubicles (MedAssociates, St. Albans, VT). Chambers were equipped with house lights, cue lights, and two retractable levers (one active; one inactive). Active lever pressing initiated the activation of the syringe pump (MedAssociates) to deliver a drug infusion (rate 60 μl in 5 s, 15 s post-infusion timeout). Drug delivery and data collection were controlled by MedAssociates software (MedPCIV). Following catheterization surgery, animals were recovered for 1 week prior to initiation of self-administration.
2.2.3. Acquisition and extinction:
For all studies animals were allowed to self-administer oxycodone (0.1 mg/kg/infusion) for on a fixed ratio 1 (FR1) schedule of reinforcement. In some studies additional females acted as yoked saline controls, receiving volume matched infusions of the saline vehicle (0.9% NaCl). For oxycodone self-administering females, the right lever was always active and the left lever was inactive. For yoked saline controls, both the left and right levers were inactive. Each session was 1 hour long. During extinction the oxycodone syringe was replaced with a saline syringe with responses on both the active and inactive lever measured. All females were weighed twice a week during the first three weeks of acquisition and then daily thereafter to monitor body weight to ensure accurate oxycodone dosing during pregnancy.
2.3. Breeding:
One week after initiating self-administration sessions, vaginal smears were conducted each morning to determine estrous cycle phase. After estrous cycles were documented for two full weeks, females determined to be in proestrus (i.e. sexually receptive females), were mated overnight with a drug naïve colony male. The catheter port was protected using a lightweight adhesive wrap (Vetrap). Pregnancy was confirmed the following day by the presence of sperm and was considered gestation day 1 (G1). Only one female failed to become pregnant in this study. An additional set of females underwent all of the same procedures, however, they were not mated overnight with a male, serving as a non-pregnancy, cycling group.
2.4. Postpartum Assessment:
Daily self-administration sessions continued as described throughout pregnancy. Beginning 22 days after mating females were monitored for parturition. The day of parturition was designated as postnatal day 0 (PND0). As females can give birth on either G22 or G23, if females had not given birth by the morning of G22, they were allowed access to oxycodone in the selfadministration chamber (1h); however, that data was not included in any analyses. No females had access to oxycodone following parturition. On PND1, dams and litters were weighed. Litters were then culled to a maximum of 10 pups (5 females: 5 males) and reweighed to allow for assessment of postnatal body weight changes.
2.5. Maternal Retrieval Behavior:
The latency for dams to actively retrieve pups and place them in the home nest was recorded following a 1h separation on PND 3, 6, 9 and 12. Prior to placing the dam back in the home cage, pups were gently scattered away from the nest. Once the dam was returned to the cage, the latency of the dam to retrieve the first pup was recorded. Dams were given a total of 8 minutes to retrieve all pups and complete the sequence of retrieving, grouping and crouching over all pups on the nest. Due to the increased mobility of the pups on PND12 an accurate assessment of dam retrieval could not be reliably determined. Thus, data from PND12 was excluded from these analyses.
2.6. Pup ultrasonic vocalizations:
Pup ultrasonic vocalizations (USVs) were analyzed on PNDs 3, 6, 9, and 12. On each testing day, one male and one female from each litter were recorded, with analysis beginning at least one hour after the mother had been reintroduced to avoid confounding the data with the self-administration session-induced separation period. On the testing day, individual pups were removed from the home cage and placed in a novel rectangular polypropylene container (11 × 13 cm). The container was placed inside a sound proof box maintained at room temperature. A heterodyne bat detector (Mini-3; Ultra Sound Advice, London, United Kingdom) was located 10 cm above the pup. Rat pups emit vocalizations in the 30–50 kHz range. The detector was connected to a PC with data recorded on Soundwave software (Softonic International, Barcelona, Spain). The number of calls was assessed over 3 minutes, starting twenty seconds after isolation to control for the expected increase in calls in response to human handling. Pups were then returned to their dam. On each test day, pups were randomly selected. Once a pup had completed testing, the right ear was punched for identification. No pup was tested more than once.
2.7. PND1 tissue harvesting:
On PND1 one male and one female from each litter was euthanized via rapid decapitation. Brains were dissected into midbrain, hypothalamic and forebrain regions. To isolate forebrain, a coronal cut directly anterior to the optic chiasm was made. Midbrain was isolated by making a ~25 °biased cut at the most rostral aspect of the cerebellum in a rostral to caudal orientation. The cerebellum was then removed from the midbrain dissection. The midbrain dissection was designed to include the tectum and tegmentum including the following nuclei: ventral tegmental area, substantia nigra, periaqueductal gray, superior colliculus, red nucleus, and corticospinal fibers. Hypothalamic tissue was excised via bilateral removal of cortical tissue in the remaining brain matter. All tissue was frozen and stored at −80° C prior to processing.
2.8. PND1 OPRM1 expression:
Total RNA was extracted using RNeasy (Qiagen) followed by cDNA conversion using RETROscript®. Real time PCR was performed on an ABI Prism 7700 (Applied Biosystems, Foster City, CA). Taqman® primers were purchased from Applied Biosystems (Oprm1 – Rn01430371_m1; Gapdh – Rn01775763_g1. Gapdh was used as the housekeeping gene based on preliminary analysis demonstrating similar expression across all groups. Final quantification of mRNA was obtained using the comparative cycle threshold (Ct) method (Pfaffl 2001) with data relative salineexposed male group.
2.9. Statistical analyses:
Data were analyzed using SPSS software (IBM SPSS Statistics, Armonk, NY) using mixed design ANOVAs with drug (saline or oxycodone) as the between subject factor for all measures. For many of the endpoints day was used as a within-subjects factor. For offspring data, males and females were analyzed separately unless otherwise stated. Additional within or between subject factors were included as described in the results. Tukey’s post hoc test was utilized for all post hoc analyses. For all data significance was defined as p<0.05.
3. Results
3.1. Female Oxycodone Self-Administration as a Function of Reproductive Status
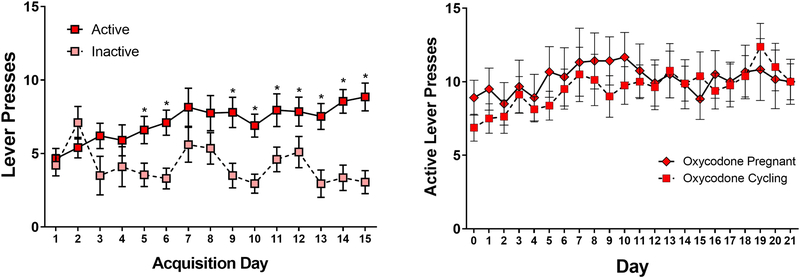
As shown in Figure 1a, females readily learn to press for oxycodone (0.1 mg/kg) during the 1h access period, with all females demonstrating increased numbers of presses on the active versus the inactive lever by the fifth session (Main Effect of Day F[14,504]=2.16, p<0.01; Main Effect of Response Lever F[1,36]=10.5, p<0.01; Day by Response Lever Interaction F[14,504]=3.05, p<0.01). After 15 sessions, a subset of females were mated with males. Data comparing the active lever responding of pregnant, as compared to non-pregnant females are shown in Figure 1b. While there was a significant main effect ofday (F[20,360]=1.79, p<0.05), there was no effect of reproductive status (F[1,18]=0.08, p=0.78) and no day by reproductive status interaction (F[20,360]=0.73, p=0.8). These findings demonstrate the feasibility of self-administration prior to and during pregnancy, and suggest that pregnancy itself does not alter the level of voluntary oxycodone intake in female Sprague Dawley rats.
Figure 1.
Left Panel: Mean (±SEM) active and inactive lever presses during oxycodone acquisition in cycling females. Right Panel: Mean (±SEM) active lever presses in pregnant and cycling females beginning on gestation day 1 for the pregnant females. *p<0.05 active as compared to inactive lever.
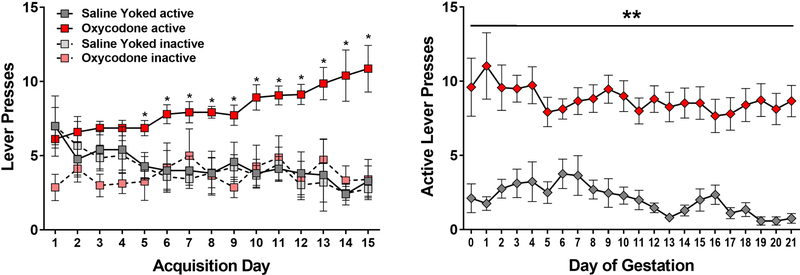
In a separate set of animals, we compared response rates for cycling females self-administering oxycodone (0.1 mg/kg) with response rates observed in yoked saline controls. As shown in Figure 2, during the 15 day acquisition period, cycling female rats quickly learned the response contingency with oxycodone self-administering rats increasing response rates on the active level (Main effect of Drug F[1,60]=11.1, p<0.01; Main Effect of Response Lever F[1,60]=13.6, p<0.001; Drug by Response Lever Interaction F[1,60]=10.3, p<0.01; Day by Drug interaction F[14, 840]=4.5, p<0.001). No differences in the number of responses on the active versus inactive lever were discerned in the saline control females. Significant differences in active lever responses between oxycodone and saline-yoked controls were observed beginning on the fifth session (p’s <0.05).
Figure 2.
Left Panel: Mean (± SEM) active and inactive lever presses during acquisition in cycling females. Right Panel: Mean (± SEM) active lever presses during pregnancy. *p<0.05 oxycodone active as compared to all other groups. **p<0.05 main effect of oxycodone versus saline.
Following 15 days of acquisition, all females were mated overnight with males and responding was monitored daily during pregnancy. Rates of active lever pressing in oxycodone self-administering females remained stable across pregnancy and was significantly higher than saline yoked controls across all days (Main Effect of Drug F[1,30]=55.6, p<0.001). As expected, the number of responses declined over time in control females (Main Effect of Day F[20,600]=1.85, p<0.05; Day by Drug Interaction F[20,600]=1.71, p<0.05).
3.2. Maternal and Fetal Outcomes
All females demonstrated significant weight gain across pregnancy, with no negative effect of oxycodone on body weight gain across pregnancy (see Table 1). The day before parturition was the final day of oxycodone self-administration. On PND1 (i.e. ~48h post-withdrawal) oxycodone females show a significant reduction in bodyweight which likely reflects the physiological effects of opioid withdrawal. By PND3, this difference is no longer observed. With regard to the pups, there were no significant differences in the number of pups or sex distribution of the litters as a function of maternal oxycodone exposure (mean ± SEM number of male pups; 6.0±0.7 saline - 6.8±.7 oxycodone: t[23]=.78, p=0.44 and mean ± SEM number of female pups; 6.8±0.7 saline - 7.0±0.5 oxycodone: t[23]=.23, p=0.81). These was also no significant difference in the body weight of males and females as measured prior to culling on PND1 (mean ± SEM body weight (g) of male pups; 43.4±5.1 saline – 49.8±5.4 oxycodone: t[23]=.8, p=0.43 and mean ± SEM body weight of female pups; 45.4±4.6 saline – 47.8±3.6 oxycodone: t[23]=.35, p=0.72).
Table 1.
The effect of oxycodone as compared to saline on mean (SEM) dam body weight (g) during gestation and during the postpartum period following withdrawal on the day prior to parturition.
| GD1 | GD22 | BW Gain | PND1* | PND3 | |
|---|---|---|---|---|---|
| Saline | 247 (3.3) | 389 (6.0) | 142 (3.9) | 280 (4.6) | 289 (4.5) |
| Oxycodone | 245 (4.5) | 382 (4.8) | 138 (3.7) | 267 (3.9) | 281 (3.7) |
| t[31]=−.36, p=0.72 |
t[31]=−.85, p=0.4 |
t[31]=−.85, p=0.4 |
t[31]=−2.2, p=0.04 |
t[31]=−1.5, p=0.16 |
significant difference in dam bodyweight
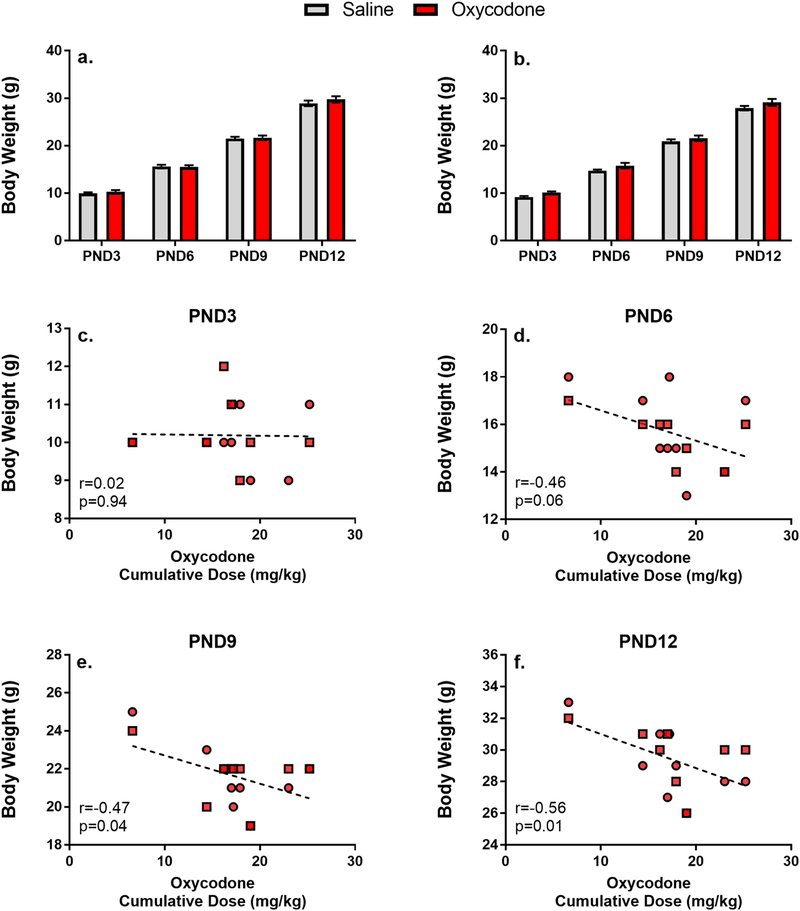
Body weight growth was also examined on PND3, 6, 9, and 12 at the time of USV testing. One male and one female from each litter was weighed at each time point and were then marked such that no pup as weighed at more than one time point. However, as the litter is considered the sample unit, a within-subjects design was used to analyze body weight changes across days. Males and females were examined separately as they represent distinct subject pools within the litter. As shown in Figure 3 (panels a and b), all offspring demonstrated significant body weight increases during the postnatal period (Main Effect of Day in Males F[3,98]=1061.4, p<0.001 and Females F[3,98]=1265.6, p<0.001). There was no significant effect of Maternal Drug (Main Effect of Drug in Males F[1,98]=1.19, p=0.29 and Females F[1,98]=4.04, p=0.06) and no significant interaction (Day by Drug Interaction F[3,98]=0.33, p=0.8 and Females F[3,98]=0.63, p=0.59).
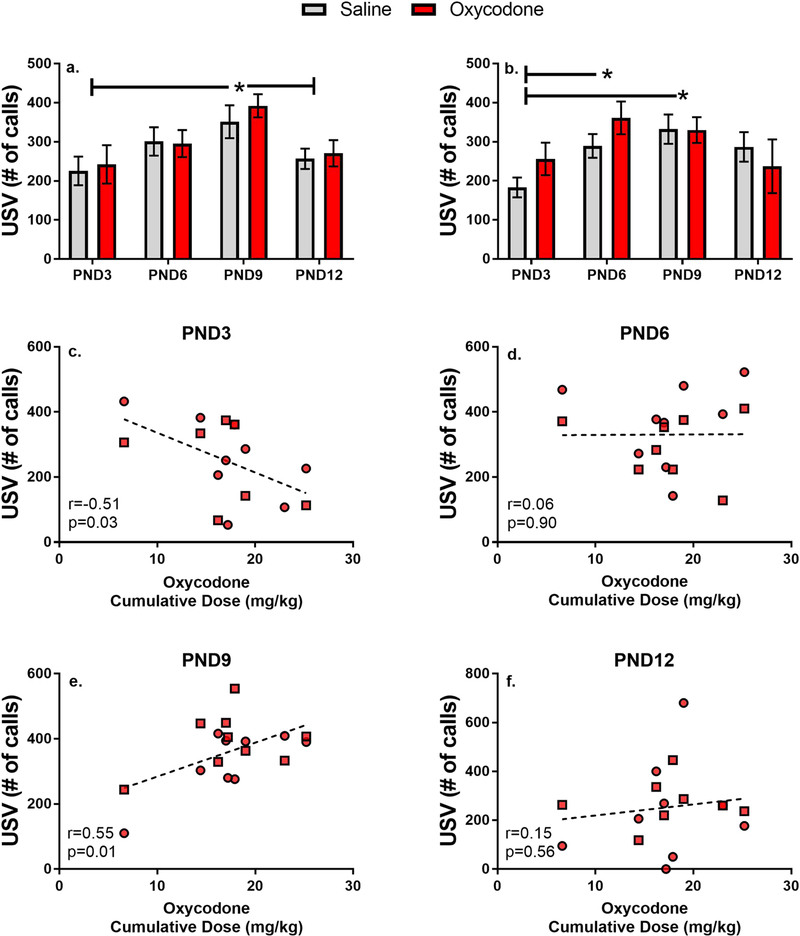
Figure 3.
Mean (±SEM) body weight (in grams) of male (a) and female (b) offspring across postnatal days Pearson’s correlations between cumulative oxycodone dose during pregnancy and pup body weight (male=square;female=circle)
To further investigate the potential effects of oxycodone intake on body weight gain. We also conducted Pearson’s correlations on the amount of total oxycodone self-administered by each female during gestation and pup body weight at each time point. These data, shown in Figure 3 (panels c-f), demonstrate a significant negative correlation, with higher cumulative intake of oxycodone associated with lower body weights. It is important to note that this effect appears to emerge over the first two postpartum weeks, with significant effects observed at PND9 and 12, but not PND3 and 6.
3.3. Maternal Retrieval
The latency to retrieve all pups back to the nest was assessed on PND3, 6, and 9 following a 1h separation. As a maximum latency score of 480 seconds was assigned to any subject that did not retrieve all pups back to the nest within the 8 minute test period, these data are non-parametric. Therefore, each postnatal day was examined separately using the Mann-Whitney U. As shown in Table 2, females self-administering oxycodone during pregnancy demonstrated significantly longer latencies to retrieve both the first and last pup back to the nest on PND3 (saline and oxycodone litters were of equal sizes). A similar trend was observed on PND6, however, by PND9 retrieval times were similar between the two groups.
Table 2.
The effect of oxycodone as compared to saline on the median (25%:75%) latency in seconds for dams to retrieve the first and last pup back to the nest following a 1h separation.
| PND3* | PND6 | PND9 | ||||
|---|---|---|---|---|---|---|
| First Pup | Last Pup | First Pup | Last Pup | First Pup | Last Pup | |
| Saline (n=12) | 32 (11:52) | 179 (116:300) | 41 (8:80) | 179 (69:441) | 75 (26:250) | 203 (76:447) |
| Oxycodone (n=9) | 145 (84:480) | 447 (245:480) | 119 (79:480) | 405 (244:480) | 111 (14:454) | 241 (77:480) |
| Mann-Whitney U | 12 | 19 | 26.5 | 29 | 53 | 53 |
| P value | 0.005 | 0.02 | 0.05 | 0.08 | 1.0 | 0.97 |
significant difference in latency to retrieve pups
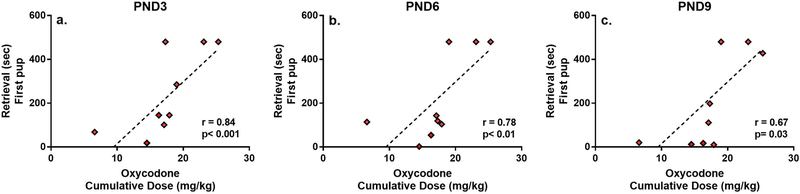
To determine whether the amount of drug self-administered by females during pregnancy correlated with postpartum retrieval behavior, Spearman’s rank correlations were performed between cumulative maternal oxycodone intake across pregnancy (mg/kg) and the latency to retrieve the first and last pup back to the nest. As shown in Figure 4, higher intake of oxycodone correlated with delayed maternal retrieval and this effect was maintained through PND9.
Figure 4.
Mean (±SEM) Spearman’s correlations between cumulative oxycodone dose during pregnancy and maternal retrieval latencies across postnatal days.
3.4. Pup Separation-Induced Ultrasonic Vocalizations
As expected the number of separation-induced calls increased over the first postnatal week in both males and females (Main effect of Day in Males F[3,94]=5.45, p<0.01 and Females F[3,98]=4.29, p<0.01). Overall, however, prenatal oxycodone exposure had no significant effect on the number of calls emitted on any of the days examined (Main effect of Drug in Males F[1,94]=0.29, p=0.59 and Females F[3,98]=0.68, p=0.41; Day by Drug interaction in Males F[4,94]=0.26, p=0.84 and Females F[3,98]=1.31, p<0.28). As shown in Figure 5, the peak response in males was observed on PND9 (p<0.05 as compared to PND3 and 12), while in females elevated responding was observed on both PND6 and PND9 (p<0.05 as compared to PND3).
Figure 5.
Mean (±SEM) number of separation-induced calls emitted by male (a) and female (b) pups across postnatal day. Pearson’s correlations between cumulative oxycodone dose during pregnancy and number of calls (c-f: male=square; female=circle). *p<0.05 compared across days.
To further investigate the potential effects of oxycodone intake on separation-induced calls we conducted Pearson’s correlations on the amount of total oxycodone self-administered by each female during gestation and the number of calls emitted by male and female pups at each time point. These data, shown in Figure 5 (panels c-f), demonstrate a significant correlation between cumulative intake of oxycodone and the number of calls, the direction of which changes over the early developmental period. Thus, on PND3, higher oxycodone intake is associated with fewer calls, while by PND9 higher oxycodone intake is associated with a greater number of calls.
3.5. PND1 OPRM1 Expression
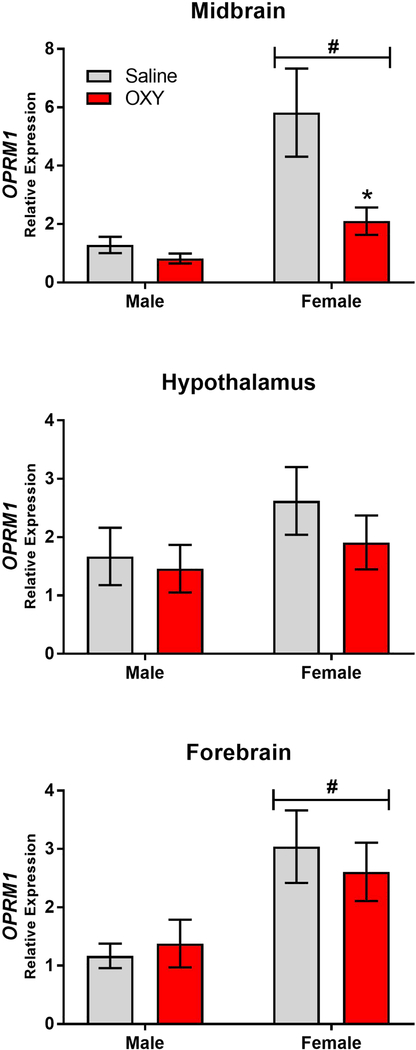
As previous findings have implicated differences in OPRM1 expression in the expression of NAS, OPRM1 expression was examined in 3 brain regions. These data, which are shown in Figure 6, were analyzed for both sex and drug effects. In the midbrain, a two-way ANOVA revealed significant main effects of both Sex and Drug (Main Effect of Sex F[1,46]=11.25, p<0.01; Main Effect of Drug F[1,46]=5.75, p<0.05) as well as a trend toward an interaction (Sex x Drug Interaction F[1,46]=3.5, p=0.06). These effects were due to decreased expression of OPRM1 in oxycodone exposed offspring which achieved significance in female offspring, as well as higher expression of OPRM1 in females which achieved significant in Saline offspring. No effects on OPRM1 expression were observed in the hypothalamus (Main Effect of Sex F[1,48]=1.8, p=0.2; Main Effect of Drug F[1,48]=0.77, p=0.4; Sex x Drug Interaction F[1,48]=0.23, p=0.6). Finally, in the Forebrain there was a significant main effect of sex (F[1,47]=8.56, p<0.01) with no main effect of drug (F[1,47]=0.04, p<0.8) and no interaction (Sex x Drug Interaction F[1,47]=.036, p=0.55). Thus, the primary effect of prenatal oxycodone exposure as measured on PND1 was decreased OPRM1 transcription in the midbrain.
Figure 6.
Mean (±SEM) PND1 expression of OPRM1 as measured in male (a) and female (d) midbrain, male (b) and female (e) hypothalamus, and male (c) and female (f) forebrain. #p < 0.01 main effect of sex and *p<0.05 as compared to saline controls within sex. N=9–14/group.
4. Discussion
The current set of findings demonstrate the feasibility of modeling voluntary opioid use during gestation using i.v. self-administration. Given the significant physiological changes observed during pregnancy, including dramatic fluctuations in circulating hormones (Soma-Pillay, Nelson-Piercy et al. 2016), we first examined whether pregnancy would alter the amount of oxycodone females self-administered. When compared to cycling females, no differences in daily intake of oxycodone were observed as a function of pregnancy. Moreover, when examining both the acquisition (i.e. first fifteen days) and maintenance data, no variability as a function of estrous cycle could be discerned. These data align with previous findings regarding both heroin and morphine self-administration in cycling female rats, which also reported no significant effects of estrous cycle phase on self-administration across a range of doses (Cicero, Aylward et al. 2003). Additionally, recent findings using oxycodone self-administration also report no significant on estrous cycling (Mavrikaki, Pravetoni et al. 2017). Thus, unlike the effects of cocaine, which vary significantly as a function of the estrous cycle (Feltenstein and See 2007) and can be significantly modulated by estrogens (Lynch, Roth et al. 2001), our findings suggest that fluctuations in ovarian hormones, either across the estrous cycle or during pregnancy, do not significantly impact the reinforcing effects of oxycodone when using a short access paradigm. It is unknown whether effects on the estrous cycle would emerge with longer access sessions.
An important feature of this model is the initiation of drug use prior to pregnancy. In the current set of data, no negative effects of oxycodone intake on fertility were observed. These results are similar to previous reports following high dose oral oxycodone administered via gavage (Davis, Franklin et al. 2010). The majority of studies examining the effects of opioids during gestation begin exposure at the time of conception or at some period during gestation (for review see (Byrnes and Vassoler 2017)). While such studies can be informative, they can also be difficult to interpret due to the significant effects of opioids on the hypothalamic pituitary adrenal (HPA) axis in rodents (Buckingham and Cooper 1984, Gonzalvez, Milanes et al. 1991). Indeed, opioids initially induce a significant increase in circulating levels of corticosterone (Eisenberg 1982, Bartolome and Kuhn 1983, Iyengar, Kim et al. 1987); however, these effects demonstrate tolerance with repeated exposures, with stimulatory effects no longer observed after 4–7 days in both mouse and rat models (Milanes, Puig et al. 1993, Esmaeili Mahani, Motamedi et al. 2005, Esmaeili-Mahani, Vahedi et al. 2007, Yamamoto, Kiguchi et al. 2011). As increased corticosterone during the prenatal period can induce significant effects on offspring (Salomon, Bejar et al. 2011, Bingham, Sheela Rani et al. 2013, Cuffe, Turton et al. 2017), some of the outcomes attributed to gestational opioids could be mediated by these HPA effects. By initiating oxycodone intake several weeks prior to gestation this potential confound is eliminated. Moreover, as our goal is to model human use patterns, it would be relatively rare for a women to initiate opioid use during pregnancy.
Another feature of the current model is the use of self-administration which allows one to examine both the responses of the pregnant females as well as offspring outcomes. Using the 1h session, all of the females demonstrated a relatively consistent and stable level of intake throughout pregnancy. Individual variations in cumulative dose, however, can be calculated using this approach. In the current set of findings we compared the cumulative dose experienced by the dam with her pup retrieval latencies and observed a significant correlation, with increased intake associated with prolonged latencies to retrieve pups. Such delayed responding is likely associated with opioid withdrawal, although it should be noted that acute effects of withdrawal on body weight are no longer observed by PND3 and yet delayed retrieval is observed even on PND6. Unlike the physical effects observed in male rats, opioid withdrawal in females is associated with few somatic signs (Craft, Stratmann et al. 1999, Radke, Holtz et al. 2013). Females do, however, demonstrate emotional signs of withdrawal as measured using acoustic startle and conditioned place aversion (Radke, Holtz et al. 2013, Radke, Gewirtz et al. 2015). In the current context, only a brief decrease in body weight was observed in oxycodone withdrawn females. The significant delay in pup retrieval, however, suggests more persistent effects on motivational systems.
The retrieval of pups back to the nest is a critical motivated behavior mediated by activity of dopamine D1 receptors in the nucleus accumbens (Keer and Stern 1999, Numan, Numan et al. 2005). In addition, retrieval behavior is influenced by the activity of the medial preoptic area and several regions of the amygdala (Numan, Bress et al. 2010). Opioid withdrawal induces significant molecular modifications in the amygdala (Ju, Long et al. 2015, Rosen, Zunder et al. 2016) and in regions of the extended amygdala, including the nucleus accumbens (Reti and Baraban 2003, Hamlin, Buller et al. 2004, Harris and Aston-Jones 2007). Thus, the effects of oxycodone withdrawal on maternal retrieval behavior could be mediated by neural alterations induced by withdrawal.
Oxytocin may also play a critical role in the disruption of maternal retrieval. Indeed, it is well known that oxytocin is a critical mediator in mother-infant bonding and motivation in maternal behaviors. In addition, there is evidence that oxytocin and drugs of abuse influence social behaviors via impacting similar neural substrates, namely the mesolimbic dopamine system (Neumann 2008, Baskerville and Douglas 2010). While it is clear that drugs of abuse impact maternal behavior, there is a body of literature that also indicates that withdrawal from such drugs also significantly alters maternal care and disrupts the maternal-infant dyad (Johns, Noonan et al. 1994, Kinsley, Turco et al. 1994, Sobrian, Ali et al. 1995, McMurray, Joyner et al. 2008). Finally, oxytocin can modulate symptoms of withdrawal from varying drugs of abuse including opioids (Kovacs, Borthaiser et al. 1985, Cui, Bowen et al. 2001, Carson, Cornish et al. 2010, Manbeck, Shelley et al. 2014). Together, this evidence suggests that the disruptions in maternal retrieval observed in this NAS model may be a result of maternal oxycodone withdrawal influencing levels of oxycodone.
Maternal retrieval is also influenced by the pups, with increased pup calls stimulating more rapid retrieval (Curry, Egeto et al. 2013). It is interesting to note that pup retrieval times were significantly delayed on PND3, a time at which there was a significant correlation between cumulative oxycodone dose and both the number of calls and maternal retrieval. Thus, females with higher intake levels of oxycodone had pups that made fewer calls and demonstrated slower retrieval times. While the association between cumulative dose and longer retrieval latencies continued to be observed at later time points, the level of oxycodone exposure in pups was associated with increased numbers of calls on PND9. Thus, it remains unclear whether the number of calls emitted by the pups influenced the motivated responding of the dam. Indeed, while the number of pup calls correlated with the level of oxycodone exposure, it was not significantly different from the number observed in saline control offspring. Nonetheless, these data demonstrate a significant effect of oxycodone withdrawal on motivated maternal responding in the early postpartum period and suggest a relationship between the amounts of oxycodone self-administered by the female with pup calls.
Offspring body weight also correlated with the cumulative dose of oxycodone and this effect became more pronounced across the early postnatal period. To what extent such changes are mediated by alternations in the maternal environment or by physiological modifications in the pups’ remains to be determined. These data, however, do reflect a strength of the current model, which allows for the examination of how the nature and/or magnitude of offspring effects may be a function of the amount of drug self-administered by the dam across a continuum. Moreover, future studies could begin to parse these influences on pup outcomes by using donor mother to rear exposed pups or using yoked oxycodone controls to begin to dissociate voluntary intake from involuntary intake. Such studies may help determine whether it is primarily the level of drug exposure, rather than some other factor that underlies the dam’s preference for higher levels of oxycodone, that most influences the pup outcomes.
In addition to alterations in maternal and pup responses during the postnatal period, we also examined expression of OPRM1 in specific brain regions of PND1 males and females. As these brains were randomly collected from litters during the culling process across multiple studies, cumulative intake levels of the dam were not recorded. In the current study we observed oxycodone-induced downregulation in OPRM1 transcription specific to the midbrain. Moreover, this effect only reached significance in female offspring. Interestingly, non-oxycodone exposed females demonstrated higher expression of OPRM1 in the midbrain, with oxycodone exposure serving to diminish this robust sex difference. A similar sex difference was observed in the forebrain, however, this effect was not altered by oxycodone exposure. Decreased OPRM1 expression in the female midbrain could be due to oxycodone-induced changes in the prenatal environment. Indeed, previous studies have found that females, but not males, demonstrate alterations in MOR expression in the midbrain in response to changes in the hormonal milieu (Morley-Fletcher, Palanza et al. 2003, Kren, Haller et al. 2008). Moreover, opioids have well-documented effects on the hypothalamic pituitary gonadal axis and also regulate the release of lactogenic hormones, including prolactin (Matera, Freda et al. 1995, Soaje and Deis 1999, Bottcher, Seeber et al. 2017). Thus, effects of oxycodone exposure on OPRM1 expression in females could be due to changes in prenatal hormones, although direct effects of oxycodone exposure on OPRM1 transcription are also possible. Overall, these findings demonstrate region and sex-specific effects of oxycodone exposure that are evident at an early developmental time point. The possibility remains that these changes alter the developmental trajectory of processes within the central nervous system or neuroendocrine axis that may drive later abnormalities and could thus influence maternal care.
5. Conclusions
The current set of findings demonstrates the feasibility and utility of using i.v. self-administration to study prenatal oxycodone use. The data suggest significant effects of oxycodone on both the dam and pup at doses that are voluntarily self-administered throughout pregnancy. The use of i.v. administration, as opposed to oxycodone in water bottles, avoids the significant issues of first pass metabolism which is more robust in females, resulting in poor bioavailability (Chan, Edwards et al. 2008). Moreover, how the robust physiological changes associated with pregnancy impact pharmacokinetics remains unknown. Thus, by allowing the female to intravenously self-administer, the dosing is based on reward efficacy such that any shifts in bioavailability can be moderated by the female. In this limited access model no significant effects on fertility or fecundity were observed, which helps to limit potential confounds on data interpretation. And yet, even with a limited exposure, significant effects could be discerned, and often were associated with the amount of drug self-administered. Future studies using prolonged exposure could help examine a wider range of doses with regard to neonatal outcomes. In addition, studies that investigate both long term outcomes and the effects of transitioning animals from oxycodone to methadone or buprenorphine during gestation would be valuable. Given the dramatic rise in the number of infants born to women misusing prescription opioids, additional preclinical studies are needed to identify potential neural mechanisms underlying outcomes associated with specific patterns of maternal opioid use.
Highlights.
Pregnant females self-administer oxycodone (0.1 mg/kg; intravenously, i.v.) at the same rate as cycling females when using a short (1h) access period.
I.V. oxycodone (0.1 mg/kg) self-administration during pregnancy does not impact fertility or fecundity.
I.V. oxycodone (0.1 mg/kg) self-administration during pregnancy followed by postpartum withdrawal impairs maternal behavior.
I.V. oxycodone (0.1. mg/kg) self-administration dose-dependently reduces postnatal body weight gain across the early postnatal period and alters ultrasonic vocalizations.
I.V. oxycodone (0.1 mg/kg) self-administration decreases OPRM1 expression in the midbrain of female but not male offspring as measured on postnatal day 1.
Acknowledgments
funding source: This work was funded by NIH awards 5R01DA025674 and 5T35RR029724.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartolome MB and Kuhn CM (1983). “Endocrine effects of methadone in rats; acute effects in adults.” Eur J Pharmacol 95(3–4): 231–238. [DOI] [PubMed] [Google Scholar]
- Baskerville TA and Douglas AJ (2010). “Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders.” CNS Neurosci Ther 16(3): e92–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham BC, Sheela Rani CS, Frazer A, Strong R and Morilak DA (2013). “Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior.” Psychoneuroendocrinology 38(11): 2746–2757. [DOI] [PubMed] [Google Scholar]
- Buckingham JC and Cooper TA (1984). “Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine.” Neuroendocrinology 38(5): 411–417. [DOI] [PubMed] [Google Scholar]
- Byrnes EM and Vassoler FM (2017). “Modeling prenatal opioid exposure in animals: Current findings and future directions.” Front Neuroendocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE and McGregor IS (2010). “Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats.” Neuropharmacology 58(1): 38–43. [DOI] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD and Smith MT (2008). “Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat.” Clin Exp Pharmacol Physiol 35(3): 295–302. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC and Meyer ER (2003). “Gender differences in the intravenous selfadministration of mu opiate agonists.” Pharmacol Biochem Behav 74(3): 541–549. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI and King SJ (1999). “Sex differences in development of morphine tolerance and dependence in the rat.” Psychopharmacology (Berl) 143(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Cuffe JS, Turton EL, Akison LK, Bielefeldt-Ohmann H and Moritz KM (2017). “Prenatal corticosterone exposure programs sex-specific adrenal adaptations in mouse offspring.” J Endocrinol 232(1): 37–48. [DOI] [PubMed] [Google Scholar]
- Cui SS, Bowen RC, Gu GB, Hannesson DK, Yu PH and Zhang X (2001). “Prevention of cannabinoid withdrawal syndrome by lithium: involvement of oxytocinergic neuronal activation.” J Neurosci 21(24): 9867–9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry T, Egeto P, Wang H, Podnos A, Wasserman D and Yeomans J (2013). “Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness.” Genes Brain Behav 12(4): 397–404. [DOI] [PubMed] [Google Scholar]
- Davis CP, Franklin LM, Johnson GS and Schrott LM (2010). “Prenatal oxycodone exposure impairs spatial learning and/or memory in rats.” Behav Brain Res 212(1): 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg RM (1982). “Short-term tolerance to morphine: effects of indomethacin.” Life Sci 30(16): 1399–1405. [DOI] [PubMed] [Google Scholar]
- Esmaeili-Mahani S, Vahedi S, Motamedi F, Pourshanazari A, Khaksari M and Ahmadiani A (2007). “Involvement of hypothalamic pituitary adrenal axis on the analgesic cross-tolerance between morphine and nifedipine.” Pharmacol Biochem Behav 86(4): 806–812. [DOI] [PubMed] [Google Scholar]
- Esmaeili Mahani S, Motamedi F, Javan M and Ahmadiani A (2005). “Involvement of hypothalamic pituitary adrenal axis on the effects of nifedipine in the development of morphine tolerance in rats.” Pharmacol Biochem Behav 81(1): 152–157. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW and See RE (2007). “Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle.” Drug Alcohol Depend 89(2–3): 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez ML, Milanes MV and Vargas ML (1991). “Effects of acute and chronic administration of mu- and delta-opioid agonists on the hypothalamic-pituitary-adrenocortical (HPA) axis in the rat.” Eur J Pharmacol 200(1): 155–158. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Buller KM, Day TA and Osborne PB (2004). “Effect of naloxone-precipitated morphine withdrawal on c-fos expression in rat corticotropin-releasing hormone neurons in the paraventricular hypothalamus and extended amygdala.” Neurosci Lett 362(1): 39–43. [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J and Jones CM (2017). “Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health.” Ann Intern Med 167(5): 293–301. [DOI] [PubMed] [Google Scholar]
- Harris GC and Aston-Jones G (2007). “Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal.” Behav Brain Res 176(2): 251–258. http://www.cdc.gov/media/releases/2015/p0122-pregnancy-opioids.html (2015). “Opioid painkillers widely prescribed among reproductive age women.” Morbidity and Mortality Weekly Report 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K and Klaassen CD (1977). “First-pass effect of morphine in rats.” J Pharmacol Exp Ther 200(1): 236–244. [PubMed] [Google Scholar]
- Iyengar S, Kim HS and Wood PL (1987). “Mu-, delta-, kappa- and epsilon-opioid receptor modulation of the hypothalamic-pituitary-adrenocortical (HPA) axis: subchronic tolerance studies of endogenous opioid peptides.” Brain Res 435(1–2): 220–226. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ and Schoffelmeer AN (2003). “Neuroadaptive effects of active versus passive drug administration in addiction research.” Trends Pharmacol Sci 24(11): 566–573. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L and Pedersen CA (1994). “Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats.” Behav Neurosci 108(1): 107–112. [DOI] [PubMed] [Google Scholar]
- Ju YY, Long JD, Liu Y and Liu JG (2015). “Formation of aversive memories associated with conditioned drug withdrawal requires BDNF expression in the amygdala in acute morphine-dependent rats.” Acta Pharmacol Sin 36(12): 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keer SE and Stern JM (1999). “Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats.” Physiol Behav 67(5): 659–669. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J and Graham AL (1994). “Cocaine alters the onset and maintenance of maternal behavior in lactating rats.” Pharmacol Biochem Behav 47(4): 857–864. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Borthaiser Z and Telegdy G (1985). “Oxytocin reduces intravenous heroin selfadministration in heroin-tolerant rats.” Life Sci 37(1): 17–26. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL and Carroll ME (2001). “Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats.” Pharmacol Biochem Behav 68(4): 641–646. [DOI] [PubMed] [Google Scholar]
- Manbeck KE, Shelley D, Schmidt CE and Harris AC (2014). “Effects of oxytocin on nicotine withdrawal in rats.” Pharmacol Biochem Behav 116: 84–89. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D and Chartoff E (2017). “Oxycodone self-administration in male and female rats.” Psychopharmacology (Berl) 234(6): 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, Jarrett TM, Elliott DL, Black MA, Hofler VE, Walker CH and Johns JM (2008). “Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams.” Stress 11(5): 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metaxas A, Bailey A, Barbano MF, Galeote L, Maldonado R and Kitchen I (2010). “Differential region-specific regulation of alpha4beta2* nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice.” Addict Biol 15(4): 464–479. [DOI] [PubMed] [Google Scholar]
- Miguens M, Crespo JA, Del Olmo N, Higuera-Matas A, Montoya GL, Garcia-Lecumberri C and Ambrosio E (2008). “Differential cocaine-induced modulation of glutamate and dopamine transporters after contingent and non-contingent administration.” Neuropharmacology 55(5): 771–779. [DOI] [PubMed] [Google Scholar]
- Milanes MV, Puig MM and Vargas ML (1993). “Simultaneous changes in hypothalamic catecholamine levels and plasma corticosterone concentration in the rat after acute morphine and during tolerance.” Neuropeptides 24(5): 279–284. [DOI] [PubMed] [Google Scholar]
- Mutschler NH and Miczek KA (1998). “Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats.” Psychopharmacology (Berl) 136(4): 402–408. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Sahraei H, Bahadoran H, Sadooghi M, Salimi SH, Kaka GR, Imani H, Mahdavi-Nasab H and Dashtnavard H (2005). “Effects of maternal oral morphine consumption on neural tube development in Wistar rats.” Brain Res Dev Brain Res 159(1): 12–17. [DOI] [PubMed] [Google Scholar]
- Neumann ID (2008). “Brain oxytocin: a key regulator of emotional and social behaviours in both females and males.” J Neuroendocrinol 20(6): 858–865. [DOI] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, Denicola AL, Bettis JK and Knapp SE (2010). “The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats.” Behav Brain Res 214(2): 368–376. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM and Smith CD (2005). “The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats.” Behav Neurosci 119(6): 1588–1604. [DOI] [PubMed] [Google Scholar]
- Orejarena MJ, Berrendero F, Maldonado R and Robledo P (2009). “Differential changes in mesolimbic dopamine following contingent and non-contingent MDMA self-administration in mice.” Psychopharmacology (Berl) 205(3): 457–466. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Davis MM, Lehman CU and Cooper WO (2015). “Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012.” J Perinatol 35(8): 667. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM and Davis MM (2012). “Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009.” JAMA 307(18): 1934–1940. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001). “A new mathematical model for relative quantification in real-time RT-PCR.” Nucleic Acids Res 29(9): e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Gewirtz JC and Carroll ME (2015). “Effects of age, but not sex, on elevated startle during withdrawal from acute morphine in adolescent and adult rats.” Behav Pharmacol 26(5): 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC and Carroll ME (2013). “Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake.” Psychopharmacology (Berl) 227(1): 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reti IM and Baraban JM (2003). “Opiate withdrawal induces Narp in the extended amygdala.” Neuropsychopharmacology 28(9): 1606–1613. [DOI] [PubMed] [Google Scholar]
- Rosen LG, Zunder J, Renard J, Fu J, Rushlow W and Laviolette SR (2016). “Opiate Exposure State Controls a D2-CaMKIIalpha-Dependent Memory Switch in the Amygdala-Prefrontal Cortical Circuit.” Neuropsychopharmacology 41(3): 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon S, Bejar C, Schorer-Apelbaum D and Weinstock M (2011). “Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats.” J Neuroendocrinol 23(2): 118–128. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Franklin L and Serrano PA (2008). “Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training.” Brain Res 1198: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrian SK, Ali SF, Slikker W Jr. and Holson RR (1995). “Interactive effects of prenatal cocaine and nicotine exposure on maternal toxicity, postnatal development and behavior in the rat.” Mol Neurobiol 11(1–3): 121–143. [DOI] [PubMed] [Google Scholar]
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H and Mebazaa A (2016). “Physiological changes in pregnancy.” Cardiovasc J Afr 27(2): 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman EM, Hayes MJ, Brown MS, Paul J, Harvey-Wilkes K, Terrin N, Huggins GS, Aranda JV and Davis JM (2013). “Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome.” JAMA 309(17): 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman EM, Hayes MJ, Lester BM, Terrin N, Brown MS, Nielsen DA and Davis JM (2014). “Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome.” J Pediatr 165(3): 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RJ, Gomez-Flores R, Smith JE and Martin TJ (2009). “Neuronal adaptations, neuroendocrine and immune correlates of heroin self-administration.” Brain Behav Immun 23(7): 993–1002. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Kiguchi N, Kobayashi Y, Maeda T, Ueno K, Yamamoto C and Kishioka S (2011). “Pharmacological relationship between nicotinic and opioid systems in analgesia and corticosterone elevation.” Life Sci 89(25–26): 956–961. [DOI] [PubMed] [Google Scholar]