Abstract
Xenotransplantation has vast clinical potential but is limited by the potent immune responses generated against xenogeneic tissue. Immune-privileged Sertoli cells (SCs) survive xenotransplantation long term (>90 days) without immunosuppression, making SCs an ideal model to identify xenograft survival mechanisms. Xenograft rejection includes the binding of natural and induced antibodies and the activation of the complement cascade. Using an in vitro cytotoxicity assay, wherein cells were cultured with human serum and complement, we demonstrated that neonatal pig SCs (NPSCs) are resistant to complement mediated cell lysis and express complement inhibitory factors, membrane cofactor protein (MCP; CD46), and decay- accelerating factor (DAF; CD55) at significantly higher levels than neonatal pig islets (NPIs), which served as non-immune-privileged controls. After xenotransplantation into naive Lewis rats, NPSCs survived throughout the study, while NPIs were rejected within 9 days. Serum antibodies, and antibody and complement deposition within the grafts were analyzed. Compared to preformed circulating anti-pig IgM anti bodies, no significant increase in IgM production against NPSCs or NPIs was observed, while IgM deposition was detected from day 6 onward in both sets of grafts. A late serum IgG response was detected in NPSC (days 13 and 20) and NPI (day 20) recipients. Consistently, IgG deposition was first detected at days 9 and 13 in NPSC and NPI grafts, respectively. Interestingly, C3 was deposited at days 1 and 3 in NPI grafts and only at day 1 in NPSC grafts, while membrane attack complex (MAC) deposition was only detected in NPI grafts (at days 1–4). Collectively, these data suggest NPSCs actively inhibit both the alternative and classical pathways of complement-mediated cell lysis, while the alternative pathway plays a role in rejecting NPIs. Ultimately, inhibiting the alternative pathway along with transplanting xenogeneic tissue from transgenic pigs (expressing human complement inhibitory factors) could prolong the survival of xenogeneic cells without immunosuppression.
Keywords: Xenotransplantation, Sertoli cells (SCs), Complement inhibitors, Humoral immune response
INTRODUCTION
Replacement of damaged organs or tissue by transplantation is an ideal solution to ameliorate life-threatening diseases. However, difficulties associated with obtaining sufficient human donor tissue and the need for chronic immune suppression to protect the allogeneic tissue are major limitations in the field of transplantation. Xenotransplantation, specifically transplantation of tissue from pigs to humans, could potentially resolve the shortage of donor tissue. However, the immune response generated against xenogeneic tissue remains a formidable hurdle. For instance, transplantation of pig islets has successfully reversed diabetes for at least 100 days in nonhuman primates (NHPs)1,2. However, chronic immune suppression was required for long-term graft function as none of the control animals (transplanted with islets and without immunosuppression) achieved insulin independence2; these immunosuppressive drugs can result in cancer and are also toxic to the transplanted islets3.
The humoral immune response, involving preformed and induced antibodies to xenogeneic antigens, results in activation of the complement cascade and plays an important role in rejecting xenogeneic tissue. Hyperacute rejection, mediated by preformed antibodies, destroys the xenotransplanted tissue within minutes to hours. Several methods such as transient depletion of preformed anti-pig antibodies, cell encapsulation, and transplantation of cells isolated from transgenic pigs (galactosyl transferase knockout and N-acetylglucosaminyltransferase knockout or expression of human complement inhibitors) have tremendously decreased the susceptibility of early graft destruction mediated by hyperacute rejection4–9. For example, it has been shown in vitro that islets isolated from α−1,3-galactosyltransferase knockout (GTKO) transgenic pigs are less susceptible to preformed antibody (IgM and IgG) deposition, complement factor (C1q and C3) deposition, and destruction by complement-mediated cell lysis compared to wild-type (WT) pig islets8. Additionally, transplantation of GTKO-neonatal pig islets (NPIs) into diabetic NHPs resulted in lower rates of primary graft dysfunction than transplantation of WT-NPIs5,8. When hyperacute rejection is averted, xenografts become prone to acute humoral and cell-mediated rejection. Despite all the progress, acute rejection is still a daunting challenge for xenotransplantation.
Immune-privileged Sertoli cells (SCs) reside within the seminiferous tubules of the testis, where they play an important role in protecting autoimmunogenic germ cells. Furthermore, isolated primary SCs survive long term when ectopically transplanted across immunological barriers (as allo- and xenografts) without the use of immunosuppressive drugs10. For instance, when neonatal pig SCs (NPSCs) were transplanted underneath the kidney capsule of naive Lewis rats as discordant xenografts, grafts analyzed for NPSC survival revealed that 66% of the grafts contained abundant NPSCs for at least 90 days11. Similar results have been observed when NPSCs were transplanted into mice, dogs, and humans10. Collectively, these studies demonstrate that NPSCs have the capability to survive both humoral and cell-mediated immune responses, making them an ideal tool to study the mechanism(s)/factor(s) that are key in preventing immune responses generated against xenografts. Despite abundant SC transplantation survival data10, studies that delve into the mechanism for their survival are limited.
In this study, we analyzed the humoral immune response generated against immune-privileged NPSCs and compared it to non-immune-privileged NPIs. The susceptibility of NPSCs and NPIs to complement mediated cell lysis in vitro and expression of complement inhibitory factors by NPSCs and NPIs was examined. Furthermore, survival of NPSCs and NPIs after xenotransplantation in naive Lewis rats, along with generation of a humoral immune response against the transplanted cells, was analyzed. The results suggest that expression of complement inhibitors by NPSCs inhibit the alternative and classical pathways of complement-mediated cell lysis resulting in prolonged NPSC xenograft survival.
MATERIALS AND METHODS
Animals
Male neonatal pigs (1–3 days old, Texas Tech University Research and Experimental Farm, New Deal, TX, USA) were used as SC and islet cell donors. Male Lewis rats, aged 6–8 weeks (151–175 g; Charles River Laboratories, Wilmington, MA, USA), were used as transplant recipients. Care and maintenance of all animals were in accordance with the Institute for Laboratory Animal Research Care and Use of Laboratory Animals, Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee (IACUC)-approved protocols, and the guidelines of the National Institutes of Health (NIH; Bethesda, MD, USA).
Cell Isolation
NPSCs were isolated as described previously11,12. Briefly, pig testes were decapsulated and chopped into 1- to 2-mm fragments in Hank’s balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO, USA). To remove contaminating cells, tissue was digested with 2 mg/ml of sterile type V collagenase (Sigma-Aldrich), followed by trypsin 0.25% (v/v) (Roche, Madison, WI, USA) and 800 U/ml of DNAse I (Roche). Cells were cultured on non-tissue culture-treated petri dishes in supplemented Ham’s F10 media (Thermo Fisher Scientific, Waltham, MA, USA) and 10% (v/v) heat-inactivated neonatal pig serum (NPS; obtained from pigs used for islet isolation) for 48 h at 37°C to allow formation of NPSC aggregates prior to transplantation.
For isolation of NPIs, pancreases were collected from neonatal pigs and chopped using into 1- to 2-mm fragments in HBSS13. Tissue was digested with 2.5 mg/ml of sterile type XI collagenase (Sigma-Aldrich) and cultured for 7–9 days on non-tissue culture-treated petri dishes in supplemented Ham’s F10 media at 37°C to purify NPIs from acinar cells13.
In Vitro Cytotoxicity Assay
The in vitro human antibody and complement- mediated cytotoxicity assay was performed in a similar manner to that described previously12. Briefly, 3–4 × 105 NPSCs (n = 7) or dissociated NPIs (n = 4; dissociated as described in Rayat et al.14) were plated and cultured overnight in 1 ml of supplemented Ham’s F10 media and 10% NPS. The next morning, 0.5 ml of media was removed, and cells were incubated at 37°C in one of four groups. For cells in group 1 [50% (v/v) human serum plus complement], 0.5 ml of heat-inactivated pooled human AB serum (Nabi BioPharmaceuticals Inc., Boca Raton, FL, USA) was added. After 1 h, 200 μl of media was removed and replaced with 200 μl of rabbit complement from 3- to 4-week-old rabbits (Pel-Freeze, Brown Deer, MI, USA). Rabbit complement from 3- to 4-week-old rabbits was used as a source of complement because it does not contain xenoreactive antibodies to porcine cells14. Cells were incubated with complement for an additional 30 min. For cells in group 2 (media alone, i.e., no human serum or complement was added), 0.5 ml of fresh supplemented Ham’s F10 media with 10% NPS was added, and cells were cultured for 1.5 h. For cells in group 3 (human serum alone, i.e., no complement was added), 0.5 ml of heat-inactivated pooled human AB serum was added, and cells were cultured for 1.5 h. For cells in group 4 (complement alone, i.e., no human serum added), 0.5 ml of fresh supplemented Ham’s F10 media with 10% NPS was added. After 1 h, 200 μl of media was removed and replaced with 200 μl of rabbit complement. Cells were incubated with complement for an additional 30 min. At the end of the cytotoxicity assay, media were removed from all the groups, and cell survival was analyzed using MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assays (R&D Systems, Minneapolis, MN, USA) as previously described12. As a control for the MTT assay, a group of cells were lysed by incubating the cells for 1.5 h in 1% (v/v) Triton X-100.
RNA Isolation and qRT-PCR
NPSCs or NPIs (n = 3) were dissolved in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and RNA was extracted according to the manufacturer’s protocol. The RNA was DNAse treated (Invitrogen), and cDNA was synthesized from 100 ng of RNA using the SuperScript VILO™ kit (Invitrogen). Real-time PCR for complement factors was performed using TaqMan Gene expression assay from Applied Biosystems (Thermo Fisher Scientific) [clusterin, assay ID: Ss03391129_m1; MCP, assay ID: Ss03392461_u1; DAF, assay ID: Ss03392383_m1; CD59, assay ID: Ss03394252_m1; and glyceraldehyde 3- phosphate dehydrogenase (GAPDH), assay ID: Ss03375629_ u1]. The real-time PCR was conducted in triplicate for the three biological samples. Nontemplate controls contained water instead of cDNA. The expression level of the gene of interest was evaluated using the comparative Ct method. Threshold values (Ct) for the gene of interest and the housekeeping gene GAPDH were determined using QuantStudio 12K Flex software (Applied Biosystems Technology). Ct values for the gene of interest were normalized to Ct values for GAPDH in each sample, and then the fold change for the gene of interest was calculated relative to the level in the reference sample (NPSCs).
Protein Isolation and Western Blot Analysis
Total cellular proteins (n ≥3) were isolated from NPSCs and NPIs by lysis with radioimmunoprecipitation assay (RIPA) buffer as described previously15. Protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Cellular lysates were separated by electrophoresis in a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel under nonreducing [membrane cofactor protein (MCP), decay-accelerating factor (DAF), CD5916–18] or reducing (clusterin19) conditions. The membranes were incubated with the following primary antibodies: sheep anti-rat clusterin (1:250 dilution; Cedarlane Laboratories Limited, Hornby, Ontario, Canada), mouse anti-pig MCP (10 μg/ml; MS from Dr. B. Paul Morgan, Cardiff University, School of Medicine, Cardiff, UK), mouse anti-pig DAF (10 μg/ml; PD4 from Dr. Morgan), and mouse anti-pig CD59 (10 μg/ml; Mel3 from Dr. Morgan). To determine equal protein loading, the same membranes were stripped (0.2 M NaOH for 5 min at 66°C) and reprobed for mouse anti-actin (1:2,000 dilution; Chemicon International, Temecula, CA, USA).
Transplantation and Cell Survival Analysis
For transplantation, the number of NPSCs or NPIs was calculated as described previously13, based on 7.1 pg of DNA/cell, using the Quant-iT PicoGreen dsDNA quantification assay (Invitrogen). Aliquots consisting of 11 × 106 cells were transplanted underneath the left renal subcapsular space of isofluorane-anesthetized Lewis rats. Graft (NPSCs or NPIs)-bearing kidneys were collected at days 1, 3, 4, 6, 9, 13, 20, and 40 posttransplantation (n ≥3), immersed in Z-Fix (Anatech Ltd., Battle Creek, MI, USA), and embedded in paraffin. Tissue sections were immunostained for GATA4 or Wilms’ tumor 1 (WT1; to identify NPSCs) and insulin (to identify NPIs). Primary antibodies included mouse monoclonal anti-human GATA4 (1:10 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-human WT1 (1:10 dilution; Dako, Carpinteria, CA, USA), and guinea pig polyclonal anti-swine insulin (1:1,000 dilution; Dako). All sections were counterstained with hematoxylin. Negative controls put through the same procedure without primary antibody lacked a positive reaction. Graft-bearing kidneys were also collected to perform PCR for cell survival (n ≥3) (Fig. 4 and Supplementary Fig. 2). Primers specific to the porcine mitochondrial gene encoding cytochrome oxidase II (COII) were used to confirm the presence of porcine DNA semiquantitatively, as described previously11. GAPDH was used as a control (data not shown). Real-time PCR was performed using TaqMan Gene Expression assay (Applied Biosystems, Thermo Fisher Scientific). Probes (Applied Biosystems, Thermo Fisher Scientific) detecting genomic DNA (gDNA) specific to pig and rat were used (pig actin, assay ID: Ss03376081_ u1; rat GAPDH, assay ID: Rn01775763_g1). The ribosomal 18S (assay ID: Hs99999901_s1) detecting both pig and rat gDNA was used as an endogenous control. Real-time PCR was conducted in triplicate for the three biological samples. Nontemplate controls contained water instead of gDNA. The amount of porcine and rat gDNA in NPSC and NPI grafts was determined using the comparative Ct method. Threshold values (Ct) for the porcine actin, rat GAPDH, and ribosomal 18S were determined using QuantStudio™ 12K Flex software (Applied Biosystems Technology). Ct values for the porcine actin and rat GAPDH were normalized to Ct values for 18S in each sample. To determine the amount of pig and rat gDNA in the grafts, the fold change for porcine actin and rat GAPDH was calculated relative to the level in the reference sample (day 4 NPSC graft for NPSC survival and day 4 NPI graft for NPI survival). To validate the specificity of probes, gDNA isolated from NPSCs, NPIs (collected prior to transplant), and rat kidney was used. NPSC and NPI gDNA showed no cross-reactivity with rat GAPDH probe and vice versa.
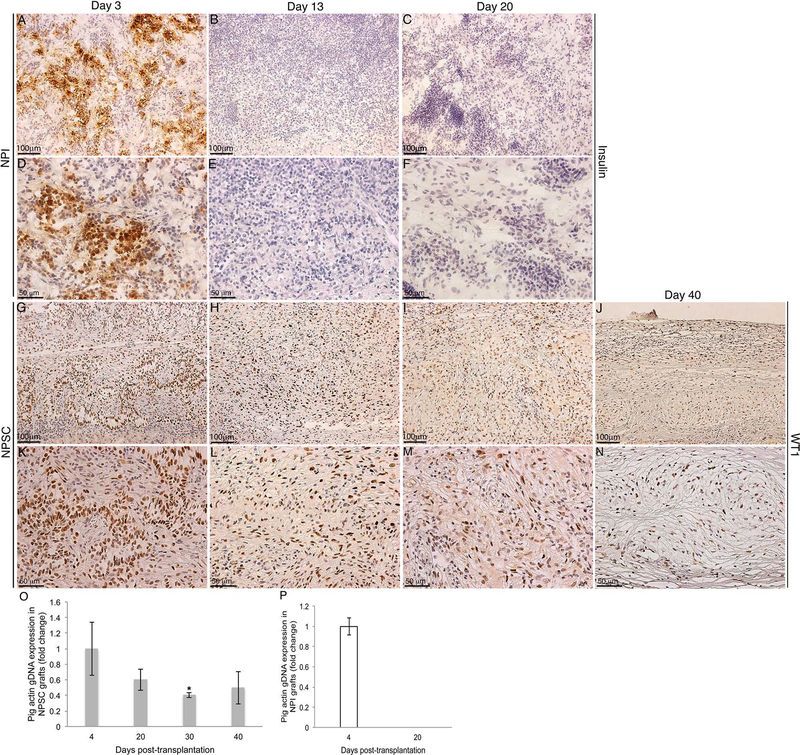
Figure 4.
In vivo NPI and NPSC xenograft survival. Grafts were collected at days 3 (A, D, G, and K), 13 (B, E, H, and L), 20 (C, F, I, and M), and 40 (J and N) posttransplantation and immunostained for NPI marker, insulin (brown color, A–F), or NPSC marker, WT1 (brown color, G–N). (D–F) and (K–N) Higher magnification images of (A–C) and (G–J), respectively. Sections were counterstained with hematoxylin (blue color). (O, P) DNA isolated from NPSC grafts (gray bars: collected at days 4, 20, 30, and 40) or NPI grafts (white bar: collected at days 4 and 20) was used for qPCR. *Significant difference in pig gDNA expression at day 30 compared to day 4 NPSC grafts (set to 1) as determined by ANOVA followed by Tukey’s post hoc test (p ≤ 0.05).
Serum Antibody (IgM and IgG) Production
Antibodies (IgM and IgG) generated specifically against the transplanted NPSCs or NPIs were measured by performing enzyme-linked immunosorbent assays (ELISAs) on serum collected from rats at 0, 1, 3, 6, 13, and 20 days posttransplantation (n ≥3). Serum was stored at −80°C until analyzed. Freshly isolated NPSCs or NPIs were fixed with 4% (w/v) paraformaldehyde (PFA) for 10 min and used as antigen for indirect ELISA. The cells were incubated with block solution [50 mM Tris (Thermo Fisher Scientific), 0.14 M NaCl (Thermo Fisher Scientific), and 1% bovine serum albumin (BSA; Sigma-Aldrich)] to prevent nonspecific binding. The cells were washed with phosphate-buffered saline (PBS) containing 0.05% (v/v) Tween 20 (Thermo Fisher Scientific), and then incubated with rat sera (1:100 dilution) followed by incubation with horseradish peroxidase (HRP)-labeled goat anti-rat IgG (1:75,000; Bethyl Laboratories Inc., Montgomery, TX, USA) or IgM (1:100,000; Bethyl laboratories) antibody. To develop the reaction, TMB substrate was added, and after 15 min the reaction was terminated via addition of 2 M of H2SO4. OD values were read at 450 nm.
Antibody and Complement Factor Deposition
Tissue sections from paraffin-embedded graft (NPSCs or NPIs)-bearing kidneys collected at days 1, 3, 4, 6, 9, 13, and 20 posttransplantation (n ≥3) were incubated with HRP-labeled mouse anti-rat IgG-Fc fragment (1:250 dilution; Bethyl Laboratories) or HRP-labeled mouse anti-rat IgM (1:400 dilution; Bethyl Laboratories) to detect antibody deposition within the grafts. To determine activation of the complement cascade, tissue sections were immunostained for complement factor 3 (C3; goat polyclonal anti-C3; 1:2,000 dilution; EMD Millipore, Billerica, MA, USA) and membrane attack complex (MAC; rabbit polyclonal anti-C5b-9, 1:200 dilution, EMD Millipore). Negative controls that were put through the same procedure without primary antibody lacked a positive reaction. To determine the number of IgM-, IgG-, C3-, or MACpositive cells in the NPSC or NPI grafts, the number of positive cells per tissue section was counted by two independent individuals blinded to the identity of the samples. Arrows in Figures 6–9 point to positive cells.
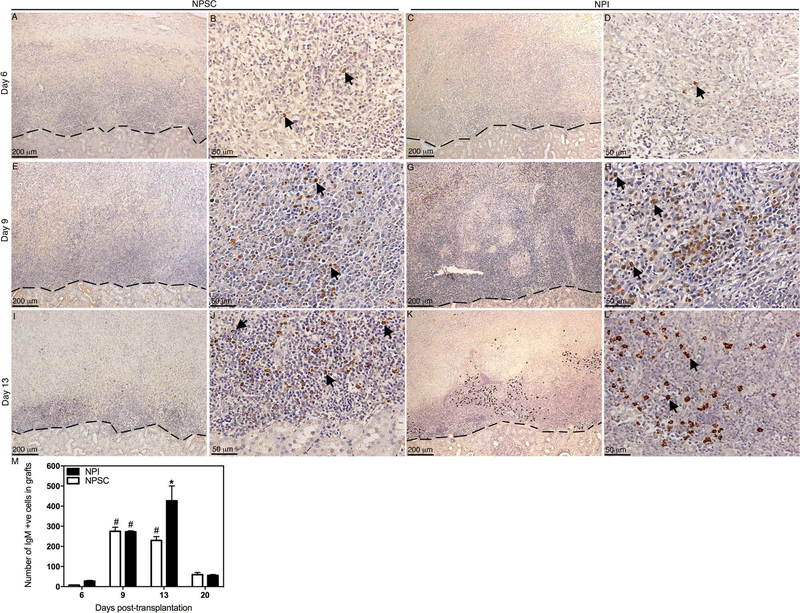
Figure 6.
Deposition and quantification of IgM in NPSC or NPI grafts. Tissue sections were collected at days 6 (A–D), 9 (E–H), and 13 (I–L) posttransplantation. NPSC (A, B, E, F, I, and J) or NPI (C, D, G, H, K, and L) grafts were immunostained for IgM (brown color, A–L). (B, D, F, H, J, and L) Higher magnification images of (A, C, E, G, I, and K), respectively. Arrows indicate positive cells. A dashed line separates the graft (above the line) from the kidney (below the line). The sections were counterstained with hematoxylin (blue color). The number of IgM-positive cells in NPI (black bar) or NPSC (white bar) grafts was counted and graphed (M). No positive cells were detected before day 6. Data shown are the mean ± SD for at least three different experiments per time point. The symbols denote a significant difference of the means as determined by ANOVA followed by Tukey’s post hoc test (#p ≤ 0.05 vs. days 6 and 20 NPSC and days 6, 13, and 20 NPI; *p ≤ 0.05 vs. days 6, 9, 13, and 20 NPSC and days 6, 9, and 20 NPI). +ve, positive cells.
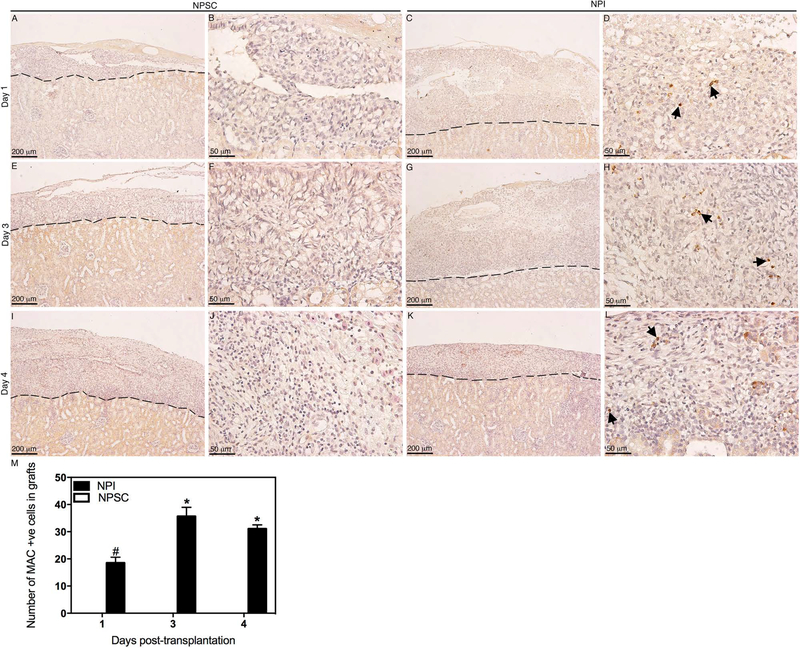
Figure 9.
Deposition and quantification of MAC in NPSC or NPI grafts. Tissue sections were collected at days 1 (A–D), 3 (E–H), and 4 (I–L) posttransplantation. NPSC (A, B, E, F, I, and J) or NPI (C, D, G, H, K, and L) grafts were immunostained for MAC (brown color, A–L). (B, D, F, H, J, and L) Higher magnification images of (A, C, E, G, I, and K), respectively. Arrows indicate positive cells. A dashed line separates the graft (above the line) from the kidney (below the line). The sections were counterstained with hematoxylin (blue color). The number of MAC-positive cells in NPI (black bar) or NPSC (white bar) grafts was counted and graphed (M). No positive cells were detected after day 4. Data shown are the mean ± SD for at least three different experiments per time point. The symbols denote a significant difference of the means as determined by ANOVA followed by Tukey’s post hoc test (#p ≤ 0.05 vs. days 1, 3, and 4 NPSC and days 3 and 4 NPI; *p ≤ 0.05 vs. days 1, 3, and 4 NPSC and day 1 NPI). +ve, positive cells.
Statistical Analysis
Data are expressed as means ± standard deviation (SD) or standard error of the mean (SEM) of n independent experiments. For the MTT assay, the final SD was calculated as % cell viability × average SD (A570)/average A570 value for each treatment. Significant differences between two independent groups were calculated by unpaired Student’s t-test using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance of difference between multiple comparisons was calculated by one-way or two-way analysis of variance (ANOVA) followed by Fisher’s PLSD or Tukey’s post hoc tests using GraphPad Prism software. A value of p ≤ 0.05 was considered significant.
RESULTS
Susceptibility of NPSCs and NPIs to Complement-Mediated Cell Lysis
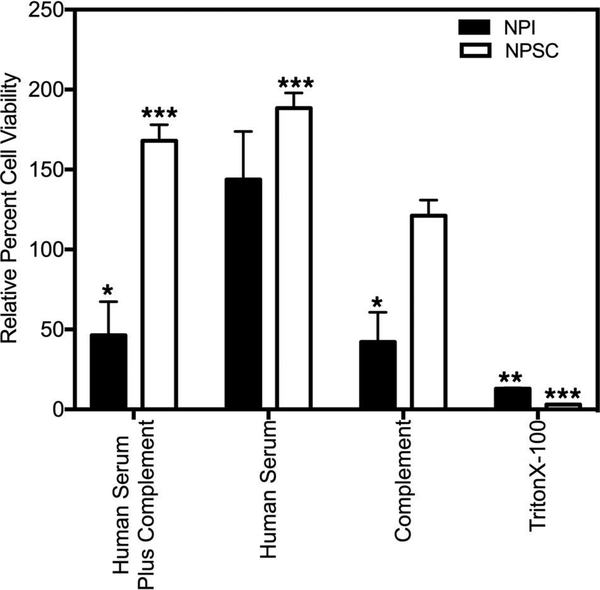
The susceptibility of NPSCs and NPIs to complementmediated cell lysis was examined by exposing the cells (NPSCs or NPIs) to 50% heat-inactivated human AB serum followed by the addition of 20% rabbit complement. Cell survival was then assessed by MTT assay (Fig. 1). When NPSCs were incubated with human serum plus complement, there was a significant increase in NPSC survival (168 ± 10% survival) (Fig. 1) compared to the control (cells cultured in media alone). In contrast, there was a significant decrease in the number of viable NPIs (46.4 ± 21% survival) (Fig. 1) after exposure to human serum plus complement. Interestingly, a significant decrease in the number of viable NPIs (42.3 ± 18.5% survival) (Fig. 1) was also observed after exposure to complement alone, while there was a significant increase in the number of viable NPSCs (188.5 ± 9.4%) (Fig. 1) after exposure to human serum alone.
Figure 1.
Susceptibility of NPSCs and NPIs to human antibody/ complement-mediated lysis. NPSCs and NPIs were exposed to human serum plus complement, human serum, complement, or Triton X-100 (as described in the Materials and Methods section), and viability was assessed by the MTT assay. Viability of cells exposed to media alone was normalized to 100%, and the relative percent cell viability (NPIs: black bar, NPSCs: white bar) for each condition was graphed. Viability is presented as the mean ± SEM for at least three different experiments performed in duplicate. Asterisks denote a significant difference from the control values. The significance was determined by ANOVA and Fisher’s PLSD (*p ≤ 0.05; **p ≤ 0.004; ***p ≤ 0.0001).
Expression of Complement Inhibitors by NPSCs and NPIs
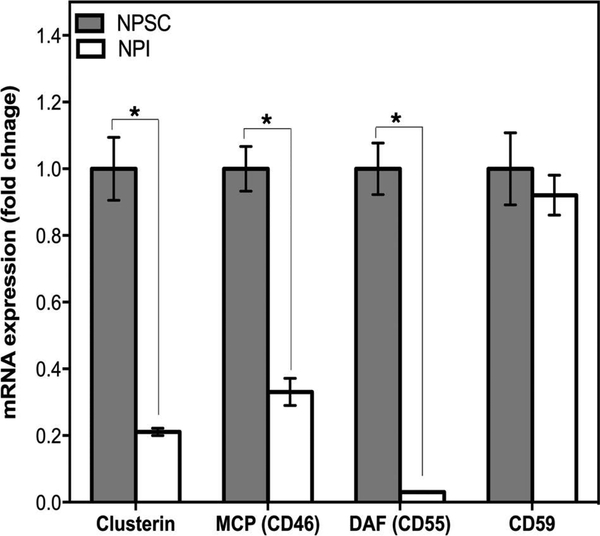
Since NPSCs were not susceptible to complement-mediated cell lysis, while NPIs were killed, the mRNA and protein expression of several complement inhibitors (clusterin, MCP, DAF, and CD59) by NPSCs and NPIs were examined by qRT-PCR and Western blot analysis. The mRNA for clusterin, MCP, DAF, and CD59 was detected in both NPSCs and NPIs (Fig. 2). NPSCs expressed significantly higher levels of clusterin, MCP, and DAF mRNA compared to NPIs (Fig. 2), while the mRNA level of CD59 was similar between NPSCs and NPIs.
Figure 2.
Quantification of clusterin, MCP, DAF, and CD59 mRNA expression in NPSCs and NPIs. qRT-PCR was performed using mRNA isolated from NPSCs (gray bars) and NPIs (white bars) for clusterin, MCP, DAF, and CD59. Real-time PCR was conducted in triplicate on three biological samples per cell type, and data shown are the mean ±SD. *Significant difference in NPI mRNA expression compared to NPSCs as determined by ANOVA followed by Tukey’s post hoc test (p ≤ 0.0001).
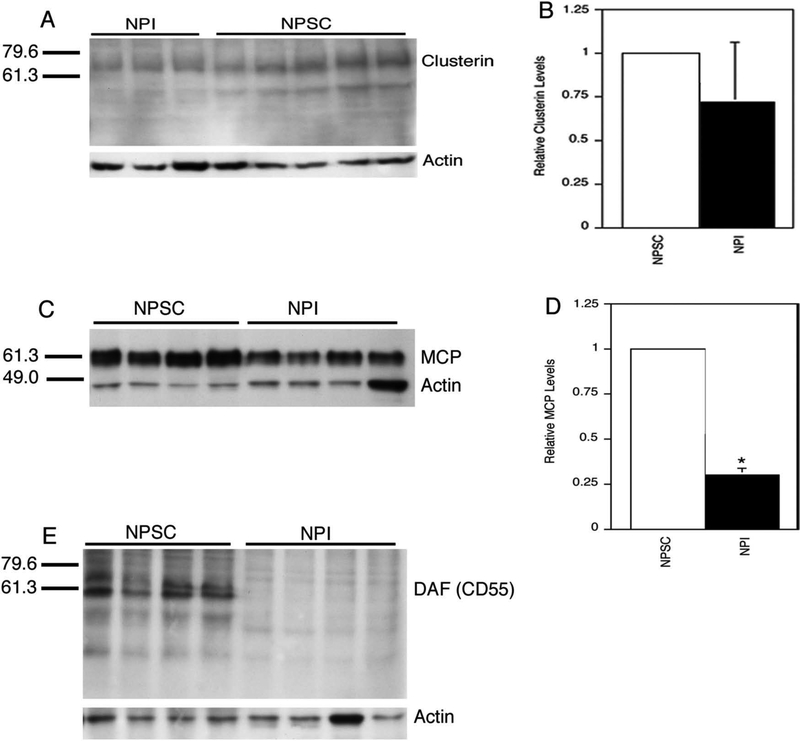
Next complement inhibitor protein levels in NPSCs were compared with the levels in NPIs. Consistent with the RT-PCR data, clusterin and MCP proteins were detected in extracts from NPSCs and NPIs (Fig. 3A and C). Clusterin is synthesized as a 64-kDa precursor protein that undergoes multiple protein modifications resulting in a broad band around 64–73 kDa. The modified protein is then cleaved into the 34- and 47-kDa chains that are held together by disulfide bonds to make up the mature protein19. Clusterin from NPSC and NPI extracts was present at low levels and consisted of both the larger precursor proteins and the smaller cleavage products. The upper bands were more intense, suggesting that clusterin in NPSCs and NPIs exists primarily as an unprocessed precursor. Comparison of the levels of clusterin precursor protein, after the band intensities were normalized to actin levels, uncovered no significant differences between NPSCs and NPIs (Fig. 3B). Comparison of the lower band levels also indicated no difference in protein levels (data not shown). A doublet of ~60 kDa was observed for MCP in NPSCs and NPIs (Fig. 3C). Interestingly, there was a significant increase (3.4-fold) in the levels of MCP in NPSCs compared to NPIs (Fig. 3D). DAF was detected as a doublet between 55 and 65 kDa in NPSCs, while NPIs were negative for DAF (Fig. 3E). The bands detected for clusterin, MCP, and DAF are all consistent with previous reports in other cell types16,18,19. CD59 has been described as a broad band of 16–22 kDa after Western blot analysis of pig erythrocytes17. This band was not observed in NPSCs but was detected in testicular extracts (data not shown). Similar to NPSCs, NPIs were also negative for CD59 protein (data not shown).
Figure 3.
Complement inhibitor production by NPSCs and NPIs. Western blot analysis for clusterin (A), MCP (C), and DAF (E) was performed using proteins extracted from NPSCs or NPIs. Each lane contains proteins extracted from separate NPSC or NPI isolations. Membranes were also immunoblotted with an anti-actin antibody. Molecular markers in kDa are shown on the left. Complement inhibitor protein levels, clusterin (B) or MCP (D), were normalized to actin levels, and then NPSC values were set to 1. NPI levels relative to NPSCs were graphed. *Significant difference from the NPSC value as determined by Student’s t-test (p < 0.05).
NPSC and NPI Survival After Xenotransplantation Into Lewis Rats
Before comparing the humoral immune response generated against NPSCs and NPIs in vivo, their survival after xenotransplantation in Lewis rats was examined. The cell survival was analyzed by immunostaining the grafted tissue for insulin, or WT1/GATA4 markers for NPIs and NPSCs, respectively (Fig. 4 and Supplementary Fig. 1; supplementary material available at http://www.ttuhsc.edu/som/cbb/documents/Wright_et_al_Supplemental_Figs.pdf). Many insulin-positive cells were detected in the NPI grafts from days 1 to 4 posttransplantation (Table 1, Fig. 4A and D, and Supplementary Fig. 1A and D). By day 6, NPI survival decreased drastically as only one out of nine grafts (11%) contained insulinpositive cells (Table 1). From day 9 onward, no insulinpositive cells were detected in the NPI grafts (Table 1, Fig. 4B, C, E, and F, and Supplementary Fig. 1B, C, E, and F). In contrast, large numbers of WT1-or GATA4positive NPSCs were detected throughout the study (i.e., day 40 posttransplantation) (Table 1, Fig. 4G–N, and Supplementary Fig. 1G–Q). The survival of NPSCs and rejection of the NPIs were further confirmed by PCR using porcine-specific primers (actin and COII) (Fig. 4O and P and Supplementary Fig. 2A). Using qPCR, porcine gDNA was detected in NPSC grafts collected at days 4, 20, 30, and 40 posttransplantation (Fig. 4O). In contrast, porcine gDNA was only detected in NPI grafts collected at day 4 posttransplantation; NPI grafts collected at day 20 posttransplantation were negative for porcine gDNA (Fig. 4P). This is consistent with the immunostaining data and semiquantitative PCR data using porcine-specific COII (Supplementary Fig. 2A).
Table 1.
Survival of NPSCs and NPIs Transplanted as Xenografts Into Lewis Rats
| Days Post-transplantation | NPSCs (WT1/ GATA4) | NPIs (Insulin) |
|---|---|---|
| 1 | 100% (6/6) | 100% (6/6) |
| 3 | 100% (6/6) | 100% (6/6) |
| 4 | 100% (9/9) | 100% (3/3) |
| 6 | 100% (8/8) | 11% (1/9) |
| 9 | 100% (3/3) | 0% (0/3) |
| 13 | 100% (4/4) | 0% (0/3) |
| 20 | 100% (6/6) | 0% (0/3) |
| 40 | 100% (4/4) | 0% (0/3) |
At least three independent experiments were performed with an n = 3 to 9 for each time point. The percent survival was determined by dividing the number of grafts with insulin or WT1/GATA4-positive cells by the total number of grafts at each time point.
In Vivo Activation of the Humoral Immune Response Against the Xenografted Tissue
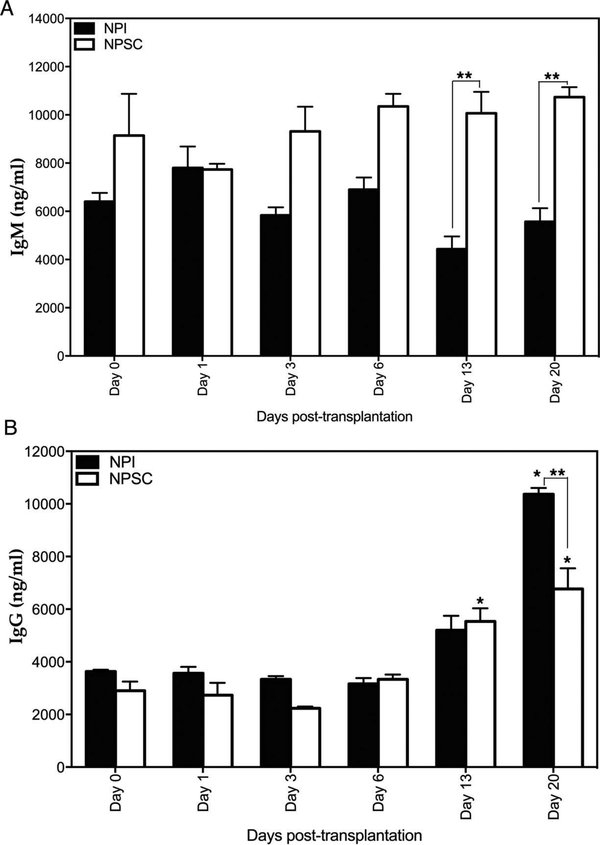
To examine the in vivo activation of the humoral immune response against NPIs and NPSCs, antibody (IgM and IgG) production and deposition, and complement factor (C3 and MAC) deposition were analyzed. Serum antibody (IgM and IgG) production specific to the grafted tissue was analyzed by ELISA. As a discordant xenograft, preformed anti-pig antibodies are present in the circulation of rats prior to transplantation. The average anti-pig IgM and IgG levels in Lewis rats prior to transplantation were 7,248 and 3,750 ng/ml, respectively (data not shown). In comparison to pretransplant IgM levels, no significant change in IgM production against NPSCs or NPIs was observed at any time point (Fig. 5A). However, serum IgM levels tended to increase in NPSC recipients and decrease in NPI recipients such that IgM levels were significantly higher in NPSC recipients compared to NPI recipients at days 13 and 20 posttransplantation (Fig. 5A). In order to activate antibody-dependent complement-mediated cell lysis (classical pathway), antibodies need to be deposited on the targeted tissue, that is, transplanted cells. Analysis of the grafts revealed that IgM deposition was first detected at day 6 posttransplantation in both NPSC and NPI grafts (Fig. 6A–D, and M). Thereafter, a significant increase in IgM deposition was detected at days 9 and 13 in both NPSC and NPI grafts compared to day 6 posttransplantation (Fig. 6E–M). Furthermore, at day 13, a significantly higher number of IgM-positive cells was detected in NPI grafts compared to NPSC grafts (Fig. 6M).
Figure 5.
Serum levels of anti-NPI or anti-NPSC IgM and IgG. The production of IgM (A) and IgG (B) against transplanted NPIs (black bar) or NPSCs (white bar) was graphed. Data shown are the mean ± SD for at least three different experiments per time point. *Significant difference from day 0 posttransplantation. **Significant difference in anti-NPI antibody (IgM and IgG) levels compared to anti-NPSC at corresponding days as determined by ANOVA followed by Tukey’s post hoc test (p ≤ 0.05).
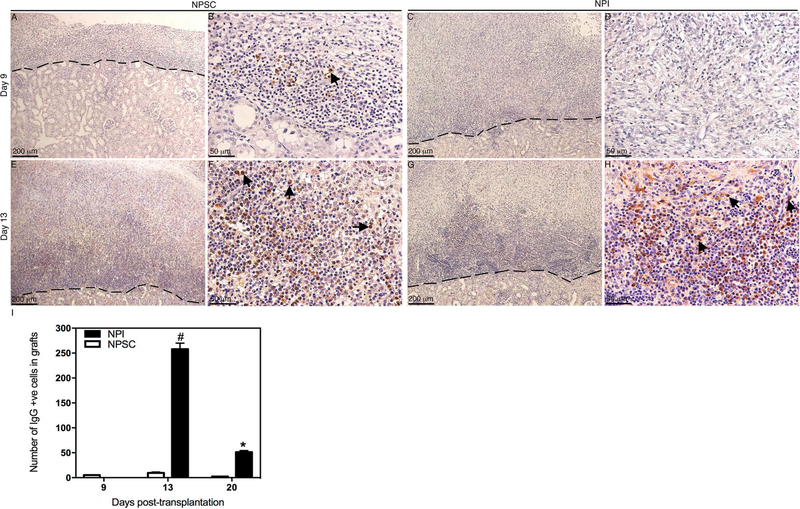
A late IgG response was mounted against the transplanted cells as evident by a significant increase in IgG production at days 13 and 20 in the case of NPSCs and day 20 posttransplantation in the case of NPIs, compared to basal levels (Fig. 5B). Additionally, anti-NPSC IgG levels were significantly lower than anti-NPI IgG at day 20 posttransplantation (Fig. 5B). The IgG deposition was first detected at day 9 posttransplantation in NPSC grafts (Fig. 7A, B, and I) and at day 13 in NPI grafts (Fig. 7G–I). Although IgG deposition was detected earlier in NPSC grafts, there were significantly fewer IgG-positive cells at days 13 and 20 in NPSC when compared to NPI grafts (Fig. 7I).
Figure 7.
Deposition and quantification of IgG in NPSC or NPI grafts. Tissue sections were collected at days 9 (A–D) and 13 (E–H) posttransplantation. NPSC (A, B, E, and F) or NPI (C, D, G, and H) grafts were immunostained for IgG (brown color, A–H). (B, D, F, and H) Higher magnification images of (A, C, E, and G), respectively. Arrows indicate positive cells. A dashed line separates the graft (above the line) from the kidney (below the line). The sections were counterstained with hematoxylin (blue color). The number of IgGpositive cells in NPI (black bar) or NPSC (white bar) grafts was counted and graphed (I). No positive cells were detected before day 9. Data shown are the mean ± SD for at least three different experiments per time point. The symbols denote a significant difference of the means as determined by ANOVA followed by Tukey’s post hoc test (#p ≤ 0.05 vs. days 9, 13, and 20 NPSC and days 9 and 20 NPI; *p ≤ 0.05 vs. days 9, 13, and 20 NPSC and days 9 and 13 NPI). +ve, positive cells.
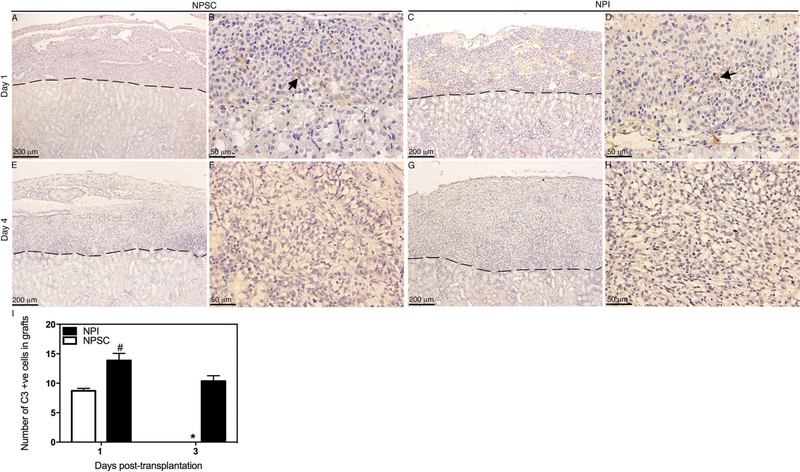
Analysis of the grafts for complement factor deposition revealed that C3 was detected at days 1 and 3 posttransplantation (Fig. 8C, D, and I) in NPI grafts, while C3 was only detected at day 1 posttransplantation in NPSC grafts (Fig. 8A, B, and I). The number of C3-positive cells in the NPI grafts was significantly higher compared to NPSC grafts (Fig. 8I). MAC deposition was also detected at early time points, that is, days 1–4 posttransplantation, in NPI grafts (Fig. 9C, D, G, H, and K–M), while NPSC grafts were negative for MAC deposition throughout the study (Fig. 9A, B, E, F, I, J, and M). Additionally, MAC deposition in NPI grafts was significantly higher at days 3 and 4 compared to day 1 posttransplantation (Fig. 9M).
Figure 8.
Deposition and quantification of C3 in NPSC or NPI grafts. Tissue sections were collected at days 1 (A–D) and 4 (E–H) posttransplantation. NPSC (A, B, E, and F) or NPI (C, D, G, and H) grafts were immunostained for C3 (brown color, A–H). (B, D, F, and H) Higher magnification images of (A, C, E, and G), respectively. Arrows indicate positive cells. A dashed line separates the graft (above the line) from the kidney (below the line). The sections were counterstained with hematoxylin (blue color). The number of C3-positive cells in NPI (black bar) or NPSC (white bar) grafts was counted and graphed (I). No positive cells were detected after day 3. Data shown are the mean ± SD for at least three different experiments per time point. The symbols denote a significant difference of the means as determined by ANOVA followed by Tukey’s post hoc test (#p ≤ 0.05 vs. days 1 and 3 NPSC and day 3 of NPI; *p ≤ 0.05 vs. day 1 NPSC and days 1 and 3 NPI). +ve, positive cells.
DISCUSSION
The focus of this study was to compare the humoral immune response against NPSCs with the response to rejecting non-immune-privileged NPIs in order to further our understanding of xenograft protection. Here we confirmed that NPSCs are resistant to complementmediated cell lysis. In fact, after exposure to human serum alone or human serum plus complement, the number of NPSCs increased. This would imply that exposure to 50% serum provides growth factors that allow these immature neonatal cells to proliferate. In contrast, NPIs were killed after exposure to human serum plus complement or complement alone, suggesting that both the alternative and classical pathways of complement-mediated cell lysis play an important role in killing NPIs, whereas NPSCs are actively inhibiting these pathways. The killing of NPIs is consistent with our in vivo data and with previously published data using adult pig islets, where it was shown that both the alternative and classical pathways play an important role in destroying in vitro cultured adult pig islets20.
Previously it was shown that despite deposition of preformed human antibodies (IgM and IgG) and complement factors (C4 and C3), the membrane attack complex (MAC/C5b-9) was not formed on NPSCs, and these cells were still resistant to complement-mediated cell lysis12. This suggests that NPSCs inhibit the complement cascade prior to the assembly of the MAC. MCP, DAF, clusterin, and CD59 are complement regulatory proteins that inhibit the complement cascade21. MCP and DAF accelerate the degradation of C3 and C5 convertases as well as limit the production of proinflammatory molecules, while clusterin and CD59 inhibit the assembly of the MAC21. The mRNA for these inhibitors has been detected in mouse and porcine SCs15,22,23 and was confirmed by this study. Here we report for the first time that NPSCs express significantly higher levels of clusterin, MCP, and DAF mRNA as well as significantly higher levels of MCP and DAF proteins compared to NPIs. Degradation of C3 and C5 convertases by MCP and DAF is critical as it prevents the generation of C3a and C5a anaphylatoxins. Binding of these anaphylatoxins to their corresponding receptors promotes maturation of antigen-presenting cells, CD4 T-cell proliferation, and decreases apoptosis of CD4 T cells24,25. Furthermore, they decrease regulatory T-cell generation and suppressive function25. This emphasizes the significance of increased expression of MCP and DAF by NPSCs.
Surprisingly, while clusterin mRNA was significantly higher in NPSCs, the protein levels were not different when compared to the levels produced by NPIs. Lee et al. demonstrated that porcine SCs express clusterin with low levels detected in the neonate and high levels from 4 weeks of age to adulthood26. However, given the similarity in expression levels by NPSCs and NPIs, this suggests clusterin alone is not responsible for NPSC survival of complement cytolysis. It is more likely that clusterin acts in combination with the other complement regulatory proteins. In vitro data indicated that NPSCs inhibit complement-mediated cell lysis prior to MAC formation. Therefore, NPSCs might use clusterin as a backup mechanism if they fail to inhibit the complement cascade at earlier steps.
Porcine CD59 effectively inhibits human complement27,28. In this study, we found that both NPSCs and NPIs express similar amounts of CD59 mRNA. However, CD59 protein was not detected in NPSCs or NPIs. Previously, flow cytometry demonstrated that fetal and adult pig islets express CD5929. In our study, NPIs were negative for CD59. This discrepancy could be explained by differences in the source of islets or the technique (flow cytometry vs. Western blot) used to detect CD59. Transgenic pigs expressing various human complement inhibitors such as hMCP, hDAF, and hCD59 have been created. However, when islets isolated from these transgenic animals were transplanted into NHPs, despite these modifications, the survival of pig islets was variable, and immune suppression was still required for long-term survival of islets4,5,8,30–32. This further highlights the importance to continue examining the mechanism(s) of NPSC survival, which could become pivotal in overcoming the hurdles of xenotransplantation.
NPSCs most likely survive as xenografts by inhibiting both the humoral and cell-mediated immune responses. In this study, we focused on the humoral immune response. To extend our in vitro observations in the presence of a fully functional immune system, NPSCs or NPIs were transplanted as xenografts into rats without any immunosuppressive drugs. NPSCs survived throughout the study (i.e., day 40), while all but one NPI graft was completely rejected by day 6 posttransplantation.
Analysis of the serum for antibody production against xenotransplanted NPSCs or NPIs revealed that rats already contained circulating preformed IgM and IgG antibodies against the xenoantigens. Serum IgM antibody levels against transplanted NPSCs or NPIs were not significantly changed from day 0 values. However, interestingly, anti-NPSC IgM levels were significantly higher at days 13 and 20 posttransplantation when compared to anti-NPI IgM levels at the same time points. Additionally, serum IgG antibodies were significantly increased at later time points in NPI (day 20)- and NPSC (days 13 and 20)-transplanted animals when compared to day 0 levels. Moreover, anti-NPSC IgG levels were significantly lower at day 20 posttransplantation when compared to anti-NPI IgG levels. In NPI grafts, complement deposition at early time points (days 1–4) with no antibody deposition suggests that the alternative rather than classical pathway of complement-mediated cell lysis is involved in rejection of islet grafts. Antibody production and deposition at later time points could be involved in clearing cell debris. No deposition of complement factors at early time points in NPSC grafts suggests that NPSCs are actively inhibiting the alternative pathway of complement-mediated cell lysis. At later time points, antibody production and deposition without complement factor deposition suggest that NPSCs are also inhibiting the classical pathway of complement-mediated cell lysis. Overall, NPSC inhibition of the complement cascade would decrease anaphylatoxin production. Besides preventing complement-mediated lysis, this could slow migration of immune cells to the graft site. Interestingly, the recipient cell infiltrate was significantly lower in NPSC compared to NPI grafts at day 20 posttransplantation (demonstrated by qPCR for recipient gDNA) (Supplementary Fig. 2B and C). Additionally, complement inhibition decreases B- and T-cell activation and increases the suppressive functions of regulatory T cells, resulting in increased graft survival24,25.
In conclusion, our data suggest that NPSCs survive the humoral immune response by actively inhibiting both the alternative and classical pathways of complementmediated cell lysis, while NPI rejection is initiated by the alternative pathway of complement-mediated cell lysis. Inhibition of the alternative pathway of complement-mediated cell lysis is not emphasized enough in xenotransplantation. Factor D is the rate-limiting step, and properdin (Factor P) stabilizes the C3 convertase (C3bBb) in the alternative pathway. BCX-1470 (synthetic inhibitor of Factor D), anti-human Factor D, and anti-properdin monoclonal antibodies have been effective in inhibiting the alternative pathway in vivo33–35. Studies examining the beneficial effect of combination therapy, that is, transplanting islets from transgenic pigs expressing complement inhibitors along with BCX-1470, anti-human Factor D, and/or anti-human Factor P, need to be carried out to further highlight the importance of inhibiting the alternative pathway of complement-m ediated cell lysis in xenotransplantation.
NPSC survival could be attributed to their significant expression of complement inhibitors, MCP and DAF. It has been suggested that complement regulatory factors are “homologously restricted” and therefore not been effective in inhibiting complement from other species28. However, porcine complement inhibitors MCP, DAF, and CD59 can effectively protect against human complementmediated lysis28. Therefore, it is more likely that porcine tissues, such as NPIs, are susceptible to human complement due to their low expression levels of the complement regulatory proteins. Future studies, quantifying the levels of MCP and DAF in NPSCs, might tell us the exact levels of these complement inhibitors required to overcome the variability in transgenic (hMCP, hDAF, and hCD59) islet cell survival.
Supplementary Material
ACKNOWLEDGMENTS:
This work was supported in part by the National Institutes of Health grant AI109398 from the National Institute of Allergy and Infectious Diseases, the Helen Jones Foundation, and the South Plains Foundation. We would like to thank Dr. B. Paul Morgan (Cardiff University, School of Medicine, Cardiff, UK) for providing MCP, DAF, and CD59 antibodies; the TTUHSC Clinical Research Institute Staff for statistical analysis; and Stanley Harris (Texas Tech University Research and Experimental Farm, New Deal) for technical assistance. The authors declare no conflicts of interest.
REFERENCES
- 1.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–3. [DOI] [PubMed] [Google Scholar]
- 2.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–6. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54(7):2060–9. [DOI] [PubMed] [Google Scholar]
- 4.Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, Bertera S, He J, Phelps C, Ayares D, Cooper DK, Trucco M. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawthorne WJ, Salvaris EJ, Phillips P, Hawkes J, Liuwantara D, Burns H, Barlow H, Stewart AB, Peirce SB, Hu M, Lew AM, Robson SC, Nottle MB, D’Apice AJ, O’Connell PJ, Cowan PJ. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am J Transplant. 2014;14(6):1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komoda H, Miyagawa S, Omori T, Takahagi Y, Murakami H, Shigehisa T, Ito T, Matsuda H, Shirakura R. Survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase-III (GnT-III) in cynomolgus monkeys. Xenotransplantation 2005;12(3):209–16. [DOI] [PubMed] [Google Scholar]
- 7.Rayat GR, Rajotte RV, Ao Z, Korbutt GS. Microencapsulation of neonatal porcine islets: Protection from human antibody/complement-mediated cytolysis in vitro and longterm reversal of diabetes in nude mice. Transplantation 2000;69(6):1084–90. [DOI] [PubMed] [Google Scholar]
- 8.Thompson P, Badell IR, Lowe M, Cano J, Song M, Leopardi F, Avila J, Ruhil R, Strobert E, Korbutt G, Rayat G, Rajotte R, Iwakoshi N, Larsen CP, Kirk AD. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11(12):2593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Lorf T, Sablinski T, Gianello P, Bailin M, Monroy R, Kozlowski T, Awwad M, Cooper DK, Sachs DH. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1–3Galbeta1–4betaGlc-X immunoaffinity column. Transplantation 1998;65(2):172–9. [DOI] [PubMed] [Google Scholar]
- 10.Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: Making allogeneic and xenogeneic transplantation feasible. Reproduction 2010;139(3):495–504. [DOI] [PubMed] [Google Scholar]
- 11.Dufour JM, Rajotte RV, Seeberger K, Kin T, Korbutt GS. Long-term survival of neonatal porcine Sertoli cells in nonimmunosuppressed rats. Xenotransplantation 2003;10(6): 577–86. [DOI] [PubMed] [Google Scholar]
- 12.Dufour JM, Hamilton M, Rajotte RV, Korbutt GS. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol Reprod. 2005;72(5):1224–31. [DOI] [PubMed] [Google Scholar]
- 13.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97(9):2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayat GR, Rajotte RV, Elliott JF, Korbutt GS. Expression of Gal alpha(1,3)gal on neonatal porcine islet beta-cells and susceptibility to human antibody/complement lysis. Diabetes 1998;47(9):1406–11. [DOI] [PubMed] [Google Scholar]
- 15.Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, Pasham MR, Kim KH, Dufour JM. Immunoprotective properties of primary Sertoli cells in mice: Potential functional pathways that confer immune privilege. Biol Reprod. 2012;86(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez de la Lastra JM, Harris CL, Hinchliffe SJ, Holt DS, Rushmere NK, Morgan BP. Pigs express multiple forms of decay-accelerating factor (CD55), all of which contain only three short consensus repeats. J Immunol. 2000;165(5):2563–73. [DOI] [PubMed] [Google Scholar]
- 17.Hinchliffe SJ, Rushmere NK, Hanna SM, Morgan BP. Molecular cloning and functional characterization of the pig analogue of CD59: Relevance to xenotransplantation. J Immunol. 1998;160(8):3924–32. [PubMed] [Google Scholar]
- 18.van den Berg CW, Perez de la Lastra JM, Llanes D, Morgan BP. Purification and characterization of the pig analogue of human membrane cofactor protein (CD46/MCP). J Immunol.1997;158(4):1703–9. [PubMed] [Google Scholar]
- 19.Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry 1987;26(12):3297–303. [DOI] [PubMed] [Google Scholar]
- 20.Schaapherder AF, Wolvekamp MC, te Bulte MT, Bouwman E, Gooszen HG, Daha MR. Porcine islet cells of Langerhans are destroyed by human complement and not by antibodydependent cell-mediated mechanisms. Transplantation 1996;62(1):29–33. [DOI] [PubMed] [Google Scholar]
- 21.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. [DOI] [PubMed] [Google Scholar]
- 22.Lee HM, Oh BC, Lim DP, Lee DS, Lim HG, Park CS, Lee JR. Mechanism of humoral and cellular immune modulation provided by porcine sertoli cells. J Korean Med Sci. 2008;23(3):514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Z, Wang L, Xiang Y, Ruan Y, Li J, Wang X, Ichim TE, Chen S, Chen G. Resistance of neonatal porcine Sertoli cells to human xenoantibody and complement-mediated lysis is associated with low expression of alpha-Gal and high production of clusterin and CD59. Xenotransplantation 2010;17(3):215–23. [DOI] [PubMed] [Google Scholar]
- 24.Ezzelarab MB, Ayares D, Cooper DK. Transgenic expression of human CD46: Does it reduce the primate T-cell response to pig endothelial cells? Xenotransplantation 2015;22:487–9. [DOI] [PubMed] [Google Scholar]
- 25.Mathern DR, Heeger PS. Molecules great and small: The complement system. Clin J Am Soc Nephrol. 2015;10(9):1636–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HM, Oh BC, Yang JH, Cho J, Lee G, Lee DS, Hwang WS, Lee JR. Age-dependent expression of immune-privilege and proliferation-related molecules on porcine Sertoli cells. Xenotransplantation 2006;13(1):69–74. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg CW, Morgan BP. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994;152(8):4095–101. [PubMed] [Google Scholar]
- 28.Morgan BP, Berg CW, Harris CL. “Homologous restriction” in complement lysis: Roles of membrane complement regulators. Xenotransplantation 2005;12(4):258–65. [DOI] [PubMed] [Google Scholar]
- 29.Bennet W, Bjorkland A, Sundberg B, Brandhorst D, Brendel MD, Richards A, White DJ, Nilsson B, Groth CG, Korsgren O. Expression of complement regulatory proteins on islets of Langerhans: A comparison between human islets and islets isolated from normal and hDAF transgenic pigs. Transplantation 2001;72(2):312–9. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T, Harb G, Rajotte RV, Korbutt GS, Mallett AG, Arefanian H, Mok D, Rayat GR. Immune mechanisms associated with the rejection of encapsulated neonatal porcine islet xenografts. Xenotransplantation 2006; 13(6):547–59. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Harb G, Rayat GR. Prolonged survival of microencapsulated neonatal porcine islets in mice treated with a combination of anti-CD154 and anti-LFA-1 monoclonal antibodies. Transplantation 2005;80(6):821–7. [DOI] [PubMed] [Google Scholar]
- 32.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland BE, Ayares D, Lakkis FG, Cooper DK, Trucco M. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–26. [DOI] [PubMed] [Google Scholar]
- 33.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50(1):59–87. [PubMed] [Google Scholar]
- 34.Undar A, Eichstaedt HC, Clubb FJ Jr., Fung M, Lu M, Bigley JE, Vaughn WK, Fraser CD Jr. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Ann Thorac Surg. 2002;74(2):355–62; discussion 362. [DOI] [PubMed] [Google Scholar]
- 35.Gupta-Bansal R, Parent JB, Brunden KR. Inhibition of complement alternative pathway function with anti-properdin monoclonal antibodies. Mol Immunol. 2000;37(5):191–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.